Abstract
Background
Intestinal injury plays a key role in the pathogenesis of severe acute pancreatitis (SAP). In this study, we investigated the protective function of downregulated Gasdermin D (GSDMD) in intestinal damage in a mouse model of severe acute pancreatitis (SAP).
Material/Methods
Twenty-four healthy male C57BL/6 mice were randomly divided into 4 groups – the NS group, the siRNA-NS group, the SAP group, and the siRNA-SAP group – with 6 mice in each group. SAP was induced in mice by intraperitoneal injection of caerulein and lipopolysaccharide. The pathological changes of pancreatic and the intestinal mucosa and the relative gene and protein expressions in each group were compared, and the levels of GSDMD and serum IL-1β and IL-18 were evaluated after induction of the SAP model.
Results
The mice in the SAP group were in more serious condition than those in the siRNA-SAP group, with various degrees of edema and hemorrhage in the intestinal tract. Under an optical microscope, the pathological changes of pancreatic tissue such as edema, inflammatory cell infiltration, and the damage of lobular structural were gradually increased in the SAP group and the siRNA-NS group. In addition, intestinal mucosal damage and intestinal villus breakage were found in the SAP group and the siRNA-NS group, and the latter was lighter than the former. Compared with the SAP group, the level of GSDMD protein expression in the siRNA-SAP group was lower, and the serum levels of IL-1β and IL-18 were higher in the SAP group and siRNA-SAP group (P<0.05). Immunohistochemical analysis showed the occludin and ZO-1 proteins in the NS group had a strong brown linear signal, while the brown-positive signals were weaker in the siRNA-SAP group and the SAP group.
Conclusions
Downregulating GSDMD protein can reduce pancreatitis associated with pyroptosis.
Keywords: Acute Disease, Intestinal Mucosa, Pancreatitis
Background
Severe acute pancreatitis is an inflammatory disorder of the pancreas that is associated with substantial morbidity and mortality. The progression of SAP is accompanied by the damage to organs such as the heart, lungs, and kidneys, and intestinal function plays a vital role in its treatment [1].
The changes of intestinal proteins in SAP may lead to bacterial translocation and secondary infection. Intestinal function is of great significance in the treatment of severe acute pancreatitis. First of all, patients with severe acute pancreas have abdominal pain and diarrhea symptoms, which are not suitable for enteral nutrition. At the initial stage of treatment, the patient is forbidden to eat, and the recovery of intestinal function is very important for treatment. According to the guidelines, the earlier enteral nutrition is restored, the better the treatment effect [2].
A variety of studies have shown that intestinal function is of great significance in SAP. Maintaining intestinal function can improve the course of SAP. When the severity of SAP is decreased, intestinal function is also enhanced [3,4]. Studies have shown that the intestinal flora is closely related to SAP. When SAP occurs, the diversity and abundance of intestinal bacteria decrease [5], so paying attention to the state of SAP intestinal function is of great significance to the study of SAP.
GSDMD is the key to pyroptosis. Several studies have revealed that caspase activation occurs in inflammatory bowel disease, and the pyroptosis mediated by GSDMD is the main reaction after caspase activation, punching holes in the cell membrane, through which many proinflammatory factors move. What is not yet clear is the impact of GSDMD on the intestinal injury in severe acute pancreatitis, so it is meaningful to explore the mechanism of GSDMD in intestinal damage in severe acute pancreatitis. In this study, we induced an SAP mouse model by intraperitoneal injection of caerulein and lipopolysaccharide, and investigated the effect of GSDMD in gut injury by downregulating its expression through siRNA.
Material and Methods
Animal Model
Healthy male C57BL/6 mice were supplied by the Laboratory Animal Center of Qingdao University (Qingdao, China). Mice were randomly divided into 4 groups – the NS group (control group), the siRNA-NS group, the SAP group, and the siRNA-SAP group – with 6 mice in each group. All mice were acclimatized to the new condition for 2 weeks before the experiment. Animals were free to drink but were fasted for about 18 h before modeling. The induction of SAP in mice was made by intraperitoneal injection of caerulein, with a weight ratio of 50 ug/kg, once per hour for 6 consecutive hours, with lipopolysaccharide (weight ratio 10 mg/kg) given by intraperitoneal injection in the same timepoint. The siRNA-SAP group was intravenously injected in the tail with siRNA 3 times before being induced in the same way as for the SAP mice. The NS group received intraperitoneal injection of saline at the same timepoints as the SAP group. The siRNA-NS group was administered siRNA by intravenous injection in the tail, and then received the same treatment as the NS group. Mice in each group were killed at 12 h after induction of the model. Serum was isolated from blood sampled by eyeball extraction. Ileal and pancreatic tissues were fixed in 10% formaldehyde solution, and part of ileal tissue was stored at −80°C for further testing.
Histological Examination
Pancreatic and ileal tissues were stained with hematoxylin and eosin (HE) and sections were observed under a light microscope. Double-blind method was used in this experiment to evaluate the pancreatic pathology according to scoring criteria proposed by Schmidt [6]. The grades of ileum damage were according to the classification of gut injury described by Chiu [7].
Reverse Transcription Polymerase Chain Reaction and Real-time Polymerase Chain Reaction
Total RNA was isolated from the ileum using TRIzol reagent. cDNA was synthesized from 2 μg of total RNA with the TaKaRa PrimeScript RT reagent kit. Specific primers (uc.173: forward-5′ACTTTTTATTGCATGGTGTGAACT-3′ and reverse-5′-CACTTGGAAAAAAATACAAACAGG-3′) and 2× PCR reagent were used for 25–35 cycles of PCR. SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) was used for real-time PCR. The levels of uc.173 were normalized to those of actin. The relative quantification was measured by the 2−ΔΔCt method.
Western Blot Analysis of GSDMD-N
Protein was extracted from intestinal tissue and the protein concentration was measured. We loaded 10 ug of total protein onto SDS gel and transferred it to PVDF membranes and blocked it with nonfat milk. The primary antibody was applied overnight in a shaking table at 4°C. After extensive washing, the secondary antibody was applied for 1 h, finally, the protein bands were scanned and quantified by image development and ImageJ software.
Immunohistochemical Analysis of ZO-1 and Occludin
For IHC staining, paraffin-embedded ileal tissue (3-μm thickness) was heated, deparaffinized using xylene, and rehydrated in a graded series of ethanol. Antigen retrieval was performed and hydrogen peroxide was used to inhibit endogenous peroxidase activity. Sections were then incubated with primary antibody at 4°C overnight and HRP-conjugated rabbit IgG for 20 min. Color development time was controlled under the microscope. Samples were dehydrated and mounted after counterstaining, then the results were recorded and analyzed.
Measurement of Serum IL-1β and IL-18
The serum was separated from whole blood collected by the eyeball method through a centrifuge at 3000 rpm for 15 min. The levels of serum IL-1β and IL-18 were measured using enzyme assay kit. (Elabscience Biotechnology).
Statistical Analysis
One-way ANOVA was used to analyze the statistical significance of data from independent samples. All statistical calculations were performed in SPSS version 22.0 for Windows (SPSS, Chicago, IL), and the results are expressed as mean±SD. Differences were considered statistically significant at P<0.05.
Results
Visual Observation of Abdominal Cavity
The NS group and siRNA-NS group were normal, without necrosis and hemorrhage. Pancreatic edema, ascites, and intestinal edema were observed in the SAP group. Compared to the SAP group, the degree of pancreatic edema and intestinal edema was significantly less in the siRNA-SAP group.
Morphological Changes in the Pancreas and Intestine
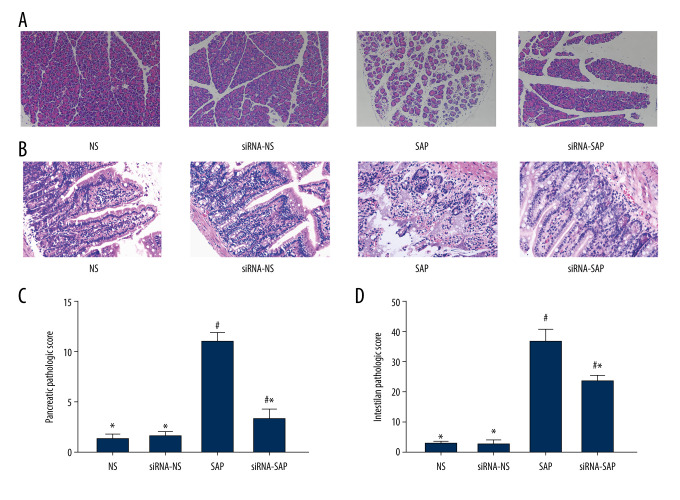
There was no pancreas edema or hemorrhage in the NS group and siRNA-NS group; however, the SAP group and siRNA-SAP group suffer various kinds of pancreas interstitial edema and leukocyte infiltration (Figure 1A). Compared with the SAP group, the siRNA-SAP group showed less severe morphological changes, and the pathological scores of the pancreas were significantly different in these groups (P<0.05) (Figure 1C). The SAP group and the siRNA-NS group showed intestinal mucosal damage and intestinal villus breakage, with less severe damage in the siRNA-NS group (Figure 1B, 1D).
Figure 1.
Pathological changes of pancreas and intestine in mice and quantitative analysis. (A) Pathological changes of pancreas in mice (HE×200). (B) Pathological changes of intestine in mice (HE×200). (C) Quantitative analysis of pancreatic pathological score. (D) Quantitative analysis of intestinal pathological score. * P<0.05 vs SAP group; # P<0.05 vs NS group
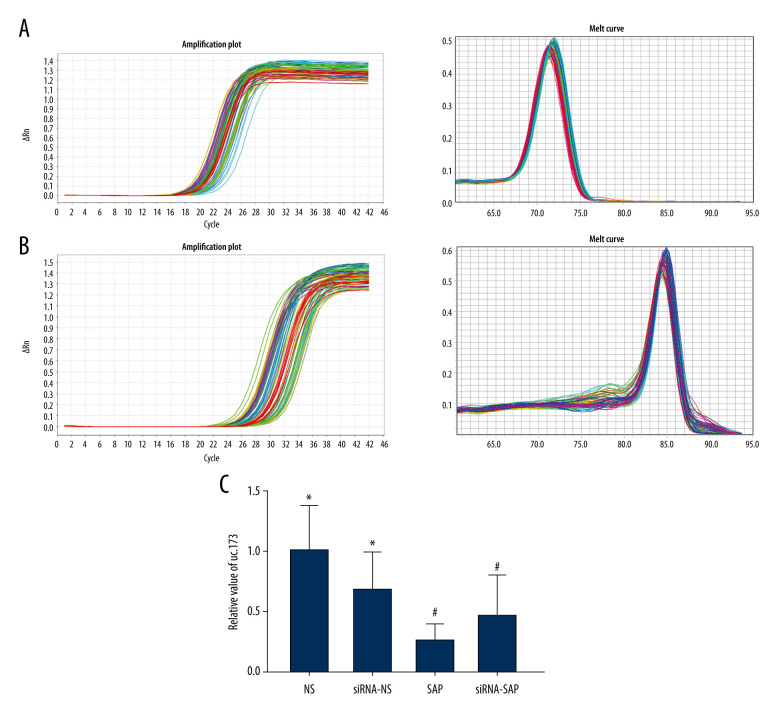
The Relative Quantification of Long Non-coding RNA uc.173 and GSDMD-N in the Intestine
We analyzed the long non-coding RNAuc.173 in ileum tissue. The data showed that the level of lncuc.173 was significantly downregulated in the SAP group and siRNA-SAP group compared to the NS group (P<0.05) (Figure 2). There was almost no expression of GSDMD-N in the intestinal tissues of the NS group and siRNA-NS group. In addition, GSDMD-N was increased in the siRNA-SAP group but was lower than that in the SAP group (P<0.05) (Figure 3A).
Figure 2.
Analysis of fluorescence quantitative results of long non-coding uc.173. (A) Real-time amplification curve and product dissolution curve of long non-coding RNA UC.173. (B) Real-time amplification curve and product dissolution curve of actin. (C) Relative quantitative analysis of uc.173. * P<0.05 vs SAP group; # P<0.05 vs NS group
Figure 3.
The expression of GSDMD-N and serum levels of IL-1β and IL-18. (A) The expression of GSDMD-N in the intestine. (B) Quantitative analysis of serum IL-1β. (C) Quantitative analysis of serum IL-1. * P<0.05 vs SAP group; # P<0.05 vs NS group.
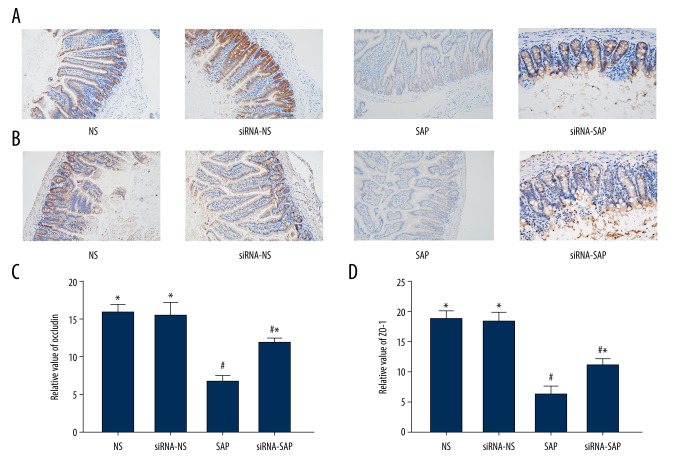
The Expression of ZO-1 and Occludin
Immunohistochemical analysis showed the occludin and ZO-1 proteins in the NS group were distributed along the intestinal mucosal epithelial cells, showing a strong brown linear signal; however, the localization of occludin and ZO-1 in the siRNA-SAP group and the SAP group was not significantly different from that of the NS group, but the brown positive signal was weakened, and there were significantly fewer positive cells in the SAP group and siRNA-SAP group (P<0.05) The difference in the expression of the 2 proteins was statistically significant (Figure 4).
Figure 4.
Immunohistochemical staining to detect occludin protein and ZO-1 and quantitative scoring in intestinal tissue of mice. (A) Immunohistochemical staining was performed on ileum sections to detect occludin. Original magnification (×200). (B) Immunohistochemical staining was performed on ileum sections to detect ZO-1 (×200). (C) Quantitative analysis of occludin. (D) Quantitative analysis of ZO-1. * P<0.05 vs SAP group; # P<0.05 vs NS group.
The Concentration of serum IL-1β and IL-18
IL-1β and IL-18 can flow out of the holes caused by GSDMD. As shown in Figure 4B, levels of serum IL-1β and IL-18 were both significantly higher in the SAP group compared with the NS group (all P<0.05). The levels of serum IL-1β and IL-18 in the siRNA-SAP group were lower than in the SAP group (P<0.05) (Figure 3B, 3C).
Discussion
Severe acute pancreatitis is currently one of the most common gastrointestinal disorders, with a high fatality rate, which is related to the actions of specific inflammatory cytokines [8]. Injured acinar cells can release cytokines to propagate the inflammation [9]. In the early stage of severe acute pancreatitis, there is infiltration of macrophages and leukocytes. Excessive activation of leukocytes leads to the release of many proinflammatory factors into the blood, generating oxygen free radicals and causing systemic inflammatory cascades, damaging the intestines, lungs, and kidneys. Studies have shown that lung injury during AP can be manifested as lung tissue interstitial edema and inflammatory cell infiltration, as well as reduced lung compliance [10]. Kidney injury can be manifested as glomerular degeneration and swelling, renal tubular epithelial cell necrosis, and interstitial inflammatory cell infiltration [11]. When SAP occurs, the intestinal mucosa is ischemic, the villi become short and fall off, the intestinal mucosa shrinks, and the normal flora is imbalanced, which further leads to ectopic bacterial toxins in the intestine. The wall of the ileum is thinner than in the jejunum, and the number of bacteria is higher than in the jejunum. When pancreatitis occurs, the ileum damage is more obvious than in the jejunum.
The intestines perform normal absorption and digestion functions and are an important organ of the human body, and the barrier function of the intestines is closely related to patient prognosis The pathological changes of the intestines, including intestinal barrier injury and changes in the microbiome, can worsen the process of severe acute pancreatitis [12]. The intestinal barrier consists of 4 parts: a mechanical barrier, an immune barrier, a chemical barrier, and a biological barrier. The mechanical barrier plays an important role in intestinal function, and the change of tight junctions between the intestinal mucosa are the key to increasing intestinal permeability in SAP [13].
In this study, we investigated changes in the intestinal barrier in the 4 groups. We demonstrated that the expressions of ZO-1 and occludin were decreased in intestinal epithelial cells in the SAP group. A previous study showed that uc.173 enhances intestinal epithelial barrier function and decreases the level of uc.173, which leads to intestinal epithelial barrier dysfunction [14]. In this study, we assessed the degree of damage and repair ability of the intestinal tissue indirectly by detecting uc.173 levels.
Pyrolysis was observed by Zychlinsky and colleagues in macrophages infected with Shigella flexneri [15]. Pyrostatic cells appear as holes formed in the cell membrane, then the cell membrane loses its integrity and barrier function, causing the cell to swell and rupture, with the nucleus intact [16]. Recent studies have shown that GSDMD is mainly expressed in immune cells (such as macrophages) and intestinal epithelial cells [17,18]. Gasdermin D (GSDMD) is the main executor of pyrolysis [19,20]. Activation of inflammasomes cuts gasdermin D (53 kDa) into an N-terminal fragment (31kDa) and a C-terminal fragment (22 kDa). The N-terminal fragment can make holes in the cell membrane [21,22] and promotes the secretion of interleukin 1β (IL-1β) and IL-18 [19]. Cytokines IL-1β and IL-18 play a particularly critical role in the process of local tissue destruction and the damage of remote organs in severe acute pancreatitis [23–25]. A study showed that the release of IL-1β in GSDMD(−/−) cells was diminished and the inflammation was reduced [26]. Previous studies found that activation of other related proteins of pyrolysis, such as caspase1 and caspase11, can aggravate the degree of pancreatitis damage, while inhibiting related proteins can reduce the degree of pancreatitis damage [27].
In this study, we induced an SAP model and downregulated the expression of GSDMD protein by injecting siRNA through the tail vein. We found that the levels of serum IL-1β and IL-18 were reduced, indicating that GSDMD protein may be related to the systemic inflammatory response caused by SAP.
Studies showed that pyroptosis is related to the occurrence and development of infectious diseases, neurological diseases, vascular diseases, inflammatory bowel diseases, and AIDS [28,29]. However, it is not yet known whether there is pyrolysis in the intestinal injury caused by severe acute pancreatitis. In the present study, we detected the expression of GSDMD in the intestine by western blot analysis, showing that the level of GSDMD in the SAP group was significantly higher than in the NS group. In addition, the expression levels of intestinal adhesion protein ZO-1 and occludin were lower than in the NS group, which proves that downregulation of GSDMD expression can alleviate intestinal damage caused by SAP and can reduce the symptoms of pancreatitis, as well as benefit the treatment of pancreatitis.
Conclusions
Downregulating the level of gasdermin D can reduce severe acute pancreatitis by mediating pyroptosis, which is related to the inflammatory reaction. The present results may provide a new therapeutic target for the treatment of severe acute pancreatitis in intestinal injury.
Acknowledgments
We thank all the participants for their cooperation. We are very grateful for the help of the Qingdao University Central Laboratory in performing this research.
Footnotes
Source of support: National Natural Science Foundation of China (no. 81870440)
References
- 1.Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156(7):2008–23. doi: 10.1053/j.gastro.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenner S, Baillie J, DeWitt J, Vege SS American College of Gastroenterology. American College of Gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–15. 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 3.Piao X, Liu B, Sui X, et al. Picroside II improves severe acute pancreatitis-induced intestinal barrier injury by inactivating oxidative and inflammatory TLR4-Dependent PI3K/AKT/NF-kappaB signaling and improving gut microbiota. Oxid Med Cell Longev. 2020;2020:3589497. doi: 10.1155/2020/3589497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su YR, Hong YP, Mei FC, et al. High-fat diet aggravates the intestinal barrier injury via TLR4-RIP3 pathway in a rat model of severe acute pancreatitis. Mediators Inflamm. 2019;2019:2512687. doi: 10.1155/2019/2512687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan YD, Zhu RX, Bian ZZ, Pan XT. Improvement of gut microbiota by inhibition of p38 mitogen-activated protein kinase (MAPK) signaling pathway in rats with severe acute pancreatitis. Med Sci Monit. 2019;25:4609–16. doi: 10.12659/MSM.914538. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215(1):44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu CJ, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. II. The protective effect of intraluminal glucose as energy substrate. Archiv Surg. 1970;101(4):484–88. doi: 10.1001/archsurg.1970.01340280036010. [DOI] [PubMed] [Google Scholar]
- 8.Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175(1):76–83. doi: 10.1016/s0002-9610(97)00240-7. [DOI] [PubMed] [Google Scholar]
- 9.Jakkampudi A, Jangala R, Ratnakar Reddy B, et al. NF-κB in acute pancreatitis: Mechanisms and therapeutic potential. Pancreatology. 2016;16(4):477–88. doi: 10.1016/j.pan.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhu R, Zhao Y, Li X, et al. Effects of penehyclidine hydrochloride on severe acute pancreatitis-associated acute lung injury in rats. Biomed Pharmacother. 2018;97:1689–93. doi: 10.1016/j.biopha.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Wajda J, Dumnicka P, Maraj M, et al. Potential prognostic markers of acute kidney injury in the early phase of acute pancreatitis. Int J Mol Sci. 2019;20(15):3714. doi: 10.3390/ijms20153714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pooran N, Indaram A, Singh P, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis. Correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3(3):252–62. doi: 10.1016/s1091-255x(99)80067-5. [DOI] [PubMed] [Google Scholar]
- 13.Galipeau HJ, Verdu EF. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol Motil. 2016;28(7):957–65. doi: 10.1111/nmo.12871. [DOI] [PubMed] [Google Scholar]
- 14.Jun-Yao W, Yu-Hong C, Lan X, et al. Regulation of intestinal epithelial barrier function by long noncoding RNA uc.173 through interaction with microRNA 29b. Mol Cell Biol. 2018;38(13):e00010–18. doi: 10.1128/MCB.00010-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–69. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 16.Sborgi L, Ruhl S, Mulvihill E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–78. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeki N, Usui T, Aoyagi K, et al. Distinctive expression and function of fourGSDMfamily genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer. 2009;48(3):261–71. doi: 10.1002/gcc.20636. [DOI] [PubMed] [Google Scholar]
- 18.Tamura M, Tanaka S, Fujii T, et al. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007;89(5):618–29. doi: 10.1016/j.ygeno.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 19.He W-t, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–98. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–71. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 21.Jingjin D, Kun W, Wang L, et al. Erratum: Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;540(7631):150. doi: 10.1038/nature20106. [DOI] [PubMed] [Google Scholar]
- 22.Xing L, Zhibin Z, Jianbin R, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–58. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda T, Takeyama Y, Yasuda T, et al. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol. 2006;41(2):158–65. doi: 10.1007/s00535-005-1735-4. [DOI] [PubMed] [Google Scholar]
- 24.Wereszczynska-Siemiatkowska U, Mroczko B, et al. The importance of interleukin 18, glutathione peroxidase, and selenium concentration changes in acute pancreatitis. Dig Dis Sci. 2004;49(4):642–50. doi: 10.1023/b:ddas.0000026312.47460.a3. [DOI] [PubMed] [Google Scholar]
- 25.Yuan BS, Zhu RM, Braddock M, et al. Interleukin-18: A pro-inflammatory cytokine that plays an important role in acute pancreatitis. Expert Opin Ther Targets. 2007;11(10):1261–71. doi: 10.1517/14728222.11.10.1261. [DOI] [PubMed] [Google Scholar]
- 26.Jianjin S, Yue Z, Kun W, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–65. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 27.Rau B, Paszkowski A, Lillich S, et al. Differential effects of caspase-1/interleukin-1beta-converting enzyme on acinar cell necrosis and apoptosis in severe acute experimental pancreatitis. Lab Invest. 2001;81(7):1001–13. doi: 10.1038/labinvest.3780312. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Qiu H, Pei X, et al. Low-dose sinapic acid abates the pyroptosis of macrophages by downregulation of lncRNA-MALAT1 in Rats with diabetic atherosclerosis. J Cardiovasc Pharmacol. 2018;71(2):104–12. doi: 10.1097/FJC.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 29.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]