Abstract
Retinopathy of prematurity (ROP) is a leading cause of preventable childhood blindness worldwide. Barriers to ROP screening and difficulties with subsequent evaluation and management include poor access to care, lack of physicians trained in ROP, and issues with objective documentation. Digital retinal imaging can help address these barriers and improve our knowledge of the pathophysiology of the disease. Advancements in technology have led to new, non-mydriatic and mydriatic cameras with wider fields of view as well as devices that can simultaneously incorporate fluorescein angiography, optical coherence tomography (OCT), and OCT angiography. Image analysis in ROP is also being employed through smartphones and computer-based software. Telemedicine programs in the United States and worldwide have utilized imaging to extend ROP screening to infants in remote areas and have shown that digital retinal imaging can be reliable, accurate, and cost-effective. In addition, tele-education programs are also using digital retinal images to increase the number of healthcare providers trained in ROP. Although indirect ophthalmoscopy is still an important skill for screening, digital retinal imaging holds promise for more widespread screening and management of ROP.
Keywords: imaging, retinopathy of prematurity, telemedicine
Retinopathy of prematurity (ROP) is a vasoproliferative disorder characterized by an arrest of normal retinal vascular development and subsequent disorganized vascular growth. It is the leading cause of preventable blindness globally and affects thousands of infants. The risk factors include, among others, younger gestational age, low birth weight, and supplemental oxygen.1 The International Committee for the Classification of Retinopathy of Prematurity (ICROP) classifies ROP based on zone or location of disease, stage or severity of disease at the border of the vascular and avascular retina, extent of disease described by clock hour(s), as well as plus disease which is characterized by the presence of dilated and/or tortuous vessels in the posterior pole.2 It is further classified into aggressive posterior ROP, pre-threshold (type 1 or type 2 ROP), and threshold disease according to various findings of zone, stage, extent, and plus disease as initially described in the Early Treatment For Retinopathy Of Prematurity study (ETROP).3
Treatment of type 1 ROP may include cryotherapy, laser photocoagulation, and anti–vascular endothelial growth factor (VEGF) therapy. The Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study was a randomized controlled trial which showed that ablation of the avascular retina through transscleral cryotherapy reduced unfavorable outcomes in severe ROP.4 However, indirect laser photocoagulation has largely replaced cryotherapy. The Bevacizumab Eliminates the Angiogenic Threat for Retinopathy of Prematurity (BEAT-ROP) study as well as other reports in the literature showed that anti-VEGF therapy is a developing treatment option for ROP, but there are still concerns regarding its safety.5
The American Academy of Pediatrics (AAP), American Association for Pediatric Ophthalmology and Strabismus (AAPOS), and the American Academy of Ophthalmology (AAO) recommend a retinal screening examination for infants with a birth weight of ≤1500 g or gestational age of ≤30 weeks who are at high risk for ROP.6,7 The use of retinal imaging for ROP screening is a quickly evolving approach. Imaging in ROP provides a cost-effective and accurate way to diagnose and monitor ROP. It should be applied to ROP screening worldwide as it can help increase access to care in areas with limited ophthalmologists trained in ROP or in areas with few resources. Currently, ROP imaging is widely used for photo documentation and telemedicine programs. In recent years, fluorescein angiography (FA), optical coherence tomography (OCT), and other imaging modalities have been more thoroughly used in ROP care and research. In this review, we discuss current trends and future directions of ROP imaging.
The first published human fundus photograph dates back to 1886 (Fig. 1). In 1906, the first fundus camera was available for commercial use by physicians.8 In the 1970s, the Kowa handheld direct ophthalmoscope was combined with a gonio lens and was useful in digital retinal imaging. Methods for retinal photography in the newborn were described about 5 decades ago. For example, Flynn et al9 describes the use of FA with a Zeiss retinal camera for infants with ROP who had “peculiar or puzzling fundus pictures” in the 1960s and 1970s. However, routine imaging of children had unique challenges which included positioning, keeping the infant warm, and familiarity with findings in the normal infant as compared with adults.10
FIGURE 1.
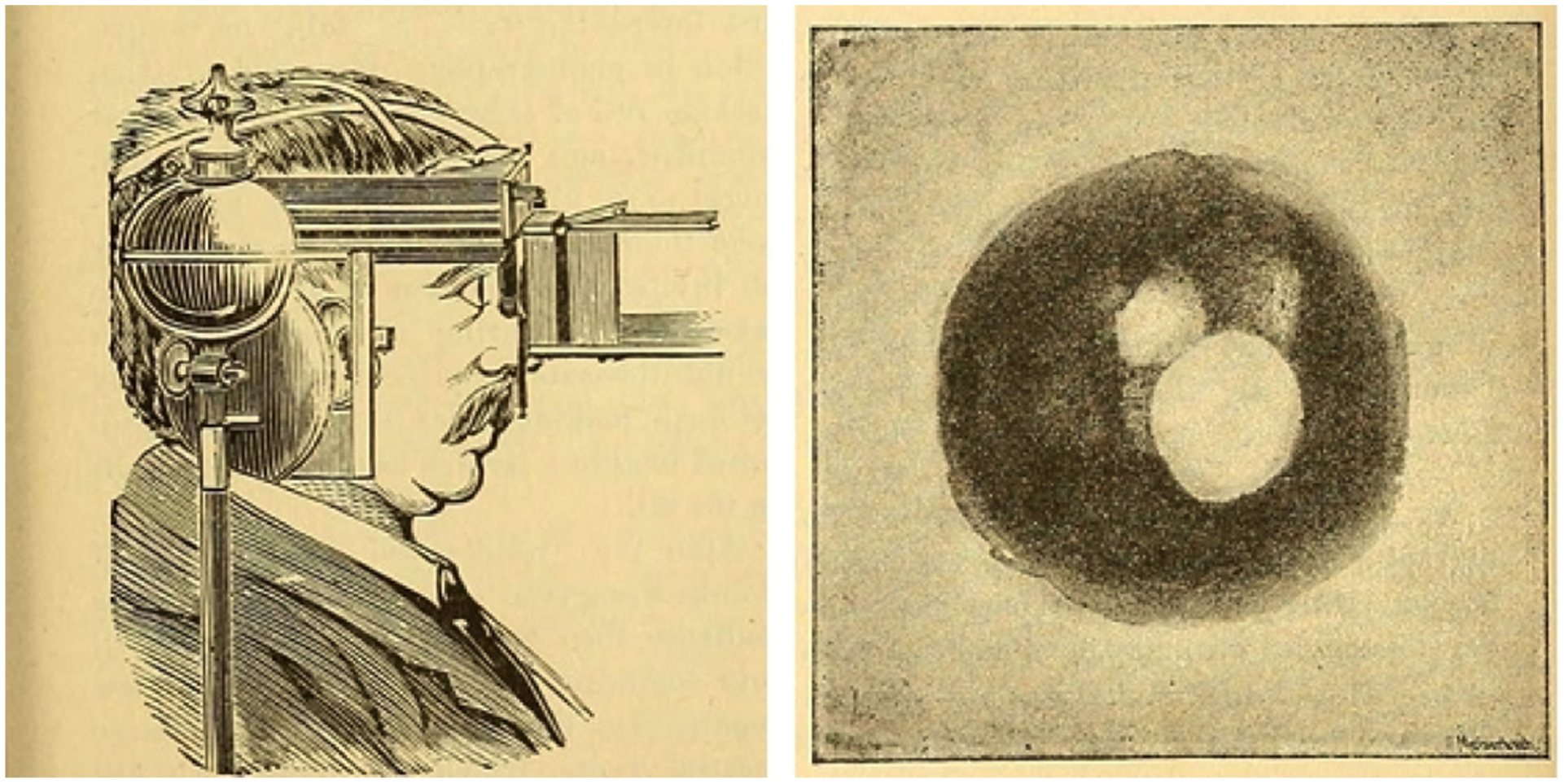
First human fundus photograph.84
IMAGING SYSTEMS FOR ROP
Multiple advancements in technology have produced numerous imaging modalities for ROP (Table 111–18).19,20 The Zeiss FF3 fundus camera was used for the ETROP study but issues with positioning were described by the original authors.21 In traditional imaging systems, patients sit upright and place their head in a headrest while directing their eye in specific directions. The patients that are imaged for ROP are infants who cannot voluntarily follow commands and require assistance in positioning of the head. Often the infant is in a supine position in a hospital bed (crib) if they are in the neonatal intensive care unit or held by the parent/staff in a supine position if they are in an outpatient clinic.
TABLE 1.
Fundus Camera and Smart Phone–Based Systems
| Camera | Pupil | Field of View, Degrees | Contact (+/−) | Handheld (+/−) | Imaging |
|---|---|---|---|---|---|
| RetCam14 | Mydriatic | 130 | + | + | FA |
| ICON15 | Mydriatic | 100 | + | + | FA |
| 3nethra neo11 | Mydriatic | 120 | + | + | – |
| 3nethra flora11 | Mydriatic | 45 | − | − | FA |
| Optos17 | Nonmydriatic | 200 | − | − | FA, OCT, ICGA |
| NIDEK18 | Nonmydriatic | 30 | − | − | – |
| Spectralis13 | Mydriatic | 102 | − | − | FA, OCT, ICGA |
| PanoCam14 | Nonmydriatic | 130 | + | + | FA |
| D-eye16 | Nonmydriatic | 20 | − | + | – |
| Ocular CellScope12 | Not specified | 55 | − | + | – |
| PEEK12 | Not specified | Not specified | − | + | – |
FA indicates fluorescein angiography; ICGA, indocyanine green angiography; OCT, optical coherence tomography; +, presence; −, absence.
In addition to unique positioning issues in infants, it is also important to use contact imaging systems given the challenges of imaging patients specifically in this age group. In this system, the camera is directly applied to the globe after topical anesthetic is instilled into the eye and gel has been applied to the probe. This method poses potential risks of injury to the eye, including corneal abrasions. Furthermore, wide-field views are essential in the evaluation of ROP and mydriasis helps obtain better views of the peripheral retina.
Wide-field contact imaging systems include the RetCam (Natus Medical Systems, Inc., Pleasanton, CA, US), which is a mydriatic, contact, handheld device that images 130° of the fundus.22 The RetCam Shuttle (Natus Medical Systems, Pleasanton, CA, US) is a more portable, lighter version of the RetCam. The RetCam also has a FA feature with various lenses (130°, 120°, 80°, 30°, and portrait) that are available for use. The use of digital imaging using the RetCam was shown to have high sensitivity and specificity when compared with the gold standard, indirect ophthalmoscopy, in terms of ROP diagnosis.23 However, potential limitations of the RetCam include relatively low resolution and capturing dark fundi.24
Over the past 10 years, a number of newer imaging modalities have emerged for ROP imaging. The ICON (Phoenix Clinical, Inc., Pleasanton, CA, US) camera system is a mydriatic, contact, handheld camera with reported improved resolution and color profile for dark fundi; FA is also available with this device.24 The 3nethra neo (Forus Health, Bangalore, India) is also available for use for retinal imaging and offers a 120° field of view and high-resolution images, as well as allows montaging of images that can be useful for ROP.11,25 The Panocam (Visunex Medical Systems, Inc., Fremont, CA, US) is a wireless, contact, handheld camera system with a 130° field of view.25 It has been used widely in newborn screening programs in Asia. The Optos California and 200-TX Optomap (Optos, Marlborough, MA, US) are non-contact, ultrawide-field imaging systems with a 200° field of view.26
Smartphone-Based Models
New developments in smartphone-based models for retinal imaging have been established, including the D-eye system, The Portable Eye Examination Kit (PEEK), Ocular CellScope, and a prototype by Harvard Medical School.12 The D-eye system has a 20° field of view, with similar principles as the direct ophthalmoscopy. The camera’s autofocus ability account for the patient’s refractive error.27 The Karnataka Internet Assisted Diagnosis of Retinopathy of Prematurity (KIDROP) program showed that the use of smartphones also allowed for efficient transfer of data to specialists and led to an earlier diagnosis of ROP. Smartphone images were also beneficial in parental education and pediatric training.28 In a study by Wang et al,29 a smartphone-based prototype device employed trans-pars-planar illumination to achieve wide-field fundus photographs. Instead of the conventional transpupillary illumination, this technique allows a greater view through the pupil, acquiring 90° fundus views in a single shot, an approach that theoretically requires less dilation.30
TELEMEDICINE
Digital retinal imaging is an important factor in telemedicine and allows remote interpretation of images. This has been pivotal in areas with an inadequate number of ophthalmologists trained in ROP screening and management. In the US, the Telemedicine Approaches to Evaluating Acute-phase ROP (e-ROP) study involved 21 clinical centers and a cohort of 2000 infants. Images were obtained using the RetCam Shuttle and graded by non-ophthalmologists.31 These were compared with diagnostic examinations by ophthalmologists and the findings were consistent with previous studies showing that telemedicine diagnosis for ROP is reliable and accurate.32 Although e-ROP was a study, there are a number of active clinical telemedicine systems in the US such as the Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP) initiative and the Focus-ROP programs.33,34 Telemedicine programs have been established in other countries as well. The Retinopathy of Prematurity Eradication – Save Our Sight (ROPE-SOS) is a mobile tele-screening program in South India. Digital retinal images of preterm infants were captured using the Retcam Shuttle and transmitted to Aravind Eye Hospital in Coimbatore, India.35 Images were then analyzed by ROP experts to determine acuity of disease, follow-up, and treatment.36 In New Zealand, the Auckland Regional Telemedicine ROP (ARTROP) network also showed that telemedicine can be beneficial in ROP screening. Digital retinal imaging was obtained with the RetCam and was the preferred tool for screening when available.37,38 Telemedicine screening programs also exist in Canada and Germany.34
ARTIFICIAL INTELLIGENCE/COMPUTER-BASED IMAGING ANALYSIS
There have been a number of approaches to use computers to quantify vascular disease in ROP over the years. These approaches have ranged from basic feature extraction (eg, ROPtool39; FocusROP, Trumbauersville, PA, US), feature-based machine learning, to deep convolutional neural networks (eg, VesselMap,40 CAIAR,41 i-ROP,42 RISA,43). Abbey et al44 reported that ROPtool showed high sensitivity (97%) and specificity (94%) in the detection of vascular abnormalities. The system has also been used to analyze images obtained from the Pictor (Volk Optical Inc, Mentor, OH, US), RetCam, or video indirect ophthalmoscopy.45,46 Other examples of computer-based imaging analysis (CBIA) include Retinal Image multiScale Analysis (RISA),47 VesselMap,39 and the Computer-Aided Image Analysis of the Retina (CAIAR) program.40
The i-ROP ASSIST system utilizes a number of defined measures of dilation and tortuosity and a machine learning classifier to diagnose pre-plus or plus disease. The system was the first to report expert-level diagnostic performance of plus disease. However, the requirement for manual segmentation of input images limits the applications in the real world.41,48 Coyner et al49 created a model utilizing a 5-fold cross-validation approach that distinguished RetCam images of acceptable quality from low/questionable quality and recognized features that grouped them into these categories. The model performed similarly to ROP experts with high intra-group and inter-group discrimination.49 In 2018, Brown et al50 reported the results of a fully automated convolutional neural network. This technique, referred to as deep learning (DL), has been reported in numerous medical imaging applications to provide diagnostic accuracy on a par with physicians. The system, referred to as i-ROP DL demonstrated an area under the curve of 0.98 for the diagnosis of plus disease, and outperformed 7 of 8 experts in the test set for consistent diagnosis compared with a reference standard diagnosis.50
FLUORESCEIN ANGIOGRAPHY
Fluorescein angiography was first utilized for retrolental fibroplasia in the 1960s by Flynn and his team with a Zeiss fundus camera (Dublin, CA, US).9 Multimodal digital retinal imaging devices allowed examiners to incorporate FA more easily for ROP screening. The technique can be used with various fundus cameras including the RetCam, 3nethra flora, Optos, Spectralis, and others (Table 1). Fluorescein angiography has been shown to be safe in children with minimal adverse effects.51 It is advantageous in that it can identify pathology that is not visible with indirect ophthalmoscopy and can potentially help identify higher severity levels of ROP earlier in the disease course (Fig. 2).52 For example, a case series by Yokoi et al53 showed that in aggressive posterior ROP, vascular changes such as greater temporal extension of vessels, shunting in the vascularized retina, or leakage from neovascular tissue were detected on FA, but not visualized on indirect ophthalmoscopy. Furthermore, various studies have shown that FA can improve diagnostic accuracy of different classifications of ROP, such as type 2 ROP, and can improve intergrader agreement when used in adjunct with digital fundus photographs.54 Fluorescein angiography can also play a role in more accurately monitoring efficacy of anti-VEGF therapy in ROP.5 This modality is highly operator-dependent and can affect image quality which in turn affects diagnostic accuracy.
FIGURE 2.
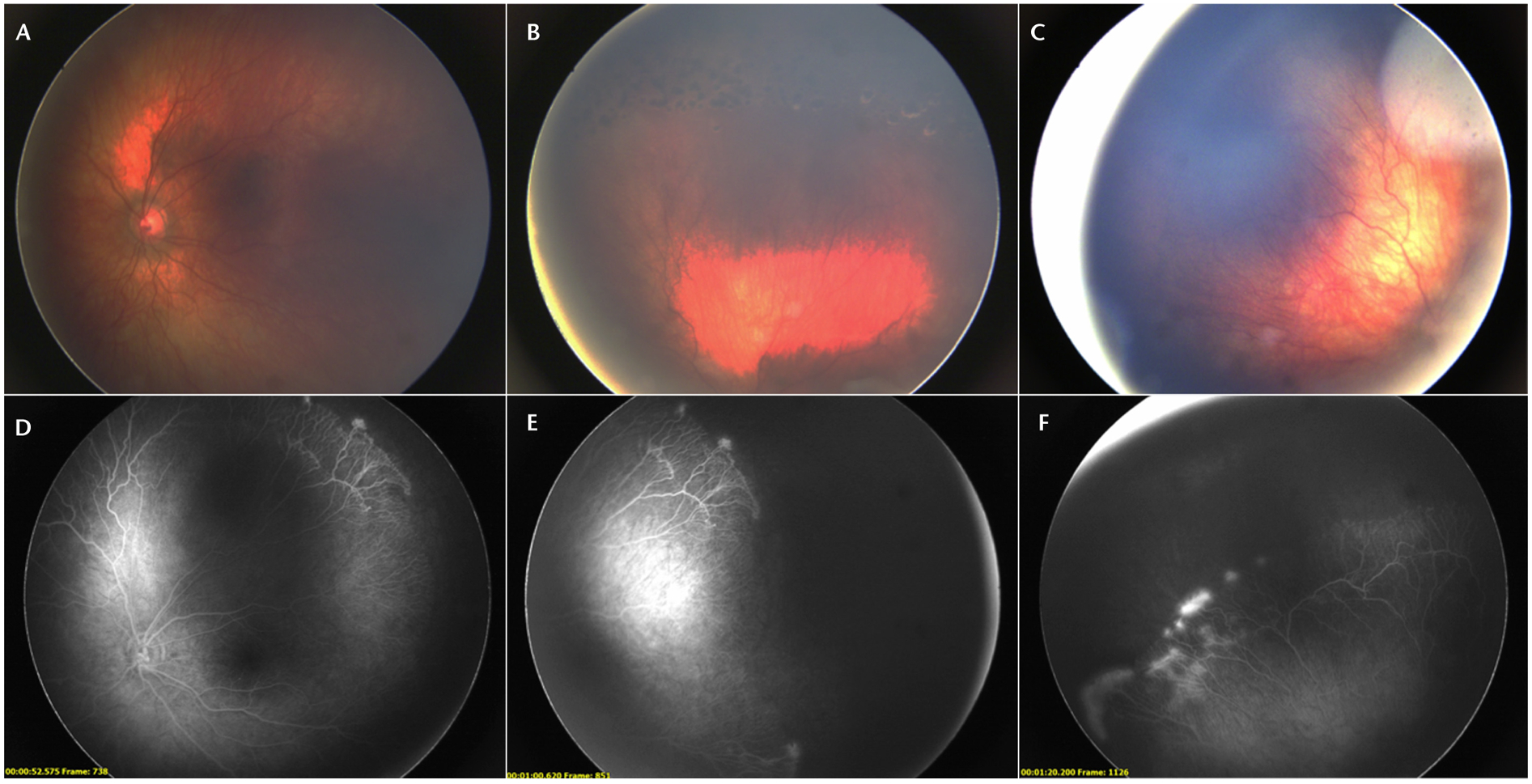
Fundus photos (A to C) and fluorescein angiography (D to F) of a patient with retinopathy of prematurity who had previously been treated with laser. In the fluorescein angiography, signs of neovascularization and leakage are more obvious compared with the fundus photographs.
OCT AND OCT ANGIOGRAPHY
There have been several early attempts to use handheld OCT and optical coherence tomography angiography (OCTA) as an adjunct to ROP screening and fundus photography.55,56 Several 3-dimensional (3D) morphological features including thickened retinal ridge, vascular tufts, and neovascularization at the avascular-vascular junction can be identified by OCT.57 Mangalesh et al58 used colorized 3D visualization and extensions of OCT processing, which had benefits in understanding extraretinal features as well as longitudinal care of ROP.
In addition to features in the avascular-vascular zone, subclinical findings in the macula and vitreous have been visualized on OCT in ROP including pre-retinal tissue (popcorn retinopathy), epiretinal membranes, cystoid macular changes, retinoschisis, precise localization of retinal detachment, vascular changes representative of plus disease, and cellular and subcellular changes related to ROP.59,60 Maldonado et al61 demonstrated that 3D topographic features correlated with ophthalmoscopic signs of plus disease. Furthermore, early identification of vitreoretinal traction led to earlier surgical intervention in infants with ROP progressing towards retinal detachment.62 Images of OCT can also provide an ultrawide-field view which is pertinent in ROP (Fig. 3). Therefore, OCT is currently a useful adjunct to ROP screening.63 Campbell et al55 demonstrated the ability to obtain OCTA using a handheld device, but the clinical utility of this technology remains to be seen (Fig. 4).
FIGURE 3.
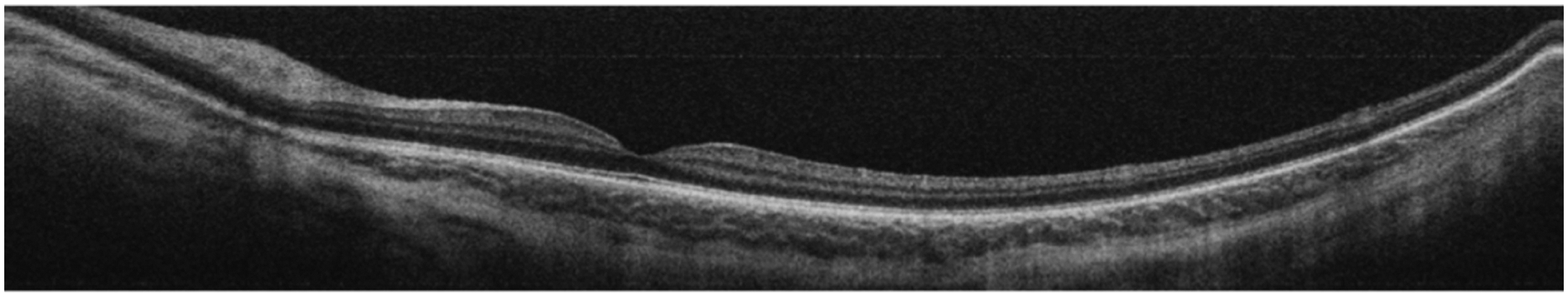
Ultrawide-field optical coherence tomography of a patient undergoing routine retinopathy of prematurity screening.
FIGURE 4.
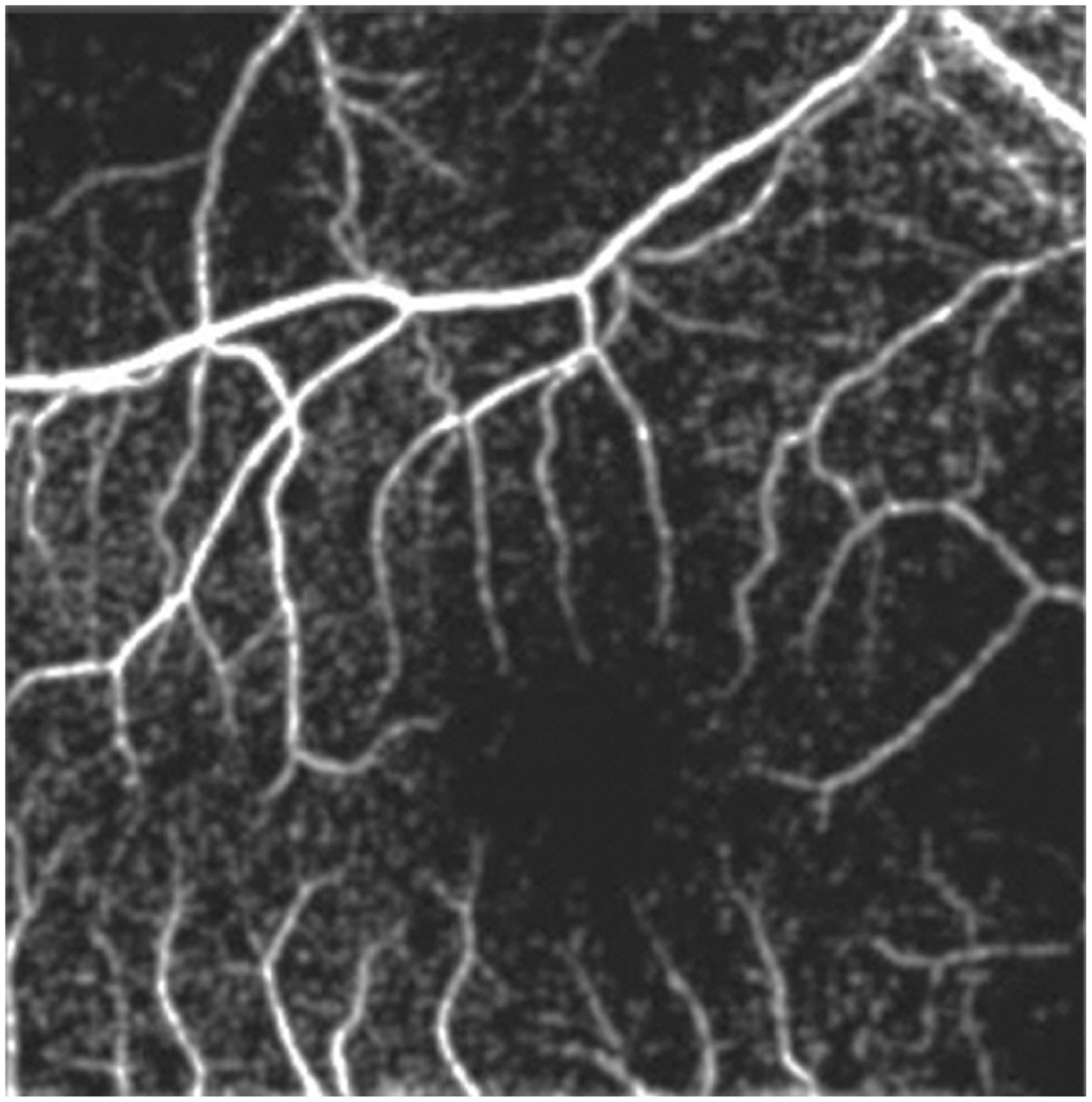
Optical coherence tomography angiography of a patient with an intact foveal avascular zone obtained during routine retinopathy of prematurity screening.
TELE-EDUCATION
Digital retinal imaging is beneficial for tele-education and training more ophthalmologists who can manage ROP. Multiple training systems have been developed. For example, the Global Education Network for Retinopathy of Prematurity (GEN-ROP) is a web-based tele-education system and utilizes over 12,000 images. Pretests and posttests were administered to ophthalmology residents in the US and Canada, and results showed improved diagnostic accuracy and reliability of ROP diagnosis with the use of this system.64 Furthermore, international applications of tele-education systems have also shown the potential to improving ROP diagnosis among ophthalmology residents and fellows in Mexico, Brazil and the Philippines, and was preferred to standard educational methods.65,66 Online training modules with digital retinal images of ROP are offered by the AAO and were developed in coordination with i-ROP and GEN-ROP.67
Education should also be provided to the parents and staff taking care of infants with ROP. A video educational and consenting system was developed by Arnold68 who showed that parents and staff had increased understanding after watching the video or viewing online material regarding ROP. Providing information remotely can improve the consenting process and be helpful in areas where it is not possible to have face-to-face education time with an ophthalmologist.
ADVANTAGES OF IMAGING
Documentation
Documentation of retinal findings in ROP was historically recorded with hand-drawn sketches based on disease classification but this led to significant interexpert variability.42,69 Digital images of ROP, however, can provide a means for objective documentation and can be stored as single images, montages, or videos.70 In a statement by the AAP, AAO and AAPOS, digital retinal imaging is described as a useful tool for objective documentation and also for education of parents and staff in the neonatal intensive care unit.7 Furthermore, cloud-based software allows information to be stored and shared as needed in the management of ROP. Arnold et al20 describes how communication and scheduling is maintained for ROP through a software called ROP-Check.
Disease Classification and Diagnosis
Digital retinal imaging offers advantages in disease classification and diagnosis. For example, several studies have shown that CBIA can help in identifying and differentiating plus from pre-plus disease, although diagnosis of plus disease among experts can be variable.41,44,47 Klufas et al54 found that the addition of FA to retinal imaging increased sensitivity of diagnosis of stage 2 ROP or worse, stage 3 or worse, pre-plus or worse, and type 2 or worse, and improved intergrader agreement for treatment-requiring ROP diagnosis. Computer-generated mosaic photographs instead of multiple single photographs could also further improve accuracy of digital retinal imaging especially in treatment-requiring ROP.71
Several studies have shown the accuracy of digital photography for diagnosis of ROP. In a study, digital imaging using the RetCam-120 system showed a sensitivity of 100% and specificity of 96% for detection of referral-warranted ROP. In addition, the positive predictive value was 92% and negative predictive value was 100% for digital photography.72 Digital retinal imaging systems offer an advantage in that they can be used by nurses and other non-physician staff, and electronically transmitted to readers and have high intragrader reliability.32,73 However, image quality is an important factor in diagnostic sensitivity.74 Wide-angle digital retinal imaging can provide high accuracy of clinically significant ROP and more objective measures of monitoring progression of disease by comparing previous photographs or videos.75
Physiologic Advantage
There are also advantages to the infant physiologically with digital retinal imaging. For example, indirect ophthalmoscopy resulted in an increased stress response as indicated by a significantly higher heart rate and respiratory rate compared with digital retinal imaging.76 Non-mydriatic cameras also eliminate the potential risks with systemic absorption of dilation drops.
Cost-effectiveness
There have been a few studies that compared the cost-effectiveness of telemedicine and digital retinal imaging with traditional indirect ophthalmoscopy for ROP screening. In the US, telemedicine was shown to be more cost-effective in terms of costs per quality-adjusted life year.77 In the UK, it was shown that trained nurses utilizing portable retinal cameras were more cost-effective than traditional imaging.78 In India, a study evaluated the costs of a tele-screening program and concluded that it was cost-effective.79
LIMITATIONS OF ROP IMAGING
Challenges to utilizing digital retinal imaging include the definition of a gold standard imaging modality and a gold standard photograph. Fundus photography has been used in the ETROP study but several potential issues have been described with the standard photograph including a narrow field of view (2–3 disc diameters) and image magnification that is larger than indirect ophthalmoscopy, which can affect diagnostic accuracy of plus disease and intergrader agreement.80 However, the emergence of wide-field fundus cameras may help address this problem by providing a wider field of view without exaggerated magnification.81 The standard photograph also displays more dilation and less tortuosity than what is typically seen in the majority of eyes with plus disease. Additional photographs have to be provided by the ICROP committee to demonstrate the minimum abnormality for plus disease.82
There are also limitations involving access to camera systems and the need for mydriasis for most of these systems. Mydriatic cameras can be expensive and may not be available for use in certain areas or hospitals with limited resources.37 Such cameras also require pharmacologic dilation of infants and expose infants to the risks of these medications. Further, digital retinal imaging produces a transient stress response in the infant although potentially less often than indirect ophthalmoscopy.76
Another important factor in the use of digital retinal imaging for ROP is the quality of images. Motion artifact, poor contrast, and pressure on the globe can affect image quality.22 The PHOTO-ROP study concluded that sensitivity, specificity, and accuracy of ROP diagnosis were dependent on image quality and for this reason, perhaps less likely to replace conventional indirect ophthalmoscopy screening for ROP.74 As described previously however, new artificial intelligence systems are developing that can differentiate between acceptable and low-quality images.49
Limitations of CBIA algorithms include quantifying plus or pre-plus disease and specifying a cutoff of normal versus abnormal.80 This is also closely linked to the concern whether CBIA systems should be restricted to existing clinical definitions or whether they should “fit the data”, simulating real-world experience where there is interexpert diagnostic variability. For example, various experts are focusing on retinal features such as arterial tortuosity and venous dilation differently to arrive at their diagnosis.48
FUTURE OF ROP IMAGING
Advancements in the existing camera models are currently being developed.13–16,19,20,79 This will most likely decrease the cost of older models and make them affordable for use in areas that have no imaging devices. In India, the 3nethra neo has recently been approved for commercial use. A pilot study showed that it has similar potential as a ROP screening tool given its excellent sensitivity (97–99%) and good specificity (75–81%).83
In addition, as resolution and clarity of the camera systems improve, higher-quality images will allow for more accurate analysis of images. It is likely that DL and CBIA algorithms will be further refined and new algorithms created to improve diagnostic accuracy of ROP. As we continue to learn more about the pathophysiology of ROP, FA, OCT and OCTA will be more frequently incorporated into the ROP screening examinations and more devices will offer this multimodal imaging functionality.
CONCLUSION
There are several modalities for imaging in ROP from digital retinal images to smartphones to artificial intelligence systems. Digital retinal imaging in ROP has been fundamental to the diagnosis and early management of ROP, applications in telemedicine and tele-education worldwide, and our growing knowledge of the pathophysiology of ROP. The future of ROP will continue to see further developments in the existing technology with incorporation of other modalities such as FA, OCT, and OCTA.
Acknowledgments
Supported by grants R01EY19474, K12EY027720, and P30EY10572 from the National Institutes of Health (Bethesda, MD), by grants SCH-1622679, SCH-1622542, and SCH-1622536 from the National Science Foundation (Arlington, VA), and by unrestricted departmental funding and a Career Development Award (J.P.C.) from Research to Prevent Blindness (New York, NY). M.F.C. is an unpaid member of the Scientific Advisory Board for Clarity Medical Systems (Pleasanton, CA), a Consultant for Novartis (Basel, Switzerland), and an initial member of Inteleretina (Honolulu, HI). R.V.P.C. is on the Scientific Advisory Board for Visunex Medical Systems (Pleasanton, CA) and a Consultant for Genentech (South San Francisco, CA).
REFERENCES
- 1.Kim SJ, Port AD, Swan R, et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63: 618–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–999. [DOI] [PubMed] [Google Scholar]
- 3.Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. [DOI] [PubMed] [Google Scholar]
- 4.Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Pediatrics. 1988;81:697–706. [PubMed] [Google Scholar]
- 5.Vural A, Perente İ, Onur İU, et al. Efficacy of intravitreal aflibercept monotherapy in retinopathy of prematurity evaluated by periodic fluorescence angiography and optical coherence tomography. Int Ophthalmol. 2018. November 26 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–195. [DOI] [PubMed] [Google Scholar]
- 7.Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142: pii: e20183061. [DOI] [PubMed] [Google Scholar]
- 8.Museum of Vision – American Academy of Ophthalmology. https://www.aao.org/museum-of-vision. Accessed December 26, 2018.
- 9.Flynn JT, Cassady J, Essner D, et al. Fluorescein angiography in retrolental fibroplasia: experience from 1969–1977. Ophthalmology. 1979;86: 1700–1723. [DOI] [PubMed] [Google Scholar]
- 10.Baum D Retinal photography in the newborn. Arch Dis Child. 1969;44:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Future of ophthalmic care. Forus Health Web site. https://www.forushealth.com/. Accessed April 6, 2019.
- 12.Panwar N, Huang P, Lee J, et al. Fundus photography in the 21st century— a review of recent technological advances and their implications for worldwide healthcare. Telemed J E Health. 2016;22:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spectralis: Multimodal imaging platform optimized for the posterior segment. Heidelberg Engineering Web site. https://business-lounge.heidelbergengineering.com/us/en/products/spectralis/. Accessed January 20, 2019.
- 14.Pediatric Digital Retinal Imaging Systems. OphthalmologyWeb Web site. https://www.ophthalmologyweb.com/Pediatric-Ophthalmology/5818-Pediatric-Retinal-Imaging/Compare/?compare=5186570,9698287,9698289,55444,54765&catid=5818. Accessed January 20, 2019.
- 15.ICON Specs. Phoenix Technology Group Web site. http://phoenix-clinical.com/icon-specs/. Accessed January 20, 2019.
- 16.Digital retinal camera. The Direct Ophthalmoscope for Your iPhone | Portable digital retinal camera | D-EYE Web site. https://www.d-eyecare.com/#videos. Accessed January 20, 2019.
- 17.Products. Optos Web site. https://www.optos.com/en/products/. Accessed January 20, 2019.
- 18.Non-mydriatic automated fundus camera. https://usa.nidek.com/products/fundus-camera/. Accessed April 18, 2019.
- 19.Cernichiaro-Espinosa LA, Tran KD, Berrocal AM. Imaging modalities in pediatric vitreoretinal disorders. Curr Ophthalmol Rep. 2018;6:17–23. [Google Scholar]
- 20.Arnold RW, Grendahl RL, Kevin Winkle R, et al. Outpatient, wide-field, digital imaging of infants with retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. 2017;48:494–497. [DOI] [PubMed] [Google Scholar]
- 21.Multicenter Trial of Cryotherapy for Retinopathy of Prematurity (CRYO-ROP). Manual of Procedures. National Institutes of Health/PHS, National Eye Institute, Bethesda, MD. [Google Scholar]
- 22.Salcone EM, Johnston S, VanderVeen D. Review of the use of digital imaging in retinopathy of prematurity screening. Semin Ophthalmol. 2010; 25:214–217. [DOI] [PubMed] [Google Scholar]
- 23.Tejada-Palacios P, Zarratea L, Moral M, et al. Comparative study of RetCamRetCam II vs. binocular ophthalmoscopy in a screening program for retinopathy of prematurity. Arch Soc Esp Oftalmol. 2015;90:373–378. [DOI] [PubMed] [Google Scholar]
- 24.Park CH, Rahimy E, Shahlaee A, et al. Telemedicine in ophthalmology. Retina Today. 2017: 55–58. Retina Today Web site: http://retinatoday.com/pdfs/0417RT_Cover_Federman.pdf. Accessed December 26, 2018. [Google Scholar]
- 25.Gupta M, Chan RVP. Pediatric retina: advances in diagnosis and treatment. Retina Specialist. Published March 22, 2016. Retina Specialist Web site. http://www.retina-specialist.com/article/pediatric-retina-advances-in-diagnosis-and-treatment. Accessed December 26, 2018.
- 26.Theodoropoulou S, Ainsworth S, Blaikie A. Ultra-wide field imaging of retinopathy of prematurity (ROP) using Optomap-200TX. BMJ Case Rep. 2013;2013 Pii: bcr2013200734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo A, Morescalchi F, Costagliola C, et al. A novel device to exploit the smartphone camera for fundus photography. J Ophthalmol. 2015;2015: 823139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinekar A, Gilbert C, Dogra M, et al. The KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reporting. Indian J Ophthalmol. 2014;62:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B, Toslak D, Alam MN, et al. Contact-free trans-pars-planar illumination enables snapshot fundus camera for nonmydriatic wide field photography. Sci Rep. 2018;8:8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toslak D, Thapa D, Chen Y, et al. Trans-palpebral illumination: an approach for wide-angle fundus photography without the need for pupil dilation. Opt Lett. 2016;41:2688–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham E Quinn, e-ROP Cooperative Group. Telemedicine approaches to evaluating acute-phase retinopathy of prematurity: study design. Ophthalmic Epidemiol. 2014;21:256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn GE, Ying G, Daniel E, et al. Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol. 2014;132:1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fijalkowski N, Zheng LL, Henderson MT, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): four-years of screening with telemedicine. Curr Eye Res. 2013;38:283–291. [DOI] [PubMed] [Google Scholar]
- 34.Chee RI, Darwish D, Fernandez-Vega A, et al. Retinal telemedicine. Curr Ophthalmol Rep. 2018;6:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah PK, Ramya A, Narendran V. Telemedicine for ROP. Asia Pac J Ophthalmol (Phila). 2018;7:52–55. [DOI] [PubMed] [Google Scholar]
- 36.Valikodath N, Shah P, Chan R, et al. Evaluation of birth weight and gestational age in infants with treatment requiring retinopathy of prematurity in ROPE-SOS trial Poster presentation at: Association for Research in Vision and Ophthalmology 2018 Annual Meeting; April 30-May 3, 2018; Seattle, WA; 2018. [Google Scholar]
- 37.Breidenstein BG, Dai S. Wide-field digital retinal imaging for retinopathy of prematurity screening in Australasia: a survey of practicing ophthalmologists. J Pediatr Ophthalmol Strabismus. 2014;51:375–378. [DOI] [PubMed] [Google Scholar]
- 38.Dai S Screening for retinopathy of prematurity in New Zealand. Retina Today. 2014: 44–46. Retina Today Web site. http://retinatoday.com/pdfs/0314RT_F4_Dai.pdf. Accessed April 18, 2019. [Google Scholar]
- 39.Kiely AE, Wallace DK, Freedman SF, et al. Computer-assisted measurement of retinal vascular width and tortuosity in retinopathy of prematurity. Arch Ophthalmol. 2010; 128:847–852. [DOI] [PubMed] [Google Scholar]
- 40.Rabinowitz MP, Grunwald JE, Karp KA, et al. Progression to severe retinopathy predicted by retinal vessel diameter between 31 and 34 weeks of postconception age. Arch Ophthalmol. 2007;125:1495–1500. [DOI] [PubMed] [Google Scholar]
- 41.Wilson CM, Cocker KD, Moseley MJ, et al. Computerized analysis of retinal vessel width and tortuosity in premature infants. Invest Opthalmol Vis Sci. 2008;49:3577–3585. [DOI] [PubMed] [Google Scholar]
- 42.Ataer-Cansizoglu E, Bolon-Canedo V, Campbell JP, et al. Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity: performance of the “I-ROP” system and image features associated with expert diagnosis. Transl Vis Sci Technol. 2015;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiang MF, Jiang L, Gelman R, et al. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125: 875–880. [DOI] [PubMed] [Google Scholar]
- 44.Abbey AM, Besirli CG, Musch DC, et al. Evaluation of screening for retinopathy of prematurity by ROPtool or a lay reader. Ophthalmology. 2016;123:385–390. [DOI] [PubMed] [Google Scholar]
- 45.Wu KY, Wallace DK, Freedman SF. Predicting the need for laser treatment in retinopathy of prematurity using computer-assisted quantitative vascular analysis. J AAPOS. 2014;18:114–119. [DOI] [PubMed] [Google Scholar]
- 46.Raufi NN, Wallace DK, Freedman SF, et al. Computer-assisted quantification of pre-plus and plus disease in images obtained using Pictor versus video indirect ophthalmoscopy: a pilot study. J AAPOS. 2017;21: 322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelman R, Jiang L, Du YE, et al. Plus disease in retinopathy of prematurity: Pilot study of computer-based and expert diagnosis. J AAPOS. 2007;11:532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell JP, Ataer-Cansizoglu E, Bolon-Canedo V, et al. Expert diagnosis of plus disease in retinopathy of prematurity from computer-based image analysis. JAMA Ophthalmol. 2016;134:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coyner A, Swan R, Campbell JP, et al. Automated fundus image quality assessment in retinopathy of prematurity using deep convolutional neural networks. Ophthalmol Retina. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown JM, Campbell JP, Beers A, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chee RI, Gupta MP, Orlin A, et al. Evaluation of potential systemic adverse events related to fluorescein angiography in pediatric patients Invest Ophthalmol Vis Sci. 2017;58:2431 Paper presented at: 2017 ARVO Annual Meeting; May 7–11, 2017; Baltimore, MD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng EY, Lanigan B, O’Keefe M. Fundus fluorescein angiography in the screening for and management of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2006;43:85–90. [DOI] [PubMed] [Google Scholar]
- 53.Yokoi T, Hiraoka M, Miyamoto M, et al. Vascular abnormalities in aggressive posterior retinopathy of prematurity detected by fluorescein angiography. Ophthalmology. 2009;116:1377–1382. [DOI] [PubMed] [Google Scholar]
- 54.Klufas MA, Patel SN, Ryan MC, et al. Influence of fluorescein angiography on the diagnosis and management of retinopathy of prematurity. Ophthalmology. 2015;122:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell JP, Nudleman E, Yang J, et al. Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. JAMA Ophthalmol. 2017;135:977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zepeda EM, Shariff A, Gillette TB, et al. Vitreous bands identified by handheld spectral-domain optical coherence tomography among premature infants. JAMA Ophthalmol. 2018;136:753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Mangalesh S, Dandridge A, et al. Spectral-domain OCT findings of retinal vascular-avascular junction in infants with retinopathy of prematurity. Ophthalmol Retina. 2018;2:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mangalesh S, Bleicher ID, Chen X, et al. Three-dimensional pattern of extraretinal neovascular development in retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2019;257:677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maldonado RS, Toth CA. Optical coherence tomography in retinopathy of prematurity: looking beyond the vessels. Clin Perinatol. 2013;40:271–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vinekar A, Avadhani K, Sivakumar M, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Opthalmol Vis Sci. 2011;52: 5183–5188. [DOI] [PubMed] [Google Scholar]
- 61.Maldonado RS, Yuan E, Tran-Viet D, et al. Three-dimensional assessment of vascular and perivascular characteristics in subjects with retinopathy of prematurity. Ophthalmology. 2014;121:1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell JP. Why do we still rely on ophthalmoscopy to diagnose retinopathy of prematurity? JAMA Ophthalmol. 2018;136:759–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31:1470–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan RV, Patel SN, Ryan MC, et al. The Global Education Network for Retinopathy of Prematurity (Gen-Rop): Development, Implementation, and Evaluation of A Novel Tele-Education System (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2015;113:T2. [PMC free article] [PubMed] [Google Scholar]
- 65.Patel SN, Martinez-Castellanos MA, Berrones-Medina D, et al. Assessment of a tele-education system to enhance retinopathy of prematurity training by international ophthalmologists-in-training in Mexico. Ophthalmology. 2017;124:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell JP, Swan R, Jonas K, et al. Implementation and evaluation of a tele-education system for the diagnosis of ophthalmic disease by international trainees. AMIA Annu Symp Proc. 2015;2015:366–375. [PMC free article] [PubMed] [Google Scholar]
- 67.Chan RV, Chiang MF, Jonas K. Retinopathy of prematurity: case-based training. November 10, 2015. American Academy of Ophthalmology Web site. https://www.aao.org/interactive-tool/retinopathy-of-prematurity-case-based-training. Accessed April 18, 2019. [Google Scholar]
- 68.Arnold RW. A video educational and consenting system for ROP. J Pediatr Ophthalmol Strabismus. 2012;49:382. [DOI] [PubMed] [Google Scholar]
- 69.Wallace DK, Quinn GE, Freedman SF, et al. Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. J AAPOS. 2008;12:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mackeen L, Ells A. Dynamic documentation of the evolution of retinopathy of prematurity in video format. J AAPOS. 2008;12:349–351. [DOI] [PubMed] [Google Scholar]
- 71.Patel SN, Klufas MA, Douglas CE, et al. Influence of computer-generated mosaic photographs on retinopathy of prematurity diagnosis and management. JAMA Ophthalmol. 2016;134:1283–1289. [DOI] [PubMed] [Google Scholar]
- 72.Ells AL, Holmes JM, Astle WF, et al. Telemedicine approach to screening for severe retinopathy of prematurity: a pilot study. Ophthalmology. 2003; 110:2113–2117. [DOI] [PubMed] [Google Scholar]
- 73.Chiang MF, Wang L, Busuioc M, et al. Telemedical retinopathy of prematurity diagnosis: accuracy, reliability, and image quality. Arch Ophthalmol. 2007;125:1531–1538. [DOI] [PubMed] [Google Scholar]
- 74.Photographic Screening for Retinopathy of Prematurity (Photo-ROP) Cooperative Group. The photographic screening for retinopathy of prematurity study (photo-ROP). Primary outcomes. Retina. 2008;28(3 Suppl):S47–54. [DOI] [PubMed] [Google Scholar]
- 75.Chiang MF, Melia M, Buffenn AN, et al. Detection of clinically significant retinopathy of prematurity using wide-angle digital retinal photography: a report by the American Academy of Ophthalmology. Ophthalmology. 2012;119:1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moral-Pumarega MT, Caserío-Carbonero S, De-La-Cruz-Bértolo J, et al. Pain and stress assessment after retinopathy of prematurity screening examination: indirect ophthalmoscopy versus digital retinal imaging. BMC Pediatr. 2012;12:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson KM, Scott KE, Graff Zivin J, et al. Cost-utility analysis of telemedicine and ophthalmoscopy for retinopathy of prematurity management. Arch Ophthalmol. 2008;126:493–499. [DOI] [PubMed] [Google Scholar]
- 78.Castillo-Riquelme MC, Lord J, Moseley MJ, et al. Cost-effectiveness of digital photographic screening for retinopathy of prematurity in the United Kingdom. Int J Technol Assess Health Care. 2004;20:201–213. [DOI] [PubMed] [Google Scholar]
- 79.Kelkar J, Kelkar A, Sharma S, et al. A mobile team for screening of retinopathy of prematurity in India: Cost-effectiveness, outcomes, and impact assessment. Taiwan J Ophthalmol. 2017;7:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wittenberg LA, Jonsson NJ, Chan RV, et al. Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2012;49:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghergherehchi L, Kim SJ, Campbell JP, Ostmo S, Chan RVP, Chiang MF. Plus disease in retinopathy of prematurity: more than meets the ICROP? Asia Pac J Ophthalmol (Phila). 2018;7:152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davitt BV, Wallace DK. Plus disease. Surv Ophthalmol. 2009;54:663–670. [DOI] [PubMed] [Google Scholar]
- 83.Vinekar A, Bhende P. Innovations in technology and service delivery to improve Retinopathy of Prematurity care. Community Eye Health. 2018;31: S20–S22. [PMC free article] [PubMed] [Google Scholar]
- 84.Jackman WT, Webster JD. On photographing the retina of the living human eye. The Philadelphia Photographer. 1886;23:340–341. [Google Scholar]


