Dear Editor,
Tuberculosis (TB) preventive therapy (TPT) has been recommended as the standard of care for people living with HIV (PLHIV) for over a decade (1), yet global implementation has been inadequate. A three-month (12-dose) regimen of weekly isoniazid and rifapentine (3HP) has similar efficacy and better tolerability versus 6–9 months daily isoniazid (2, 3). Self-administered therapy (SAT) for 3HP was noninferior to directly observed therapy (DOT) in a large randomized trial (4), but SAT performed least well in South Africa, and completing TPT will likely necessitate additional clinic visits for most PLHIV, especially as visits for antiretroviral therapy become less frequent with differentiated service delivery (5). The costs associated with clinic visits (6), including for transportation, food and lost income (7), may therefore present an important barrier to TPT completion. Providing monetary reimbursement for clinic visits may help overcome this barrier. The willingness of patients to accept varying levels of reimbursement for costs associated with 3HP-related clinic visits is unknown. We therefore conducted a willingness-to-accept survey from January 10 to May 8, 2019 among adults ≥18 years accessing HIV/AIDS care at the Mulago AIDS Clinic in Kampala, Uganda.
Participants were asked to estimate costs of completing a typical clinic visit and time/income lost from work. The survey also assessed participants’ stated willingness to accept a 12-week directly observed TPT regimen under different levels of weekly monetary reimbursement. Using a double-bounded contingent valuation format, we first asked, without any mention of reimbursement, “If your doctor recommended that you take this treatment, do you think that you would successfully be able to come to the clinic every week for 12 weeks to take the pills?”. Respondents who answered affirmatively were presumed to be willing to accept the treatment course with no reimbursement. Respondents who answered negatively entered a negotiation starting with an offer of 10,000 Ugandan Shillings (USh)/week ($2.70 US Dollars (USD)) (8). Participants who accepted 10,000 USh were subsequently offered 5,000 USh, whereas those who declined were offered 20,000 USh. Participants who stated an unwillingness to attend clinic visits were asked to state the minimum per-visit reimbursement they would accept to complete the 12-week treatment. All participants were asked to name a fair level of reimbursement per visit. We evaluated cumulative proportions of patients accepting any reimbursement below each willingness-to-accept threshold. We measured associations using logistic regression; multivariable analyses adjusted for age, sex, and income quartile. All analyses were conducted using Stata 14 (StataCorp LP, College Station, TX).
We surveyed 378 consecutive consenting adults. Median age was 39 years (Interquartile Range (IQR): 31–45), 65% were women, and median personal income was 50,000 USh (~$13.57USD) (IQR: 20,000–120,000)/week. Participants reported living a median eight kilometers away from clinic (IQR: 5–15km), requiring a median of one hour of time and 4,000 USh (IQR: 2,500–5,000) each way on transportation. 250 participants (66%) incurred additional costs for food while attending clinic (median 3,000USh, IQR: 2,000–4,000). 229 participants (61%) reported that they would accept treatment under the advice of their healthcare provider without any mention of reimbursement. An additional 83 (22%) reported that they would attend if offered 10,000 USh/visit; only 18 (5%) would accept 5,000 USh. 66 participants (17%) would not accept 10,000 USh, but 19 (5%) reported willingness to attend if the offer were increased to 20,000 USh (Figure: Panel A, solid line). Minimum per-visit reimbursement was valued between 3,500–10,000 USh by seventy participants (19%), between 10,001–20,000 USh by 28 participants (7%), and between 20,001–60,000 USh by 16 participants (4%); 35 participants (9%) stated unwillingness to complete treatment regardless of reimbursement. The median per-visit reimbursement deemed fair was 10,000 USh (IQR: 7,000–15,000) (Figure: Panel A, dashed line). Individuals who lived further from clinic and/or spent more time in transit were less willing to accept reimbursement levels less than 10,000 USh/visit (Figure: Panel B). For 5,000 USh/visit, 55% of individuals spending ≥2 hours in one-way transit to clinic reported willingness to attend versus 70% of those spending <2 hours; similarly, 50% of those living >15km from clinic would attend versus 70% of those living ≤15km. These differences persisted after adjustment for age, sex, and income quartile (adjusted odds ratio [95% confidence interval]: 0.55 [0.35–0.87] for time from clinic, 0.46 [0.28–0.75] for distance from clinic).
Figure. Cumulative willingness to accept financial reimbursement for tuberculosis preventive therapy clinic visits.
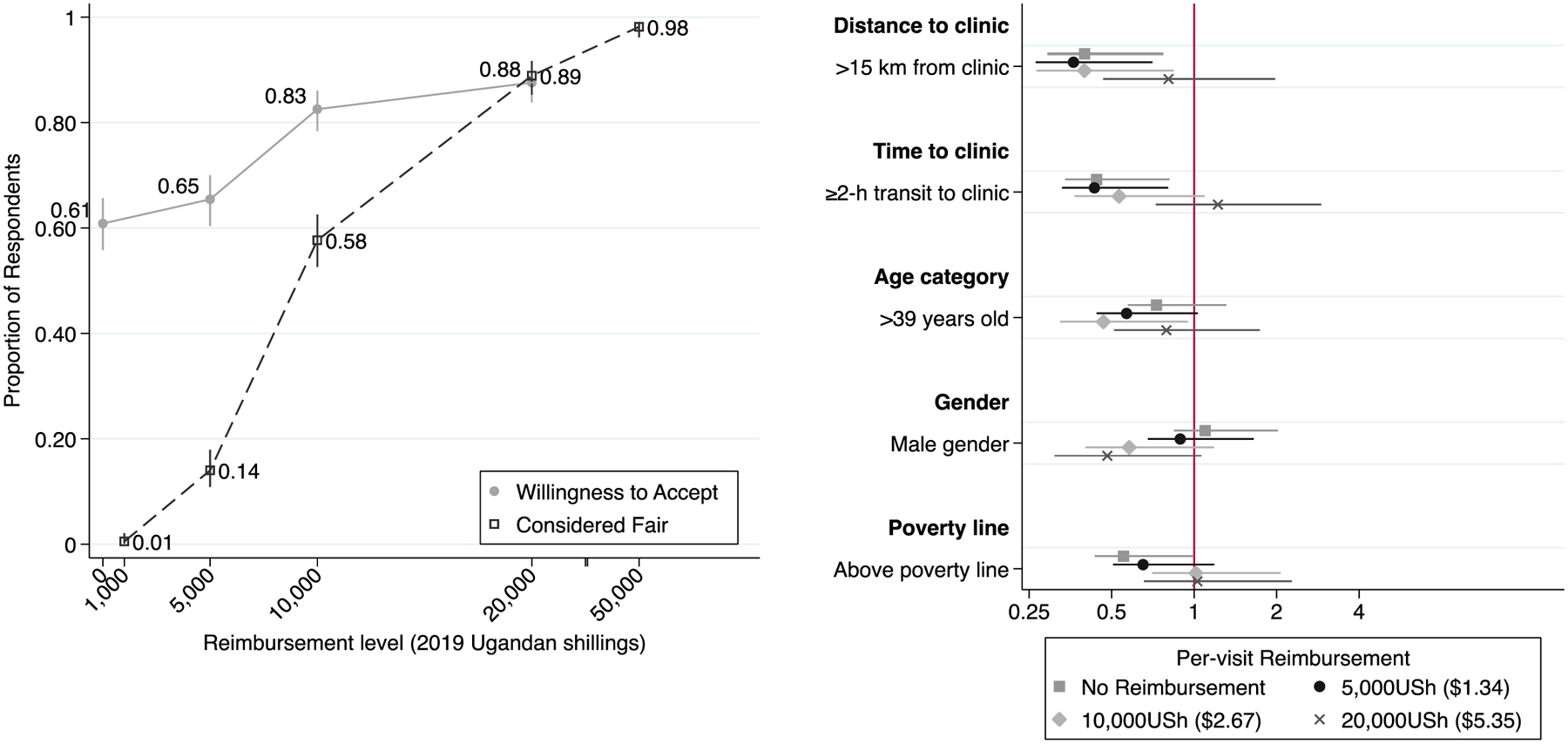
Panel A – Overall: The solid line and corresponding markers indicate the proportion (and corresponding 95% confidence interval) of patients attending an HIV/AIDS clinic in Kampala, Uganda, who stated a willingness to attend weekly clinic visits for tuberculosis preventive therapy if a per-visit financial reimbursement were offered at the value listed on the x-axis on a contingent valuation survey. The dashed line and corresponding markers indicate the proportion of patients who, when asked to state a per-visit reimbursement they would consider fair, provided a value at or below that listed on the x-axis. All financial reimbursement levels are provided in 2019 Ugandan shillings (5,000 Ugandan shillings = 1.35 US dollar in March 2019 (8)). The distance between the 20,000 and 50,000 shilling labels is reduced to enhance readability. Panel B – Patient sub-groups: The square, circle, diamond and cross-shaped markers and corresponding lines indicate the unadjusted odds ratio (and corresponding 95% confidence intervals) comparing participants’ willingness to attend clinic visits for tuberculosis preventive therapy for no reimbursement, 5,000 Ugandan shillings (USh), 10,000 USh, or 20,000 USh, respectively. Odds ratios compare subgroups of age (>39 years old vs. ≤39 years), gender (male vs. female), time to clinic (≥2 hours in transit time vs. <2 hours), distance to clinic (>15 kilometers to clinic vs. ≤15 kilometers), and poverty threshold (above poverty line ($1.90USD/day) vs. below).
Our findings highlight the financial realities that patients in low-income settings must consider when recommended to take TPT. Even in this densely populated urban setting, the median cost of two-way transport (8,000USh) represented 16% of the median weekly personal income (50,000USh), and two-thirds of patients incurred additional costs (median 3,000USh) for food. The reported median fair reimbursement level (10,000USh) was congruent with these expenses. Despite these costs, 61% of patients reported a willingness to attend clinic on the recommendation of their physician – but given this substantial financial burden, stated willingness to attend might overestimate real-life (“revealed”) behavior (9). Social desirability bias may also have led participants to overestimate their willingness to attend clinic without reimbursement. The actual impact of reimbursement on improving clinic attendance for TPT might therefore be greater. Our results also underscore the challenges faced by patients living farther from clinic (>15km or two hours’ transit). These patients reported substantially lower willingness to attend clinic for TPT – a difference that was overcome with an offer of larger reimbursements. Our findings are consistent with other studies conducted in similar contexts that have documented distance to clinic as a major barrier to patient adherence (6), including TPT completion (10). Lack of funds for transportation to clinic is a barrier that is not specific to TPT; this structural barrier is a broad issue for patients in sub-Saharan Africa seeking a variety of routine (11, 12) and emergency healthcare services (13, 14). Our findings suggest that patient-centered strategies, such as providing reimbursements, might help to lessen the burden of DOT or clinic visits for refills and defray costs of accessing TB care, particularly for those traveling greater distances. Future analyses could compare the cost-effectiveness of providing monetary reimbursements to patients against home-based delivery and monitoring of TPT by lay health workers or digital adherence technologies.
In summary, we provide preliminary evidence that offering patients small monetary reimbursements of US$2–3 (lower than the current cost of the weekly 900mg rifapentine dose (15)) for attending clinic visits could be a powerful tool for improving uptake of TPT among PLHIV in high-burden, resource-limited settings. Larger reimbursements may be appropriate for patients living long distances from clinic. Future studies should consider measuring revealed preferences among patients actively taking TPT. Nevertheless, these results suggest that small reimbursements could serve as a low-cost and effective means to substantially enhance uptake of TB preventive therapy among PLHIV in high TB/HIV-burden settings.
ACKNOWLEDGEMENTS
The authors thank the administration, staff and patients at the Mulago ISS clinic for their time and participation, and the research administration of the Uganda TB Implementation Research Consortium (U-TIRC) and the Infectious Diseases Research Collaboration (IDRC). J.K. and D.D. analyzed the data, and J.K., A.K. and D.D. wrote the original letter. A.C., F.S., and C.B. provided input on the letter draft. A.C., F.S., D.D., J.N., M.K. and A.K. conceived of the study. A.M., F.W., J.N., and J.S. collected the data. The study was supported by a grant from the U.S. National Institutes of Health (R01HL144406). The authors have no conflicts of interest to declare.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report. Geneva, Switzerland; 2018. [Google Scholar]
- 2.Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–66. [DOI] [PubMed] [Google Scholar]
- 4.Belknap R, Holland D, Feng PJ, Millet JP, Cayla JA, Martinson NA, et al. Self-administered Versus Directly Observed Once-Weekly Isoniazid and Rifapentine Treatment of Latent Tuberculosis Infection: A Randomized Trial. Ann Intern Med. 2017;167(10):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathmanathan I, Pevzner E, Cavanaugh J, Nelson L. Addressing tuberculosis in differentiated care provision for people living with HIV. Bull World Health Organ. 2017;95(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4(7):e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–65. [DOI] [PubMed] [Google Scholar]
- 8.Currency Converter [Available from: https://www.exchange-rates.org/Rate/USD/UGX/3-1-2019.
- 9.Urama KC, Hodge ID. Are stated preferences convergent with revealed preferences? Empirical evidence from Nigeria. Ecological Economics. 2006;59(1):24–37. [Google Scholar]
- 10.Munseri PJ, Talbot EA, Mtei L, Fordham von Reyn C. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis. 2008;12(9):1037–41. [PubMed] [Google Scholar]
- 11.Fisher E, Lazarus R, Asgary R. Attitudes and Perceptions Towards Access and Use of the Formal Healthcare Sector in Northern Malawi. J Health Care Poor Underserved. 2017;28(3):1104–15. [DOI] [PubMed] [Google Scholar]
- 12.Sibeudu FT, Uzochukwu BS, Onwujekwe OE. Investigating socio-economic inequity in access to and expenditures on routine immunization services in Anambra state. BMC Res Notes. 2017;10(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine AC, Presser DZ, Rosborough S, Ghebreyesus TA, Davis MA. Understanding barriers to emergency care in low-income countries: view from the front line. Prehosp Disaster Med. 2007;22(5):467–70. [DOI] [PubMed] [Google Scholar]
- 14.Geleto A, Chojenta C, Musa A, Loxton D. Barriers to access and utilization of emergency obstetric care at health facilities in sub-Saharan Africa: a systematic review of literature. Syst Rev. 2018;7(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLuca A, Frick M, Lessem E, Kanouse J, Wegener D, Mingote LR. Activism on rifapentine pricing: removing cost barriers to improve the uptake of tuberculosis research innovations. Public Health Action. 2014;4(4):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


