Abstract
This study comprehensively evaluated the association between known circulating tumor necrosis factor (TNF) superfamily ligands and receptors and the development of early progressive renal decline (PRD) leading to end-stage kidney disease (ESKD) in Type 1 diabetes (T1D). The Macro-Albuminuria Study comprised of 198 individuals, and the Micro-Albuminuria Study consisted of 148 individuals. All individuals had normal renal function and were followed for 7–15 years to determine estimate glomerular filtration rate (eGFR) slopes and to ascertain onset of ESKD. Plasma concentrations of 25 TNF superfamily proteins were measured using proximity extension assay applied in the OLINK proteomics platform. In the both studies risk of early PRD, determined as eGFR loss greater or equal to 3 ml/min/1.73m2/year, was associated with elevated circulating levels of 13 TNF receptors out of 19 examined. In the Macro-Albuminuria Study, we obtained similar findings for risk of progression to ESKD. These receptors comprised: TNF-R1A, -R1B, -R3, -R4, -R6, -R6B, -R7, -R10A, -R10B, -R11A, -R14, -R21, and -R27. Serial measurements showed that circulating levels of these TNF receptors had increased before the onset of PRD. In contrast, none of the 6 measured TNF ligands showed association with risk of early PRD. The disease process that underlies PRD which leads to ESKD in T1D is enriched with up-regulated levels of multiple TNF receptors, a profile also seen in autoimmune disorders. The mechanisms of this enrichment may be causally related to the development of PRD in T1D and must be investigated further. Some of these receptors may be used as new predictors of risk of ESKD.
Keywords: Tumor necrosis factor (TNF) superfamily, TNF receptors, progressive renal decline, type 1 diabetes, diabetic kidney disease, end-stage kidney disease
INTRODUCTION
Over the last 30 years an important role of the tumor necrosis factor (TNF) superfamily proteins has been established in inflammation and in the etiology of autoimmune diseases such as rheumatoid arthritis (RA), Crohn’s disease (CD), multiple sclerosis (MS) and systemic lupus erythematosus (SLE) (1–7). We recently demonstrated the importance of some of these proteins in the development of diabetic kidney disease (DKD) (8–10). The TNF superfamily proteins comprise 19 structurally related ligands (TNF-Ls) and their 30 receptors (TNF-Rs) (1, 3). Almost all the TNF superfamily proteins can be measured in plasma and serum.
Our previous studies firmly established an association between levels of circulating TNF-R1A (TNF-R1) and TNF-R1B (TNF-R2) and risk of the development of progressive renal decline (PRD) leading to end-stage kidney disease (ESKD) in Type 1 diabetes (T1D) and Type 2 diabetes (T2D) (9, 10). Our findings about the two TNF receptors were replicated in multiple subsequent studies (11–15). Recently, we examined a much larger number of circulating inflammatory proteins (n= 194) in a follow-up study of several cohorts of T1D and T2D and identified 17 KRIS (Kidney Risk Inflammatory Signature) proteins that were strongly associated with 10-year risk of ESKD; among them 2 were previously discovered and 4 were new TNF receptors (8). Since that study included mainly individuals with late PRD, i.e. individuals with impaired renal function, it is unknown whether elevated levels of these receptors are associated with the development of early PRD, i.e. individuals with normal renal function. Confirmation of such a hypothesis would imply that elevated levels of these receptors may be causally related to the development of PRD in T1D. To test this hypothesis, we conducted two nested case-control studies: one in individuals with Macro-Albuminuria and the other in individuals with Micro-Albuminuria. Similar findings in both studies would indicate that the effect of TNF proteins on early PRD is independent from the levels of albuminuria.
In our previous study we used the SOMAscan platform to identify 17 KRIS proteins (8). That platform used unique single stranded sequences of DNA or RNA, referred to as aptamers, to recognize folded protein epitopes; usually 1 aptamer is used to recognize 1 protein. Recently a high-throughput OLINK proteomics platform that applies proximity extension assay (PEA) became available (16). The basis of PEA is a dual-recognition immunoassay where two matched antibodies labeled with unique DNA oligonucleotides simultaneously bind to a target protein in solution. This brings the two antibodies into proximity, allowing their DNA oligonucleotides to hybridize, serving as a template for a DNA polymerase-dependent extension step. This creates a double-stranded DNA “barcode” which is unique for the specific antigen and quantitatively proportional to the initial concentration of target protein. These unique properties of the OLINK provided excellent specificity and precise measurements of TNF superfamily proteins.
In the present study we applied this newer proteomics platform to measure 19 circulating TNF receptors and 6 TNF ligands. We aimed to validate the previous findings obtained using SOMAscan and search in a comprehensive way for new TNF proteins so a profile of TNF ligands and receptors that is associated with risk of early and late PRD could be established. Once the profile was established, we compared it with profiles of TNF proteins in autoimmune disorders where abnormalities in circulating TNF proteins are observed and involved in their etiologies (1–7).
RESULTS
Study design and selection of study subjects
Our investigation comprised two nested case-control studies. Participants for these studies were selected from among 526 individuals with Macro-albuminuria and 563 individuals with Micro-albuminuria participating in the T1D Joslin Kidney Study (JKS). These participants were followed for 7–15 years to determine estimated glomerular filtration rate (eGFR) slope and onset of ESKD. From among those with Macro-albuminuria, we selected 103 cases with ESKD and 95 randomly selected non-cases without ESKD to be included in the Macro-Albuminuria Study. From among those with Micro-Albuminuria, we randomly selected 74 decliners (cases) and 74 non-decliners (non-cases) to be included in the Micro-Albuminuria study. PRD was recognized if the patient’s long-term eGFR loss was greater than or equal to 3 ml/min/1.73m2/year or they developed ESKD within 7–15 years of follow-up. Figure 1 outlines the study design and selection of individuals.
Figure 1.
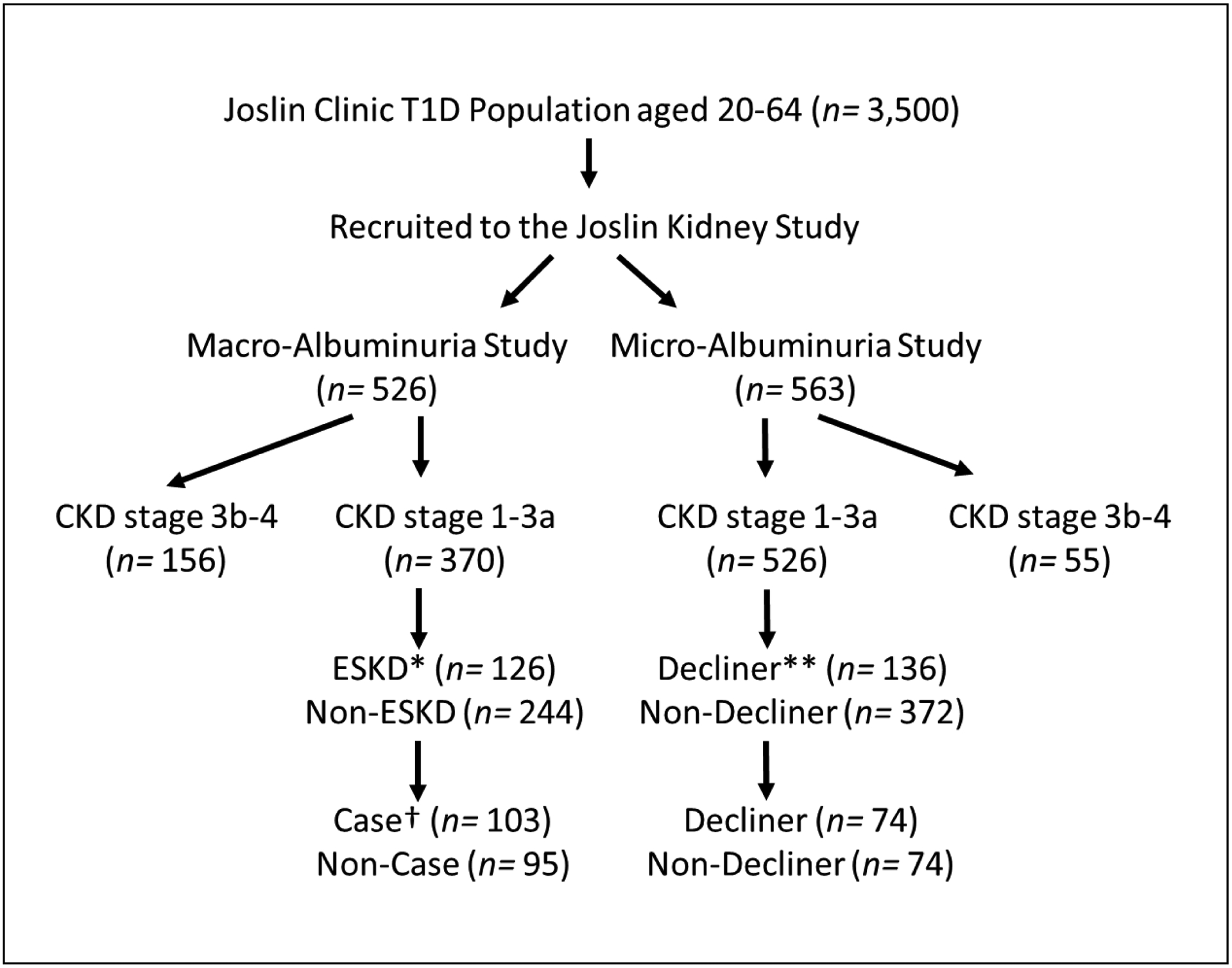
Selection of type 1 diabetes (T1D) patients for the two nested case-control studies. Joslin Kidney Study (JKS) enrolled 526 individuals with Macro-Albuminuria and 563 with Micro-Albuminuria. They were followed for 7–15 years to determine eGFR slope and onset of ESKD. From among those with Macro-Albuminuria, 103 individuals with ESKD (Cases) and 95 randomly selected patients without ESKD (Non-Cases) were selected. From among patients with Micro-Albuminuria, 74 decliners (Cases) and 74 non-decliners (Non-Cases) were randomly selected. *“ESKD” indicates individuals who developed end-stage kidney disease (ESKD) during the follow-up period. **“Decliners” indicates individuals who had eGFR loss ≥3 ml/min/1.73m2/year. †“Cases” represents the patients with T1D who developed ESKD within 15 years of follow-ups. CKD, chronic kidney disease. eGFR, estimated glomerular filtration rate.
Characteristics of study groups
In total, there were 198 individuals in the Macro-Albuminuria Study and 148 in the Micro-albuminuria study. Ninety five percent of participants included in these studies were Caucasian. The first study included 24 individuals who had multiple examinations with plasma specimens obtained before, around, and after onset of PRD. Individuals in this sub-cohort were used to examine changes in concentration of circulating TNF proteins with relation to the onset of PRD. Table 1 compares characteristics of the cases versus non-cases in each study. Many of the clinical characteristics were very similar between cases and non-cases and did not differ between studies. In the both studies, cases had significantly higher HbA1c and urinary albumin/creatinine ratio (ACR). In addition, cases in the Macro-albuminuria Study had lower baseline eGFR than non-cases. During follow-up, by design, cases in the both studies had very fast annual eGFR loss (eGFR slope) in comparison with non-cases.
Revised Table 1.
Clinical characteristics of Macro- and Micro-Albuminuria Studies.
| Macro-Albuminuria Study | Micro-Albuminuria Study (n= 148) | ||||
|---|---|---|---|---|---|
| Main cohort (n= 198) | Subcohort (n= 24) | ||||
| Cases* (n= 103) | Non-Cases (n= 95) | Decliners** (n= 74) | Non-Decliners (n= 74) | ||
| At baseline | |||||
| Age, year | 36 (32, 42) | 38 (32, 42) | 39 (33, 47) | 35 (26, 39) | 36 (28, 45) |
| Men, % | 56.3 | 61.1 | 62.5 | 48.7 | 64.9 |
| Duration of diabetes, year | 21 (17, 29) | 24 (17, 31) | 26 (21, 33) | 15 (9, 27) | 23 (17, 31) |
| Systolic BP, mmHg | 132 (118, 143) | 130 (120, 143) | 132 (118, 142) | 128 (119, 140) | 118 (111, 122) |
| Diastolic BP, mmHg | 80 (74, 88) | 80 (71, 85) | 82 (78, 87) | 78 (70, 86) | 77 (70, 80) |
| ACE inhibitor/ARB use, % | 61.5 | 58.3 | 60.2 | 64.5 | 76.0 |
| HbA1c, % | 9.6 (8.6, 10.9) | 8.8 (8.0, 9.8) | 9.3 (7.9, 10.7) | 9.7 (8.7, 11.4) | 8.6 (7.4, 9.9) |
| eGFR, ml/min/1.73m2 | 86 (66, 109) | 107 (85, 117) | 102 (76, 112) | 115 (99, 126) | 111 (99, 122) |
| ACR, mg/g | 1210 (494, 2280) | 503 (343, 1158) | 586 (343, 1694) | 84 (33, 286) | 33 (19, 63) |
| During Follow-up | |||||
| eGFR slope, ml/min/1.73m2/year | −9.7 (−15.2, −6.6) | −2.2 (−4.0, −1.1) | −7.1 (−9.8, −5.9) | −7.1 (−10.7, −5.1) | −1.0 (−1.7, −0.4) |
| All ESKD during 15 years, n (%) | 103 (52.0) | 12 (50.0) | 11 (7.4) | ||
| Death unrelated to ESKD, n (%) | 0 (0.0) | 17 (17.9) | 2 (8.3) | 10 (13.5) | 5 (6.8) |
“Cases” represents the patients with T1D who developed ESKD within 15 years of follow-ups.
“Decliners” indicates the patients whose eGFR loss were greater than or equal to 3 ml/min/1.73m2/year. BP, blood pressure; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; ACR, albumin/creatinine ratio; ESKD, end-stage kidney disease.
Circulating levels of TNF receptors and ligands and risk of early PRD
Our OLINK platform measured plasma concentration of 19 TNF receptors and 6 TNF ligands (Table 2). Using logistic regression 17 TNF receptors were found to be associated with progression to ESKD in the Macro-Albuminuria Study. In the Micro-Albuminuria Study, 13 out of these 17 TNF receptors were confirmed to be associated with as eGFR loss ≥3 ml/min/1.73m2/year. Notice that the results were similar when we used eGFR loss ≥5 ml/min/1.73m2/year (Supplementary Table S1). Following is the list of these TNF receptors that includes abbreviated genetic names and frequently used protein names: TNF-R1A (TNF-R1), TNF-R1B (TNF-R2), TNF-R3 (LTBR), TNF-R4 (OX4), TNF-R6 (FAS), TNF-R6B (DcR3), TNF-R7 (CD27),TNF-R10A (TRAIL-R1), TNF-R10B (TRAIL-2), TNF-R11A (RANK), TNF-R14 (LIGHTR), TNF-R21 (DR6) and TNF-R27 (EDA2R). In contrast, none of the 6 TNF ligands was associated with early PRD in either study. Since the magnitude and direction of associations of TNF receptors with the risk of ESKD in the Macro-Albuminuria Study and renal decline measured as eGFR loss ≥3 ml/min/1.73m2/year in the Micro-Albuminuria Study were similar, the 2 studies were combined in further analyses.
Table 2.
Comparison of effect estimates of tumor necrosis factor (TNF) proteins on risk of earl progressive renal decline (PRD) between Macro- and Micro-Albuminuria Studies.
| Macro-Albuminuria Study Risk of ESKD 15years n= 198 |
Micro-Albuminuria Study eGFR loss ≥3 ml/min/year n= 148 |
||||
|---|---|---|---|---|---|
| Gene Name | Protein name | OR | FDR | OR | P value |
| TNF receptors associated with the both outcome | |||||
| TNF-R1A | TNF-R1 | 1.79 | <.0001 | 1.64 | 0.0015 |
| TNF-R1B | TNF-R2 | 1.80 | <.0001 | 1.86 | 0.0001 |
| TNF-R3 | LTBR | 1.92 | <.0001 | 1.46 | 0.0140 |
| TNF-R4 | OX40 | 1.66 | <.0001 | 1.64 | 0.0017 |
| TNF-R6 | FAS | 1.37 | 0.0249 | 1.49 | 0.0090 |
| TNF-R6B | DcR3 | 1.96 | <.0001 | 1.56 | 0.0043 |
| TNF-R7 | CD27 | 1.87 | <.0001 | 1.73 | 0.0006 |
| TNF-R10A | TRAIL-R1 | 1.90 | <.0001 | 1.61 | 0.0024 |
| TNF-R10B | TRAIL-R2 | 2.43 | <.0001 | 1.40 | 0.0290 |
| TNF-R11A | RANK | 2.07 | <.0001 | 1.43 | 0.0200 |
| TNF-R14 | HVEM | 1.94 | <.0001 | 1.60 | 0.0024 |
| TNF-R21 | DR6 | 1.47 | 0.0066 | 1.49 | 0.0100 |
| TNF-R27 | EDA2R | 1.98 | <.0001 | 1.45 | 0.0150 |
| TNF Receptors inconsistent associations | |||||
| TNF-R10C | TRAIL-R3 | 1.21 | 0.1672 | 1.22 | 0.1900 |
| TNF-R11B | OPG | 1.19 | 0.2155 | 1.36 | 0.0410 |
| TNF-R12A | TWEAKR | 1.76 | 0.0002 | 1.24 | 0.1600 |
| TNF-R13B | TACI | 1.51 | 0.0038 | 1.30 | 0.0820 |
| TNF-R19 | TROY | 2.03 | <.0001 | 1.29 | 0.0890 |
| TNF-R19L | RELT | 1.90 | <.0001 | 1.24 | 0.1400 |
| Findings for TNF ligands | |||||
| TNF-L5 | CD40-L | 1.23 | 0.1575 | 0.93 | 0.6300 |
| TNF-L6 | FASLG | 1.24 | 0.1394 | 1.13 | 0.4100 |
| TNF-L7 | CD70 | 1.21 | 0.1816 | 1.39 | 0.0320 |
| TNF-L10 | TRAIL | 1.17 | 0.2401 | 1.36 | 0.0460 |
| TNF-L13 | APRIL | 1.19 | 0.2167 | 1.18 | 0.2700 |
| TNF-L13B | BAFF | 1.12 | 0.3812 | 1.33 | 0.0580 |
Odds ratio (OR) was estimated as one quartile change in baseline concentration of TNF proteins and stratified by each batch in order to consider the inter-variation between batches. False discovery rate (FDR) of q value <0.05 in the Macro-albuminuria Study and nominal p value <0.05 in the Micro-Albuminuria Study were considered as statistical significance. eGFR, estimated glomerular filtration rate. ESKD, end-stage kidney disease.
Inter-correlation among circulating levels of TNF receptors and with eGFR slope
To characterize inter-correlation among the TNF receptors, we performed hierarchical clustering analysis (Figure 2A) and Spearman rank correlation (Supplementary Figure S1) among individuals in the combined studies. The cluster analysis grouped the 13 TNF receptors into 4 clusters (Figure 2A). Spearman correlation coefficients were very high among the receptors in clusters #1 and #2 and lower in #3 and #4 (Supplementary Figure S1). To identify the TNF receptors most strongly associated with eGFR slope in the combined studies, variable importance of predicting annual eGFR decline was performed by partial least squares (PLS). Among the 13 TNF receptors, 6 fulfilled the variable importance: TNF-R1A, TNF-R7, TNF-R6, TNF-R6B, TNF-R10A and TNF-R11A (Figure 2B).
Figure 2.
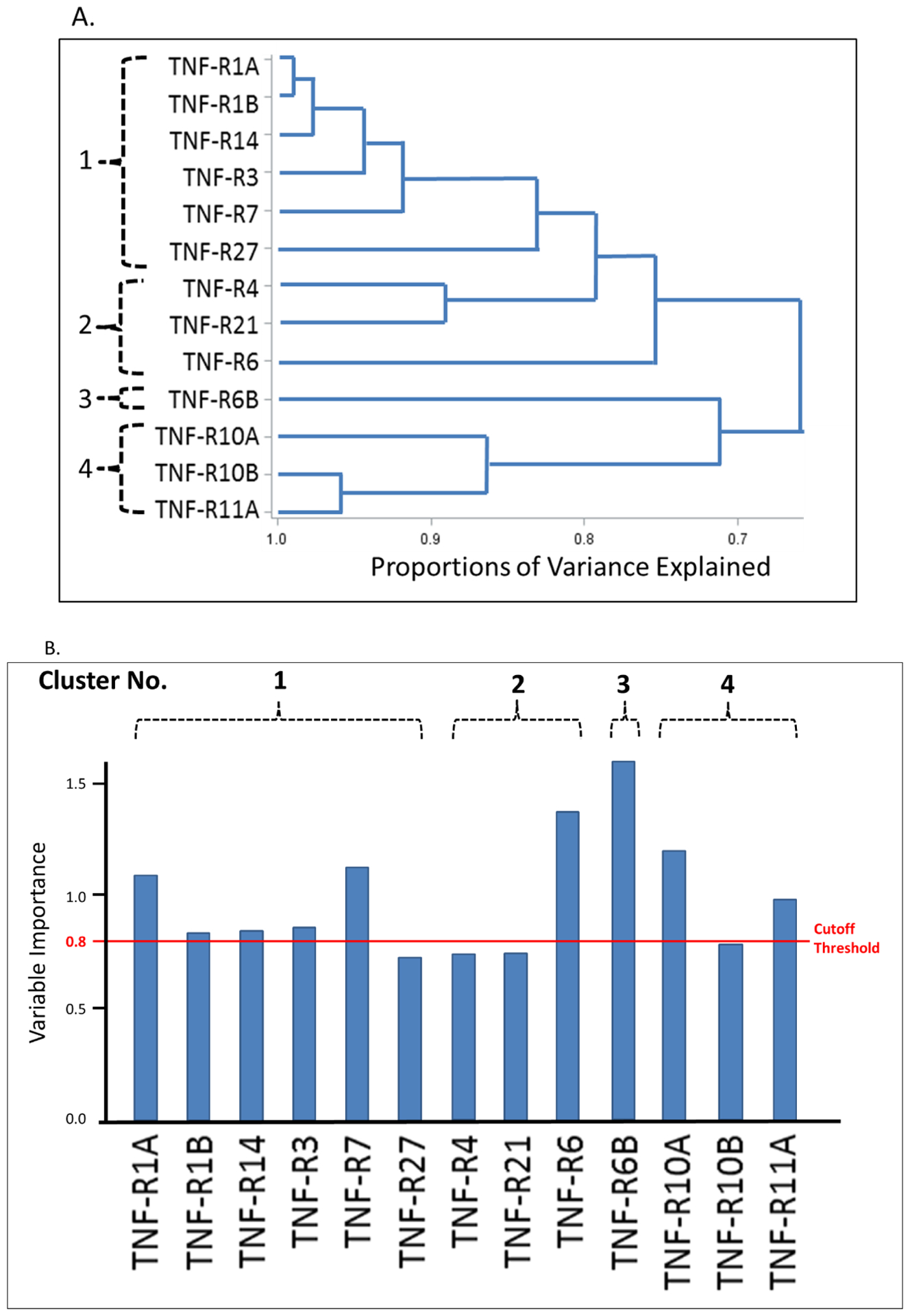
Associations among 13 tumor necrosis factor (TNF) receptors and eGFR slope in the combined studies (n = 343); (A) Hierarchical clustering analysis of the selected TNF receptors; (B) Importance of selected TNF receptors with respect to eGFR slope from partial least square regression; Cutoff threshold of variable importance in partial least square regression is 0.8. The variables above the cutoff are considered important. The TNF receptors are ordered by clusters in (A). eGFR, estimated glomerular filtration rate.
Circulating TNF receptors as predictors of progression to ESKD
The ability of the TNF receptors to predict development of PRD, independent from TNF-R1A and TNF-R1B, was examined in Macro-Albuminuria Study. Table 3 presents the sequential predictive logistic models according to the variables considered. In addition to the clinical variables, TNF-R1A and TNF-R1B contributed equally to the prediction of ESKD (Model #2 and #3). However, when the model included the remaining TNF receptors, the odds ratio for TNF-R1A was still greater than 1 but not statistically significant, and odds ratio for TNF-R1B was less than 1 and also not significant. From among the remaining 11 TNF receptors, TNF-R6B and TNF-R11A contributed independently and significantly into the predictive power of the model (Model #4). It is possible that other TNF receptors might contribute to the predictive model if the study group was much larger.
New Table 3.
Predictive logistic models for ESKD according to baseline variables considered. Models were developed using 196 individuals selected for Macro-Albuminuria Study.
| Model #1 | Model #2 | Model #3 | Model #4* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| eGFR | 0.98 | (0.96–0.99) | 0.0007 | 0.98 | (0.97–1.00) | 0.0545 | 0.98 | (0.97–1.00) | 0.0203 | 0.99 | (0.97–1.01) | 0.1696 |
| HbA1c | 1.57 | (1.26–1.96) | <.0001 | 1.60 | (1.28–2.00) | <.0001 | 1.60 | (1.28–2.00) | <.0001 | 1.50 | (1.19–1.89) | 0.0006 |
| ACR | 1.70 | (1.26–2.30) | 0.0005 | 1.62 | (1.20–2.20) | 0.0018 | 1.63 | (1.20–2.21) | 0.0016 | 1.66 | (1.20–2.30) | 0.0024 |
| TNF-R1A | – | – | – | 1.49 | (1.04–2.13) | 0.0279 | – | – | – | 1.24 | (0.69–2.22) | 0.4706 |
| TNF-R1B | – | – | – | – | – | – | 1.43 | (1.02–2.00) | 0.0373 | 0.73 | (0.40–1.36) | 0.3246 |
| TNF-R6B | – | – | – | – | – | – | – | – | – | 1.49 | (1.03–2.17) | 0.0357 |
| TNF-R11A | – | – | – | – | – | – | – | – | – | 1.73 | (1.08–2.76) | 0.0227 |
| C statistics | 0.774 | 0.788 | 0.784 | 0.815 | ||||||||
Model #4: This model was developed by including 5 markers (eGFR, ACR, HbA1c, TNF-R1A and –R1B) and adding the TNF receptors that were selected from remaining 11 TNF receptors using backward elimination (p-for-stay = 0.1).
The effects of eGFR and HbA1c on development of ESKD were estimated as 1 ml/min/1.73m2 increase and as 1% increase, respectively. The effects of ACR, TNF-R1A, TNF-R1B, TNF-R6B, and TNF-R11A on development of ESKD were estimated as one quartile increase. ESKD, end-stage kidney disease. OR, odds ratio
Levels of circulating TNF receptors before and after onset of PRD
To investigate whether levels of the 13 TNF receptors were elevated before or after onset of early PRD, we examined longitudinal changes in concentration of these receptors in 24 individuals in the sub-cohort of Macro-Albuminuria. In these individuals we were able to observe a clear breakpoint in eGFR trajectory separating a period of no decline of renal function (median 2.6 years after baseline) and subsequent fast decline (median 2.7 years after 1st follow-up). The mean eGFR was 97 ml/min/1.73m2 at baseline, 92 ml/min/1.73m2 at the 1st follow-up (around the onset of the decline), and it dropped to 53 ml/min/1.73m2 at the 2nd follow-up (Figure 3A). The mean eGFR slope from baseline to the 1st follow-up was −0.96 ml/min/1.73m2/year and −6.66 ml/min/1.73m2/year from the 1st to the 2nd follow-up. No changes were observed in urinary ACR (Figure 3A) and HbA1c (Supplementary Figure S2). The 13 TNF receptors were measured at the 3 time points and Figure 3B shows crude changes in concentration for each protein. Concentration of most TNF receptors increased significantly between baseline and 1st follow-up (before onset of eGFR decline). The increase between 1st and 2nd (after onset of eGFR decline) was almost twice as high as before onset for all the TNF receptors. The largest changes in 4 receptors are shown in Figure 3C.
Figure 3.
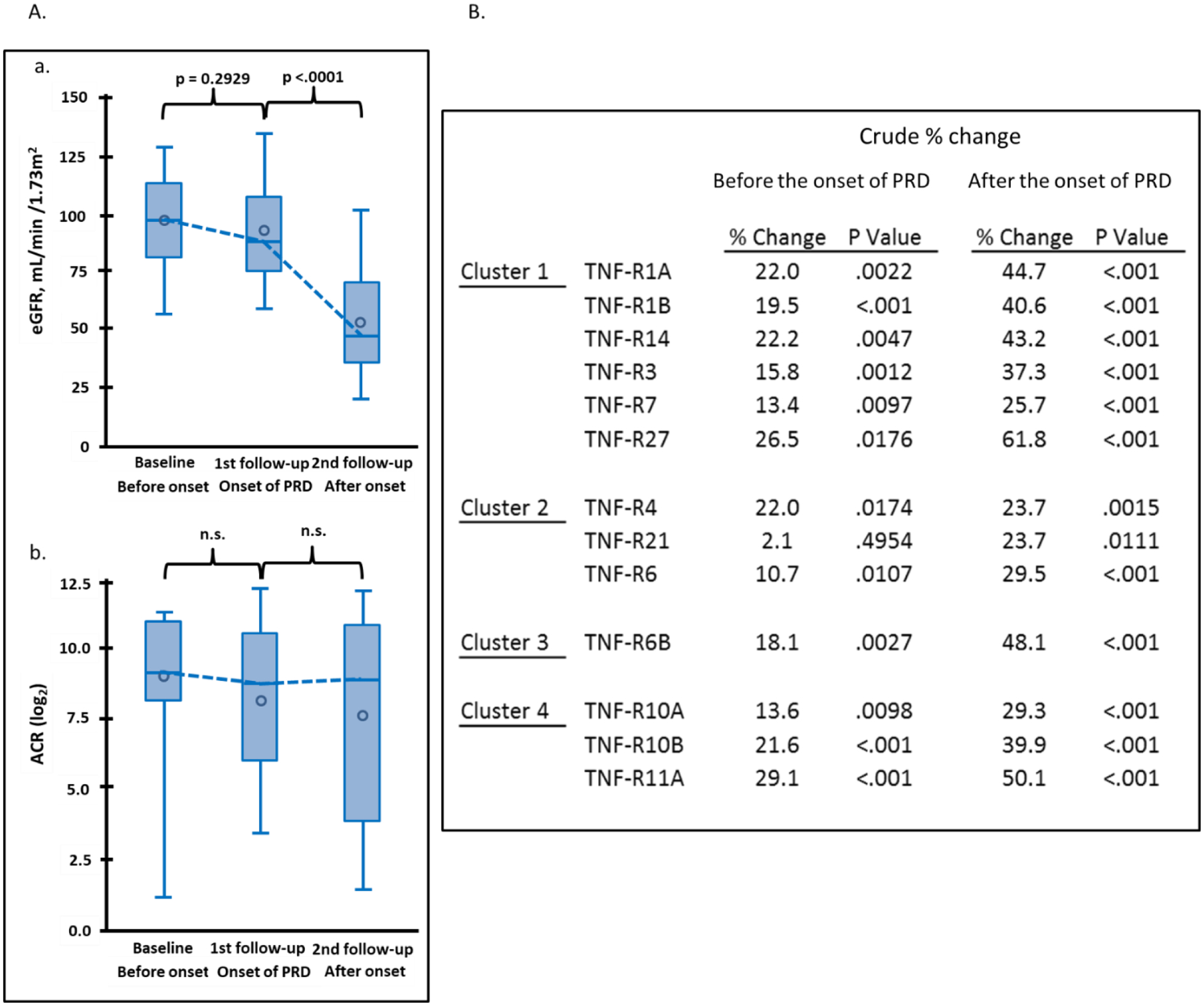
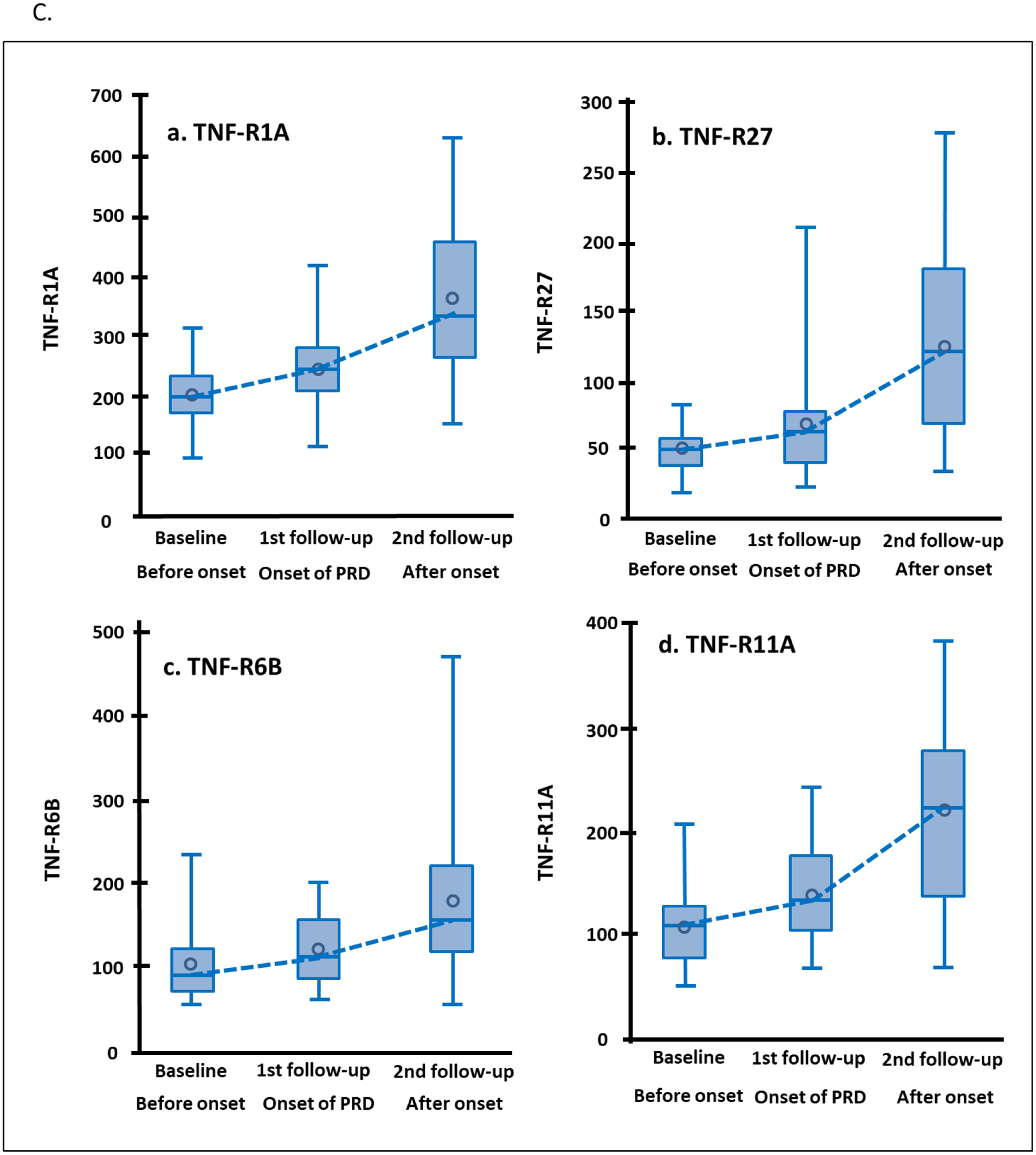
Trajectories of clinical covariates and 13 tumor necrosis factor (TNF) receptors in 24 individuals in Macro-Albuminuria sub-cohort who had measurements performed 2.6 years before (baseline) onset, at onset (1st follow-up) and 2.7 years after onset of progressive renal decline (PRD) (2nd follow-up). (A) Trajectories of eGFR (ml/min/1.73m2) and ACR (log2) according to three examinations. (B) Crude percent changes in plasma concentration of 13 TNF receptors during period before and after onset of PRD. Percent changes before onset of PRD: plasma concentration of TNF receptor at 1st follow-up – plasma concentration for the same TNF receptor at baseline expressed as % change. Percent changes after onset of PRD: plasma concentration of TNF receptor at 2nd follow-up – plasma concentration for the same TNF receptor at 1st follow-up expressed as % change. The TNF receptors are ordered according to clusters in Figure 2A. (C) Trajectories of plasma concentrations of 4 TNF receptors with the most significant increases over time. To easily capture the changes in the TNF receptors over time, the NPX values of TNF receptors in log2-scale were transformed: 2 to (NPX values) -th (power) as if they were measured as “absolute” values (vertical axes). eGFR, estimated glomerular filtration rate. ACR, urinary albumin creatinine ratio.
Increases in concentration of the TNF receptors before onset of early PRD indicate these proteins are involved or indicate the disease process that underlies the development of PRD. To exclude the possibility that these increases might be confounded by eGFR decline during the same period, we fitted a linear repeated measures model simultaneously estimating the effect of eGFR decline on concentration and an additional change in concentration that remains unexplained by eGFR slope. The changes in concentration after adjusting for eGFR decline were significant for all the TNF receptors except TNF-R21 (Figure 4). To further test whether the % changes in concentration of these TNF receptors might be co-regulated by the same mechanisms/factors, we examined Spearman correlations among these % changes (Figure 5). This analysis revealed that % changes in TNF receptors in clusters #1 and #2 were highly inter-correlated. In contrast % change in concentration of TNF-R6B (cluster #3) was least correlated with other TNF receptors. Similarly, % changes in the 3 TNF receptors in cluster #4 were correlated only moderately with % changes in other TNF receptors but they were highly correlated among themselves.
Figure 4.
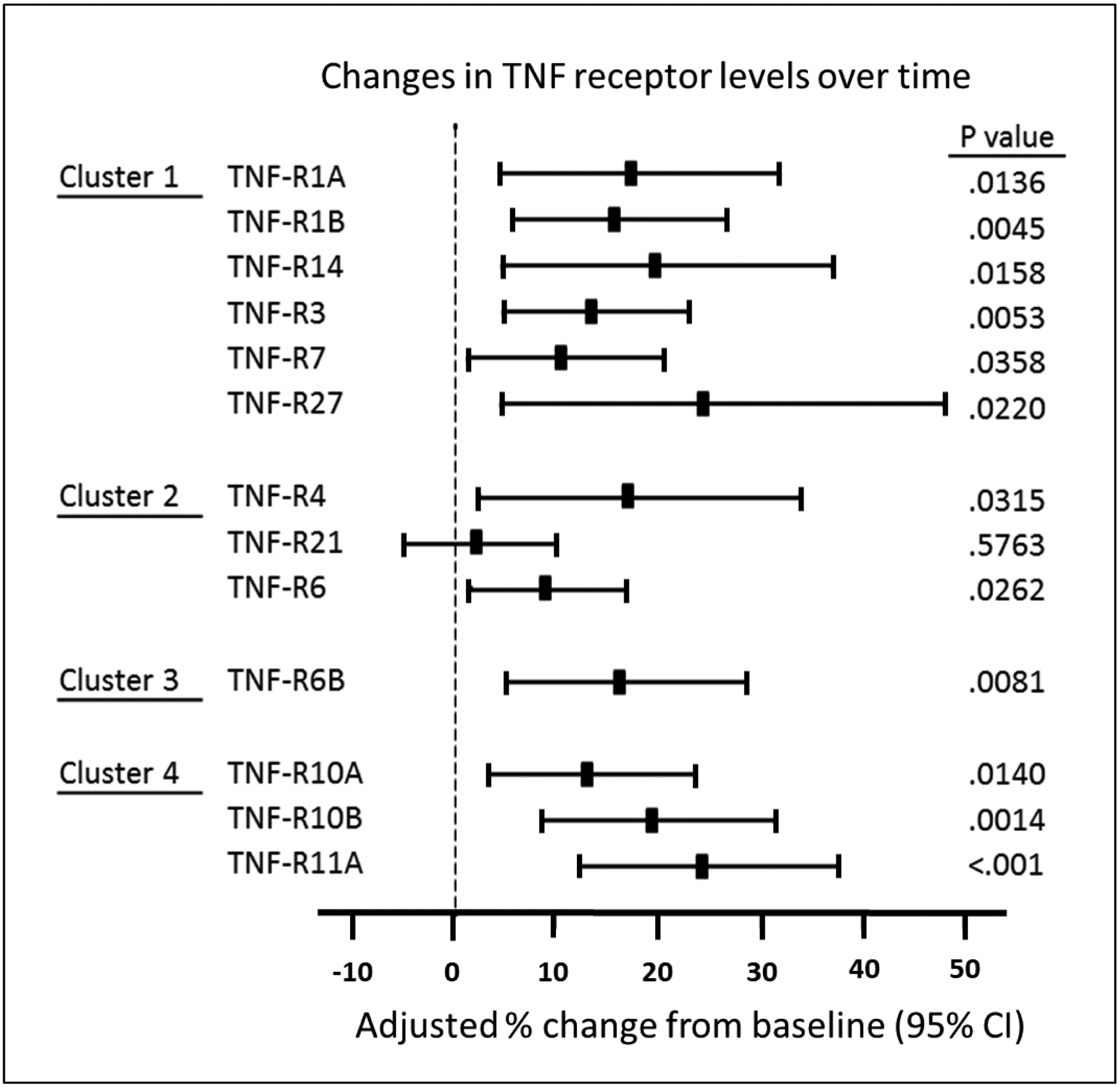
eGFR slope adjusted percent changes in plasma concentration of 13 tumor necrosis factor (TNF) receptors before onset of progressive renal decline (PRD) in individuals in Macro-Albuminuria sub-cohort. The TNF receptors are ordered according to clusters in Figure 1A. eGFR, estimated glomerular filtration rate.
Figure 5.
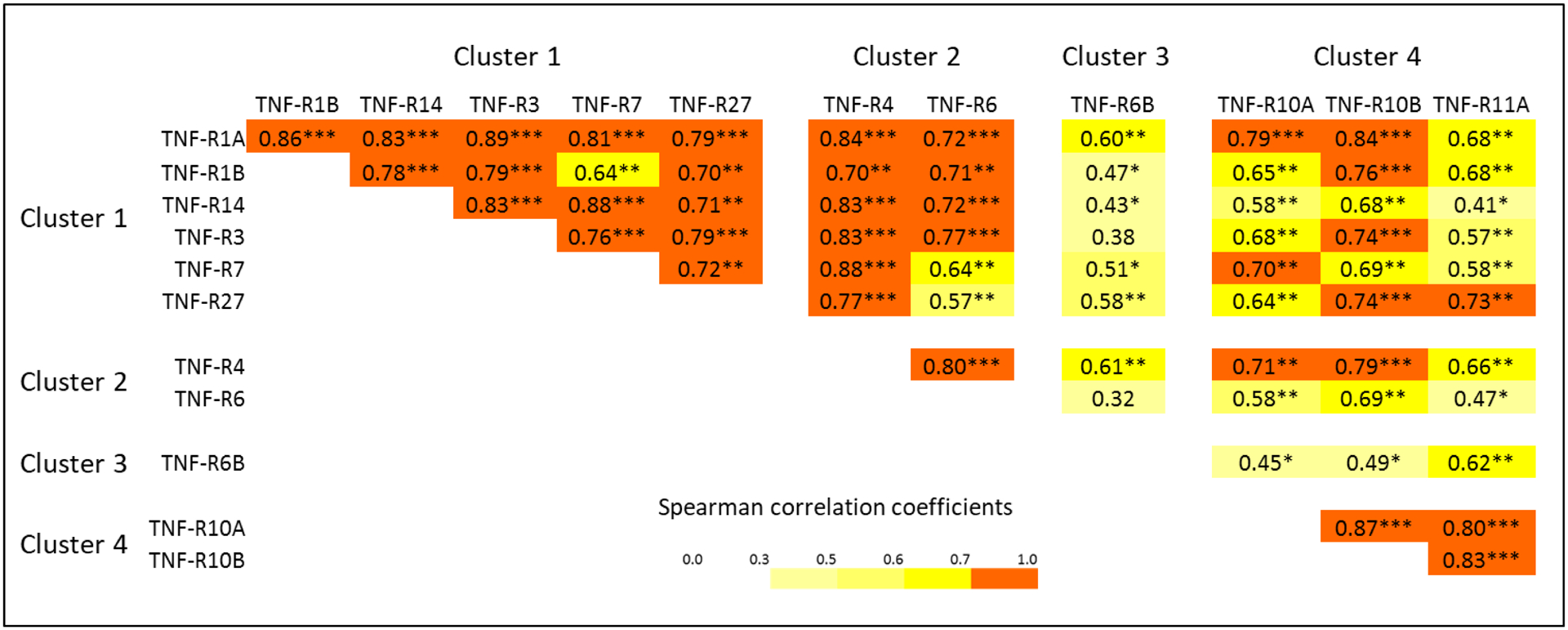
Matrix of Spearman rank correlation coefficients among baseline plasma concentrations of 12 tumor necrosis factor (TNF) receptors that were elevated before the onset of progressive renal decline (PRD).
Profiles of TNF superfamily proteins in late and early PRD
The results for early PRD obtained in the Macro-Albuminuria Study were compared with results for late PRD reported previously (8). While the results for the present study were obtained using the OLINK platform, the results for the previous study were obtained using the SOMAscan. The effects of TNF superfamily proteins on risk of ESKD in late and early stages of PRD were estimated by time-to-onset of ESKD using Cox proportional hazards model and the results are shown in Figure 6. Out of 17 TNF ligands examined by SOMAscan, only 3 proteins TNF-L7, TNFL12 and TNF-L15 were associated with risk of late PRD. Out of 6 TNF ligands examined by the OLINK, only TNF-L7 was marginally associated with risk of early PRD. In striking contrast, multiple TNF receptors were strongly associated with late and/or early PRD. Ten TNF receptors measured by SOMAscan were associated very strongly with late PRD, and 7 of them also had associations with risk of early PRD measured by OLINK. In contrast there were 6 TNF receptors measured by OLINK and associated with risk of early PRD, but none was associated with late PRD when SOMAscan was used.
Figure 6.
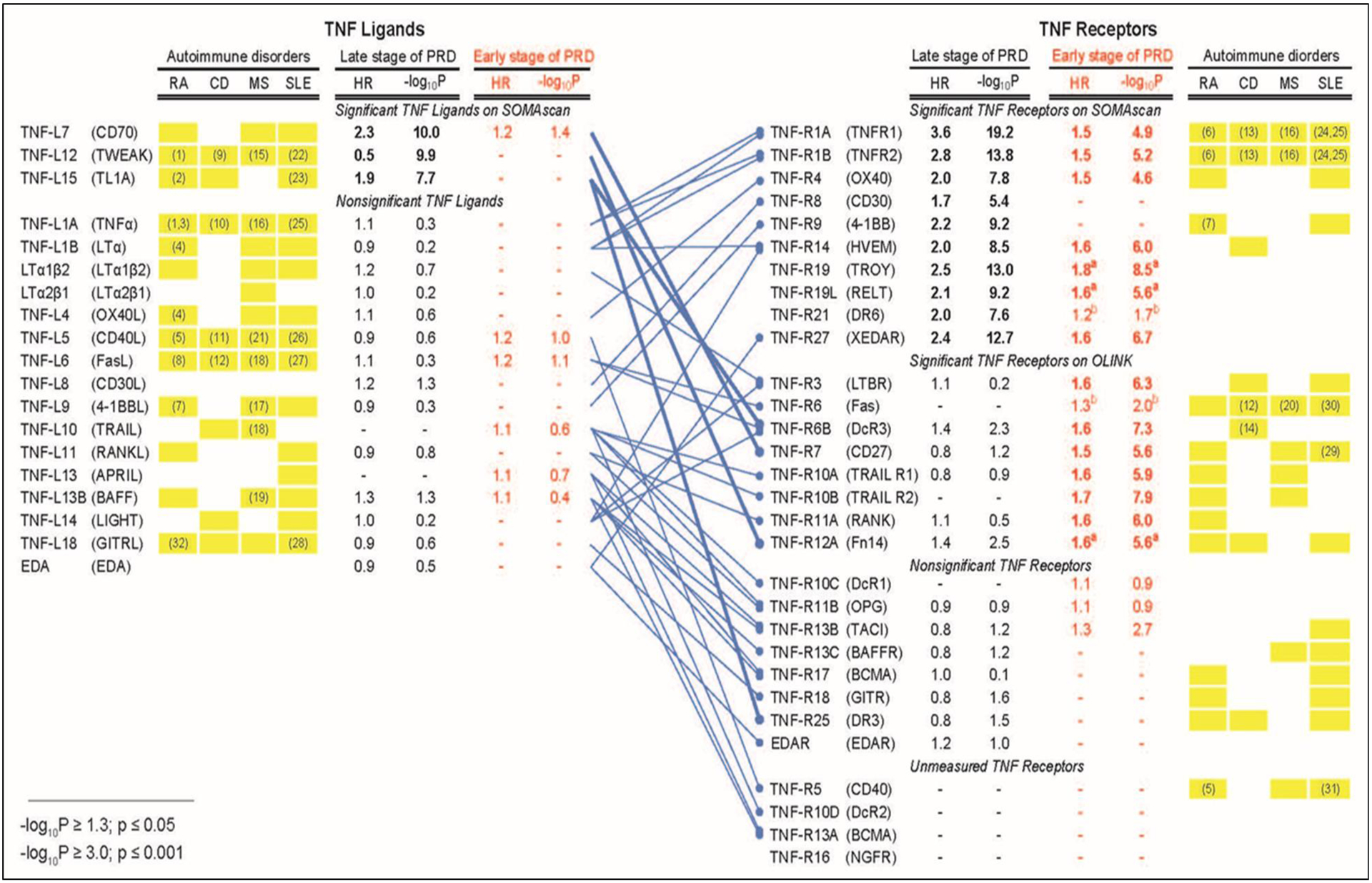
Profiles of tumor necrosis factor (TNF) proteins associated with late and early stages of progressive renal decline (PRD) in type 1 diabetes (T1D) compared with profiles of TNF proteins associated with autoimmune disorders. The binding patterns of TNF ligands to TNF receptors are as reported in our previous publication (8). Cox proportional hazards model was used to estimate effect (hazard ratio, HR) of TNF proteins on time-to-onset of ESKD in two T1D studies: 1) previous study (n= 219) that focused on late PRD (8) and 2) present Macro-Albuminuria Study (n= 198) that focused on early PRD. Baseline plasma concentration of TNF proteins in the previous study were determined by SOMAscan and by OLINK in the present study. HRs were estimated as one quartile change in TNF superfamily proteins. Univariate HRs are represented.
Bonferroni adjusted statistical significance is considered if –log10 p-value ≥3.00 (equivalent of p ≤0.001) on either platform (adjustment was made for 19 TNF ligands and 30 receptors). The results from the previous study and the present study are represented in black and red, respectively. The HRs and p values for TNF ligands and receptors whose effects were statistically significant are shown as bold letters. Subscript a) indicates 3 TNF receptors that were statistically significant in Macro- but not in Micro-Albuminuria Study, and subscript b) indicates 2 TNF receptors that were not significant in Macro-Albuminuria Study but were significant when Macro- and Micro-Albuminuria were analyzed together.
Yellow boxes indicate profiles of TNF proteins observed in autoimmune disorders: RA (Rheumatoid Arthritis), CD (Crohn’s Disease), MS (Multiple Sclerosis), and SLE (Systemic Lupus Erythematosus). These profiles were obtained from review articles (3–7) and the Database for Annotation, Visualization and Integrated Discovery (David) Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/). Associations supported by studies with measurements of plasma and/or serum concentrations of the TNF superfamily proteins are denoted by reference numbers in yellow boxes (For references see Supplementary References).
The discrepant findings may reflect different profiles of TNF receptors in late versus early PRD or could be due to different abilities of the two platforms to accurately measure concentration of the TNF receptors. To distinguish between these possibilities, we analyzed data obtained from 130 individuals participating in the Macro-Albuminuria Study in whom both platforms were used to measure TNF receptors (Supplementary Table S2). Out of 16 TNF receptors measured by both platforms, 8 receptors were giving similar results (6 positive and 2 negative) and 8 were giving discrepant results (positive when OLINK and negative when SOMAscan was used). One of the former TNF receptors (TNF-R1A) and two of the latter TNF receptors (TNF-R6B and TNF-R7) were examined further using commercially available ELISAs. All these receptors showed strong association with risk of ESKD, similar to the results obtained using OLINK. However, TNF-R6B and TNF-R7 still showed discrepancies in SOMAscan findings (Supplementary Table S2). To further evaluate the accuracy of the assays, we computed Spearman rank correlations pairwise among measurements of the 6 TNF receptors on each platform. As shown in Supplementary Figure S3A and S3B, there was a high correlation among proteins measured on OLINK compared to minimal or no correlation on SOMAscan platform. Therefore, we concluded that the discrepant findings with regard to TNF receptor profiles between late and early PRD are most likely due to SOMAscan’s inability to accurately measure concentration of certain receptors.
Profiles of TNF superfamily proteins in PRD and in autoimmune disorders
To explore possible implications/interpretations of our findings, we compared profiles of TNF proteins associated with PRD with those associated with 4 autoimmune disorders (Figure 6). Multiple TNF ligands were reported to be associated with RA, CD, MS and SLE but only 3 were associated with the development of late PRD in T1D. On the other hand, we observed striking similarities/overlaps between profiles of circulating TNF receptors associated with the development of PRD and profiles of these receptors in the 4 autoimmune disorders.
DISCUSSION
Combining the results of the present study that focused on early PRD with the previous findings for late PRD (8), we were able to comprehensively evaluate the association between baseline circulating levels of TNF proteins and risk of PRD leading to ESKD in T1D. In total, we found 3 TNF ligands and 18 TNF receptors associated with the development of PRD. Most of these receptors were associated with risk of early PRD in individuals with Macro- and Micro-Albuminuria and elevated levels of these receptors preceded the clinical manifestation of PRD. The latter finding supports the hypothesis that these receptors are involved in the disease process that underlies the development of PRD in T1D.
It has been shown that multiple elevated levels of TNF superfamily proteins are associated with the development and progression of autoimmune disorders (3–7). In this report we demonstrated some similarity between profiles of circulating TNF proteins associated with risk of PRD in T1D and risk of certain autoimmune disorders. In the latter up-regulation of multiple TNF ligands was observed, whereas abnormal levels of only 3 of these ligands were associated with risk of PRD in our studies. It is important to notice that while many authors postulate a role of TNF-L1 (TNFα) in the development of autoimmune disorders (3–7) as well as in DKD (17), we have not found any association between circulating levels of this ligand and PRD in our studies using the SOMAscan platform (8) or sensitive ELISA assays that measured bound and free TNF-L1 (9, 10). In striking contrast to TNF ligands, we observed similarities between profiles of circulating TNF receptors associated with PRD and autoimmune disorders. At present it is difficult to explain this finding, however, it may suggest that disease processes similar to those in autoimmune disorders are also involved in the development of PRD in T1D.
The involvement of TNF superfamily proteins in the etiology of autoimmune disorders is complex (3–7). The mechanisms that are responsible for association of some of these proteins with the development of PRD are even more multifaceted. As was recently reviewed, expression of TNF superfamily proteins is quite broad (3). Many-ligand-receptor pairs are constitutive or inducible on lymphocytes and participate in promoting T and B cell responses. In addition, many TNF ligands and receptors are also expressed in non-lymphoid cells including epithelial cells, fibroblasts, smooth muscle cells, and endothelial cells. Currently there is no knowledge regarding the origin (organ/tissue/cells) of the circulating TNF receptors associated with risk of PRD. Our previous report showed the kidney was an unlikely source of increased levels of the TNF receptors (8). Some authors argued that an increase in serum concentration of TNF-R1A is a result of renal function impairment (18–20). However, this study clearly showed that elevations of the TNF receptors in circulation occurred before onset of PRD.
Several mechanisms may contribute to these increases. This includes overexpression of these TNF receptors in tissues/cells, elevated activities of sheddases that cleave these receptors off the cell membranes or increased exocytosis of an intact form of the receptors from specific tissues/cells. Among the expected mechanisms, it has been recognized that sheddases play an important role in regulation of TNF proteins. They cleave extracellular parts of these proteins before they are released into circulation (20, 21). Indeed, TNF-L1, TNF-R1A, TNF-R1B, TNF-R5, TNF-R8, TNF-R16 and TNF-L11 among other proteins become soluble and enter the circulation by the cleavage activity of the matrix metalloproteinase TNF-converting enzyme (ADAM17) (22–26). ADAM17 modulation was shown to impact the generation of the soluble TNF-L1 and its 2 receptors in monocytes, neutrophils and proximal tubules (27, 28). The mechanisms and specific enzymes of shedding the other TNF proteins have not yet been elucidated. In our study almost all TNF ligands and receptors were measured in plasma. However, we do not know whether we measured these proteins as cleaved or intact forms or a combination of both. For example, an increased exocytosis of intact TNF receptors might contribute to the elevation of circulating TNF receptors in individuals at risk of PRD. Future studies are required to elucidate these questions to understand the mechanisms responsible for increased levels of circulating TNF receptors and possibly their causal role in the development of PRD.
Levels of circulating TNF receptors associated with risk of early PRD were inter-correlated. Clustering analysis grouped them into 4 clusters: #1) TNF-R1A, -R1B, -R3, -R7, -R14 and -R27; #2) TNF-R4, -R6 and -R21; #3) TNF-R6B; and #4) TNF-R10A, -R10B and -R11A. Clusters #1 and #2 were similar according to both the proportions of variance and inter-correlations. As to forming an independent cluster, TNF-R6B appears different from other TNF receptors, indicating that TNF-R6B, the Decoy receptor, may have a very distinct role in the development of early PRD compared to the other receptors. Cluster #4 includes 3 receptors tightly inter-correlated but less correlated with the other TNF receptors. Interestingly a similar pattern of clustering was observed when the changes in concentration of the TNF receptors over time were analyzed. These similar patterns of clustering may indicate that there are at least 3 different mechanisms of regulation of these proteins. Although unknown as of now, these mechanisms may be related to different origin and intensity of synthesis of these proteins and/or different mechanisms of releasing these proteins into circulation, i.e. by cleavage or exocytosis. Also, one cannot exclude the possibility that the proteins in the 3 clusters have different mechanisms of up-stream regulation by nuclear transcription factors or by miRNAs.
Out of the 13 TNF receptors only 6, TNF-R1A, TNF-R7 in cluster #1, TNF-R6 in cluster #2, TNF-R6B in cluster #3, and TNF-R10A, TNF-R11A in cluster #4 had an independent important impact on risk of early PRD. This finding is particularly interesting for two reasons. First, concentrations of these 6 important TNF receptors in circulation can be used for the development of prognostic algorithms to identify patients at risk of early PRD. In fact, we identified the significant predictive abilities of TNF-R6B and TNF-R11A, as representatives of clusters #3 and #4, independent from TNF-R1A and TNF-R1B whose predictive abilities were previously established (9–15). However, a reliable prognostic algorithm based on these proteins needs to be further developed using much larger cohorts. Second, the 6 important receptors may point to etiological drivers of the development of early PRD so they can be used as new therapeutic targets.
Several strengths of our study deserve consideration. First, the prospective study designs and size of the study groups were appropriate for a comprehensive assessment of associations between circulating levels of TNF proteins and risk of PRD and progression to ESKD in T1D. Second, it is important to emphasize that our findings are derived from longitudinal observations, whereas most of the studies regarding autoimmune disorders were cross-sectional designs. Third, the similarity of findings in individuals with Macro- and Micro-Albuminuria, and for different definitions of early PRD, i.e. eGFR slopes and progression to ESKD, assures the robustness of our findings.
Finally, the following limitations should be noted. In this study of early PRD, we used the OLINK platform. OLINK had better specificity and sensitivity than the aptamer-based SOMAscan platform that was used in the previous study on late PRD. While both platforms gave many similar results, the use of the OLINK platform resulted in our finding of an association between PRD and 8 more TNF receptors. Seventeen TNF ligands were measured using SOMAscan and only 3 showed positive findings. Since 11 of these ligands were not measured using the OLINK platform, one cannot exclude the possibility that, had they been measured using OLINK, some might have been found to be associated with risk of PRD. This possibility needs to be studied further. This report summarizes our findings regarding circulating TNF superfamily proteins in early and late PRD in T1D. It is not clear whether our findings can be generalized to T2D. Furthermore, this is a clinic-based cohort rather than a population-based study; therefore our results may not be representative of all individuals with T1D.
METHODS
Joslin Kidney Study
The Joslin Diabetes Center Committee on Human Studies approved the informed consent, recruitment and examination procedures for the Joslin Kidney Study (JKS). The JKS is a longitudinal observation that aims to investigate the determinants and to describe the natural history of PRD in T1D. Results from this study and protocols used were previously published (8, 29–31). Participants were recruited from among 3,500 adults with T1D, 95% Caucasians, who attended the Joslin clinic between 1991 and 2009. According to the median values of ACR from 2 or more consecutive urine samples obtained during the 2-year period preceding enrollment (baseline), 3 study groups were assembled: Macro-Albuminuria (ACR ≥300 mg/g) (n= 526), Micro-Albuminuria (30≤ ACR <300 mg/g) (n= 563), and Normo-Albuminuria (ACR <30 mg/g) (n= 795). This investigation comprises 2 case-control studies nested in the JKS: Macro-albuminuria and Micro-Albuminuria study. Figure 1 outlines the study design and selection of individuals for each study. More information about the JKS is provide in Appendix #1.
Proteomics platforms
The OLINK Proseek Multiplex panels® (Uppsala, Sweden) were used to measure proteins in plasma by real-time qPCR through PEA technology (16). In total the OLINK platform measures 1,061 proteins. These proteins are organized into 13 panels and each OLINK panel includes 92 measured proteins. For this work, we used 11 panels available at the time measuring a total of 979 markers. In the present study only measurements of TNF superfamily proteins were used: 19 TNF receptors and 6 TNF ligands were measured as part of 460 proteins using 5 OLINK panels (Supplementary Table S3). Among the other 6 panels, the Inflammation panel includes 6 TNF superfamily proteins, however, they were not correlated with eGFR slope in a pilot study and therefore that panel was not used (Supplementary Table S3). The measurements were expressed as relative values on a log2-scale. Quality control (QC) was performed in 2 steps: 1) each sample plate was evaluated on the standard deviation of the internal controls, and 2) the quality of each sample was assessed by evaluating the deviation from median value of the internal controls. The proportions of samples passing QC were 92–100% and 98–100% in the discovery and replication panels, respectively. Average intra-assay % coefficients of variation in the discovery and replication panels were 4–21% and 4–6%, respectively.
To examine the influence of duration of sample storage on values in TNF receptors, we computed Spearman rank correlation coefficients between duration of storage and value of TNF receptors at each examination of participants included in the Macro-Albuminuria subgroup (n= 24) (Supplementary Table S4). The correlations were weak and the corresponding p values were non-significant, except for TNF-R21 and TNF-R6. However, these correlations were weakly positive, i.e. longer duration of storage, the higher values of these receptors. By adjusting for this effect our findings would become even stronger. Overall we can conclude that plasma values of TNF receptors were not affected by duration of sample storage.
The SOMAscan platform® (Somalogic, Denver, CO) uses aptamers, recognizing folded protein epitopes with high affinity and specificity. This platform was used to measure plasma concentration of TNF circulating proteins in a subset of 130 subjects with Macro-Albuminuria and T1D who were participants of the present study and had OLINK measurements performed (Supplementary Table S2). To further examine proteins that gave discrepant results between these two platforms, we used commercially available ELISAs from Abcam® (Abcam plc, Cambridge, UK) for TNF-R6A and TNF-R11A, and Meso Scale Diagnostics® (Meso Scale Diagnostics, LLC., Rockville, Maryland, USA) for TNF-R7.
Statistical analysis
Baseline characteristics were presented as median and interquartile range or number and percent, as applicable. To correct for multiple testing, we applied the Benjamini-Hochberg procedure to control the false discovery rate (FDR) with q-value (32). FDR q value <0.05 were considered statistically significant for the findings in the Macro-Albuminuria Study and two-sided p-value <0.05 was considered statistically significant in the Micro-Albuminuria Study. Univariate and multivariable logistic regression models were used to estimate the effect of TNF superfamily proteins on PRD. Multivariable models were adjusted for baseline eGFR, HbA1c and for Macro/Micro-Albuminuria studies if necessary. We did not adjust for ACR because ACR is an intermediate factor between renal function and ESKD in the causal diagram. The inter-correlation of the selected TNF receptors was examined by hierarchical cluster analysis using Ward’s method in the combined cohorts after quantile normalization in order to correct the batch and cohort variabilities. To identify the TNF receptors most strongly associated with eGFR slope which was used as a quantitative measure of PRD, variable importance was performed by PLS with Wold’s method. The Macro-Albuminuria Study was used to examine which TNF receptors contributed most to the prediction of development of ESKD during 15 years of follow-up. For this purpose, logistic models were sequentially developed: model #1 included only clinical variables (eGFR, ACR, HbA1c), model #2 included model #1 adding TNF-R1A, model #3 included model #1 adding TNF-R1B, and model #4 included clinical variables, TNF-R1A and TNF-R1B and 2 TNF receptors that were selected from remaining 11 TNF receptors using backward elimination (p-for-stay = 0.1).
The sub-cohort from the Macro-Albuminuria Study was used to examine whether plasma concentration of TNF receptors increased before the onset of eGFR decline. For this purpose, we used a repeated measures model with unstructured covariance matrix in PROC MIXED. The model included a linear effect of eGFR change on protein concentration, and an additional indicator variable for the change in protein concentration that occurred before the inflection in eGFR trajectory and was absent at baseline measurement. All analyses were performed by SAS 9.4 (SAS Institute Inc, Cary, NC, USA).
Supplementary Material
Supplementary Reference. References for Figure 6.
ACKNOWLEDGEMENTS:
We acknowledge grants support from: the National Institutes of Health (NIH) (DK041526 and DP3DK112177) to A.S.K; the Novo Nordisk Foundation grant NNF14OC0013659 (PROTON) to A.S.K.; JDRF (3-SRA-2015-106-Q-R) to A.S.K; JDRF (5-CDA-2015-89-A-B) to M.A.N.; The Sunstar Foundation, Japan (Hiroo Kaneda Scholarship) and the Foundation for Growth Science from Japan to E.S.; the Uehara Memorial Foundation, and the Japan Society for the Promotion of Science (Overseas Research Fellowship) to H.K.; and by NIH DERC grant to Joslin Diabetes Center (P30 DK036836).
We would like to acknowledge E. Mills and N. Rashidi from OLINK Proteomics Inc. for their assistance with protein measurements.
Footnotes
DISCLOSURE:
ASK and MAN are co-inventors of the TNF-R1 and TNF-R2 patent for predicting risk of ESRD. This patent was licensed by the Joslin Diabetes Center to EKF Diagnostics.
The other authors of this report declare no competing conflicts of interest.
REFERENCES
- 1.Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012; 119: 651–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005; 115: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dostert C, Grusdat M, Letellier E, et al. The TNF Family of Ligands and Receptors: Communication Modules in the Immune System and Beyond. Physiol Rev. 2019; 99: 115–160. [DOI] [PubMed] [Google Scholar]
- 4.Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017; 17: 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft M, Siegel RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017; 13: 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ślebioda TJ, Kmieć Z. Tumour necrosis factor superfamily members in the pathogenesis of inflammatory bowel disease. Mediators Inflamm. 2014; 2014: 325129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonar S, Lal G. Role of Tumor Necrosis Factor Superfamily in Neuroinflammation and Autoimmunity. Front Immunol. 2015; 6: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niewczas MA, Pavkov ME, Skupien J, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019; 25: 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012; 23: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESKD in type 2 diabetes. J Am Soc Nephrol. 2012; 23: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsblom C, Moran J, Harjutsalo V, et al. Added value of soluble tumor necrosis factor-α receptor 1 as a biomarker of ESKD risk in patients with type 1 diabetes. Diabetes Care. 2014; 37: 2334–2342. [DOI] [PubMed] [Google Scholar]
- 12.Saulnier PJ, Gand E, Ragot S, et al. Association of serum concentration of TNFR1 with all-cause mortality in patients with type 2 diabetes and chronic kidney disease: follow-up of the SURDIAGENE Cohort. Diabetes Care. 2014; 37: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 13.Pavkov ME, Nelson RG, Knowler WC, et al. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015; 87: 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coca SG, Nadkarni GN, Huang Y, et al. Plasma Biomarkers and Kidney Function Decline in Early and Established Diabetic Kidney Disease. J Am Soc Nephrol. 2017; 28: 2786–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barr ELM, Barzi F, Hughes JT, et al. High Baseline Levels of Tumor Necrosis Factor Receptor 1 Are Associated with Progression of Kidney Disease in Indigenous Australians With Diabetes: The eGFR Follow-up Study. Diabetes Care. 2018; 41: 739–747. [DOI] [PubMed] [Google Scholar]
- 16.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014; 9: e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009; 76: 262–276. [DOI] [PubMed] [Google Scholar]
- 18.Brockhaus M, Bar-Khayim Y, Gurwicz S, et al. Plasma tumor necrosis factor soluble receptors in chronic renal failure. Kidney Int. 1992; 42: 663–667. [DOI] [PubMed] [Google Scholar]
- 19.Ward R, McLeish KR. Soluble TNF alpha receptors are increased in chronic renal insufficiency and hemodialysis and inhibit neutrophil priming by TNF alpha. Artif Organs. 1996; 20: 390–395. [DOI] [PubMed] [Google Scholar]
- 20.Bemelmans MH, Gouma DJ, Buurman WA. Tissue distribution and clearance of soluble murine TNF receptors in mice. Cytokine. 1994; 6: 608–615. [DOI] [PubMed] [Google Scholar]
- 21.Bemelmans MHA, van Tits LJH, Buurman WA. Tumor Necrosis Factor: Function, Release and Clearance. Crit Rev Immunol. 2017; 37: 249–259. [DOI] [PubMed] [Google Scholar]
- 22.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997; 385: 729–733. [DOI] [PubMed] [Google Scholar]
- 23.Levine SJ, Adamik B, Hawari FI, et al. Proteasome inhibition induces TNFR1 shedding from human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2005; 289: L233–243. [DOI] [PubMed] [Google Scholar]
- 24.Contin C, Pitard V, Itai T, et al. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J Biol Chem. 2003; 278: 32801–32809. [DOI] [PubMed] [Google Scholar]
- 25.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008; 29: 258–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheller J, Chalaris A, Garbers C, et al. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011; 32: 380–387. [DOI] [PubMed] [Google Scholar]
- 27.Bell JH, Herrera AH, Li Y, et al. Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J Leukoc Biol. 2007; 82: 173–176. [DOI] [PubMed] [Google Scholar]
- 28.Kefaloyianni E, Muthu ML, Kaeppler J, et al. ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight. 2016; 18: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krolewski AS Progressive Renal Decline: The New Paradigm of Diabetic Nephropathy in Type 1 Diabetes. Diabetes Care. 2015; 38: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skupien J, Warram JH, Smiles AM, et al. The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int. 2012; 82: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krolewski AS, Niewczas MA, Skupien J, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care. 2014; 37: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990; 9 (7): 811–818. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007; 18 (4): 1353–1361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Reference. References for Figure 6.


