Abstract
Purpose of review:
Adrenarche is the pubertal maturation of the innermost zone of the adrenal cortex, the zona reticularis. The onset of adrenarche occurs between 6–8 years of age when dehydroepiandrosterone sulfate (DHEAS) concentrations increase. This review provides an update on adrenal steroidogenesis and the differential diagnosis of premature development of pubic hair.
Recent findings:
1) The complexity of adrenal steroidogenesis has increased with recognition of the alternative “backdoor pathway” and the 11-oxo-androgens pathways. 2) Traditionally, sulfated steroids such as DHEAS have been considered to be inactive metabolites. Recent data suggest that intracellular sulfated steroids may function as tissue specific intracrine hormones particularly in the tissues expressing steroid sulfatases such as ovaries, testes, and placenta.
Summary:
The physiologic mechanisms governing the onset of adrenarche remain unclear. To date, no validated regulatory feedback mechanism has been identified for adrenal C19 steroid secretion. Available data indicate that for most children, premature adrenarche is a benign variation of development and a diagnosis of exclusion. Patients with premature adrenarche tend to have higher BMI values. Yet, despite greater knowledge about C19 steroids and zona reticularis function, much remains to be learned about adrenarche.
Keywords: Adrenarche, Premature Adrenarche, Pubarche, Adrenal, DHEAS
Introduction
Adrenarche represents the peri-pubertal maturation of the adrenal cortex. The onset of adrenarche is arbitrarily defined as occurring when dehydroepiandrosterone sulfate (DHEAS) concentrations are detectable using standard laboratory techniques, usually, between 6–8 years of age. The physical manifestation of adrenarche, pubarche, is characterized by the development of pubic or axillary hair, adult apocrine odor, increased oiliness of the skin and hair, and acne. In the National Health and Nutrition Survey (NHANES), the mean ages for pubic hair development among girls were 9.5 years for non-Hispanic blacks, 10.3 years for Mexican-Americans, and 10.5 years for non-Hispanic whites [1]. For boys, means ages for pubic hair development were 11.1 years for non-Hispanic blacks, 12.3 years for Mexican-Americans, and 12.0 years for non-Hispanic whites [1].
Adrenarche occurs in individuals with hypogonadotropic hypogonadism and in those with primary gonadal failure [2]. The occurrence of adrenarche in patients with hypothalamic-pituitary-gonadal (HPG) axis disorders indicates that the regulatory mechanisms responsible for adrenarche and HPG axis function differ [3]. Adrenarche appears to be a phenomenon limited to humans and higher primates [4].
Adrenal steroidogenesis
Steroidogenesis is the process through which cholesterol is converted to steroid hormones (Figure 1). The adrenal cortex and the gonads are the primary sources of steroid hormones [5]. Only minimal amounts of steroid hormone are stored within the adrenal gland. Enzymes expressed in liver, adipose tissue, and other peripheral tissues can modify steroid hormones; such tissue specific modifications activate and inactivate the secreted steroid hormones.
Figure 1.
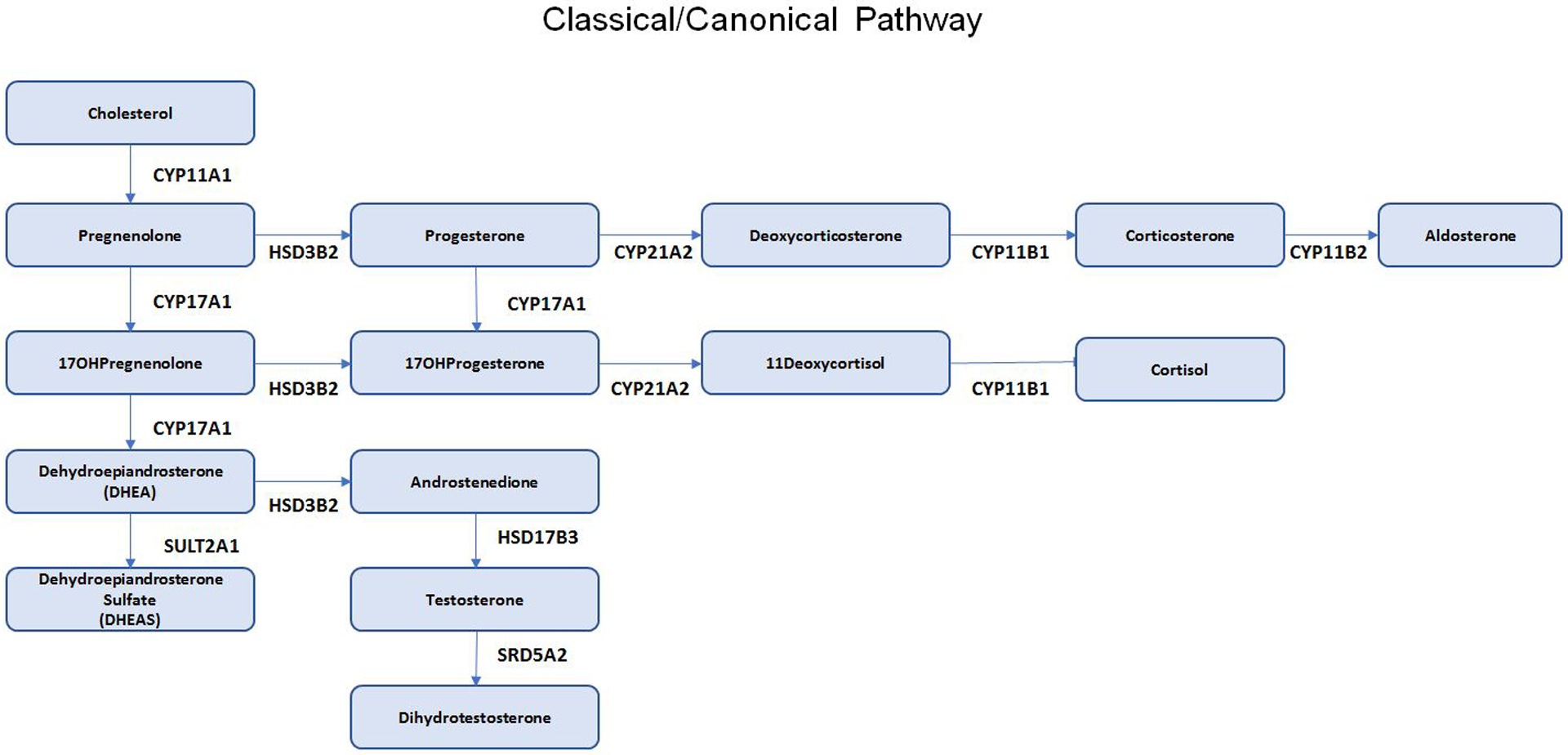

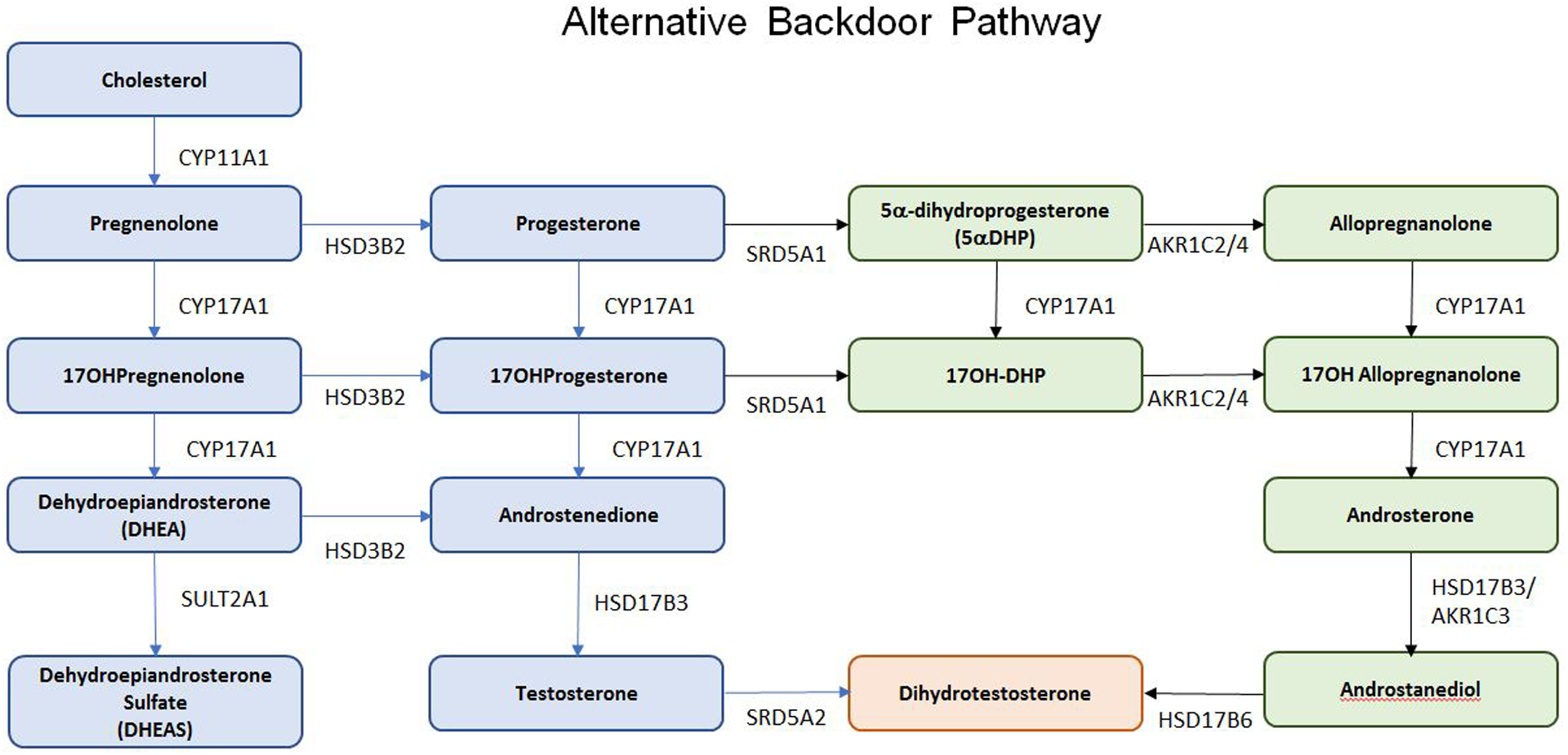
Steroid Pathways. A. Classical/Canonical Pathway; B. 11-oxo-Androgen Pathway; C. Alternative Backdoor Pathway. Abbreviations: CYP11A1: cholesterol side chain cleavage; HSD3B2, 3-beta hydroxysteroid dehydrogenase type 2; CYP17A1, 17-alpha hydroxylase/17,20 lyase; CYP21A2, 21-hydroxylase; CYP11B1, 11beta-hydroxylase; CYP11B2, aldosterone synthase; SRD5A1: 5a-reductase type 1, SRD5A2: 5a-reductase type 2, AKR1C1: Aldo-keto reductase type 1 C1, AKR1C2: Aldo-keto reductase type 1 C2, AKR1C3: Aldo-keto reductase type 1 C3, AKR1C4: Aldo-keto reductase type 1 C4, HSD17B3: 17B-hydroxysteroid dehydrogenase type 3, HSD17B6: 17B-hydroxysteroid dehydrogenase type 6
The adrenal cortex consists of three distinct zones. The outer zone, the zona glomerulosa, synthesizes mineralocorticoids and is principally regulated by the renin-angiotensin pathway and serum potassium concentrations. Expression of the enzyme aldosterone synthase, encoded by CYP11B2, is limited to the zona glomerulosa. The middle zone, the zona fasciculata, synthesizes cortisol and is regulated by pituitary adrenocorticotrophin (ACTH) secretion. Acting through the ACTH receptor, encoded by the melanocortin-2 receptor (MC2R) gene, ACTH has chronic and acute actions. Its chronic actions promote transcription and translation of the adrenal steroidogenic enzymes. Acutely, ACTH stimulates cortisol secretion. The hypothalamic-pituitary-adrenal (HPA) axis governs cortisol secretion by negative feedback inhibition to limit ACTH secretion. ACTH and cortisol manifest diurnal variation with the highest concentrations in the early morning.
The inner zone, the zona reticularis secretes C19 steroids such as DHEA and DHEAS. DHEAS is the steroid hormone circulating in the greatest abundance, has a long half-life, and shows minimal diurnal variation. Evidence is accumulating that intracellular sulfated steroids may function as intracrine hormones in tissues expressing steroid sulfatases [6,7*]. Despite attempts to characterize an adrenal androgen stimulating factor, no proposed substance has been validated [8]. Further, no feedback loops regulating zona reticularis C19 androgen secretion have been substantiated. Hence, the physiologic mechanisms governing zona reticularis C19 secretion remain an enigma.
Detailed description of the classic/canonical pathway for adrenal steroidogenesis is beyond the scope of this review (Figure 1A)). The reader is referred to reviews of adrenal steroidogenesis [5,9]. However, two additional pathways for androgen biosynthesis warrant mention: the alternative “backdoor pathway” and the 11-oxo-androgen pathway. In the “backdoor pathway”, 17-hydroxyprogesterone can be converted to dihydrotestosterone (DHT) without prior conversion to testosterone (Figure 1B). This pathway is particularly relevant in individuals with 21-hydroxylase and P450-oxidoreductase deficiencies [10].
In the 11-oxo-androgen pathway, the 11β-hydroxylase enzyme, encoded by CYP11B1, participates in the synthesis of 11-ketoandrostenedione, 11β-hydroxyandrostenedione, 11β-hydroxytestosterone, and 11-ketotestosterone (Figure 1C) [11**,12]. Among girls with adrenarche and premature adrenarche, DHEA, DHEAS, 11-ketotesterone, testosterone, and 5-androstenediol-3-sulfate (Adiol-S) concentrations rise with adrenarche [13]. DHEAS and Adiol-S concentrations rise in parallel [14].
While testosterone and DHT are the most potent androgens, 11-ketotestosterone and 11-ketodihydrotestosterone are also potent androgen receptor agonists [15]. In peripheral tissues such as the testes, 11-ketotestosterone can be converted to 11-ketodihydrotetosterone by 5α-reductase type 2. The other 11-oxo-androgens, 11β-hydroxytestosterone, 11-ketoandrostenedione and 11β-hydroxyandrostenedione are less potent androgens but have in vitro androgen activity. Androstenedione, DHEA, and DHEAS do not show significant androgen receptor agonist activity.
Using LC-MS/MS, DHEA, DHEAS, and 11-oxo-androgen concentrations were measured in serum samples obtained from 18 different species. As anticipated, DHEA, DHEAS, and 11-oxo-androgen concentrations were an order of magnitude higher in humans and non-human primates. These studies also confirmed that the adrenal gland is the primary source of circulating 11-ketotestosterone among humans [16].
Adrenarche
During fetal life, the fetal adrenal cortex secretes DHEA and DHEAS, which serve as substrates for placental estrogen biosynthesis [5]. Following birth, the fetal adrenal cortex involutes and measurable DHEA and DHEAS concentrations decline. With onset of adrenarche, DHEA and DHEAS concentrations rise with DHEAS concentrations peaking during the second decade of life.
Traditionally, adrenarche is considered to occur between 6–8 years of age. However, this notion is being questioned because urinary excretion of DHEA and its metabolites begins to increase as early as age 3 years [17]. One small study of healthy Finnish children found that DHEAS concentrations were measurable at 1 year of age and correlated with DHEAS concentrations at 6 years of age [18*].
The molecular trigger for adrenarche remains unclear. Environmental exposures such as stress and nutrition may modulate the timing of adrenarche [19]. Studies in monozygotic and dizygotic twins showed heritability to be 58%−61% for adrenarche [20,21]. However, no distinct genetic markers were identified in these small case-control candidate gene studies [22]. At adrenarche, DHEAS concentrations rise independently of ACTH and cortisol concentrations. Yet, the lack of adrenarche in patients with MC2R mutations or ACTH deficiency indicates a partial or permissive role for ACTH in adrenarche. Nevertheless, despite the availability of new tools to investigate the serum and urine steroid metabolome profiles, the proximate signals to initiate adrenarche remain unknown [23].
Differential Diagnosis of Premature Adrenarche
The appearance of pubic or axillary hair before age 8 years in females and 9 years in males is arbitrarily defined as premature pubarche. The most common etiology is premature adrenarche. Other causes of early pubarche need to be eliminated before premature adrenarche is diagnosed (Figure 2).
Figure 2.
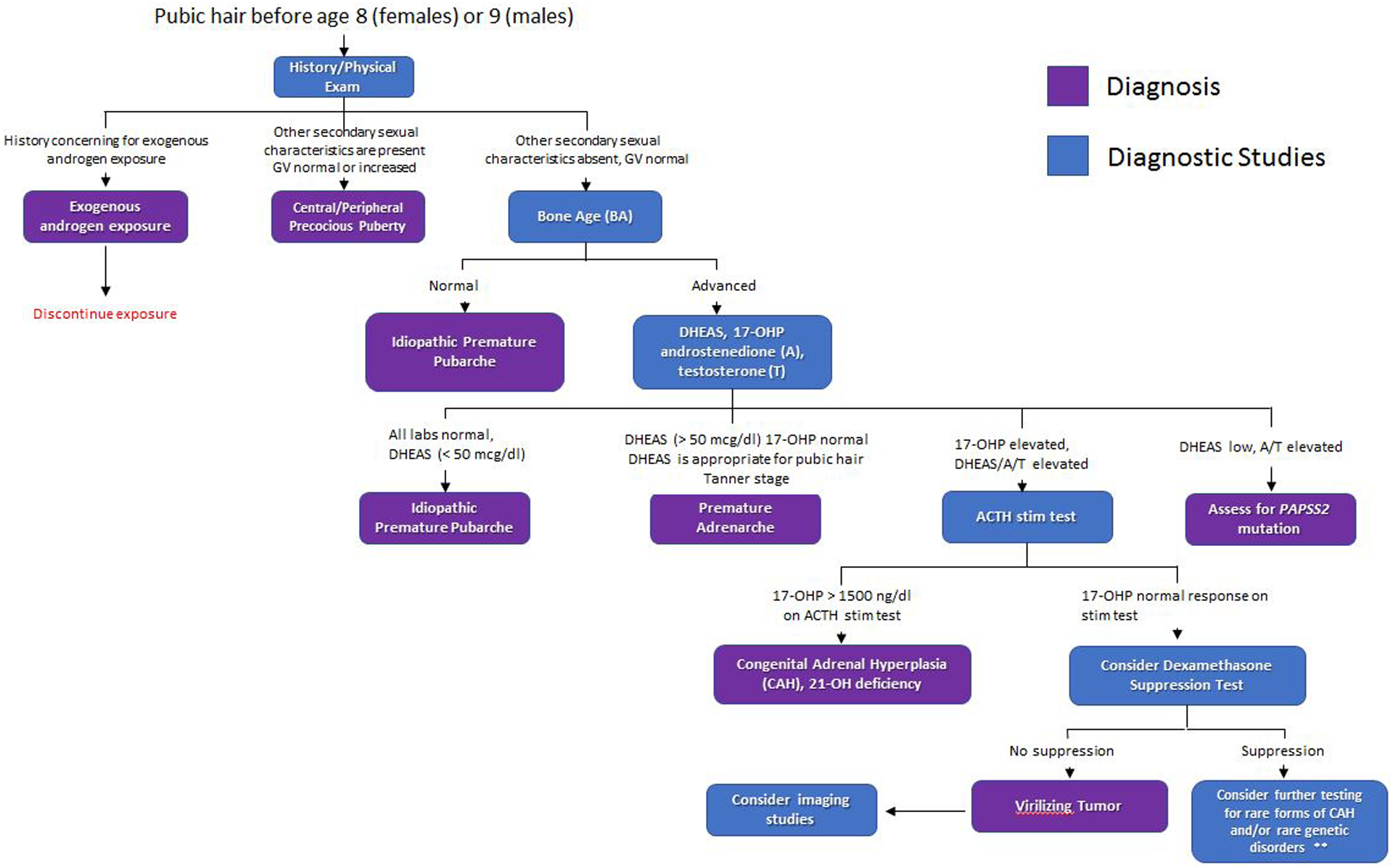
Algorithm to evaluate adrenarche. This algorithm provides guidance for evaluation of children with premature pubarche. For patients with CPP, breast development in girls and testicular enlargement in boys usually precedes pubic hair development. Rare forms of CAH include 3-hydroxysteroid dehydrogenase deficiency due to HSD3B2 mutations and 11-hydroxylase deficiency due to CYP11B1 mutations. Another rare genetic disorder is apparent cortisone reductase deficiency due to H6PD mutations.
The most frequent differential diagnosis is congenital adrenal hyperplasia (CAH). The virilizing congenital adrenal hyperplasias encompass a group of autosomal recessive disorders. These disorders are characterized by impaired glucocorticoid biosynthesis, loss of negative feedback inhibition, increased ACTH secretion, and subsequent increased adrenal C19 steroid secretion [24]. The most common form is 21-hydroxylase deficiency due to mutations in the 21-hydroxylase (CYP21A2) gene. The phenotypic spectrum ranges from severe to mild reflecting the consequences of the specific mutation. The current estimate regarding prevalence of non-classic 21-hydroxylase deficiency in American Caucasians is 1 in 200 [25]. Whether measured by radioimmunoassay or LC-MS/MS, early morning 17-OHP values greater than 200 ng/dl have greater than 96% sensitivity and 96% specificity to detect children with non-classic 21-hydroxylase deficiency [26,27]. Nevertheless, when symptoms suggest CAH, ACTH stimulation tests should be performed despite normal morning 17-OHP concentrations [28*].
Typically, patients with GnRH-dependent precocious puberty (CPP) follow the normal sequence of pubertal development with breast development in girls and testicular enlargement in boys preceding the appearance of sexual hair. Secondary CPP can occur in patients with advanced skeletal maturation attributed to untreated CAH or androgen secreting tumors.
Apparent cortisone reductase and PAPS synthase 2 (PAPSS2) deficiencies are very rare genetic disorders associated with premature pubarche. Apparent cortisone reductase deficiency is due to mutations in the hexose-6-phosphate dehydrogenase (H6PD) gene [29]. Loss of function PAPSS2 mutations impair conversion of DHEA to DHEAS leading to low DHEAS concentrations and elevated unconjugated adrenal C19 steroids [30]. Patients with PAPSS2 mutations may also manifest spondyloepimetaphyseal dysplasia characterized by short stature and mild brachydactyly [31].
Androgen secreting adrenal or gonadal tumors are very rare causes of premature pubarche and virilization. The tempo for pubertal changes is often rapid and accompanied by accelerated linear growth and skeletal maturation. Adrenal cortical, Leydig cell, and ovarian androgen secreting tumors can present with premature pubarche or virilization [32,33,34,35]. Testicular, brain, and hepatic tumors may secrete β-human chorionic gonadotropin (β-hCG) which stimulates testicular LH receptors to secrete testosterone; affected boys typically present with virilization and testicular enlargement. Girls generally do not present with precocious puberty associated with β-hCG secreting tumors because estradiol is not synthesized in the absence of FSH-induced aromatase expression.
Exposure to exogenous androgens in creams or gels containing testosterone, DHT, or androstenedione can lead to premature pubarche and/or virilization. Transfer of exogenous androgens can occur by direct application or skin-skin contact [36].
Laboratory Data
In premature adrenarche DHEA and DHEAS concentrations are elevated, typically above 50 mcg/dl, and consistent with the stage of pubic hair development. However, circulating DHEA, DHEAS, and androstenedione concentrations may not correlate with the clinical features. Traditionally, steroid hormones have been measured using radioimmunoassays. Limitations of this methodology include being able to measure only a single hormone and lack of specificity. Newer methods include LC-MS/MS and GC/MS. Advantages of these methods include the ability to measure multiple hormones simultaneously while providing greater specificity [37]. Knowing the specific reference ranges for the laboratory is important to avoid the misinterpretation of the laboratory results [38]. Since some hormones show diurnal variation, early morning samples, i.e. between 7:30–8:30 AM, are preferred.
Bone age
Premature adrenarche can be associated with advanced skeletal maturation and tall stature [39]. Skeletal maturation, also known as bone age, represents the expected change in ossification centers over time. Determination of bone age typically involves an X-ray of the left hand and wrist. The current methods of evaluating bone age are imperfect because existing standards are largely derived from a white population and are not always applicable to children of other ethnicities [40,41,42].
When the bone age is advanced, concerns arise regarding the potential for adult short stature. However, available data from outcome studies conflict. Some studies report that predicted adult heights are decreased in this population whereas other studies indicate that most children achieve an appropriate adult height [43,44,45,46]. Importantly, significantly advanced bone age results (> 2SD) should prompt evaluation for other disorders, e.g., non-classic CAH [47].
Linear growth during childhood is driven by growth plate chondrogenesis [48]. Androgens and estrogens affect skeletal maturation and bone health through their actions on osteoblasts, osteocytes, and osteoclasts [49]. In premature adrenarche, the rising DHEAS, testosterone and androstenedione concentrations are positively correlated with bone age advancement [50,51]. Obesity can further accelerate the rate of skeletal maturation in premature adrenarche; increased insulin, IGF-1, and leptin concentrations likely contribute to this acceleration [52,53]. Genetics, nutritional status, hormones, medications, and various disease states influence the tempo of skeletal maturation.
Neurobiology
Both DHEA and DHEAS are also considered to be neurosteroids because they can be synthesized de novo in the brain. The temporal proximity of increasing DHEAS secretion and cerebral cortex maturation suggests the hypothesis that these hormones enhance brain development and enable the pre-adolescent brain to adapt to the social challenges of approaching adulthood [54,55*].
What are the evolutionary benefits of adrenarche and the long childhood pause before achieving reproductive maturity? Potential developmental tasks occurring during this time frame include learning about gender specific activities, socialization, and cultural awareness [56,57,58*]. Earlier onset of adrenarche appears to be associated with increased risk to develop anxiety symptoms during puberty and young adulthood [59**,60]. Available data suggest that the pattern and timing of hormone exposure during adrenarche interacts with brain development, biologic sex, and psychological stress to influence risk for mental health issues during adolescence [61,62].
Polycystic ovary syndrome
Polycystic ovary syndrome is a common heterogeneous disorder characterized by clinical or biochemical hyperandrogenism and irregular menses [63*,64]. The relationship of premature adrenarche to PCOS remains unresolved. Inquiry into this question is problematic because the diagnostic criteria for PCOS include features typical for peri-pubertal development such as irregular menses, polycystic ovary morphology, and mild hyperandrogenism [65**].
Based on a report that 45% of a cohort of Catalan girls with a history of premature pubarche had developed functional ovarian hyperandrogenism, it was suggested that girls with premature pubarche might have an increased risk to develop PCOS [66]. Subsequent data led to the concept of a developmental sequence beginning with prenatal growth restriction (SGA infants), rapid post-natal growth in early childhood, hyperinsulinemia, increased hepato-visceral fat stores, and premature pubarche that culminated in functional ovarian hyperandrogenism [67,68,69*,70]. Yet, available data are inconsistent. Not all girls with PCOS have a history of premature adrenarche. Similarly, not all girls with premature adrenarche will eventually develop PCOS. Since definitive answer remains unclear, longitudinal follow-up of girls with premature adrenarche might help to identify the risk factors associated with development of PCOS [65].
Obesity/metabolism
The molecular basis of the hyperinsulinemia and/or insulin resistance noted in children with premature adrenarche is likely multi-factorial. Obesity exacerbates insulin resistance and is often accompanied by compensatory hyperinsulinemia [71]. Hyperinsulinemia promotes increased adrenal and gonadal steroid secretion. One prospective study involving young Finnish adult women with a history of premature adrenarche concluded that premature adrenarche was not associated with dyslipidemia or metabolic syndrome [72*]. Rather adiposity was associated with insulin resistance or metabolic syndrome independent of pubertal history [73].
The interrelationships between insulin resistance, hyperinsulinemia, and hyperandrogenism are being scrutinized through urinary steroid metabolome by GC-MS analysis of 24-hour urine collections. Specific patterns of the urinary steroid metabolome have been associated with non-syndromic obesity [74]. When the urinary steroid metabolome of children with insulin resistance was compared to those without insulin resistance, children with insulin resistance showed increased excretion of C19 androgens, glucocorticoids, and mineralocorticoid metabolites [75*].
Evaluation and Treatment
Premature adrenarche is a diagnosis of exclusion and is the most common of prepubertal androgen excess [76*]. Following diagnosis, no specific intervention with medication is generally needed. Nevertheless, regular re-evaluations to monitor linear growth velocity, weight gain, skeletal maturation, and pubertal progression are helpful. Annual monitoring of adrenal C19 steroids can be considered. Given the potential consequences of premature adrenarche monitoring physical and psychological development with a focus on healthy lifestyle interventions may be beneficial.
Conclusion
The proximate physiologic mechanisms governing the onset of adrenarche remain unclear. Although DHEAS is the most abundant circulating steroid hormone, its function remains to be established. Available data indicate that for most children premature adrenarche is a benign variation of development. The importance of the 11-oxo-androgen pathway to adrenal function has become increasingly apparent. Despite greater knowledge about zona reticularis function, much remains to be learned about adrenarche.
Key Points.
Adrenarche represents adrenal pubertal maturation and is accompanied by increased adrenal C19 steroid secretion
Three pathways for C19 steroid biosynthesis have been characterized and likely play roles in adrenarche
Premature adrenarche is a diagnosis of exclusion
Long-term longitudinal follow-up is important to assess for metabolic and reproductive consequences associated with premature adrenarche
Acknowledgements
Acknowledgements: No assistance
Financial support and sponsorships: The authors acknowledge support of grants from the following NIH grants: T32DK007729 (BP/PI: Radhika Muzumdar) and R01DK069950 (SFW/PI: William E. Rainey).
Footnotes
Conflicts of interest: No conflicts of interest, financial or otherwise exist. The authors have agreed on the order of authorship. No competing interests.
Reference
- 1.Sun SS, Schubert CM, Chumlea WC, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics 2002;110:911–919. [DOI] [PubMed] [Google Scholar]
- 2.Sklar CA, Kaplan SL, Grumbach MM. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab 1980;51:548–556. [DOI] [PubMed] [Google Scholar]
- 3.Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler GB Jr, Glenn M, Bush M, et al. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology 1978;103:2112–2118. [DOI] [PubMed] [Google Scholar]
- 5.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32:81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labrie F, Martel C, Bélanger A, Pelletier G. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J Steroid Biochem Mol Biol 2017;168:9–18. [DOI] [PubMed] [Google Scholar]
- 7.▪.Schiffer L, Arlt W, Storbeck KH. Intracrine androgen biosynthesis, metabolism and action revisited. Mol Cell Endocrinol 2018;465:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprehensive overview of human C19 steroid biosynthesis, C19 steroid metabolism represented in the context of intracrine pathways for tissue-specific steroid hormone activation and inactivation.
- 8.Anderson DC. The adrenal androgen-stimulating hormone does not exist. Lancet 1980;2(8192):454–456. [DOI] [PubMed] [Google Scholar]
- 9.Rege J,Turcu AF,Else T, et al. Steroid biomarkers in human adrenal disease. J Steroid Biochem Mol Biol. 2019;190:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Shaughnessy PJ, Antignac JP, Le Bizec B, et al. Alternative (backdoor) androgen production and masculinization in the human fetus. PLoS Biol. 2019. February 14;17(2):e3000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.▪▪.Turcu AF, Rege J, Auchus RJ, Rainey WE. 11-Oxygenated androgens in health and disease. Nat Rev Endocrinol 2020. March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]; This reviews provides an extensive update on the biosynthesis and relevance of 11-oxo-androgens in humans.
- 12.Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab 2013;98:1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rege J, Turcu AF, Kasa-Vubu JZ, et al. 11-Ketotestosterone Is the Dominant Circulating Bioactive Androgen During Normal and Premature Adrenarche. J Clin Endocrinol Metab 2018;103:4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rege J, Karashima S, Lerario AM, et al. Age-dependent Increases in adrenal cytochrome b5 and serum 5-Androstenediol-3-sulfate. J Clin Endocrinol Metab. 2016;101:4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campana C, Rege J, Turcu AF, et al. Development of a novel cell based androgen screening model. J Steroid Biochem Mol Biol 2016;156:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rege J, Garber S, Conley AJ, et al. Circulating 11-oxygenated androgens across species. J Steroid Biochem Mol Biol 2019;190:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab 2005;90:2015–2021. [DOI] [PubMed] [Google Scholar]
- 18.▪.Liimatta J, Jääskeläinen J, Karvonen AM, et al. Tracking of Serum DHEAS Concentrations from Age 1 to 6 Years: A Prospective Cohort Study. J Endocr Soc. 2020;4:bvaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors conclude that adrenarche may begin at younger ages. They also found that DHEAS concentrations at 1 year of age correlated with DHEAS concentrations at 6 years of age.
- 19.Houghton LC, Cooper GD, Booth M, et al. Childhood environment influences adrenarcheal timing among first-generation Bangladeshi migrant girls to the UK. PLoS One 2014;9:e109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratt JH, Manatunga AK, Li W. Familial influences on the adrenal androgen excretion rate during the adrenarche. Metabolism 1994;43:186–189. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Ji C, Yang L, Zhuang C. Heritability of serum dehydroepiandrosterone sulphate levels and pubertal development in 6–18-year-old girls: a twin study. Ann Hum Biol 2017;44:325–331. [DOI] [PubMed] [Google Scholar]
- 22.Utriainen P, Laakso S, Liimatta J, et al. Premature adrenarche--a common condition with variable presentation. Horm Res Paediatr 2015;83:221–31 [DOI] [PubMed] [Google Scholar]
- 23.Storbeck KH, Schiffer L, Baranowski ES, et al. Steroid Metabolome Analysis in Disorders of Adrenal Steroid Biosynthesis and Metabolism. Endocr Rev 2019;40:1605–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witchel SF. Congenital Adrenal Hyperplasia. J Pediatr Adolesc Gynecol 2017;30(5):520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannah-Shmouni F, Morissette R, Sinaii N, et al. Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians. Genet Med 2017;19:1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armengaud JB, Charkaluk ML, Trivin C, et al. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab 2009;94:2835–2840. [DOI] [PubMed] [Google Scholar]
- 27.Chesover AD, Millar H, Sepiashvili L, et al. Screening for Nonclassic Congenital Adrenal Hyperplasia in the Era of Liquid Chromatography-Tandem Mass Spectrometry. J Endocr Soc 2019;4:bvz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.▪.Baranowski ES, Arlt W, Idkowiak J. Monogenic Disorders of Adrenal Steroidogenesis. Horm Res Paediatr 2018;89:292–310. [DOI] [PMC free article] [PubMed] [Google Scholar]; This thorough review describes the pathophysiology, biochemistry, and clinical aspects of steroidogenic disorders.
- 29.White PC. Alterations of Cortisol Metabolism in Human Disorders. Horm Res Paediatr 2018;89:320–330. [DOI] [PubMed] [Google Scholar]
- 30.Oostdijk W, Idkowiak J, Mueller JW, et al. PAPSS2 deficiency causes androgen excess via impaired DHEA sulfation--in vitro and in vivo studies in a family harboring two novel PAPSS2 mutations. J Clin Endocrinol Metab 2015;100:E672–E680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tüysüz B, Yılmaz S, Gül E, et al. Spondyloepimetaphyseal dysplasia Pakistani type: expansion of the phenotype. Am J Med Genet A 2013;161A:1300–1308. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, Dou J, Zhang X, et al. Increased 3β-hydroxysteroid dehydrogenase 2 and 17α-hydroxylase activities in a virilized adolescent female with adrenal adenoma: A case report. Exp Ther Med 2016;11:530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marti N, Malikova J, Galván JA, et al. Androgen production in pediatric adrenocortical tumors may occur via both the classic and/or the alternative backdoor pathway. Mol Cell Endocrinol 2017;452:64–73. [DOI] [PubMed] [Google Scholar]
- 34.Fresneau B, Orbach D, Faure-Conter C, et al. Sex-Cord Stromal Tumors in Children and Teenagers: Results of the TGM-95 Study. Pediatr Blood Cancer 2015;62:2114–2119. [DOI] [PubMed] [Google Scholar]
- 35.Yen E, Deen M, Marshall I. Youngest reported patient presenting with an androgen producing sclerosing stromal ovarian tumor. J Pediatr Adolesc Gynecol 2014;27:e121–e124. [DOI] [PubMed] [Google Scholar]
- 36.Cabrera SM, Rogol AD. Testosterone exposure in childhood: discerning pathology from physiology. Expert Opin Drug Saf 2013;12:375–388. [DOI] [PubMed] [Google Scholar]
- 37.Wudy SA, Schuler G, Sánchez-Guijo A, Hartmann MF. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J Steroid Biochem Mol Biol 2018;179:88–103. [DOI] [PubMed] [Google Scholar]
- 38.Dušková M, Kolátorová L, Stárka L. Androgens in women - critical evaluation of the methods for their determination in diagnostics of endocrine disorders. Physiol Res 2018;67(Suppl 3):S379–S390. [DOI] [PubMed] [Google Scholar]
- 39.Oberfield SE, Tao RH, Witchel SF. Present Knowledge on the Etiology and Treatment of Adrenarche. Pediatr Endocrinol Rev. 2018;15:244–254. [DOI] [PubMed] [Google Scholar]
- 40.Creo AL, Schwenk WF 2nd. Bone Age: A Handy Tool for Pediatric Providers. Pediatrics 2017; 140(6). pii: e20171486. [DOI] [PubMed] [Google Scholar]
- 41.Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed. Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 42.Tanner JM, Whitehouse RH, Cameron N, et al. Assessment of Skeletal Maturity and Prediction of Adult Height (TW2 Method). 2nd ed. Cambridge, MA: Academic Press; 1983 [Google Scholar]
- 43.Gurnurkar S, Arheart KL, Messiah SE, et al. A. Skeletal maturation and predicted adult height in children with premature adrenarche. J Pediatr Endocrinol Metab 2014;27:69–74. [DOI] [PubMed] [Google Scholar]
- 44.Diaz A, Bhandari S, Sison C, Vogiatzi M. Characteristics of children with premature pubarche in the New York Metropolitan Area. Horm Res 2008; 70:150–154. [DOI] [PubMed] [Google Scholar]
- 45.DeSalvo DJ, Mehra R, Vaidyanathan P, Kaplowitz PB. In children with PA, bone age advancement by 2 or more years in common and generally benign. J Pediatr Endocrinol Metab 2013;26:215–221. [DOI] [PubMed] [Google Scholar]
- 46.Oron T, Lebenthal Y, de Vries L, et al. Interrelationship of extent of precocious adrenarche in appropriate for gestational age girls with clinical outcome. J Pediatr 2012;160:308–313. [DOI] [PubMed] [Google Scholar]
- 47.Bizzarri C, Crea F, Marini R, et al. Clinical features suggestive of non-classical 21-hydroxylase deficiency in children presenting with precocious pubarche. J Pediatr Endocrinol Metab 2012; 25:1059–1064. [DOI] [PubMed] [Google Scholar]
- 48.Jee YH, Baron J. The Biology of Stature. J Pediatr 2016. ;173:323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almeida M, Laurent MR, Dubois V, et al. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol Rev 2017;97:135–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon JH, Lee HA 3, Kim YJ, et al. Effects of adrenal androgen levels on bone age advancement in prepubertal children: using the EWHA birth and growth cohort study. J Korean Med Sci. 2017;32:968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Na JH, Kim YH, Hong SJ, Kim JK. Association between Body Mass Index and Serum Dehydroepiandrosterone Sulfate Level in 8-Year-Old Girls. Obes Metab Syndr 2018;27:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sopher AB, Jean AM, Zwany SK, et al. Bone age advancement in prepubertal children with obesity and PA: possible potentiating factors. Obesity (Silver Spring) 2011;19: 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein KO, Newfield RS, Hassink SG. Bone maturation along the spectrum of normal weight to obesity: a complex interplay of sex, growth factors, and weight gain. J Pediatr Endocrinol Metab 2016;29:311–318. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen TV, Wu M, Lew J, et al. Dehydroepiandrosterone impacts working memory by shaping cortico-hippocampal structural covariance during development. Psychoneuroendocrinology 2017;86: 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.▪.Greaves RF, Wudy SA, Badoer E, et al. A tale of two steroids: The importance of the androgens DHEA and DHEAS for early neurodevelopment. J Steroid Biochem Mol Biol 2019;188:77–85. [DOI] [PubMed] [Google Scholar]; This article reviews how DHEA and DHEAS could potentially modulate early brain development from fetal life to onset of adrenarche..
- 56.Del Giudice M Middle childhood: an evolutionary-developmental synthesis. Child Dev Perspect, 2014: 8: 193–200. [Google Scholar]
- 57.Campbell BC. Adrenarche and middle childhood. Hum Nat 2011;22:327–349. [DOI] [PubMed] [Google Scholar]
- 58.▪.Campbell B DHEAS and Human Development: An Evolutionary Perspective. Front Endocrinol (Lausanne) 2020. March 3;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]; The process of adrenarche is limited to humans and a few nonhuman primate species. This article considers adrenarche in the context of nutrition, weaning, and cortical maturation.
- 59.▪▪.Livadas S, Bothou C, Kanaka-Gantenbein C, et al. Unfavorable Hormonal and Psychologic Profile in Adult Women with a History of Premature Adrenarche and Pubarche, Compared to Women with Polycystic Ovary Syndrome. Horm Metab Res 2020;52:179–185. [DOI] [PubMed] [Google Scholar]; This study involved girls with history of premature adrenarche and appropriate birthweight for gestation age. Although ~40% of girls features typical of PCOS, only 14% fulfilled the Rotterdam diagnostic criteria for PCOS. As a group, the girls with a history of premature adrenarche had greater anxiety and decreased insulin sensitivity compared to the control group. These authors recommend longitudinal re-evaluations of girls with premature adrenarche.
- 60.Barendse MEA, Simmons JG, Patton G, et al. Adrenarcheal Timing Longitudinally Predicts Anxiety Symptoms via Amygdala Connectivity During Emotion Processing. J Am Acad Child Adolesc Psychiatry. 2019. May 2 pii: S0890–8567(19)30286–2. [DOI] [PubMed] [Google Scholar]
- 61.Sontag-Padilla LM, Dorn LD, Tissot A, et al. Executive functioning, cortisol reactivity, and symptoms of psychopathology in girls with premature adrenarche. Dev Psychopathol 2012;24:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byrne ML, Whittle S, Vijayakumar N, et al. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev Cogn Neurosci 2017;25:12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.▪.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2018;89:251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript presents the evidence-based international guidelines for the diagnosis and management of PCOS.
- 64.▪.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270–284. [DOI] [PubMed] [Google Scholar]; This article provides updated information regarding pathophysiology, diagnosis, and management of PCOS.
- 65.▪▪.Peña AS,Witchel SF, Hoeger KM, et al. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med 2020;18(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]; Based on the International Evidence Based Guidelines, this manuscript details the PCOS guidelines specific for adolescent girls to improve diagnostic accuracy while avoiding over-diagnosis.
- 66.Ibañez L, Potau N, Virdis R, et al. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab 1993;76:1599–1603. [DOI] [PubMed] [Google Scholar]
- 67.Ibáñez L, Potau N, Francois I, de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab 1998;83:3558–3562. [DOI] [PubMed] [Google Scholar]
- 68.Ibáñez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab 2006;91:2153–2158. [DOI] [PubMed] [Google Scholar]
- 69.▪.de Zegher F, López-Bermejo A, Ibáñez L. Central Obesity, Faster Maturation, and ‘PCOS’ in Girls. Trends Endocrinol Metab 2018;29:815–818. [DOI] [PubMed] [Google Scholar]; This article presents the hypothesis regarding mismatches between prenatal and postnatal weight gain potentially leading to ectopic hepatic fat storage and, possibly, PCOS.
- 70.Ibáñez L, Del Río L, Díaz M, et al. Normalizing Ovulation Rate by Preferential Reduction of Hepato-Visceral Fat in Adolescent Girls With Polycystic Ovary Syndrome. J Adolesc Health 2017;61:446–453. [DOI] [PubMed] [Google Scholar]
- 71.Kaya G, Yavas Abali Z, Bas F, et al. Body mass index at the presentation of premature adrenarche is associated with components of metabolic syndrome at puberty. Eur J Pediatr 2018;177:1593–1601. [DOI] [PubMed] [Google Scholar]
- 72.▪▪.Liimatta J, Utriainen P, Laitinen T, Voutilainen R, Jääskeläinen J. Cardiometabolic Risk Profile Among Young Adult Females With a History of Premature Adrenarche. J Endocr Soc 2019;3:1771–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]; This prospective study reports the outcome of 30 girls with premature adrenarche and 41 controls. Girls with a history of premature adrenarche tended to have higher central fat mass. Premature adrenarche did not seem to be associated with metabolic syndrome dyslipidemia, or hypertension.
- 73.Williams KM, Oberfield SE, Zhang C, McMahon DJ, Sopher AB. The Relationship of Metabolic Syndrome and Body Composition in Children with Premature Adrenarche: Is It Age Related? Horm Res Paediatr 2015;84(6):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this cohort, PA was not associated with metabolic syndrome, dyslipidemia, or hypertension.
- 74.Gawlik A, Shmoish M, Hartmann MF, et al. Steroid Metabolomic Disease Signature of Nonsyndromic Childhood Obesity. J Clin Endocrinol Metab 2016;101:4329–4337. [DOI] [PubMed] [Google Scholar]
- 75.▪.Gawlik AM, Shmoish M,Hartmann MF, et al. Steroid Metabolomic Signature of Insulin Resistance in Childhood Obesity. Diabetes Care 2020;43:405–410. [DOI] [PubMed] [Google Scholar]; The urinary steroid metabolome was characterized in 87 obese children. The authors report that the urinary steroidal metabolomic signature was characterized by enhanced secretion of steroids from all three adrenal pathways; they hypothesize that a vicious cycle involving obesity, insulin resistance, glucocorticoids, and C19 steroids exists.
- 76.▪.Idkowiak J, Elhassan YS, Mannion P, et al. Causes, patterns and severity of androgen excess in 487 consecutively recruited pre- and post-pubertal children. Eur J Endocrinol 2019;180:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a retrospective review of 199 children who presented with androgen excess. The most common diagnosis among the prepubertal children was premature adrenarche. The differential diagnosis of androgen excess in children and adolescents is reviewed.


