Abstract
Background
Resistance to therapy and a poor outcome characterize relapsed or refractory acute myeloid leukemia (AML). There is a clear need for additional palliative approaches with acceptable toxicities. Vincristine sulfate liposome injection (VSLI) confers enhanced pharmacokinetics and activity when compared to the parent compound. It is effective and well tolerated in heavily pretreated acute lymphoblastic leukemia (ALL) patients. Preclinically VSLI has activity in vincristine-resistant cancers. As relapsed or refractory AML patients would have minimal exposure to vincristine it was hypothesized that VSLI would be well tolerated and may have activity.
Methods
A pilot phase II clinical trial was conducted. Five patients with relapsed or refractory disease were treated using the Food and Drug Administration (FDA)-approved dose and schedule.
Results
Of the five patients treated none completed more than one cycle; there were no responses and two patients did not complete one cycle of therapy. Surprisingly, three of the five patients had treatment-related constipation, and two had neuropathy consistent with the known toxicities of VSLI. Given the toxicity and lack of response, the trial was terminated early.
Conclusions
VSLI had no activity against relapsed or refractory AML in this limited, single institution dataset.
Keywords: Acute myeloid leukemia, Vincristine, Relapsed, Refractory, Therapy
Introduction
Acute myeloid leukemia (AML) is an aggressive malignancy that leads to marrow failure and death [1]. AML affects approximately 20,800 people per year in the USA resulting in over 10,000 deaths [1]. Despite decades of research, the overall 5-year survival rate remains less than 30%. The current standard of care for most patients with AML is induction chemotherapy with cytarabine and an anthracycline. Most patients will achieve a complete, but transient, remission. Once relapsed, the disease is increasingly resistant to further therapy [2].
Vincristine has been explored in AML previously and has shown activity in preclinical models but has modest clinical efficacy when used in the upfront and relapsed settings [3-6]. Studies have shown that primary patient AML isolates are sensitive to vincristine [3]. Despite encouraging preclinical data, the addition of vincristine to standard induction therapy demonstrated a reduced remission rate [4]. Vincristine is a substrate for multi-drug resistance (MDR) efflux pumps that are expressed by refractory AML, resulting in a poor therapeutic profile [7, 8]. These results led to the general cessation of use of vincristine in the treatment of AML in most cases.
Vincristine sulfate liposomal injection (VSLI, Marqibo®) is a unique liposomal preparation of vincristine that has significantly different pharmacokinetics and anticancer activity from vincristine [9]. These differing properties include a more than doubling of the plasma half-life, an improved distribution with a more than doubling of delivery to bone marrow and more than 12-fold increase in delivery to the spleen. Despite this pharmacokinetic profile, the systemic toxicity is decreased compared to traditional formulations of vincristine [10]. VSLI has minimal hematological toxicity [11]. In addition, VSLI has demonstrated activity in several tumor models that are resistant to vincristine both in vitro and in vivo [12]. VSLI is active against cancer cells that overexpress MDR efflux proteins and those that have been specifically generated by repeated exposure to be resistant to vincristine [12]. These properties led to a clinical trial of single agent VSLI in heavily pretreated acute lymphoblastic leukemia (ALL) patients [13]. In this very difficult-to-treat population, a complete remission (CR) or complete remission with incomplete count recovery (CRi) was achieved in 20% of patients, and an additional 15% of patients achieved either a partial response or a decrease in leukemia cells in the marrow. There were responses in patients receiving VSLI as third, fourth, and even fifth line therapy despite the fact that all of the treated patients had been previously exposed to vincristine. Notably, 12 of the 65 patients were bridged to a bone marrow transplant. This remarkable activity was achieved with toxicities that were entirely predictable and manageable [13]. This data led to the approval of VSLI for Philadelphia chromosome-negative adult ALL following at least two relapses or failed treatment with at least two prior anti-leukemia regimens by the Food and Drug Administration (FDA) in 2012.
Our central hypothesis was that VSLI will have clinical efficacy in AML due to the fact that this agent overcomes the common resistance mechanisms for conventional vincristine with a predictable and acceptable risk profile. Further, the primary toxicity attributed to VSLI in the ALL trials was peripheral neuropathy, a toxicity known to be associated with cumulative exposure to vincristine [13]. All of the ALL trial patients had received conventional vincristine, but AML patients will not be previously exposed. We therefore hypothesized that the observed toxicities would be no worse and possibly better than what was observed in the ALL trial. Given the activity of VSLI against cancer cells resistant to vincristine, its improved pharmacokinetics, and demonstrated safety in a heavily pretreated and similar cohort of leukemia patients, we conducted a feasibility trial of VSLI treatment for the palliation of relapsed or refractory AML patients.
Patients and Methods
Patients
Adults age 18 or older with histologically or cytologically documented relapsed and/or refractory AML were eligible for the study. They were required to have relapsed/refractory disease and be ineligible for, declined, or have failed at least one previous salvage regimen. Notably, patients were excluded if they had active central nervous system (CNS) disease, poor performance status (ECOG greater than or equal to 3), or persistent grade 3 or higher prior vincristine related neuropathy.
Objectives
The primary objective of the study was to determine the feasibility of administering VSLI to relapsed or refractory AML patients having failed, refused or not a candidate for at least one chemotherapy salvage regimen. Feasibility was defined as at least four of the first 10 patients able to complete two cycles to allow the study to continue. Secondary objectives included the hematologic improvement rate of VSLI in this patient population, the response rate (CR, CRi, partial remission (PR), and morphologic leukemia-free state (MLFS)) and overall survival of patients treated with VSLI.
Statistical design
The study was based on a Simon’s optimal two-stage design [14]. The assumptions were that the hematologic improvement rate would be non-existent without this intervention and any hematologic improvement would be beneficial to the patient population. Therefore, the null hypothesis was that the current response rate was 0.01 (approximately 0) which was to be tested against a one-sided alternative of a 10% hematologic improvement rate. Should the futility criteria for stopping not be met, 17 patients were to be accrued in the first stage. If one or more participants experience hematologic improvement then the study would continue to the second stage, otherwise the study would have stopped for lack of efficacy. During stage 2, an additional 22 patients for a total of 39 were to be enrolled. If two or more responses were observed in the 39 patients, the null hypothesis that the intervention is ineffective would have been rejected. This design yields a type I error rate of 0.04 and power of 80%.
Study approval and oversight
The study was approved by the institutional review board, and all patients were registered with the Comprehensive Cancer Center of Wake Forest University (CCCWFU) Protocol Registrar. Informed consent was obtained prior to the pre-enrollment medical screening. Pre-enrollment medical screening was used to determine the patient’s eligibility. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Treatment plan
VSLI was dosed according to the ALL label, at 2.25 mg/m2 via intravenous access, without dose cap, on days 1, 8, 15, and 22 of a 28-day cycle. Treatment was continued until loss of response, progression, unacceptable toxicity, patient withdrawal of consent, or pursuit of hematopoietic stem cell transplantation (HSCT) or additional salvage therapy.
Results
A total of five patients were enrolled in the study and treated with at least one dose of VSLI. Patient characteristics are shown in Table 1. The patients are discussed below. All toxicities attributed as at least possibly related to VSLI are shown by patient in Table 2.
Table 1. Patient Characteristics.
| Median age (range) | 78 (41 - 81) |
| Age ≥ 60 years old (%) | 4/5 (80%) |
| Male (%) | 3/5 (60%) |
| No. Caucasian (%) | 5/5 (100%) |
| ECOG performance status | |
| 0 - 1 | 1/5 (20%) |
| 2 | 4/5 (80%) |
| 3 | 0 (0%) |
| Median duration of CR1 in months (range) | 3 (3 - 11) |
| Refractory disease (%) | 2/5 (40%) |
| Previous lines of salvage | |
| 0 | 0 (0%) |
| 1 | 2/5 (40%) |
| 2 | 1/5 (20%) |
| > 2 | 2/5 (40%) |
| Antecedent hematologic disorder | 1/5 (20%) |
| Previous HDAC or IDAC-based salvage | 4/5 (80%) |
| ELN 2017 cytogenetic risk score | |
| Good risk | 0/5 (0%) |
| Intermediate risk | 2/5 (40%) |
| Poor risk | 3/5 (60%) |
| Median %blasts in marrow (range) | 29% (15-49%) |
CR1: first complete remission; HDAC: high-dose cytarabine (Ara-C); IDAC: intermediate-dose Ara-C.
Table 2. Toxicities.
| Patient number | Cycle | Toxicity | Grade |
|---|---|---|---|
| 01 | 1 | Abdominal pain | 2 |
| 01 | 1 | Bloating | 2 |
| 01 | 1 | Constipation | 2 |
| 01 | 1 | Peripheral sensory neuropathy | 1 |
| 01 | 2 | Abdominal distension | 2 |
| 02 | 1 | Constipation | 1 |
| 02 | 1 | Peripheral sensory neuropathy | 1 |
| 03 | 1 | Abdominal distension | 1 |
| 03 | 1 | Anorexia | 2 |
| 03 | 1 | Bloating | 1 |
| 03 | 1 | Constipation | 2 |
| 03 | 1 | Nausea | 2 |
| 03 | 1 | Peripheral sensory neuropathy | 1 |
| 03 | 1 | Vomiting | 1 |
| 04 | 1 | Abdominal pain | 2 |
| 04 | 1 | Nausea | 3 |
| 05 | 1 | Abdominal pain | 2 |
| 05 | 1 | Constipation | 1 |
| 05 | 1 | Nausea | 1 |
| 05 | 1 | Peripheral sensory neuropathy | 1 |
| 05 | 1 | Rash maculo-papular | 1 |
Patient 1
Patient 1 was a 64-year-old man initially diagnosed with AML (iso(7q), gain of q31, and FLT-3 TKD positive) at an outside institution in December 2013. He received induction therapy with cytarabine and daunorubicin which was followed by two cycles of high-dose cytarabine (HiDAC) consolidation. His first relapse was in March 2014 with normal cytogenetics with a single N-terminus CEBPa mutation. He then was enrolled in CCWFU 22112 using an investigational agent CPI-613 with HiDAC and mitoxantrone. He received two courses of therapy, and bone marrow following this showed persistent AML with 27% blasts. He went on to receive two cycles of mitoxantrone, etoposide, and cytarabine (MEC). Bone marrow biopsy after these two cycles in July 2014 showed no morphologic evidence of disease. Repeat bone marrow biopsies in August and October of 2014 continued to show a hypocellular marrow with no morphologic evidence of disease. In January 2015, bone marrow biopsy confirmed relapsed disease. He was treated with two cycles of azacitidine which was completed in February 2015. Extramedullary disease was identified in April 2015 with disease of the left tonsil for which he was treated with radiation therapy. Bone marrow biopsy in June 2015 was consistent with persistent AML with iso(7q). In June 2015, he was enrolled in this study. ECOG performance status (PS) was 2 at the time of enrollment. He completed cycle 1 of VSLI therapy and received days 1, 8, 15, and 22 of treatment with no delays or dose adjustments. On cycle 1 day 15, he reported grade 2 constipation and grade 1 neuropathy and was managed with supportive measures. On day 22, he reported nausea which was thought to be unrelated to therapy. After receiving his day 22 dose, he was admitted for workup and management of nausea and was found to have gastric outlet obstruction secondary to a large paraesophageal hernia. He was determined to be a poor surgical candidate, withdrew from the study and transitioned to hospice care.
Patient 2
Patient 2 was a 78-year-old man initially diagnosed with AML with complex cytogenetics (deletion -5, -7, and -3p) in April 2014. He was treated with standard induction therapy with cytarabine and daunorubicin. Recovery bone marrow biopsy showed focal aggregates of blasts, but both fluorescence in situ hybridization (FISH) and cytogenetics were normal. He received three cycles of HiDAC and end of treatment bone marrow in October 2014 was consistent with remission by morphology, FISH, and cytogenetics. He was noted to have his first relapse in April 2015 with complex cytogenetics (-5, -7, and +8). He was enrolled in a clinical trial and received re-induction with HiDAC, mitoxantrone, and an investigational agent CPI-613 for two courses. Recovery bone marrow was consistent with remission by morphology, FISH, and cytogenetics. After two cycles of consolidation, repeat bone marrow demonstrated 13% blasts consistent with relapsed disease with normal cytogenetics. FISH was positive for 5q- in 33% of cells and 7q- in 43.5% of cells. He was enrolled in this study in September 2015. ECOG PS was 2 at the time of enrollment and there was no history of preexisting neuropathy. He received cycle 1 days 1, 8, and 15 of treatment. Prior to day 22, he was admitted with septic shock of unclear origin. After several days of antibiotic therapy and persistent hemodynamic instability, he elected to withdraw from the study and transition to hospice care.
Patient 3
Patient 3 was an 80-year old woman initially diagnosed with AML in August 2014 with complex cytogenetics (-5, -7, +8, +21, and t(4;16)). She was enrolled on ECOG 2906 Arm A and received induction therapy with cytarabine and daunorubicin. Day 14 bone marrow biopsy showed poor cytoreduction, and she was removed from the protocol. She then received re-induction with HiDAC and mitoxantrone. Nadir bone marrow biopsy again showed persistent disease, but further treatment was deferred at that time due to deconditioning. In October 2014, she received re-induction therapy with 10-day decitabine. Recovery bone marrow biopsy was negative for leukemia by morphology, but FISH remained positive for -5, -7, and +8. She went on to receive seven cycles of maintenance 5-day decitabine and then repeat marrow showed recurrent morphologic disease with complex cytogenetics and clonal evolution. Therapy was changed to azacitidine in July 2015. In September 2015, bone marrow showed persistent AML with increased blasts of 29% with complex cytogenetics with FISH positive for -5, -7, and +8. In September 2015, she was enrolled in this study. ECOG PS was 2 at enrollment. Peripheral blast count on cycle 1 day 1 of VSLI was 17%. She received cycle 1 days 1 and 8 of treatment. She presented to clinic on day 15 for treatment consideration and reported significant fatigue and constipation. Peripheral blast count was increased to 55%. She was classified as a treatment failure and withdrawn from the study.
Patient 4
Patient 4 was a 41-year old woman diagnosed with AML with t(8;21) and c-Kit mutation positive in September 2014. She received induction therapy with cytarabine and daunorubicin and nadir bone marrow biopsy in October 2014 was negative. Recovery bone marrow was consistent with remission. She received consolidation with HiDAC. Her first relapse was in February 2015 with t(8;21) noted by cytogenetics. She was treated with HiDAC and mitoxantrone for two full courses. Residual disease was demonstrated on the second nadir. She then received mitoxantrone, etoposide, and cytarabine for two courses. Nadir bone marrow was negative and she achieved a brief remission with incomplete count recovery. Following a subsequent relapse in June 2015 she received azacitidine for three cycles with no response and then was enrolled in this study in September 2015. ECOG PS was 2 at enrollment. She completed cycle 1 of VSLI in October 2015 with no delays, dose adjustments, or overt toxicity. She was admitted at the end of cycle 1 for pain management. She had no response to VSLI with an increase in her blast count at the end of cycle 1. Given her deconditioning and lack of response, she opted to withdraw from the study and transition to supportive care.
Patient 5
Patient 5 was an 81-year-old man initially diagnosed with myelodysplastic syndrome (MDS) at an outside institution in June 2009. Cytogenetics from time of diagnosis were not available. His MDS was treated with lenalidomide and epoetin alfa. In October 2011, he progressed to AML with normal cytogenetics and was negative for FLT-3, NPM-1, and CEBPa. He was treated with 10-day decitabine for three courses. Recovery bone marrow showed a hypocellular marrow with 10% residual blasts. The decision was made to move forward with maintenance therapy with 5-day cycles of decitabine. Bone marrow biopsy in November 2012 after 10 cycles of decitabine revealed an increase in blasts and the therapy was changed to azacitidine. He received a total of 39 cycles of azacitidine (cycles 1 - 30 were 7-day courses and cycles 31 - 39 were 5-day courses). In January 2016, bone marrow biopsy showed persistent AML with 22% blasts. He was enrolled in this study in February 2016, and ECOG PS was 1 at the time of enrollment. He completed cycle 1 of VSLI with no dose adjustments required. On cycle 2 day 1, he was seen in clinic and reported worsening fatigue, constipation, and grade 2 peripheral neuropathy. VSLI was held due to these adverse effects. He remained pancytopenic with unchanged peripheral blood counts. At follow-up 1 week later, he reported persistent side effects and elected to transition to supportive care. He was enrolled in home hospice 2 weeks later.
In summary of the five patients enrolled there were no responses. Two patients did not complete one cycle of therapy and no patient completed two cycles. As the feasibility-stopping rule required at least four of the first 10 patients to complete two cycles combined with the observed toxicity rate and lack of efficacy signal the decision was made to terminate the study early. The median overall survival of the study population was 1.34 months (Fig. 1) and the median time on therapy was 29 days (Fig. 2).
Figure 1.
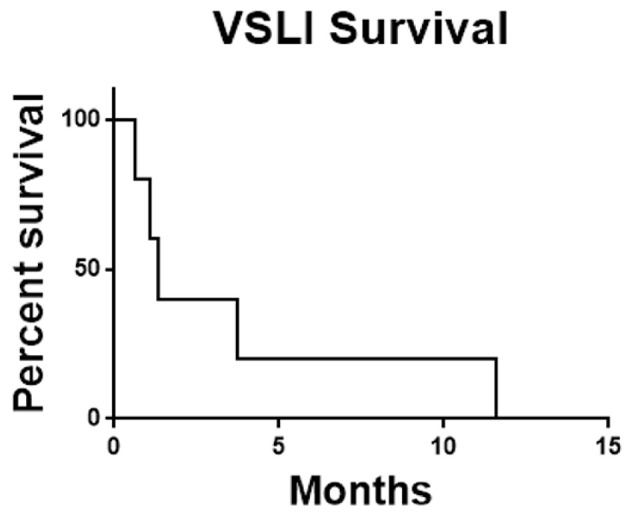
Overall survival for all patients. Survival was calculated from date of enrollment until date of death from any cause. VSLI: vincristine sulfate liposome injection.
Figure 2.
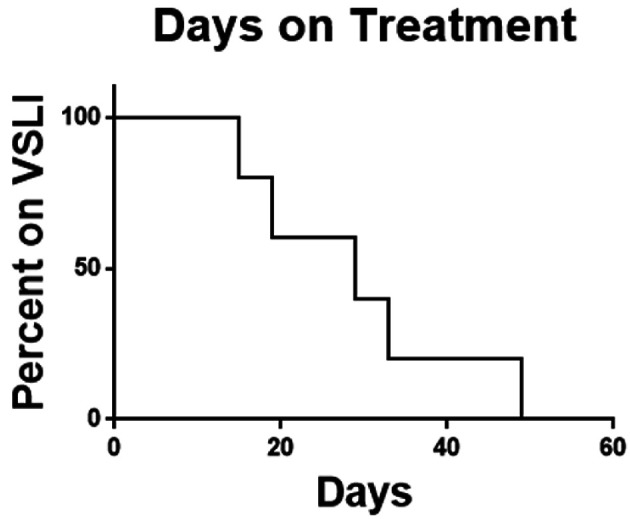
Time on therapy. Time on VSLI was calculated from time of first dose to time of discontinuation for any cause. VSLI: vincristine sulfate liposome injection.
Discussion
Five patients were enrolled in this study. All patients were heavily pretreated having received between one and three salvage regimens prior to enrollment. ECOG performance status at the time of enrollment ranged from 1 - 2. No patients had any history of preexisting peripheral neuropathy. In regards to drug exposure, three of the five patients completed cycle 1 of VSLI and no patient completed two cycles.
No responses were achieved with VSLI in this limited cohort. Given the low enrollment of this study it is difficult to draw firm conclusions. The lack of an efficacy signal may be related to the low number of patients treated, the brief exposure to the drug, or the poor efficacy of liposomal vincristine in this population. Two of five patients discontinued therapy prior to any response assessment during or shortly after cycle 1, and had significant complications during therapy (septic shock, gastric outlet obstruction). Patient 3 and patient 5 were classified as treatment failures as they both had increasing blast counts on treatment. Patient 2 transitioned to hospice due to the adverse effects of therapy.
In terms of toxicity, three of five patients experienced grade 1 fatigue, grade 1 - 2 peripheral neuropathy, and grade 1 - 2 constipation. Of the five patients, two had peripheral neuropathy (grade 1 and grade 2) which was unexpected given the low incidence of neuropathy in the ALL population treated with VSLI and additionally considering the lack of exposure of neurotoxic agents in our patients’ prior therapy.
Given the toxicity signal and complete lack of efficacy the decision was made to discontinue the study despite not formally meeting the futility criteria. This was decided, as the treating physicians did not feel comfortable exposing additional palliative patients to the toxicities in the absence of any indication of disease control. This was further bolstered by the very low median survival observed in our study of 1.34 months. This is similar to the 2-month median survival reported for relapsed AML patients receiving only supportive care [15].
Several previous reports have described resistance mechanisms of AML towards vincristine. While some of these resistance mechanisms maybe overcome by VSLI, particularly MDR efflux pumps [12], there are likely additional mechanisms not sensitive to VSLI. One of the most likely is the ability of myeloperoxidase (MPO) to degrade vincristine [16]. Since VSLI must ultimately deliver vincristine to the cytoplasm of an AML cell, it would then be a substrate for MPO-mediated degradation. Additionally, any vincristine that was released from VSLI in serum could also be subjected to MPO degradation as MPO levels in serum correlated with vincristine degradation in pediatric ALL patients [17]. If it is true one possible future approach would be to combine VSLI with an MPO inhibitor. There are several such inhibitors in development including one in clinical trials for the treatment of Parkinson’s disease [18]. These strategies will require additional study.
Our study has several important limitations. This was a single institution study and only a limited number of patients were treated. Additionally, all patients were heavily pretreated with three of five patients having two or more previous lines of therapy. It is unclear if using VSLI earlier in the course of their disease would have made a difference. Likewise, although no patient was previously treated with vincristine all of them had received prior treatment with cytidine analogues. These agents are also associated with treatment-related neuropathy [19] and may have predisposed these patients to developing neuropathy following VSLI treatment.
Conclusions
In summary, this study failed to demonstrate any antileukemic effect of VSLI. Further studies would be needed to clarify the mechanism of resistance.
Acknowledgments
The authors appreciate the support of the Comprehensive Cancer Center of Wake Forest Baptist Health.
Financial Disclosure
This work was supported by the Frances P. Tutwiler Fund, the Doug Coley Foundation for Leukemia Research, the McKay Cancer Research Foundation, and the National Institute of Health (TSP is supported by NCI 1R01CA197991-01A1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of Interest
The authors have no relevant conflicts of interest to disclose.
Informed Consent
All patients provided written informed consent.
Author Contributions
TSP designed the study and enrolled patients. TSP, MBS and RW drafted the manuscript and did critical editing. LRE, DSH, RB, MM, SD, SL, BLP and TSP enrolled patients, provided clinical care to patients on study and edited the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H. et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe JM. et al. Very poor survival of patients with AML who relapse after achieving a first complete remission: The eastern cooperative oncology group experience. Blood. 2005;106(11):162a–163a. doi: 10.1182/blood.V106.11.546.546. [DOI] [Google Scholar]
- 3.Muller MR, Lennartz K, Boogen C, Nowrousian MR, Rajewsky MF, Seeber S. Cytotoxicity of adriamycin, idarubicin, and vincristine in acute myeloid leukemia: chemosensitization by verapamil in relation to P-glycoprotein expression. Ann Hematol. 1992;65(5):206–212. doi: 10.1007/BF01703946. [DOI] [PubMed] [Google Scholar]
- 4.Ohno R, Kobayashi T, Tanimoto M, Hiraoka A, Imai K, Asou N, Tomonaga M. et al. Randomized study of individualized induction therapy with or without vincristine, and of maintenance-intensification therapy between 4 or 12 courses in adult acute myeloid leukemia. AML-87 Study of the Japan Adult Leukemia Study Group. Cancer. 1993;71(12):3888–3895. doi: 10.1002/1097-0142(19930615)71:12<3888::AID-CNCR2820711216>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Leung MF, Wong KF. The differentiating effect of retinoic acid and vincristine on acute myeloid leukemia. J Hematother. 1999;8(3):275–279. doi: 10.1089/106161299320307. [DOI] [PubMed] [Google Scholar]
- 6.Sartiano GP, Pfrimmer WJ, Turner AR. Vincristine (NSC-67574), cytosine arabinoside (NSC-63878), 6 thioguanine (NSC-752) and daunorubicin (NSC-82151) (VAT-D): a pilot study of combination chemotherapy for remission induction in acute myeloid leukemia in adults. Med Pediatr Oncol. 1978;4(3):205–212. doi: 10.1002/mpo.2950040303. [DOI] [PubMed] [Google Scholar]
- 7.Hosoi E, Hirose M, Hamano S, Morimoto M, Kuroda Y. Effect of MDR antagonists on the cidal activity of vincristine for cells expressing MDR-1 is superior to those expressing MRP. Int J Oncol. 1998;13(2):343–348. doi: 10.3892/ijo.13.2.343. [DOI] [PubMed] [Google Scholar]
- 8.Beck J, Handgretinger R, Klingebiel T, Dopfer R, Schaich M, Ehninger G, Niethammer D. et al. Expression of PKC isozyme and MDR-associated genes in primary and relapsed state AML. Leukemia. 1996;10(3):426–433. [PubMed] [Google Scholar]
- 9.Embree L, Gelmon K, Tolcher A, Hudon N, Heggie J, Dedhar C, Logan P. et al. Pharmacokinetic behavior of vincristine sulfate following administration of vincristine sulfate liposome injection. Cancer Chemother Pharmacol. 1998;41(5):347–352. doi: 10.1007/s002800050750. [DOI] [PubMed] [Google Scholar]
- 10.Silverman JA, Deitcher SR. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71(3):555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deitcher OR, Glaspy J, Gonzalez R, Sato T, Bedikian AY, Segarini K, Silverman J. et al. High-dose vincristine sulfate liposome injection (Marqibo) Is not associated with clinically meaningful hematologic toxicity. Clin Lymphoma Myeloma Leuk. 2014;14(3):197–202. doi: 10.1016/j.clml.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Leonetti C, Scarsella M, Semple SC, Molinari A, D'Angelo C, Stoppacciaro A, Biroccio A. et al. In vivo administration of liposomal vincristine sensitizes drug-resistant human solid tumors. Int J Cancer. 2004;110(5):767–774. doi: 10.1002/ijc.20174. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien S, Schiller G, Lister J, Damon L, Goldberg S, Aulitzky W, Ben-Yehuda D. et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol. 2013;31(6):676–683. doi: 10.1200/JCO.2012.46.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 15.Brandwein JM, Saini L, Geddes MN, Yusuf D, Liu F, Schwann K, Billawala A. et al. Outcomes of patients with relapsed or refractory acute myeloid leukemia: a population-based real-world study. Am J Blood Res. 2020;10(4):124–133. [PMC free article] [PubMed] [Google Scholar]
- 16.Schlaifer D, Cooper MR, Attal M, Sartor AO, Trepel JB, Laurent G, Myers CE. Myeloperoxidase: an enzyme involved in intrinsic vincristine resistance in human myeloblastic leukemia. Blood. 1993;81(2):482–489. doi: 10.1182/blood.V81.2.482.482. [DOI] [PubMed] [Google Scholar]
- 17.Ozgen U, Turkoz Y, Stout M, Ozugurlu F, Pelik F, Bulut Y, Aslan M. et al. Degradation of vincristine by myeloperoxidase and hypochlorous acid in children with acute lymphoblastic leukemia. Leuk Res. 2003;27(12):1109–1113. doi: 10.1016/S0145-2126(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 18.Jucaite A, Svenningsson P, Rinne JO, Cselenyi Z, Varnas K, Johnstrom P, Amini N. et al. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson's disease. Brain. 2015;138(Pt. 9):2687–2700. doi: 10.1093/brain/awv184. [DOI] [PubMed] [Google Scholar]
- 19.Baker WJ, Royer GL Jr, Weiss RB. Cytarabine and neurologic toxicity. J Clin Oncol. 1991;9(4):679–693. doi: 10.1200/JCO.1991.9.4.679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.


