Abstract
Objective:
To evaluate if the use of apneic oxygenation (AO) during tracheal intubation (TI) in children is feasible and would decrease the occurrence of oxygen desaturation.
Design:
Prospective pre-post observational study
Setting:
A large single-center non-cardiac pediatric intensive care unit (PICU) in North America
Patients:
All patients less than 18 years of age who underwent primary TI from August 1, 2014 - September 30, 2018
Interventions:
Implementation of AO for all primary TI as quality improvement
Measurements and Main Results:
Total of 1373 TI’s (661 pre- and 712 post-implementation) took place during study period. Within 2 months, AO use reached to pre-defined adherence threshold (>80% of primary TIs) after implementation, and sustained at >70% level throughout the post-implementation. Between the pre- and post-implementation, no significant differences were observed in patient demographics, difficult airway features, or providers. Respiratory and procedural indications were more common during pre-intervention. Video laryngoscopy devices were used more often during the post-implementation (pre 5% vs post 75%, p<0.001). Moderate oxygen desaturation <80% were observed in fewer TIs after AO implementation (pre 15.4% vs. post 11.8%, p=0.049); severe oxygen desaturation<70% was also observed in fewer TIs after implementation (pre 10.4% vs. post 7.2%, p=0.032). Hemodynamic TI associated events (i.e., cardiac arrests, hypotension, dysrhythmia) were unchanged (pre 3.2% vs. post 2.0%, p=0.155). Multivariable analyses showed AO implementation was significantly associated with a decrease in moderate desaturation <80% (adjusted odds ratio: aOR 0.55, 95% CI 0.34–0.88) and with severe desaturation<70% (aOR 0.54, 0.31–0.96) while adjusting for TI indications and device.
Conclusions:
Implementation of AO in pediatric ICU was feasible, and was associated with significant reduction in moderate and severe oxygen desaturation. Use of AO should be considered when intubating critically ill children.
Keywords: Airway management, child, pediatrics, endotracheal intubation, tracheal intubation, PICU
Introduction:
Tracheal intubation (TI) is a life-saving procedure performed in pediatric intensive care units (PICU). Although it is frequently performed, it comes with significant risk. Adverse TI associated events (TIAEs) have been reported in 16–19% of TIs [1–4]. Occurrence of adverse TIAEs and oxygen desaturation during TI is associated with longer duration of mechanical ventilation, longer ICU length of stay and mortality [4].
Oxygen desaturation during TI occurs in 16–19% of TI’s in the PICU. It is strongly associated with the occurrence of hemodynamic TIAEs (cardiac arrest with or without return of spontaneous circulation, hypotension, dysrhythmia)[5–7]. Risk factors for oxygen desaturations include infants (<1 year old), respiratory failure or upper airway obstruction as TI indication, resident participation as laryngoscopists, and multiple TI attempts [5–7].
Apneic oxygenation (AO) has been successfully implemented in adult care settings to reduce hypoxemia from the unavoidable apneic phase of airway management due to laryngoscopy [8,9]. While no ventilation is provided during this phase, oxygen provided by nasal cannula will continue to diffuse to alveoli during laryngoscopy, resulting in attenuation of alveolar deoxygenation [10,11]. A similar effect is reported in pediatric anesthesia studies [12,13].
With this knowledge, we aimed to decrease occurrence of oxygen desaturation during TI for critically ill children by implementing the use of apneic oxygenation (AO) for primary TIs in the PICU. We hypothesized: (1) Implementation of AO is feasible, and (2) Implementation of AO is associated with a decrease in moderate oxygen desaturation (SpO2<80%) and severe oxygen desaturation (SpO2<70%). We specifically evaluated the feasibility by measuring the duration to achieve a priori defined AO practice adherence at initial threshold of 80%, as well as the proportion of the months with maintenance threshold >70% during post-implementation phase.
Method:
Study Design and Setting
This was a single-center prospective pre- and post- design study for AO practice implementation as quality improvement (QI). This study took place in a large academic non-cardiac PICU at Children’s Hospital of Philadelphia, one of the largest children’s hospitals in the United States.
Subjects
Inclusion criteria were all primary TIs performed in the PICU during the study period. Primary TI is defined as initial TI, excluding an existing tracheal tube change. The first course (first set of method or approach with a set of medications) of the each TI encounter was included for the analysis [1,2]. TIs outside the PICU were excluded.
Data Collection
With Institutional Review Board approval, our local PICU TI data during August 1, 2014 - September 30, 2018 were obtained from the National Emergency Airway Registry for Children (NEAR4KIDS), a multicenter airway management QI database for critically ill children [1–7]. This study period was chosen to include 24 months in pre-implementation phase and 24 months in the post-implementation phase while excluding the implementation phase as the transition phase. These data points include TI patient, provider, and practice characteristics: i.e., TI dates, patient demographics, TI indications, difficult airway features, primary laryngoscopist discipline and training levels, and TI outcomes. Our local compliance plan ensured complete (>95%) TI data capture. The data accuracy was double checked before data entry and confirmed monthly following the approved NEAR4KIDS compliance plan [1].
Outcome Definitions
Primary Outcome:
Consistent with previous publications, moderate oxygen desaturation was defined as oxygen desaturation below 80% (pulse oximetry SpO2<80%) in TIs with highest SpO2>90% after pre-oxygenation [5–7].
Secondary Outcome:
Severe oxygen desaturation was defined as oxygen desaturation below 70% (pulse oximetry SpO2<70%) in TIs with highest SpO2>90% after pre-oxygenation. Adverse hemodynamic TIAE was also chosen as a secondary outcome, given its physiological association with peri-intubation hypoxemia [5,6]. This was defined as the occurrence of the following adverse TIAE: cardiac arrest with or without return of spontaneous circulation, hypotension, and dysrhythmia, following the NEAR4KIDS Operational Definitions [5,6].
Apneic Oxygenation (AO) Intervention
Apneic oxygenation (AO) was implemented on August 6, 2016 using a developed AO implementation toolkit by NEAR4KIDS. This toolkit approach is based on the prior multicenter TI bundle checklist implementation experience across the NEAR4KIDS [14]. Briefly, the toolkit provided the framework for AO as the QI intervention by: (1) Requiring ICU QI committee leadership endorsement, (2) Ensuring sufficient staff education prior to AO implementation, (3) Clarifying AO indication for all primary TIs, (4) Updating TI airway bundle checklist with AO instruction, and (5) Periodic (monthly) data feedback to the ICU for AO compliance, oxygen desaturation rate, and adverse TIAE data.
We used the following oxygen flow using a regular nasal cannula: 5 liters per minute for infants < 12 months, 10 liters per minute for children from 1–7 year, and 15 liters per minute for older children equal or above 8 years to adults based on multicenter NEAR4KIDS consensus and a previous adult study [8]. We educated each ICU provider to apply the nasal cannula either before induction, or soon after induction if a child did not tolerate nasal cannula placement. If a child requiring TI was already on a high flow humidified oxygen system, we used the same flow rate through the system already in place and simply increased the fraction of inspired oxygen to 100%. If a child was on non-invasive positive pressure ventilation (NIPPV), we advised that the nasal cannula be placed at the time of induction or when providers began manual bag-mask ventilation. The primary purpose of AO is to provide 100% oxygen during the apneic phase: the period when a child does not receive any ventilation for laryngoscopy. Detailed AO education was provided through specifically designed education material.
Statistical Analysis
For summary statistics, categorical variables are described as number and percentages, and non-normally distributed continuous variables as median and interquartile ranges (IQR). Differences in patient, provider, and practice between pre- and post-implementation phases were evaluated with the chi-square test for categorical or dichotomous variables and with the Wilcoxon rank-sum test for continuous variables with a skewed distribution.
We assessed the effect of the AO implementation by comparing pre-intervention and post-intervention phases with the primary outcome of the occurrence of moderate oxygen desaturation (oxygen desaturation <80%). For secondary outcomes, we also evaluated the severe oxygen desaturation (oxygen desaturation <70%) and the occurrence of hemodynamic TIAEs. To adjust for differences in patient and practice characteristics between two phases, we performed a multivariable logistic regression. Covariates associated with the phase at p<0.1 level and variables known to be associated with the primary outcome were included in the multivariable model [15]. As an exploratory analysis, we also conducted a per protocol analysis for association of study outcomes with the individual patient exposure to AO for each TI. Adequate fit of the multivariable models were assessed using the Hosmer-Lemeshow test. Subgroup analysis and assessment for effect modification was performed with Mantel-Haenszel test for homogeneity. All statistical analysis was performed using Stata 14.2 (Stata Corp, College Station, TX). A two-sided p-value <0.05 was considered to be statistically significant (For Mantel-Haenszel test, p<0.1 was considered significant).
Results
Feasibility
Apneic oxygenation was officially introduced on August 06, 2016. AO compliance reached to the adherence threshold (>80% of all primary TIs) in September, 2016. AO compliance was sustained above the maintenance threshold (>70%) throughout the next 24 months (October 2016-September 2018), shown in Figure 1.
Figure 1.
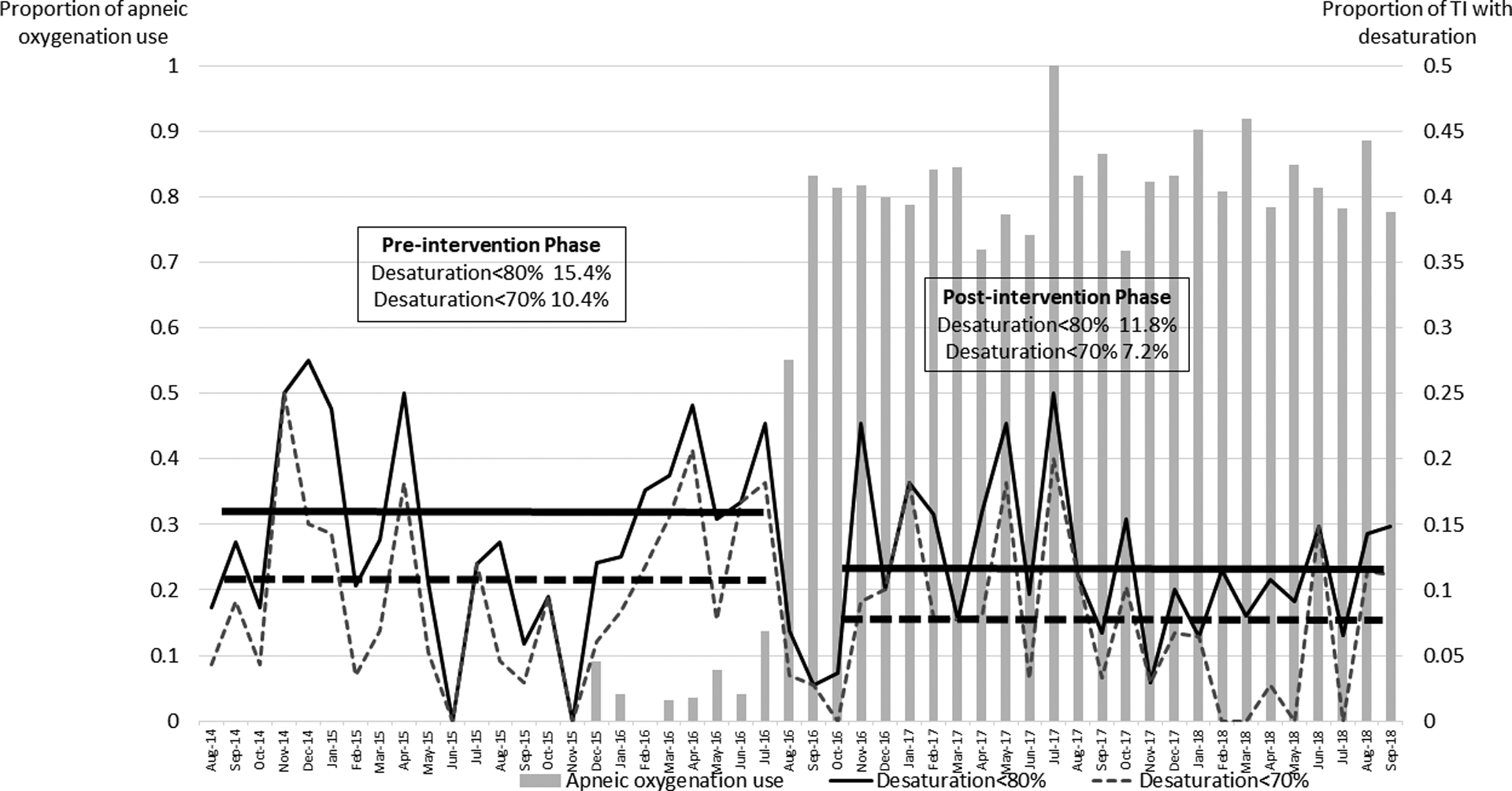
Trend of apneic oxygenation use and proportion of tracheal intubation with oxygen desaturation
Patient Demographics, Provider, and Practice Characteristics
A total of 1373 TIs were reported during the study period: 661 TIs in pre-implementation phase and 712 TIs in post-implementation phase. Between the pre- and post-implementation, no significant differences were observed in patient demographics or difficult airway features, Table 1. Respiratory and procedural indication for TI were more common during pre-implementation. Video laryngoscopy devices were used more often during the post-implementation (pre 5% vs post 75%, p<0.001).
Table 1.
Patient demographics, Provider and Practice characteristics
| Patient characteristics | Pre-implementation (n=661) | Post-implementation (n=712) | p-value |
|---|---|---|---|
| Age in years (median, IQR) | 4 (1–10) | 4 (1–12) | 0.492 |
| Age category | |||
| Infant (<1 year) | 134 (20%) | 175 (25%) | |
| Young child (1–7 year) | 296 (45%) | 262 (37%) | |
| Older child (8–17 year) | 192 (29%) | 224 (31%) | |
| Adult (≥18 year) | 39 (6%) | 51 (7%) | |
| Diagnosis | 0.169 | ||
| Respiratory | 267 (40%) | 317 (45%) | |
| Neurological | 312 (47%) | 301 (42%) | |
| Shock/sepsis | 51 (8%) | 67 (9%) | |
| Trauma/TBI/Other | 31 (5%) | 27 (4%) | |
| TI indication | |||
| Respiratory indication | 319 (48%) | 283 (40%) | 0.001 |
| Procedural indication | 296 (45%) | 260 (37%) | 0.002 |
| Shock/Hemodynamic instability | 60 (9%) | 67 (9%) | 0.832 |
| Neurological indication | 85 (13%) | 89 (13%) | 0.842 |
| Difficult Airway feature | |||
| History of difficult airway | 128 (19%) | 127 (18%) | 0.467 |
| Presence of difficult airway feature | 158 (24%) | 173 (24%) | 0.864 |
| Provider | 0.166 | ||
| PCCM Attending | 30 (5%) | 49 (7%) | |
| PCCM/EM Fellow | 421 (64%) | 447 (63%) | |
| Pediatric/EM resident | 37 (6%) | 37 (5%) | |
| Nurse Practitioner/Hospitalist | 111 (17%) | 130 (18%) | |
| Subspecialist/Other | 62 (9%) | 49 (7%) | |
| Device* | <0.001 | ||
| Direct laryngoscopy | 615 (94%) | 163 (23%) | |
| Video laryngoscopy | 36 (5%) | 535 (75%) | |
| Other | 6 (1%) | 13 (2%) |
IQR denotes interquartile range. TBI: traumatic brain injury. PCCM: pediatric critical care medicine. EM: Emergency medicine.
Device data were missing in 4 intubations in pre-intervention and 1 in post-intervention.
Primary and Secondary Outcomes
Moderate oxygen desaturation <80% was observed in fewer TIs after AO implementation (pre-implementation 102/661, 15.4% vs. post-implementation 84/712, 11.8%, p=0.049); severe oxygen desaturation<70% was also observed in fewer TIs after implementation (pre-implementation 69/661, 10.4% vs. post-implementation 51/712, 7.2%, p=0.032). Oxygen desaturation<90% was observed similarly in TIs after implementation (pre-implementation 23.0% vs. post-implementation 19.8%, p=0.149). Adverse hemodynamic TIAE rates were also unchanged (pre-implementation 21/661, 3.2% vs. post-implementation 14/712, 2.0%, p=0.155).
Multivariable Analyses
Multivariable analyses showed the post-implementation phase was significantly associated with lower occurrence of moderate oxygen desaturation<80% (adjusted odds ratio: aOR 0.55, 95% CI 0.34–0.88, p=0.013, Table 2) and with severe oxygen desaturation<70% (aOR 0.54, 95%CI 0.31–0.96, p=0.035, Table 3) while adjusting for TI indications and laryngoscopy device. Procedural indication was associated with lower occurrence of moderate and severe oxygen desaturation.
Table 2.
Multivariable logistic regression for the effect of implementation of apneic oxygenation use on moderate oxygen desaturation<80%
| Variable | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Implementation of apneic oxygenation | |||
| Pre-intervention | Reference | ||
| Post-intervention | 0.55 | 0.34–0.88 | 0.013 |
| Patient | |||
| Respiratory indication | 1.37 | 0.91–2.06 | 0.130 |
| Procedural indication | 0.55 | 0.35–0.87 | 0.010 |
| Device | |||
| Direct laryngoscopy | Reference | ||
| Video laryngoscopy | 1.45 | 0.89–2.35 | 0.132 |
| Other* | 4.32 | 1.57–11.85 | 0.004 |
Total N=1,368. Five Encounters are missing device data.
Goodness-of-fit test Chi(2)=18.07, p=0.259.
Covariates were selected based on the change in patient and practice between pre- and post-intervention phases.
Other device includes laryngeal mask airway, fiberoptic bronchoscope or combination of two devices.
Table 3.
Multivariable logistic regression for the effect of implementation of apneic oxygenation use on severe oxygen desaturation<70%
| Variable | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Implementation of apneic oxygenation | |||
| Pre-intervention | Reference | ||
| Post-intervention | 0.54 | 0.31–0.96 | 0.035 |
| Patient | |||
| Respiratory indication | 1.19 | 0.73–1.95 | 0.488 |
| Procedural indication | 0.49 | 0.28–0.86 | 0.013 |
| Device | |||
| Direct laryngoscopy | Reference | ||
| Video laryngoscopy | 1.23 | 0.69–2.22 | 0.483 |
| Other* | 5.25 | 1.82–15.12 | 0.002 |
Total N=1,368. Five Encounters are missing device data.
Goodness-of-fit test Chi(2)=18.74. p=0.226
Covariates were selected based on the change in patient and practice between pre- and post-intervention phases.
Other device includes laryngeal mask airway, fiberoptic bronchoscope or combination of two devices.
Per-protocol Analysis
The use of AO for TI was associated with lower occurrence of moderate oxygen desaturation<80% (with AO 119/778 15.3% vs. without AO 67/595 11.3%, p=0.030), and the lower occurrence of severe oxygen desaturation<70% (with AO 79/778 10.2% vs. without AO 41/595 6.9%, p=0.034). The use of AO was also associated with lower occurrence of oxygen desaturation<90% (with AO 23.7% vs. without AO 18.3%, p=0.017). Adverse hemodynamic TIAE rates were not different (with AO 23/778 3.0% vs. without AO 12/595 2.0%, p=0.274).
Multivariable analyses showed the use of AO was significantly associated with lower occurrence of moderate oxygen desaturation <80% (adjusted odds ratio: aOR 0.66, 95% CI 0.44–0.996, p=0.048). The use of AO did not reach statistical significance with severe oxygen desaturation <70% (aOR 0.70, 0.43–1.15, p=0.162) while adjusting for covariates associated with the use of AO (respiratory indication, difficult airway history, difficult airway feature, device, provider).
Subgroup Analysis by device
For the TIs with direct laryngoscopy (n=778), the use of AO was not significantly associated with moderate oxygen desaturation (with AO 11.0% vs. without AO 14.2%, p=0.315). For the TIs with video laryngoscopy (n=571), the use of AO was significantly associated with fewer moderate oxygen desaturation (with AO 11.1% vs. without AO 18.3%, p=0.034). The effect of AO was not significantly different between the TIs with direct and video laryngoscopy: test for homogeneity Chi2=0.52, p=0.47.
Discussion:
Our study demonstrated the implementation of AO as a QI intervention was feasible with the use of an implementation toolkit in a large academic PICU. The implementation of AO was significantly associated with a reduction in moderate and severe oxygen desaturation. While there was a substantial reduction in adverse hemodynamic TIAEs, the reduction did not reach statistical significance due to the small event rate. Our per-protocol analysis showed a similar result, confirming a robust association between AO implementation and reduction in oxygen desaturation.
Our AO procedure was developed based on physiological observations and results from adult studies [8,9,16,17]. Despite an emphasis on pre-oxygenation, severe oxygen desaturation was common in previously reported large pediatric multicenter data [5,7]. This is due to the higher rate of oxygen consumption relative to the oxygen reserve in children. Oxygen consumption will further increase with fever, respiratory distress, or shock state, often observed in children in PICU. Moreover more than 40% of children requiring TIs in PICU present with acute respiratory failure [1,5], resulting in substantial decrease in functional residual capacity and higher alveolar arterial oxygen gradient (A-a gradient). To prevent rapid oxygen desaturation during the apneic time necessary for laryngoscopy, we implemented AO as a QI. While adult ICU and Emergency Medicine studies typically utilized the classic rapid sequence induction (no positive pressure ventilation after induction) [8,16], critically ill children often receive mask ventilation after induction medications are provided [18]. Therefore nasal cannula placement needs to take place ideally before mask ventilation or at least immediately before laryngoscopy [19]. With AO implementation, we were able to reduce moderate and severe oxygen desaturation effectively among critically ill children high risk for deterioration. We were not able to demonstrate a significant reduction in hemodynamic TIAEs, most likely due to the rarity of these events. Currently, a multicenter QI intervention to implement AO is ongoing, which will provide sufficient sample size to evaluate the AO effect on hemodynamic outcomes.
We implemented AO practice before the introduction of video laryngoscopy as a laryngoscopy coaching device in our ICU. This is because we were concerned that the implementation of video laryngoscope would be associated with a longer procedural time and more frequent oxygen desaturation [20, 21]. This concern was also mitigated by default use of video laryngoscopy as a coaching device (i.e., laryngoscopist is expected to use the direct view), and standardized, succinct coaching language education. Our subgroup analysis showed the consistent effect of AO in reducing oxygen desaturation: it was not different in TIs with a direct or a video laryngoscope.
We chose to use a standard nasal cannula and age-specific oxygen flow rate as opposed to a high flow humidified oxygen system. This decision was made for the following two reasons: (1) Feasibility and low-cost. Our method requires only the additional oxygen source to attach nasal cannula during the pre-oxygenation phase, and (2) Previous adult study have showed that the use of regular nasal cannula was sufficient to decrease peri-intubation hypoxemia [8]. We evaluated the regular nasal cannula system to ensure the set oxygen flow being delivered through nasal cannula without pressure build-up in our respiratory laboratory. We believe this approach contributed to the rapid AO adherence and high level of sustained use in our study.
Adult studies of AO in the ICU setting remain conflicting. While a meta-analysis showed the use of AO was associated with lower occurrence of moderate oxygen desaturation (SpO2<80%) and higher level of lowest oxygen saturation during TI [20], individual study results were heterogeneous. This may be due to several factors. Heterogeneity of oxygen flow rate used for AO may contributes to conflicting results; while the study by Jaber utilized 60 liters per minute flow [9], the study by Selmer utilized 15 liters per minute flow [17]. Apneic time for laryngoscopy might be simply too long for AO to demonstrate the effectiveness in some of the critically ill high risk patients for preventing hypoxemia (e.g., obesity, respiratory failure, congestive heart failure) [21]. While critically ill children share some of the similar risk factors, the relative oxygen flow for the functional residual capacity is likely larger than that in adult patients. This may contribute to the larger effect in reduction of oxygen desaturation in our study compared to the adult studies [17, 22].
Our study result should be interpreted with important limitations. First, our study was conducted in a ‘resource rich’ PICU setting with an already existing QI structure around airway management and resuscitation. The acceptance of the new QI interventions by interdisciplinary staff is likely based on the local culture and staff experience with previous and ongoing QI efforts. Second, there was also an introduction of video laryngoscopy (C-MAC videolaryngoscope with MacIntosh 2, 3 and Miller 1 blades, Karl Storz Endoskope, Tuttingen, Germany) as a laryngoscopy coaching device in our airway management practice during the study period. This may have contributed to our positive study result. However, our study result was robust even after adjusting for the video laryngoscopy use. Moreover previous studies showed the use of video laryngoscopy was in fact associated with longer apneic time [20, 21], which could have negatively biased our result. Our study was based on a single center longitudinal cohort study, which is inherently vulnerable to practice change overtime (i.e., secular trend). This is clearly a limitation, therefore the study result needs to be validated further with a multicenter PICU cohort. Third, we occasionally heard that there was an increase in mask leak when the nasal cannula was applied during mask ventilation. We implemented a softer nasal cannula to overcome this issue along with additional staff education and use of standard two-person bag mask ventilation practice. As discussed above, the nasal cannula placement during NIPPV did not increase mask leak in an adult study [19]. At last, this study would have been a more robust if we had a time measurement for each patient to develop oxygen desaturation. This was not feasible since our AO implementation was done in the vast majority of TIs as a part of QI. With the unpredictability of TIs in the PICU, deploying a study staff for this time measurement was not feasible. Future studies using a video recording system or a bag mask system with a flow sensor should be considered.
Conclusion:
We demonstrated that implementation of AO in a large academic non-cardiac pediatric ICU is feasible with rapid practice adherence. The implementation of AO was associated with a significant reduction in moderate and severe oxygen desaturation. Based on this study result, use of AO may be considered when intubating critically ill children. A multicenter study is in progress to evaluate AO feasibility in various ICU settings and the effectiveness to reduce moderate and severe oxygen desaturation in a reproducible manner.
Acknowledgements:
We thank to the local NEAR4KIDS team and respiratory therapists for diligent data collection and following data compliance plans. We also thank Ms. Hayley Buffman, MPH for her administrative work.
Financial Support/COI:
This study was supported by Agency for Healthcare Research and Quality: AHRQ R03HS021583, AHRQ R18HS022464, R18HS024511 and Endowed Chair, Critical Care Medicine, The Children’s Hospital of Philadelphia.
Ms. Napolitano and Drs. Nadkarni and Nishisaki are supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development R21 HD089151 and Agency for Healthcare Quality and Research R18HS024511.
Copyright form disclosure: Drs. Napolitano, Craig, and Nishisaki’s institutions received funding from AHRQ R03HS021583, AHRQ R18HS022464, and R18HS024511. Drs. Napolitano and Craig’s institutions received funding from Endowed Chair, Critical Care Medicine, The Children’s Hospital of Philadelphia. Dr. Napolitano’s institution also received funding from Eunice Kennedy Shriver National Institute of Child Health and Human Development R21 HD089151; she disclosed off-label product use of simple nasal cannula with higher flow ranges than recommended by the manufacturer; and she disclosed that she has research relationships and consulting relationships with Draeger, Actuated Medical, Smiths Medical, Vero Biotech, Philips/Respironics, and Aerogen. Dr. Snyder disclosed that she received compensation from AACN (presenting at NTI in 2017) and Springer Publishing for writing a chapter on pediatric sepsis. Dr. Branca received support for article research from AHRQ R03HS021583, AHRQ R18HS022464, and R18HS024511. Dr. Nishisaki received support for article research from the AHRQ. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
No reprints will be ordered
References:
- 1.Nishisaki A, Turner DA, Brown CA 3rd, et al. A national emergency airway registry for children: Landscape of tracheal intubation in 15 PICUs. Crit Care Med. 2013;41(3):874–885. [DOI] [PubMed] [Google Scholar]
- 2.Sanders RC Jr, Giuliano JS Jr, Sullivan JE, et al. Level of trainee and tracheal intubation outcomes. Pediatrics. 2013;131(3):e821–828. [DOI] [PubMed] [Google Scholar]
- 3.Nett S, Emeriaud G, Jarvis JD, Montgomery V, Nadkarni VM, Nishisaki A. Site-level variance for adverse tracheal intubation-associated events across 15 North American PICUs: A report from the national emergency airway registry for children*. Pediatr Crit Care Med. 2014;15(4):306–313. [DOI] [PubMed] [Google Scholar]
- 4.Parker MM, Nuthall G, Brown C 3rd, et al. Relationship between adverse tracheal intubation associated events and PICU outcomes. Pediatr Crit Care Med. 2017;18(4):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Hsieh TC, Rehder KJ, et al. Frequency of desaturation and association with hemodynamic adverse events during tracheal intubations in PICUs. Pediatr Crit Care Med. 2018;19(1):e41–e50. [DOI] [PubMed] [Google Scholar]
- 6.Mokhateb-Rafii T, Bakar A, Gangadharn S, et al. Hemodynamic impact of oxygenation during tracheal intubation among critically ill children with cyanotic and noncyanotic heart disease. Pediatr Crit Care Med. 2019;20(1):19–26. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Turner DA, Kamat P, et al. : The number of tracheal intubation attempts matters! A prospective multi-institutional pediatric observational study. BMC Pediatr 2016; 16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wimalasena Y, Burns B, Reid C, Ware S, Habig K. Apneic oxygenation was associated with decreased desaturation rates during rapid sequence intubation by an Australian helicopter emergency medicine service. Ann Emerg Med. 2015;65(4):371–6. [DOI] [PubMed] [Google Scholar]
- 9.Jaber S, Monnin M, Girard M, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016; 42(12):1877–87. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson IM, Lodenius Å, Tunelli J, et al. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) - a physiological study. Br J Anaesth. 2017;118(4):610–617. [DOI] [PubMed] [Google Scholar]
- 11.Weingart SD. Preoxygenation, reoxygenation, and delayed sequence intubation in the emergency department. J Emerg Med. 2011;40(6):661–7. [DOI] [PubMed] [Google Scholar]
- 12.Riva T, Pedersen TH, Seiler S, Kasper N, Theiler L, Greif R, Kleine-Brueggeney M. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth. 2018;120(3):592–599. [DOI] [PubMed] [Google Scholar]
- 13.Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. 2017;118(2):232–238. [DOI] [PubMed] [Google Scholar]
- 14.Davis KF, Napolitano N, Li S, et a; National Airway Emergency for Children (NEAR4KIDS) and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Promoters and Barriers to Implementation of Tracheal Intubation Airway Safety Bundle: A Mixed-Method Analysis. Pediatr Crit Care Med. 2017;18(10):965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. [DOI] [PubMed] [Google Scholar]
- 16.Miguel-Montanes R, Hajage D, Messika J, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med. 2015;43(3):574–83. [DOI] [PubMed] [Google Scholar]
- 17.Semler MW, Janz DR, Lentz RJ, et al. ; FELLOW Investigators; Pragmatic Critical Care Research Group. Randomized trial of apneic oxygenation during endotracheal intubation of the critically ill. Am J Respir Crit Care Med. 2016;193(3):273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima T, Harwayne-Gidansky I, Shenoi AN, et al. ; National Emergency Airway Registry for Children (NEAR4KIDS) and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI). Cricoid pressure during induction for tracheal intubation in critically ill children: A report from National Emergency Airway Registry for Children. Pediatr Crit Care Med. 2018;19(6):528–537. [DOI] [PubMed] [Google Scholar]
- 19.Brown DJ, Carroll SM, April MD. Face mask leak with nasal cannula during noninvasive positive pressure ventilation: A randomized crossover trial. Am J Emerg Med. 2018;36(6):942–948. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Lu Y, Huang Y, Jiang H. Pediatric video laryngoscope versus direct laryngoscope: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2014;24(10):1056–65. [DOI] [PubMed] [Google Scholar]
- 21.Abdelgadir IS, Phillips RS, Singh D, Moncreiff MP, Lumsden JL. Videolaryngoscopy versus direct laryngoscopy for tracheal intubation in children (excluding neonates). Cochrane Database Syst Rev. 2017. May 24;5:CD011413 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binks MJ, Holyoak RS, Melhuish TM, et al. Apnoeic oxygenation during intubation in the intensive care unit: A systematic review and meta-analysis. Heart Lung. 2017;46(6):452–457. [DOI] [PubMed] [Google Scholar]
- 23.Mort TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med. 2005;33(11):2672–5. [DOI] [PubMed] [Google Scholar]


