ABSTRACT
Purpose
The present study explored the potential therapeutic role of oleuropein in sepsis-induced heart injury along with the role of GSK-3β/NF-kB signaling pathway.
Methods
Sepsis-induced myocardial injury was induced by cecal ligation and puncture (CLP) in rats. The cardiac injury was assessed by measuring the levels of cTnI and creatine kinase-MB (CK-MB). Sepsis-induced inflammation was assessed by measuring interleukin-6 (IL-6), IL-10 and HMGB1 levels. The different doses of oleuropein (5, 10, and 20 mg/kg) were given prior to CLP. Oleuropein (20 mg/kg) was administered after 6 hof CLP. The expressions of GSK-3β, p-GSK-3β (Ser9) and nuclear factor-κB (NF-κB) were measured in heart homogenates.
Results
Cecal ligation and puncture was associated with myocardial injury, an increase in IL-6, a decrease in IL-10 and an increase in HMGB1. Moreover, it decreased the ratio of p-GSK-3β/GSK-3β and increased the expression of p-NF-kB. Pretreatment with oleuropein attenuated CLP-induced myocardial injury and systemic inflammation in a dose-dependent manner. Administration of oleuropein after the onset of CLP also attenuated cardiac injury and inflammation. It also restored CLP-induced changes in the HMGB1 levels, the ratio of p-GSK-3β/GSK-3β and expression of p- NF-kB.
Conclusions
Oleuropein attenuates sepsis-induced systemic inflammation and myocardial injury by inhibiting NF-kB and GSK-3β signaling.
Key words: Sepsis, Inflammation, Cytokines, Myocardium, Rats
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection1. It has been suggested that the development of organ dysfunction (heart, liver, kidney or lungs) may be employed as a primary outcome variable in a shock model cecal ligation and puncture in rodents2. Accordingly, sepsis may induce widespread damage in the different body organs including the myocardium3. Indeed, myocardial dysfunction is very common in patients suffering from sepsis and it is recognized as one of the major health burdens throughout the world, which may contribute to morbidity as well as mortality. The release of heart-specific biomarkers, including cardiac troponins and creatine kinase, is employed to assess sepsis-induced myocardial injury4 - 5. However, there is specific drug therapy for the management of sepsis-induced myocardial injury. Accordingly, there is a need to explore new therapeutic agents that may be potentially employment for the successful mitigation of the myocardial injury in sepsis patients.
Oleuropein is a polyphenolic compound, present in olive oil6, and it is found to exhibit a wide range of therapeutic activities including analgesic, anti-inflammatory, antioxidant, antidepressant, anti-Parkinson, anticancer, etc.7 - 9. Moreover, oleuropein has also been shown to produce useful effects in cardiovascular diseases including hypertension, preventing cardiomyocytes death, ischemia-reperfusion injury10 - 12. For the present investigation, it was hypothesized that the combination of cardioprotective effects and anti-inflammatory actions may possibly contribute in preventing sepsis-induced myocardial injury. However, there is no such experimental study depicting the effectiveness of oleuropein in sepsis-induced myocardial injury.
HMGB1, an intracellular protein, acts as a late mediator of inflammation and organ damage13 - 14. It exerts its proinflammatory actions via binding to receptors for advanced glycation end products (RAGE). HMGB1/RAGE axis has been implicated in several inflammatory heart disorders15 - 16. Furthermore, it has also been reported that treatment with angiotensin receptor blockers significantly inhibits the HMGB1/RAGE axis in stroke and hypertensive patients17. Since the molecular mechanisms of inflammatory sepsis-induced cardiac injury are not fully understood and considering the HMGB1/RAGE axis as an important target of inflammatory disorder, the present study was aimed to identify the role HMGB1/RAGE axis in sepsis-induced myocardial injury.
Glycogen synthase kinase-3β (GSK-3β) and NF-kB are the key components of intracellular signaling cascade18 - 19. The role of GSK-3β and NF-kB in the initiation and amplification of inflammation is well documented20 - 21. Their role in sepsis-induced systemic inflammation has also been described22 - 23. Furthermore, it has been suggested that oleuropein produces diverse biological actions through modulation of GSK-3β and NF-kB24 - 25. Accordingly, it has been hypothesized that oleuropein-mediated potential useful effects in sepsis-induced myocardial injury may be possibly mediated through modulation of GSK-3β and NF-kB. Accordingly, the present study was designed to explore the therapeutic potential of oleuropein in sepsis-induced myocardial injury and the possible role of GSK-3β and NF-kB in oleuropein-mediated cardioprotective effects in rats.
Methods
Animals and drugs
Male Wistar albino rats (200–250 g) were used for this investigation. The experiments were approved by the Ethic Committee of Qingdao Municipal Hospital with ethical approval number 2020042. The experiments were conducted as per ethical guidelines in Department of Cardiology, Qingdao Municipal Hospital, Qingdao, China. The kits for the estimation of cTnT, CK-MB, IL-6, IL-10, p-GSK-3β and p-NF-kB were purchased from MyBioSource (San Diego, CA, USA).
Cecal ligation puncture (CLP)-induced sepsis
In this experimental sepsis model, the recommendations and considerations of the International expert consensus for pre-clinical sepsis studies were followed26. The rats were divided randomly into seven groups and sepsis was induced by CLP method as described previously27 - 28. Under anesthesia (pentobarbital sodium 20 mg/kg intraperitoneal – i.p.), the laparotomy was performed in which an incision of about a 2 cm was made to expose the cecum. Thereafter, a sterile 3-0 silk suture was used to ligate the cecum at its base. An 18-gauge needle was used to puncture the cecum and extrude fecal material from the punctured site. Afterwards, the cecum was placed back in the abdominal cavity and incision was closed using surgical sutures. Buprenorphine (0.05 mg/kg subcutaneous – s.c.) was administered to take care of surgery and sepsis-induced pain. The prewarmed normal saline (37 °C; 5 mL/100 g s.c.) was injected for fluid resuscitation. Thereafter, rats were placed in a temperature-controlled room (22°C) with 12-h light and dark cycles, with full access to water and food29. Twelve hours after the laparotomy, the rats were sacrificed (cervical dislocation) to remove blood (cardiac puncture method) and hearts.
Markers of myocardial damage
Sepsis-induced myocardial injury was quantified by determining the levels of CKMB and cTnI in the plasma using commercially available diagnostic kits.
Assessment of inflammatory markers in the circulation
The levels of IL-6 and IL10 were assessed in the plasma using commercially available ELISA kits and the estimations were done as per instructions.
Biochemical parameters in the heart
The heart was homogenized to quantify the levels of HMGB1, p-NF-kB, p-GSK-3 and GSK-3β using commercially available ELISA kits and the estimations were done as per instructions.
Study design
The experimental study was comprised of seven groups, with eight rats in each group. The sample size estimation was done using “resource equation” method in which the value of E was calculated30. The different experimental groups included, (i) Normal; (ii) Sham, in which only laparotomy procedures were performed without cecum ligation and puncture; (iii) CLP; (iv) Oleuropein (5 mg/kg i.p.)-pretreated CLP, in which oleuropein was given 30 min before performing CLP; (v) Oleuropein (10 mg/kg i.p.)-pretreated CLP; (vi) Oleuropein (20 mg/kg i.p.)-pretreated CLP; (vii) Oleuropein (20 mg/kg)-treated CLP, in which oleuropein was administered 6 h after CLP. The dose of oleuropein in group vii was selected on the basis of results of groups iv, v and vi.
Statistical analysis
Graph Pad Prism was employed to statistically analyze the data. The data were represented as mean ± S.D. The data were statistically analyzed using one-way ANOVA followed by Tukey’s test for post hoc analysis. The value of p < 0.05 was considered statistically significant.
Results
Cecal ligation puncture produced myocardial injury and increased systemic inflammation
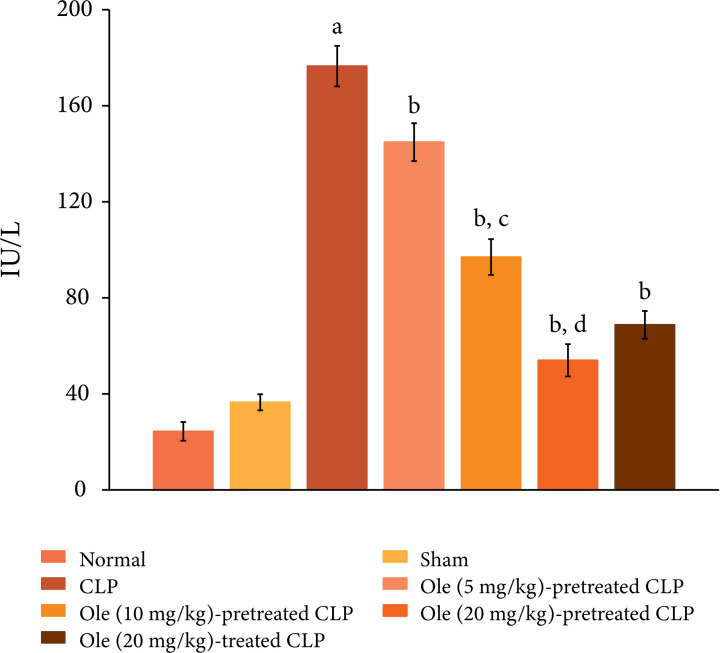
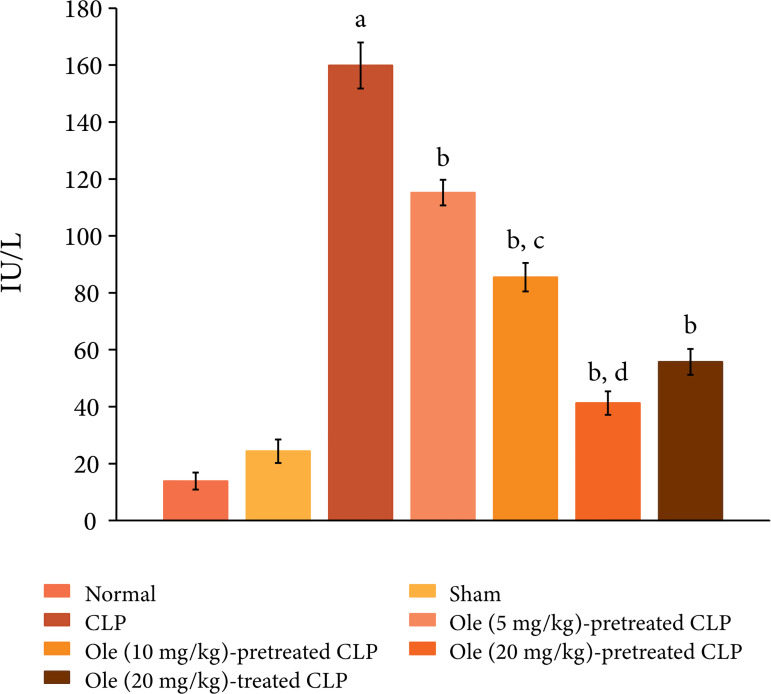
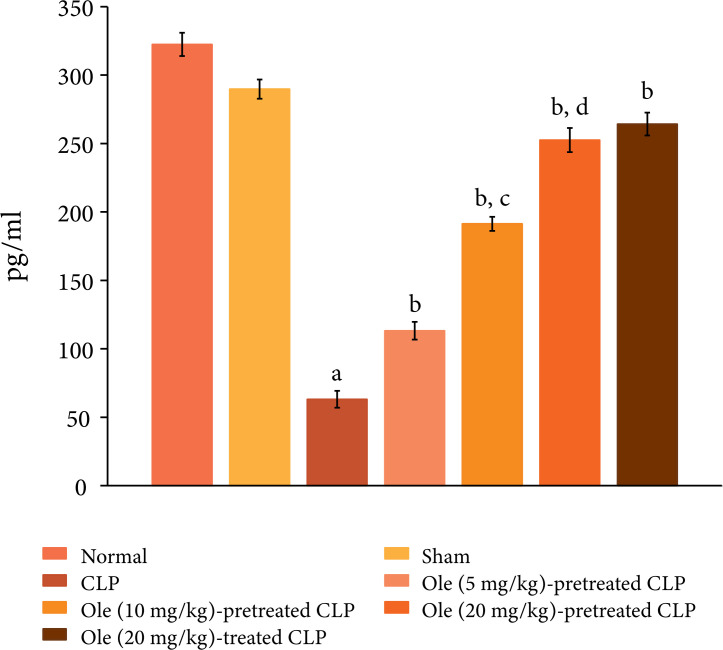
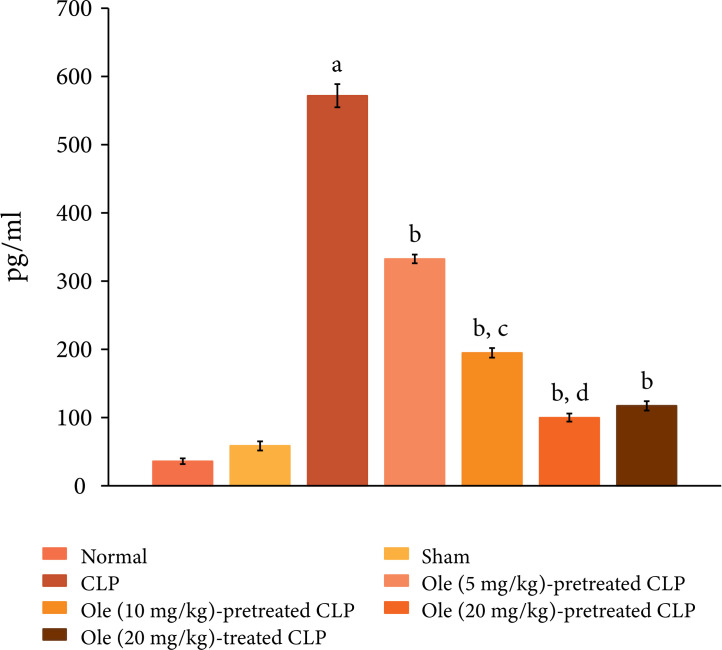
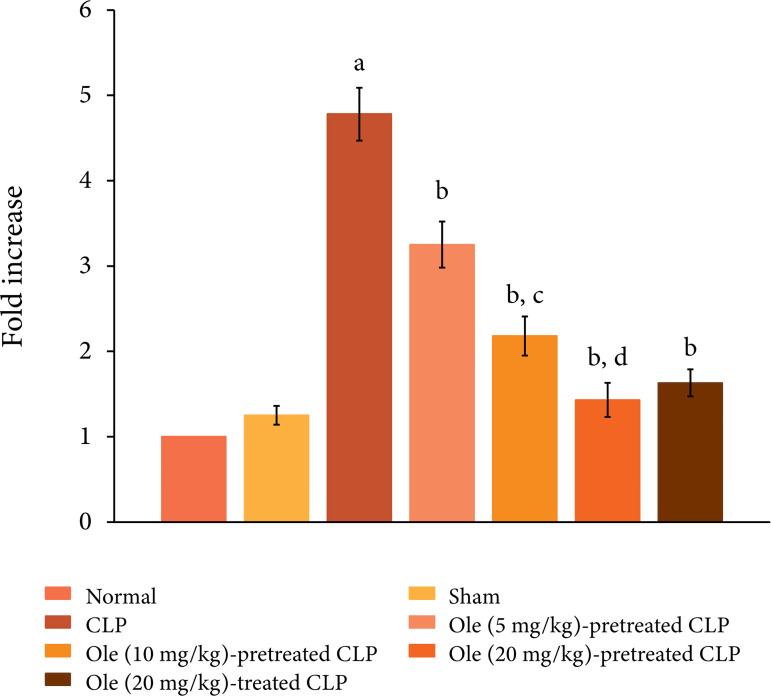
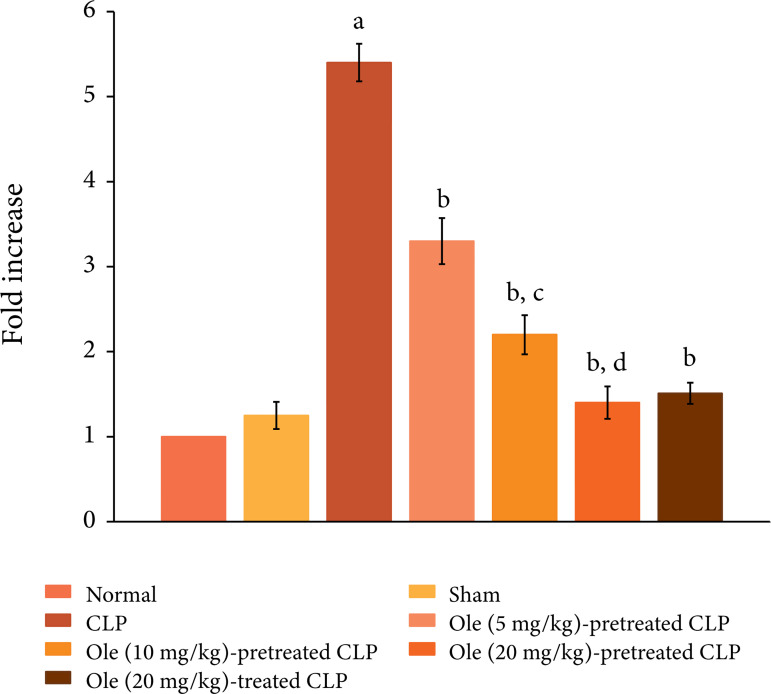
The ligation and puncture of the cecum led to marked myocardial injury, which was assessed 12 h after laparotomy. Indeed, there was a significant increase in the plasma levels of CK-MB (Fig. 1) and cTnT (Fig. 2) as compared to sham and normal control. Creatine kinase-MB and cTnT are the heart-specific biochemicals and their increase in the plasma levels indicates injury to the heart. Moreover, CLP also induced systemic inflammation and there was a significant increase in the plasma levels of proinflammatory cytokine, IL-6 (Fig. 3) and decrease in the plasma levels of anti-inflammatory cytokine, IL-10 (Fig. 4).
Figure 1. Effect of different doses of oleuropein onCLP-induced increase in plasma CK-MB levels. a = p < 0.05vs. Sham; b = p < 0.05 vs. CLP; c = p < 0.05 Ole (5 mg/kg) in CLP; d = p < 0.05 Ole (10 mg/kg) in CLP. Ole: Oleuropein.
Figura 2. Effect of different doses of oleuropein onCLP-induced increase in plasma cTnT levels. a = p < 0.05 vs. Sham; b = p < 0.05 vs. CLP; c = p < 0.05 Ole (5 mg/kg) in CLP; d = p < 0.05 Ole (10 mg/kg) in CLP.
Figure 4. Effect of different doses of oleuropein on CLP-induced increase in plasma IL-10 levels. a = p < 0.05 vs. Sham; b = p < 0.05 vs. CLP; c = p < 0.05 Ole (5 mg/kg) in CLP; d = p < 0.05 Ole (10 mg/kg) in CLP.
Oleuropein attenuated CLP-induced myocardial injury and systemic inflammation
Pretreatment with oleuropein (5, 10 and 20 mg/kg) abolished CLP-induced increase in the plasma levels of CK-MB (Fig. 1) and cTnT (Fig. 2) in a dose-dependent manner suggesting the cardioprotective actions of oleuropein. Moreover, pretreatment with oleuropein was also associated with a significant decrease in the plasma levels of IL-6 (Fig. 3) and increase in the IL-10 levels (Fig. 4) in CLP-subjected rats suggesting the anti-inflammatory actions of oleuropein. Administration of oleuropein (20 mg/kg) after the induction of CLP was attenuated myocardial injury (Figs. 1 and 2) and inflammation. There was significant decrease in the levels of IL-6 (Fig. 3) and rise in the levels of IL-10 (Fig. 4) in the plasma of oleuropein-treated rats.
Figure 3. Effect of different doses of oleuropein on CLP-induced increase in plasma IL-6 levels. a = p < 0.05 vs. Sham; b = p < 0.05 vs. CLP; c = p < 0.05 Ole (5 mg/kg) in CLP; d = p < 0.05 Ole (10 mg/kg) in CLP.
Oleuropein abolished CLP-induced biochemical changes in the heart
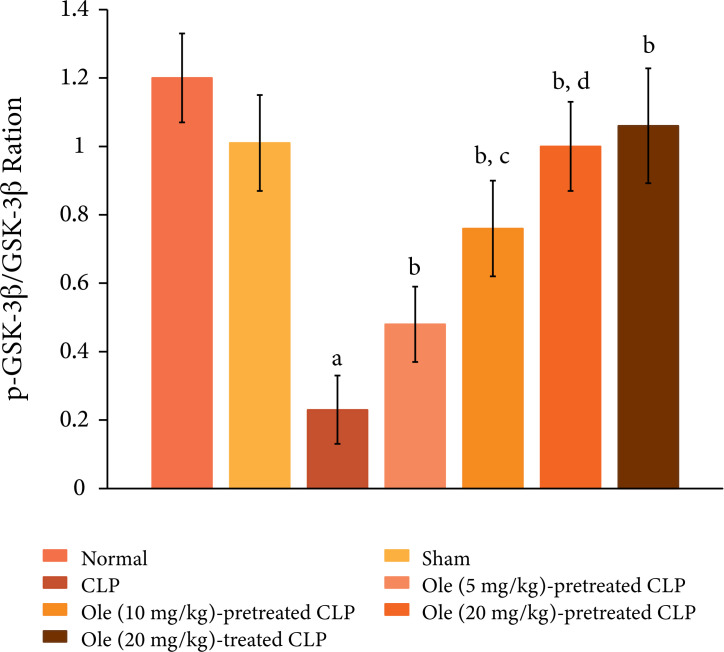
The ligation and puncture of cecum produced significant changes in the heart homogenates including an increase in protein expression of HMGB1 (Fig. 5), a decrease in the ratio of p-GSK-3β/GSK-3β (Fig. 6) and increase in the expression of p-NF-kB (Fig. 7). HMGB1 is a cytokine and is considered as a specific marker of sepsis. The decrease in the levels of p-GSK-3β indicates the activation of enzyme GSK-3β, while the increase in the levels of p-NF-kB indicates its increase inactivity. Pretreatment with oleuropein (5, 10 and 20 mg/kg) led to the restoration of CLP-induced changes in the HMGB1 levels, the ratio of p-GSK-3β/GSK-3β and expression of p-NF-kB in a dose-dependent manner. Administration of oleuropein (20 mg/kg) following the induction of CLP also led to restoration of biochemical alterations including HMGB1 levels (Fig. 5), p-GSK-3β/GSK-3β ratio and p-NF-kB levels (Fig. 6).
Figure 5. Effect of different doses of oleuropein on CLP-induced increase in HMGB1 levels in the heart homogenate. a =p < 0.05 vs. Sham; b = p < 0.05 vs. CLP;c = p < 0.05 Ole (5 mg/kg) in CLP; d = p < 0.05 Ole(10 mg/kg) in CLP.
Figure 6. Effect of different doses of oleuropein onCLP-induced changes in p-GSK-3β/GSK-3β ratio in the heart homogenate. a = p < 0.05 vs. Sham; b = p < 0.05 vs. CLP; c = p < 0.05 Ole (5 mg/kg) in CLP; d = p < 0.05Ole (10 mg/kg) in CLP.
Figure 7. Effect of different doses of oleuropein onCLP-induced changes in p-Akt levels in the heart homogenate. a = p < 0.05 vs. Sham; b = p < 0.05 vs. CLP; c = p < 0.05 Ole (5 mg/kg) in CLP; d = p < 0.05 Ole(10 mg/kg) in CLP.
Discussion
In this current study, ligation and puncture of cecum produced marked myocardial injury, which was assessed 12 h after laparotomy by measuring the heart-specific biochemicals, i.e., CK-MB and cTnT in the circulation. Cecal ligation and puncture-induced myocardial injury may be ascribed to the development of sepsis as sepsis is one of the important factors contributing to myocardial injury31. Moreover, CLP is one of the most common methods to induce sepsis in laboratory animals28. Accordingly, it is possible to suggest that CLP-induced development of sepsis may be responsible for the development of myocardial injury. Cecal ligation and puncture-induced myocardial injury, observed in this study, is consistent with the earlier published reports32 - 33. In this study, CLP also led to the development of systemic inflammation, which was assessed by decreased levels of IL-10 (anti-inflammatory cytokine) and an increase in IL-6 (proinflammatory cytokine) in the circulation. Along with it, there was a significant rise in the levels of HGMB in the heart homogenates following CLP. HMGB1 is one of the specific markers of sepsis and it has been suggested to contribute in myocardial injury. Indeed, HMGB is a late mediator of sepsis and its release has been detected after 8 h of sepsis34. There have been previous studies showing that CLP leads to development of systemic inflammation, which may contribute to myocardial injury35.
In this investigation, pretreatment with different doses of oleuropein led to significant amelioration of CLP-induced increase in the plasma levels of CK-MB and cTnT, suggesting its cardioprotective actions. Moreover, pretreatment with oleuropein also ameliorated CLP-induced increase in IL-6 and a decline in IL-10 levels in the plasma, suggesting the decline in systemic inflammation. Apart from it, oleuropein also led to a significant decline in the levels of HMGB1 in the heart homogenates suggesting its ameliorative effects on sepsis. Apart from it, administration of oleuropein after the induction of CLP (as post-treatment) also produced cardioprotection and attenuated the levels ofHMGB1. There have been previous studies documenting that oleuropein exerts anti-inflammatory actions and it serves to reduce the levels of proinflammatory cytokines in circulation36 - 37. Based on this, it may be possibly suggested that an oleuropein-mediated decrease in systemic inflammation may contribute in attenuating myocardial injury in CLP-subjected rats. There have been studies showing the widespread use of oleuropein in experimental diseases such as in pain, periodontitis, asthma, renal injury36 - 38. Moreover, oleuropein is also documented to exert a protective effect on the heart as it is found to prevent heart failure, ischemia-reperfusion myocardial injury and arrhythmia39 - 41. Nevertheless, the beneficial actions of oleuropein in sepsis-induced myocardial injury are reported for the first time in this current study.
In this investigation, CLP also led to an increase in the expression of p-NF-kB and a decrease in the ratio of p-GSK-3β/GSK-3β. NF-kB is a key transcriptional factor and its activation, as assessed by an increase in expression of its phosphorylated form, has been identified in sepsis, including in CLP model42. Activation of NF-kB plays an important role in the initiation and maintenance of inflammatory reactions43. Moreover, an increase in p-NF-kB and its subsequent activation has been associated with the development of myocardial injury44. Accordingly, it is possible that CLP-induced activation of NF-kB signaling may play a key role in initiating inflammation and producing myocardial injury. However, pretreatment as well as post-treatment of oleuropein led to the significant restoration of p-NF-kB in the heart homogenates of CLP-subjected rats. Oleuropein has been shown to attenuate the production of inflammatory cytokines by inhibiting the activation of NF-kB45. Accordingly, it may be proposed that oleuropein-mediated decrease in NF-kB signaling may contribute in reducing inflammation and producing myocardial protection.
GSK-3β is a unique enzyme which gets deactivated after its phosphorylation; hence, the decrease in the ratio of p-GSK-3β/GSK-3β represents the increase in the activation of GSK-3β46. Accordingly, in the current investigation, the decrease in the ratio of p-GSK-3β/GSK-3β represents the increase in the GSK-3β enzymatic activity in CLP-subjected rats. The activation of GSK-3β has been postulated in CLP-induced sepsis and liver injury47. However, it is the first report documenting the activation of GSK-3β in sepsis-induced inflammation and heart injury. Treatment with oleuropein abolished CLP-induced decrease in p-GSK-3β/GSK-3β ratio suggesting the inactivation of GSK-3β in response to oleuropein treatment. A preclinical study has described that oleuropein offers neuroprotective effects by inhibiting the GSK-β activity33. Accordingly, it may be proposed that oleuropein may prevent sepsis-induced cardiac injury by inhibiting systemic circulation, which may be possibly attributed to a decrease in NF-kB and GSK-3β signaling. However, future studies are required to elucidate the precise pathway involved in oleuropein-mediated beneficial effects in CLP-subjected rats. Since sepsis leads to multi-organ dysfunction, therefore, the protective role of oleuropein on different organs including brain, liver and lungs may also be investigated in CLP model in future studies.
Conclusions
Oleuropein has the potential to prevent and treat sepsis-induced systemic inflammation and myocardial injury. The protective effects of oleuropein in sepsis-subjected rats may be attributed to inhibition of NF-kB and GSK-3β signaling.
Acknowledgments
The authors acknowledge their host institute for all the support and technical services for this paper.
Footnotes
Research performed at Department of Cardiology from the Qingdao Municipal Hospital, China
Data availability statement: The data will be available on request
Funding: Not applicable.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der, Vincent J-L, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham MN, Kelly AP, Brandwein AB, Fernandes TD, Leisman DE, Taylor MD, Brewer MR, Capone CA, Deutschman CS. Use of organ dysfunction as a primary outcome variable following cecal ligation and puncture: recommendations for future studies. Shock. 2020;54(2):168–182. doi: 10.1097/SHK.0000000000001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagy M, Al-Qaqaa Y, Kim P. Definitions and pathophysiology of sepsis. Curr Probl Pediatr Adolesc Health Care. 2013;43(10):260–263. doi: 10.1016/j.cppeds.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Frencken JF, Donker DW, Spitoni C, Koster-Brouwer ME, Soliman IW, Ong DSY, Horn J, van der, van Klei, Bonten MJM, Cremer OL. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ Cardiovasc Qual Outcomes. 2018;11(2):e004040. doi: 10.1161/CIRCOUTCOMES.117.004040. [DOI] [PubMed] [Google Scholar]
- 5.Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep. 2015;12(2):130–140. doi: 10.1007/s11897-014-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badr AM, Attia HA, Al-Rasheed N. Oleuropein reverses repeated corticosterone-induced depressive-like behavior in mice: evidence of modulating effect on biogenic amines. Sci Rep. 2020;10(1):3336–3336. doi: 10.1038/s41598-020-60026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao X, Xia B, Zheng M, Zhou Z. Assessment of the anti-inflammatory, analgesic and sedative effects of oleuropein from Olea europaea L. Cell Mol Biol (Noisy-le-grand) 2019;65(1):52–55. [PubMed] [Google Scholar]
- 8.Imran M, Nadeem M, Gilani SA, Khan S, Sajid MW, Amir RM. Antitumor perspectives of oleuropein and its metabolite hydroxytyrosol: recent updates. J Food Sci. 2018;83(7):1781–1791. doi: 10.1111/1750-3841.14198. [DOI] [PubMed] [Google Scholar]
- 9.Sun W, Frost B, Liu J. Oleuropein, unexpected benefits! Oncotarget. 2017;8(11):17409–17409. doi: 10.18632/oncotarget.15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcelino G, Hiane PA, Freitas KC, Santana LF, Pott A, Donadon JR, Guimarães RCA. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients. 2019;11(8):18–26. doi: 10.3390/nu11081826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miceli C, Santin Y, Manzella N, Coppini R, Berti A, Stefani M, Parini A, Mialet-Perez J, Nediani C. Oleuropein aglycone protects against MAO-A-induced autophagy impairment and cardiomyocyte death through activation of TFEB. Oxid Med Cell Longev. 2018;2018:8067–8592. doi: 10.1155/2018/8067592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin H-X, Zhang Y-H, Guo R-N, Zhao S-N. Inhibition of MEK/ERK/STAT3 signaling in oleuropein treatment inhibits myocardial ischemia/reperfusion. Int J Mol Med. 2018;42(2):1034–1043. doi: 10.3892/ijmm.2018.3673. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock. 2009;32(4):348–357. doi: 10.1097/SHK.0b013e3181a551bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bangert A, Andrassy M, Müller A-M, Bockstahler M, Fischer A, Volz CH, Leib C, Göser S, Korkmaz-Icöz S, Zittrich S, Jungmann A, Lasitschka F, Pfitzer G, Müller OJ, Katus HA, Kaya Z. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc Natl Acad Sci USA. 2016;113(2):E155–E164. doi: 10.1073/pnas.1522288113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volz HC, Kaya Z, Katus HA, Andrassy M. The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin Thromb Hemost. 2010;36(2):185–194. doi: 10.1055/s-0030-1251503. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi K, Tancharoen S, Ito T, Morimoto-Yamashita Y, Miura N, Kawahara K-I, Maruyama I, Murai Y, Tanaka E. Potential of the angiotensin receptor blockers (ARBs) telmisartan, irbesartan, and candesartan for inhibiting the HMGB1/RAGE axis in prevention and acute treatment of stroke. Int J Mol Sci. 2013;14(9):18899–18924. doi: 10.3390/ijms140918899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golpich M, Amini E, Hemmati F, Ibrahim NM, Rahmani B, Mohamed Z, Raymond AA, Dargahi L, Ghasemi R, Ahmadiani A. Glycogen synthase kinase-3 beta (GSK-3β) signaling: Implications for Parkinson’s disease. Pharmacol Res. 2015;97:16–26. doi: 10.1016/j.phrs.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86–86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgart S, Chen N-M, Zhang J-S, Billadeau DD, Gaisina IN, Kozikowski AP, Singh SK, Fink D, Ströbel P, Klindt C, Zhang L, Bamlet WR, Koenig A, Hessmann E, Gress TM, Ellenrieder V, Neesse A. GSK-3β governs inflammation-induced NFATc2 signaling hubs to promote pancreatic cancer progression. Mol Cancer Ther. 2016;15(3):491–502. doi: 10.1158/1535-7163.MCT-15-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Zhu H, Liu C, Wang Y, Wang D, Liu H, Cao W, Hu Y, Lin Q, Tong C, Lu M, Sachinidis A, Li L, Peng L. GSK‐3β inhibition protects the rat heart from the lipopolysaccharide‐induced inflammation injury via suppressing FOXO3A activity. J Cell Mol Med. 2019;23(11):7796–7809. doi: 10.1111/jcmm.14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvaraj V, Nepal N, Rogers S, Manne NDPK, Arvapalli R, Rice KM, Asano S, Fankhanel E, Ma JJ, Shokuhfar T, Maheshwari M, Blough ER. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials. 2015;59:160–171. doi: 10.1016/j.biomaterials.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Ahn KS, Shanmugam MK, Wang H, Shen H, Arfuso F, Chinnathambi A, Alharbi SA, Chang Y, Sethi G, Tang FR. Oleuropein induces apoptosis via abrogating NF‐κB activation cascade in estrogen receptor-negative breast cancer cells. J Cell Biochem. 2019;120(3):4504–4513. doi: 10.1002/jcb.27738. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Liu X, Li Q. Protective effects of oleuropein against cerebral ischemia/reperfusion by inhibiting neuronal apoptosis. Med Sci Monit. 2018;24:6587–6598. doi: 10.12659/MSM.912336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon J-M, Chaudry IH, Coopersmith CM, Deutschman C, Drechsler S, Efron P, Frostell C, Fritsch G, Gozdzik W, Hellman J, Huber-Lang M, Inoue S, Knapp S, Kozlov AV, Libert C, Marshall JC, Moldawer LL, Radermacher P, Redl H, Remick DG, Singer M, Thiemermann C, Wang P, Wiersinga WJ, Xiao X, Zingarelli B. Minimum quality threshold in pre-clinical sepsis studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Intensive Care Med Exp. 2018;6(1):26–26. doi: 10.1186/s40635-018-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock—A review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 28.Ge C, Liu J, Dong S. miRNA-214 protects sepsis-induced myocardial injury. Shock. 2018;50(1):112–118. doi: 10.1097/SHK.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 29.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Yang W, Sun X, Xie L, Yang Y, Sang M, Jiao R. SS31 Ameliorates sepsis-induced heart injury by inhibiting oxidative stress and inflammation. Inflammation. 2019;42(6):2170–2180. doi: 10.1007/s10753-019-01081-3. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Zeng Z, Wu C, Liu H. Tropisetron inhibits sepsis by repressing hyper-inflammation and regulating the cardiac action potential in rat models. Biomed Pharmacother. 2019;110:380–388. doi: 10.1016/j.biopha.2018.11.142. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Yao J, Wang X, Li H, Liu T, Zhao W. Poly (ADP-ribose) synthetase inhibitor has a heart protective effect in a rat model of experimental sepsis. Int J Clin Exp Pathol. 2015;8(9):9824–9835. [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 35.Qian Y, Qian F, Zhang W, Zhao L, Shen M, Ding C, Guo J. Shengjiang Powder ameliorates myocardial injury in septic rats by downregulating the phosphorylation of P38-MAPK. J Biosci. 2019;44(2):40–40. doi: 10.1007/s12038-019-9857-7. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y-H, Choi Y-J, Kang M-K, Lee E-J, Kim DY, Oh H, Kang Y-H. Oleuropein curtails pulmonary inflammation and tissue destruction in models of experimental asthma and emphysema. J Agric Food Chem. 2018;66(29):7643–7654. doi: 10.1021/acs.jafc.8b01808. [DOI] [PubMed] [Google Scholar]
- 37.Taskan MM, Yuce HB, Karatas O, Gevrek F, Toker H. Evaluation of the effect of oleuropein on alveolar bone loss, inflammation, and apoptosis in experimental periodontitis. J Periodont Res. 2019;54(6):624–632. doi: 10.1111/jre.12662. [DOI] [PubMed] [Google Scholar]
- 38.Yin M, Jiang N, Guo L, Ni Z, Al-Brakati AY, Othman MS, Moneim AEA, Kassab RB. Oleuropein suppresses oxidative, inflammatory, and apoptotic responses following glycerol-induced acute kidney injury in rats. Life Sci. 2019;232:116–634. doi: 10.1016/j.lfs.2019.116634. [DOI] [PubMed] [Google Scholar]
- 39.Esmailidehaj M, Bajoovand S, Rezvani ME, Sherifidehaj M, Hafezimoghadam Z, Hafizibarjin Z. Effect of oleuropein on myocardial dysfunction and oxidative stress induced by ischemic-reperfusion injury in isolated rat heart. J Ayurveda Integr Med. 2016;7(4):224–230. doi: 10.1016/j.jaim.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janahmadi Z, Nekooeian AA, Moaref AR, Emamghoreishi M. Oleuropein attenuates the progression of heart failure in rats by antioxidant and antiinflammatory effects. Naunyn-Schmiedeberg’s Arch Pharmacol. 2017;390(3):245–252. doi: 10.1007/s00210-016-1323-6. [DOI] [PubMed] [Google Scholar]
- 41.Esmailidehaj M, Mirhosseini S-J, Rezvani ME, Rasoulian B, Mosaddeghmehrjardi MH, Haghshenas D. Prolonged Oral Administration of Oleuropein Might Protect Heart against Aconitine-induced Arrhythmia. Iran J Pharm Res. 2012;11(4):1255–1263. [PMC free article] [PubMed] [Google Scholar]
- 42.Al Zoubi, Chen J, Murphy C, Martin L, Chiazza F, Collotta D, Yaqoob MM, Collino M, Thiemermann C. Linagliptin attenuates the cardiac dysfunction associated with experimental sepsis in mice with pre-existing type 2 diabetes by inhibiting NF-κB. Front Immunol. 2018;9:2996–2996. doi: 10.3389/fimmu.2018.02996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nisr RB, Shah DS, Ganley IG, Hundal HS. Proinflammatory NFkB signalling promotes mitochondrial dysfunction in skeletal muscle in response to cellular fuel overloading. Cell Mol Life Sci. 2019;76(24):4887–4904. doi: 10.1007/s00018-019-03148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du J, Liu L, Yu B, Hao F, Liu G, Jing H, Xu H. Clusterin (apolipoprotein J) facilitates NF-κB and Bax degradation and prevents I/R injury in heart transplantation. Int J Clin Exp Pathol. 2018;11(3):1186–1196. [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Z, Li X, Lin J, Zheng W, Hu Z, Xuan J, Ni W, Pan X. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017;8(10):3737–3744. doi: 10.1039/C7FO00823F. [DOI] [PubMed] [Google Scholar]
- 46.Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E, Giampietri C. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxid Med Cell Longev. 2017;2017:4629495–4629495. doi: 10.1155/2017/4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H, Wang W, Fang H, Yang Y, Li X, He J, Jiang X, Wang W, Liu S, Hu J, Liu A, Dahmen U, Dirsch O. GSK-3βinhibition attenuates CLP-induced liver injury by reducing inflammation and hepatic cell apoptosis. Mediators Inflamm. 2014;2014:629507–629507. doi: 10.1155/2014/629507. [DOI] [PMC free article] [PubMed] [Google Scholar]