The recent use of chimeric antigen receptor T cells (CAR-T) products has led to a shift in the management of chemotherapy refractory high grade B cell lymphomas as well as B acute lymphoblastic leukaemia. Currently, there are 2 licensed products in the United Kingdom, including axicabtagene ciloleucel (axi-cel/Yescarta) and tisagenlecleucel (Kymriah). The most common toxicities include cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).1 However, prior intensive therapy, hypogammaglobulinemia, and cytopenias render these patients more susceptible to infectious toxicities that are typically seen in post allograft patients. Human herpesvirus 6 (HHV6) encephalitis is an important cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation.2
In patients with hematological malignancies, there is little evidence that HHV6 causes significant disease,2 even in the post-autograft setting. However, following allogeneic transplants, HHV6 can reactivate causing post-transplant acute limbic encephalitis. Patients display distinctive clinical features, including anterograde amnesia, syndrome of inappropriate antidiuretic hormone secretion (SIADH), mild cerebrospinal fluid (CSF) pleocytosis, and temporal electroencephalogram abnormalities, with magnetic resonance imaging (MRI) identifying hyperintensities within the uncus amygdala and hippocampus on T2, fluid attenuated inversion recovery (FLAIR), and diffusion weighted imaging sequences.3 We present a case of HHV6 encephalitis in a patient who is post axi-cel treatment.
A 55-year-old male was admitted electively under our care for axi-cel CD19 CAR-T cells. He had a background of primary refractory stage IVB diffuse large B cell lymphoma, originally diagnosed 15 months earlier. His initial treatment consisted of 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone, with 3 cycles of high-dose methotrexate, resulting in progressive disease by positron emission tomography (PET) on completion. He went on to receive 2 cycles of rituximab, dexamethasone, (high dose) cytarabine, cisplatin but progressed with extensive pelvic, abdominal, and axillary disease. Post leukapheresis while waiting for axi-cel manufacture, he had bridging chemotherapy with 1 cycle of Gemcitabine/oxaliplatin, followed by pulses of dexamethasone, which were stopped prior to starting lymphodepletion.
Lymphodepletion with fludarabine and cyclophosphamide was initiated on day –5 for 3 days. The patient remained well during this period, in particular, with no febrile episodes; however, he had a rising C-reactive protein (CRP) following cessation of steroids to a peak of 296 mg/L at day –2. His septic screen was negative, and he received his cells back on day 0 as scheduled. Within 24 hours, he developed grade 1 CRS and was started on broad-spectrum antibiotics. CRS continued until day +5, when he developed fluid unresponsive hypotension consistent with grade 2 CRS. This required treatment with tocilizumab (of which he received 2 doses in total) as well as a dose of intravenous dexamethasone. This resulted in temporary improvement in his blood pressure, however, fevers persisted. Throughout this period, his immune effector cell-associated encephalopathy (ICE) score1 had remained 10/10 with no features of neurotoxicity.
On day +8, he developed a tremor and first dropped his ICE score to 9/10 due to deterioration in his handwriting. At day +14, he acutely dropped his ICE score to 0/10, with confusion, disorientation, and aphasia. He was admitted to critical care and commenced on dexamethasone 10 mg qds for grade 3 ICANS. computed tomography and MRI brain were unremarkable. A lumbar puncture (LP) was not performed at this stage due to a thrombocytopenia of <20. Within 48 hours, his ICE score had improved to 8/10 but had not normalized. He continued to have evidence of mild dysphasia with moderate ataxia due to postural hypotension. He was slowly weaned off his steroids, which were stopped on day +23.
On day +24, he had a further deterioration in his ICE score to 6/10 with mini-mental state examination (MMSE) of 12/30. He was restarted on dexamethasone for presumed recurrence of neurotoxicity. Despite the reinitiation of steroids, his cognitive function continued to decline, in particular, with evidence of both anterograde and retrograde amnesia. He was commenced on high-dose intravenous aciclovir and broad-spectrum antibiotics for suspected meningoencephalitis.
During this period, his CRP remained low (compounded by recent use of tocilizumab), and developed acute severe hyponatremia (nadir of 116 mmo/L) with osmolalities suggestive of an underlying SIADH. He remained profoundly cytopenic. The patient was readmitted to critical care for management of acute hyponatremia and worsening cognitive state. He was started on hypertonic saline and continued on broad-spectrum antimicrobials.
An MRI head was performed (Figure 1) on day +31, which identified symmetrical T2/FLAIR hyperintensity with associated restricted diffusion involving the entirety of the hippocampal bodies, head, and amygdala. This was also illustrated on his routine day +28 PET scan, which also identified a complete metabolic response, with no detectable uptake in residual soft tissue at previous sites of disease, but noted symmetrical intense hippocampal avidity, corresponding to the MRI abnormalities. Both of these changes are consistent with an encephalitic process.
Figure 1.
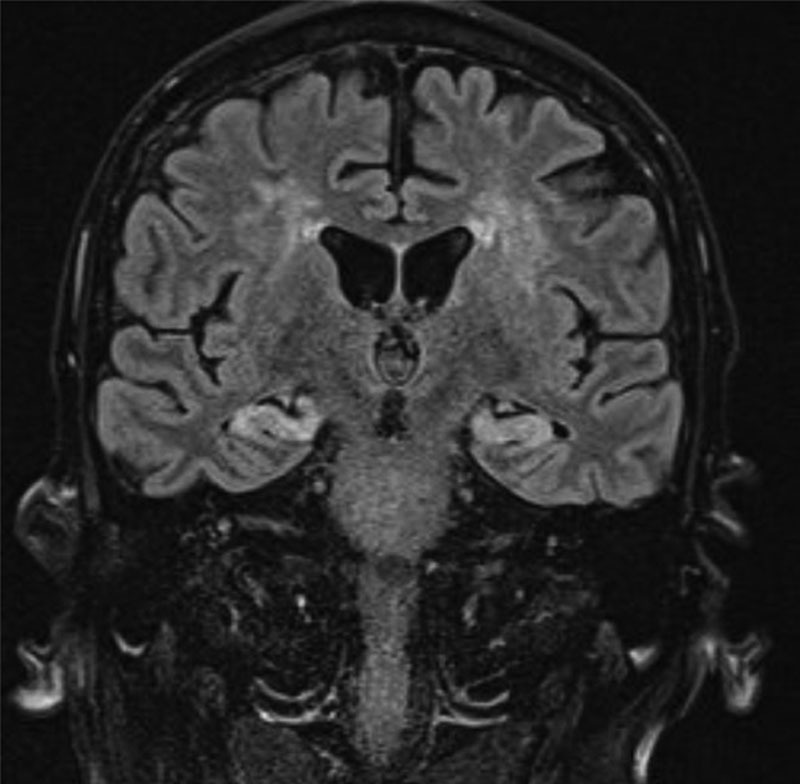
MRI head on day +31 identifying bilateral hippocampal and periventricular hyperintensities. MRI = magnetic resonance imaging.
Lumbar puncture identified a high CSF protein of 1.06 g/L, glucose of 4.5 mmol/L, red cells at 315/mm3, and no detectable white cells. There were no clonal B cells identified. CSF was positive for HHV6 DNA (qualitative assay), which was confirmed on retesting. Whole blood taken a week after the first LP was HHV6 DNA positive but below the lower limit of quantification at 5000 copies/mL.
At this point in time, clinically and radiologically, the patient had encephalitis with the positive CSF HHV6 DNA suggesting this as the causative agent.
The patient was subsequently started on intravenous foscarnet treatment on day +37, which was continued over the subsequent 3 weeks. CSF HHV6 DNA by qualitative assay was negative 12 days after initiation of treatment; whole blood HHV6 DNA persisted at the same level below the lower limit of quantification at 5000 copies/mL. This was not subsequently repeated. During and after this period, he had further infectious complications with Escherichia coli septicemia, hospital-acquired pneumonia, rhinovirus, as well as BK-related hemorrhagic cystitis. BK virus DNA was detectable in both urine and blood, with levels of 6,658,947 international unit (IU)/mL and 256 IU/mL, respectively. This recovered spontaneously and did not require treatment with cidofovir. He had hypogammaglobulinemia, which predated CAR-T treatment; this continued to fall post infusion and was treated with intravenous immunoglobulins on day +77. His cytopenias persisted until day +70 with grade 3 to 4 neutropenia4 (unresponsive to granulocyte colony-stimulating factor) and grade 3 to 4 thrombocytopenia4 requiring platelet supplementation.
Cognitively the patient continued to improve, with the exception of near-total anterograde amnesia and lesser retrograde amnesia. His MMSE improved to 16/30, his formal Addenbrooke’s Cognitive Examination score being 66/100 with severe deficits in memory tasks. There was also significant impairment in performance of verbal fluency tasks, although in part confounded by amnesia, that is, difficulty remembering the task parameters. A repeat MRI at day +82 identified resolution of hippocampal signal changes and swelling, but now with bilateral hippocampal atrophy (Figure 2). Day +82 PET imaging, however, identified small-volume recurrence of disease in the stomach and small bowel. The patient was transferred to a local nursing home for rehabilitation but died from progressive disease on day +130 without significant improvement in cognitive function.
Figure 2.
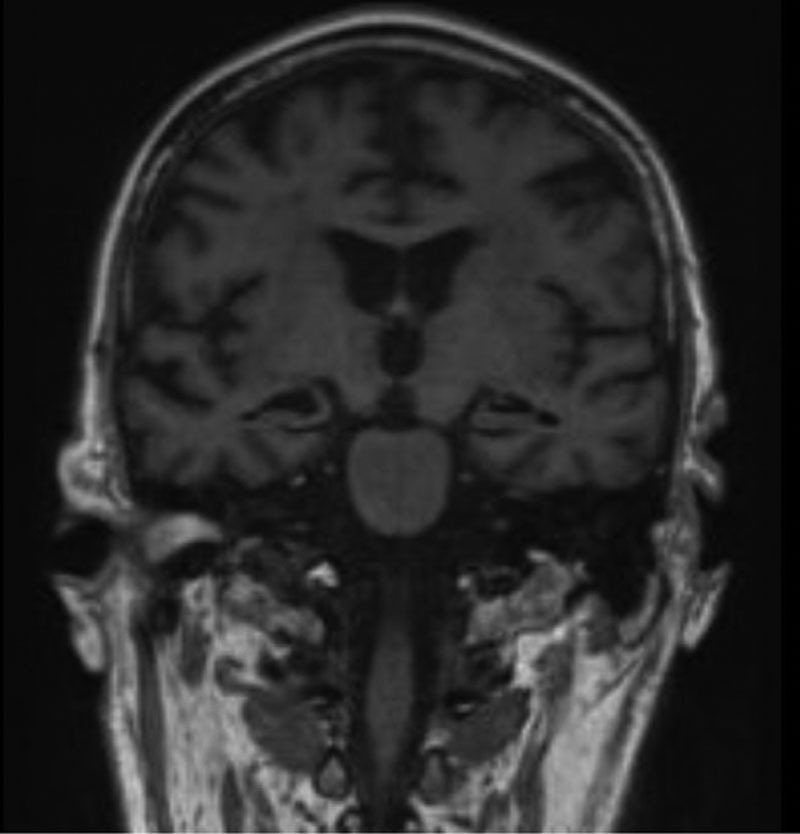
MRI head on day +82 identifying bilateral hippocampal atrophy. MRI = magnetic resonance imaging.
Our case identifies the importance of infectious complications in patients receiving CAR-T therapy. These are comparable to patients receiving allogeneic stem cell transplants, especially as these patients are heavily pretreated, with severe cytopenias and hypogammaglobulinemia. Moreover, a single-center retrospective study5 identified four MRI patterns of ICANS; of these, an autoimmune encephalitis characterized by symmetric white matter changes of pons and hippocampus was identified.5 HHV6 encephalitis would also explain these findings and should be actively considered as an important differential when evaluating a patient with possible neurotoxicity.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurological toxicity associated with immune effector cells Biol Blood Marrow Transplant. 2019; 25:625–638 [DOI] [PubMed] [Google Scholar]
- 2.Ogata M, Oshima K, Ikebe T, et al. Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017; 52:1563–1570 [DOI] [PubMed] [Google Scholar]
- 3.Gorniak RJT, Young GS, Wiese DE, et al. MR imaging of human herpesvirus-6 associated encephalitis in 4 patients with anterograde amnesia after allogeneic haematopoietic stem cell transplantatation AJNR Am J Neuroradiol. 2006; 27:887–891 [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer A, Saygin C, Maakaron J, et al. Cytopenias after chimeric antigen receptor T-cells (CAR-T) infusion; patterns and outcome Biol Blood Marrow Transplant. 2019; 25:S171 [Google Scholar]
- 5.Strati P, Tummala S, Nastoupil J, et al. Clinical and radiological correlates of neurotoxicity after standard of care axicabtagene ciloleucel in patients with relapsed/refractory large B cell lymphoma Blood. 2019; 134(Supplement_1):76531262781 [Google Scholar]


