Abstract
Introduction
Patients with severe COVID-19 develops an acute respiratory distress syndrome (ARDS), requiring admission to the intensive care unit. COVID-19 also reports an increased prevalence of comorbidities, similar to patients with Sleep disorder breathing (SDB).
Objectives
To evaluate the association between undiagnosed SDB and the risk of ARDS and pulmonary abnormalities in a cohort of patients’ survivors of COVID-19 between 3 and 6 months after diagnosis.
Methods
Prospective cohort study of patients who developed ARDS during hospitalization due to COVID-19 compared with a control group of patients who had COVID-19 with mild to moderate symptoms. All patients were evaluated between the 12th and 24th week after SARS-CoV-2 infection. The evaluation includes persistent symptoms, lung diffusing capacity of carbon monoxide (DLCO), chest CT scan and home sleep apnea test. SDB was diagnosed by the respiratory disturbance index ≥5 ev/h. The association between SDB and ARDS, the hazards of lung impairment and the hazard ratios (HR) were analyzed.
Results
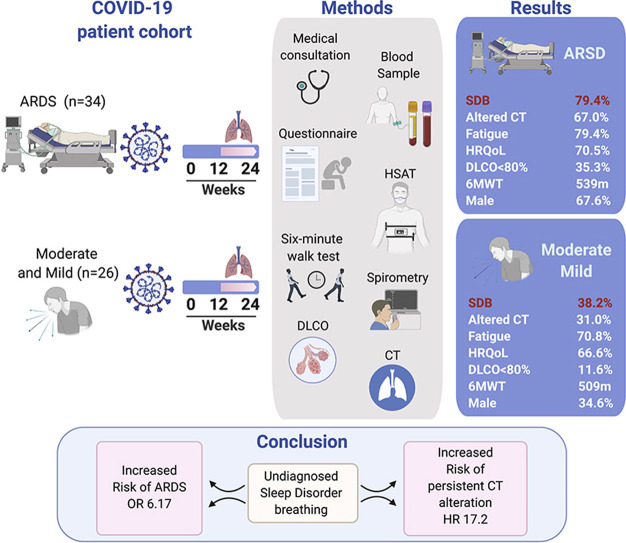
A total of 60 patients were included (ARDS: 34 patients, Control: 26 patients). The mean follow-up was 16 weeks (range 12–24). ARDS reported a high prevalence of SDB (79% vs. 38% in control group). A total of 35% reported DLCO impairment, and 67.6% abnormal chest CT. SDB was independently associated to ARDS, OR 6.72 (CI, 1.56–28.93), p < 0.01, and abnormal Chest CT, HR 17.2 (CI, 1.68–177.4, p = 0.01). Besides, ARDS, days in mechanical ventilation, male gender were also associated with an increased risk of abnormal chest CT.
Conclusion
Undiagnosed SDB is prevalent and independently associated with ARDS. In addition, undiagnosed SDB increased the hazard of abnormal Chest CT in the midterm.
Study register
ISRCTN16865246.
Keywords: COVID-19, SARS-CoV-2, Pulmonary function test, Sleep disorder breathing, Sleep apnea, Obstructive
Graphical abstract
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) [1]. During 2020, this emergent disease has rapidly spread on different continents. The clinical diagnosis usually involves detecting SARS-CoV-2 nucleic acid utilizing a protein chain reaction assay [2]. To date, 94, 309, 742 confirmed cases have been reported worldwide, including 661,180 new cases and 17,369 deaths in Chile [3].
According to the Centers for Disease Control and Prevention [2], COVID-19 involves broad clinical manifestations, including asymptomatic disease, mild illness, moderate illness, severe illness (including acute respiratory distress syndrome [ARDS]), and intensive care unit (ICU) admission. The management of this disease includes clinical monitoring and supportive care in mild to moderate cases and hospitalization, oxygen therapy, and critical care management in those with severe and critical illness [1].
After acute COVID-19, the clinical evolution in surviving patients is not fully described. Previous data from China and Europe reported that, in the midterm (between 1 and 3 months post-infection) [4,5], pulmonary and radiological features in survivors of critical COVID-19 showed a high prevalence of pulmonary structural abnormalities and functional impairment [6,7]. Although the primary explanation for this impairment are the development of ARDS and critical care support, it is possible that other factors such as sleep disordered breathing (SDB) play a role in COVID-19 severity and concomitant sequalae [8]. Previous observational studies have reported a common risk factor and comorbidities between SDB and poor COVID-19 outcomes, such as cardiometabolic comorbidities [8]. However, despite the increasing reports of late sequelae of COVID-19, it is still unknown whether COVID-19 patients who had different levels of severity will suffer pulmonary impairment in the midterm post-discharge period and whether SDB may increase the risk of having severe COVID-19 or concomitant pulmonary sequelae.
The purpose of this study was to evaluate the association between SDB and COVID-19-mediated ARDS during the acute phase of the disease and analyze the evolution of the post-discharge patients after a follow-up period between 3 and 6 months in a cohort of patient survivors.
2. Material and methods
2.1. Study design
We performed a prospective observational cohort study including 2 clinical centers located in Chile (Hospital Regional Dr. Guillermo Grant Benavente, Concepcion, and Complejo Asistencial Dr. Victor Rios Ruiz, Los Angeles) following the current recommendations from the STROBE statement [9]. The study protocol was previously registered in the ISRCTN registry (ID: ISRCTN16865246) and was approved by the Institutional Review Boards (IRBs) from Servicio de Salud Bio Bio (IRB: CEC113) and Servicio de Salud Concepcion (IRB: CEC-SSC: 20-07-26). Signed informed consent was acquired prior to inclusion in the study.
We included patients >18 years old with positive real-time reverse transcription polymerase chain reaction (rRT-PCR) test for SARS-CoV-2 nucleic acid from April 2020 to July 2020. Our study design included 2 groups:
-
1)
Patients who developed ARDS during hospitalization due to COVID-19. This group was admitted to the ICU, they suffered severe hypoxemia and their medical records fulfilled ARDS according to the Berlin criteria.
-
2)
Patients who had COVID-19 with mild to moderate symptoms, which were used as control group. Patients with mild symptoms had fever, cough, and change in taste or smell, but no dyspnea, received clinical outpatient monitoring and supportive care. Patients with moderate symptoms required hospitalization without connection to invasive mechanical ventilation (IMV) and they exhibited clinical or radiographic evidence of lower respiratory tract disease.
Patients who were not available for follow-up, had previous respiratory disease, were transferred to another hospital or city after discharge, were in palliative care or had a mental disability that prevented the completion of evaluations were excluded from the study.
A subgroup of 30 participants with mild to moderate illness were telephonically invited to participate in the study, from which 26 agreed to participate and their data was extracted from the local register of patients with confirmed COVID-19. Finally, we were unable to enroll patients with non-COVID-19 ARDS (or ARDS due to other viral pneumonias) as a comparison during this period of follow-up.
2.2. Data extraction
2.2.1. Baseline and intensive care unit stay
We extracted data about demography (age, gender, and rural area), anthropometry (body mass index [BMI, in kg/m2] and neck, waist and hip circumferences), social habits (tobacco and alcohol usage), and comorbidities (hypertension, insulin resistance, diabetes mellitus, hypothyroidism, arrhythmia, coronary heart disease or stroke). We also extracted laboratory parameters, including ferritin (mg/dL), C-reactive protein (mg/dL), white blood cell count (x109/L), lymphocyte count (x109/L), D-dimer (mg/dL), fibrinogen (mg/dL), and PaO2/FIO2 ratio, and critical care support during the critical care stay, including high-flow nasal canula (HFNC), vigil prone, steroid use (intravenous dexamethasone), anti-interleukin 6 therapy (tocilizumab), antibiotics, invasive mechanical ventilation (IMV), days on IMV, neuromuscular blockade (NMB), prone positioning, tracheostomy, days in the ICU, total days in the hospital, and need for O2 treatment post-discharge. For the control group, we extracted data from participants with at least one medical visit and initial laboratory parameters after the COVID-19 diagnosis. This group received clinical monitoring by telephone and supportive care.
All laboratory tests were evaluated at ICU admission for ARDS or at intending admission for the mild and moderate group.
2.2.2. Midterm follow-up
The details of the medical evaluation and procedures are available in E-Appendix 1. Between the 12th and 24th weeks after SARS-CoV-2 diagnosis, all participants underwent a clinical evaluation exploring new symptoms, such as muscle fatigue, achieved by the binary Chalder fatigue questionnaire [10]. A cut-off ≥ 4 points was considered severe fatigue. Dyspnea was assessed by the modified Medical Research Council (mMRC) scale, and depression was assessed using the Beck Depression Inventory (BDI) [11]. During the evaluation, all participants answered the Hospital Anxiety and Depression Scale (HADS) [12]. Finally, the health-related quality of life (HRQoL) personal change was evaluated by a visual analog scale, with a range of 0% (worse HRQoL) and 100% (best HRQoL) prior to SARS-CoV-2 infection and during the follow-up. A change ≥10% was indicative of a change in HRQoL, which is similar to previous reports [5].
2.2.3. Pulmonary function tests (PFTs)
All participants underwent forced spirometry following the current guidelines of the American Thoracic Society (ATS) [17,18]. Data such as the forced vital capacity (FVC, %), forced expiratory volume in the first second (FEV1, %), and FEV1/FVC ratio were obtained. In addition, the diffusion capacity of the lungs for carbon monoxide (DLCO) and a 6-min walk test (6MWT) were performed. The DLCO was adjusted according to hemoglobin levels (DLCOc), (% mL/min/mmHg) [19], and we categorized participants by DLCOc < 80%, alveolar volume (AV, %), and DLCO/AV ratio (%). The 6MWT was performed following current ATS guidelines (meters, %) [20]. Finally, all PFTs were reported as a % of the predictive value, following the Chilean population's predictive values [18,19,21].
2.2.4. Computed tomography (CT) scan of the chest
All images were acquired using a high-resolution CT scan (SOMATOM, Siemens, Germany), with the patients in a supine position and in a cephalic-caudal direction with slices achieved at the end of inspiration and end of expiration. A radiologist blinded to the medical records evaluated the CT images and classified them as normal or abnormal. The following findings were extracted according to the Fleischner Society [22]: ground-glass opacities, mixed ground-glass opacities, consolidation, interlobular thickening, bronchiectasis, atelectasis, solid nodules, non-solid nodules, reticular lesions, fibrotic lesions, air trapping, and the number of lobes affected.
In addition, the persistence of CT alterations was quantified using the total severity score (TSS). This score includes the visual inspection of each lobe, reporting the % impairment of each lobe (0–25%: 1 point, 26%–50%: 2 points, 51%–75%: 3 points, and 76%–100%: 4 points), and the sum of each lobe represents the TSS. This method was previously reported in patients with ARDS during acute and convalescent periods by Ooi et al. [23].
2.2.5. Sleep study
Participants completed the following sleep-related questionnaires: Pittsburgh Sleep Quality Index (PSQI); Epworth Sleepiness Scale (ESS); Satisfaction, Alertness, Timing, Efficiency, and Duration (SATED) [13]; and STOP-BANG questionnaire [14]. In addition, participants completed a home sleep apnea test (HSAT) using an ApneaLink Air device (ResMed, Australia) following the current recommendations and requirements of the American Academy of Sleep Medicine (AASM) for level III studies [15]. The HSAT analysis was scored manually by a blinded researcher (GL). The study included a nasal cannula, a pulse oximeter sensor and one respiratory effort sensor with a thoracoabdominal band.
In addition, we included the following variables of the HSAT for analysis: respiratory disturbance index (RDI) (apneas or hypopneas associated with 3% oxygen desaturation per hour), mean oxygen saturation (mean SpO2), minimum oxygen saturation (min SpO2), total time with oxyhemoglobin saturation below 90% (T90%), and oxygen desaturation index (ODI-3%). SDB was defined by an RDI ≥5/h, and moderate to severe SDB was defined by an RDI ≥15/h [16].
2.2.6. Statistical analysis and confounder assessment
Differences in demographics and characteristics during both the acute and follow-up phases between the ARDS and control groups were reported using the means (standard deviations [SDs]) for numerical variables and frequencies for categorical variables. Differences between the groups were established by the t-test or chi-squared test for parametric variables and the Mann–Whitney U-test, Kruskal–Wallis test or Fisher test for non-parametric variables.
2.2.7. Association between undiagnosed SDB and ARDS
The primary outcome was a cross-sectional analysis between the prevalence of SDB within groups. The association between undiagnosed SDB and ARDS was assessed using unadjusted and adjusted odds ratios (ORs) with respective confidence intervals (CIs) through a logistic regression model following a stepwise analysis. The independent variable for this analysis was ARDS (yes/no), and we included all the sample sizes. As covariables, we included confounding variables related to an increased risk of ARDS, such as age, gender, hypertension, obesity (BMI, neck circumference, and abdominal circumference), smoking status, ferritin levels, and D-dimer levels during the acute phase.
2.2.8. Association between undiagnosed SDB and pulmonary outcomes at follow-up
The secondary outcome of this study was to evaluate the association between undiagnosed SDB and posterior impairment in PFTs (abnormal DLCOc) and persistence of CT alterations after follow-up within the groups. For this purpose, we developed a Kaplan–Meier survival analysis calculating the log rank (Mantel–Cox) model. The independent variables for this analysis were persistence of CT alterations (yes/no) and DLCO anomalies (yes/no). The incidence ratio of persistence of CT alterations was evaluated using unadjusted and adjusted hazard ratios (HRs) through a Cox proportional HR. The multivariate analysis was adjusted by the following covariables associated with persistence of CT alterations or DLCO anomalies: follow-up (weeks), age, gender, hypertension, BMI, smoking status, ARDS, ICU management (HFNC, vigil prone, steroid usage, IMV, NMB, tracheotomy, and days on IMV), and markers of nocturnal hypoxemia (T90%, ODI-3%, and lowest SpO2). All the tests were two-tailed, and p-values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS software version 25 (IBM, Chicago, USA).
3. Study results
3.1. Characteristics of the study population in the acute phase
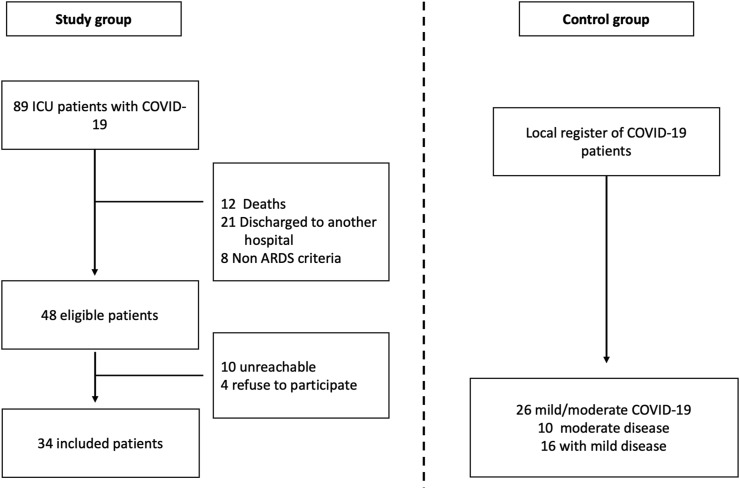
A summary of the study's flowchart is shown in Fig. 1 . During the study period, a total of 81 patients with critical COVID-19 were admitted to both hospitals. A total of 48 patients were eligible for follow-up, and 34 patients agreed to participate in the study. The control group included 26 patients with mild to moderate COVID-19 (10 moderate illness and 16 mild illness). Sixteen patients with mild COVID-19 were discharged without supplementary O2, and those with moderate COVID-19 received O2 therapy in the medical ward. We did not use CPAP-BiPAP therapy in these patients. Moreover, no patient in both the control and ARDS groups reported supplementary O2 or CPAP-BiPAP therapy after discharge. A summary of the baseline and acute COVID-19 characteristics is shown in Table 1 .
Fig. 1.
Study flowchart.
Table 1.
Baseline and acute COVID-19 characteristics.
| Control (n = 26) | ARDS (n = 34) | p-value | |
|---|---|---|---|
| Follow up (weeks), mean (SD) | 15.9 (±3.4) | 16.4 (±4.0) | 0.61 |
| Age, mean (SD) | 40.4 (±23.6) | 51 (±11.6) | < 0.01 |
| Gender, Male (%) | 9 (34.6%) | 23 (67.6%) | 0.01 |
| Rural area, N (%) | 1 (3.8) | 5 (14.7) | 0.17 |
| Anthropometry | |||
| BMI (kg/m2), mean (SD) | 30.0 (±4.9) | 31.9 (±5.0) | 0.73 |
| Neck circumference (cms), mean (SD) | 39.8 (±4.9) | 43.2 (±4.9) | 0.01 |
| Waist circumference (cms), mean (SD) | 97.8 (±11.7) | 107.8 (±12.1) | < 0.01 |
| Tobacco | |||
| Nonsmoker, N (%) | 17 (65.3) | 20 (58.8) | 0.252 |
| Current, N (%) | 5 (19.2) | 3 (8.8) | |
| Former, N (%) | 4 (15.3) | 11 (32.3) | |
| Pack/year, mean (SD) | 7.5 (±6.5) | 8.6 (±9.3) | 0.80 |
| Alcohol | |||
| None, N (%) | 11 (42.3) | 14 (41.1) | 0.45 |
| Usually, N (%) | 15 (57.6) | 18 (52.9) | |
| Frequent, N (%) | 0 | 2 (5.8) | |
| Comorbidities | |||
| Hypertension, N (%) | 5 (19.2) | 14 (41.1) | 0.04 |
| Insulin resistance, N (%) | 1 (3.8) | 10 (29.4) | 0.01 |
| Type 2 diabetes mellitus, N (%) | 4 (15.3) | 3 (8.8) | 0.34 |
| Hypothyroidism, N (%) | 1 (3.8) | 4 (11.7) | 0.27 |
| Arrythmia, N (%) | 1 (3.8) | 0 | 0.43 |
| Stroke, N (%) | 0 | 1 (2.9) | 0.56 |
| Acute COVID-19 Laboratory | |||
| Ferritin, mg/dL, mean (SD) | 734 (±1127) | 2299 (±1586) | 0.01 |
| CPR, mg/dL, mean (SD) | 75.5 (±97.3) | 168.9 (±121.7) | 0.02 |
| WBC, x 109/L, mean (SD) | 5371.7 (±3119) | 11756 (±5042) | 0.01 |
| Lymphocyte count, x109/L, mean (SD) | 1255 (±1019) | 859 (±321) | 0.28 |
| D-Dimer, mg/dL, mean (SD) | 639 (±519) | 1629 (±1155) | 0.01 |
| Fibrinogen, mg/dL, mean (SD) | 511.2 (±264) | 713.0 (±230) | 0.10 |
| Worse PaO2/FiO2, mean (SD) | 306.8 (±106) | 179.5 (±35.8) | 0.01 |
Abbreviation: ARDS: Acute respiratory distress syndrome, SD: Standard deviation, BMI: Body mass index, CPR: C reactive protein, WBC: White cell count, Highlights: statistically significant.
We documented a mean follow-up time of 15.9 weeks (range 12–24 weeks) in the control group and 16.4 weeks (range 12–24 weeks) in the ARDS group (p = 0.60). Patients in the ARDS group reported an older age of 51 (±11.6) years old vs. 40.4 (±23.6) years old, and 67.6% of the sample was male. In the univariate analysis, we found differences in neck circumference of 43.2 (±4.9) cm vs. 39.8 (±4.9) cm and waist circumference of 107.8 (±12.1) cm vs. 97.8 (±11.7) cm. Moreover, the ARDS group had higher prevalence of hypertension and insulin resistance than the control group, but no difference in BMI was observed between groups (Table 1).
Regarding acute COVID-19 laboratory parameters, the comparison between the ARDS and the control group showed significant differences in the following parameters: ferritin (2299 [±1586] mg/dL vs. 734 [±1127] mg/dL), white blood cell count (11,756 [±5042] x 109/L vs. 5371 [±3119] x 109/L), and D-dimer (1629 [±1155] mg/dL vs. 639 [±519] mg/dL) levels and a worse PaO2/FIO2 ratio (179.5 [±519] vs. 306.8 [±106]) (Table 1). Finally, the summary of hospitalization in the ICU during the acute phase is shown in Table E1.
3.2. Clinical symptoms, pulmonary function tests and chest CT scans at follow-up
In terms of the follow up clinical symptoms at 3–6 months post-infection, we observed a total of 16/26 (61.5%) patients in the control group and 24/34 (70.5%) patients in the ARDS group exhibiting COVID-19-related symptoms (Table 2 ). We found no difference in the mMRC dyspnea scale (p = 0.27). Both groups reported decreased HRQoL (70.5% of patients in the ARDS group and 66.6% of patients in the control group) and high prevalence of fatigue (70.8% in the control group and 79.4% in the ARDS group, using the Chalder fatigue scale) (Table 2). A summary of the clinical differences and physical examination are shown in Table 2. Results obtained from the BDI and HADS questionnaire to identify changes in anxiety and depression are shown in Table E2.
Table 2.
Follow up clinical symptoms.
| Control (n = 26) | ARDS (n = 34) | p-value | |
|---|---|---|---|
| Symptoms | |||
| Asymptomatic, N (%) | 10 (38.4) | 10 (29.4) | 0.32 |
| Headache, N (%) | 9 (34.6) | 12 (35.2) | 0.09 |
| Thoracic pain, N (%) | 1 (2.9) | 3 (8.8) | 0.41 |
| Sore throat, N (%) | 1 (2.9) | 4 (11.7) | 0.27 |
| Cough, N (%) | 6 (17.6) | 6 (17.6) | 0.42 |
| Dyspnea, N (%) | 5 (14.7) | 10 (29.4) | 0.27 |
| mMRC, N (%) | |||
| 0 | 8 (30.7) | 8 (23.5) | |
| 1 | 17 (65.3) | 23 (67.6) | |
| 2 | 1 (2.9) | 1 (2.9) | |
| 3 | 0 (0) | 2 (5.8) | |
| Polypnea, N (%) | 2 (7.6) | 2 (5.8) | 0.58 |
| Myalgia, N (%) | 3 (11.5) | 4 (11.7) | 0.65 |
| Change in smell, N (%) | 2 (7.6) | 2 (5.8) | 0.58 |
| Change in taste, N (%) | 1 (2.9) | 0 (0) | 0.43 |
| Change in QoL | |||
| Basal HRQoL (%), mean (SD) | 88.07 (±11.05) | 90.5 (±9.72) | 0.36 |
| Post HRQoL (%), mean (SD) | 69.42 (±23.4) | 68.23 (±22.52) | 0.84 |
| Change in HRQoL (>10%), N (%) | 16 (66.6) | 24 (70.5) | 0.32 |
| Fatigue, N (%) | 17 (70.8) | 27 (79.4) | 0.17 |
| Chalder fatigue scale, mean (SD) | 4.96 (±3.4) | 5.61 (±2.3) | 0.41 |
| Physical examination | |||
| Heart Rate (bpm), mean (SD) | 76.7 (±11.6) | 79.3 (±11.6) | 0.36 |
| SBP (mmHg), mean (SD) | 123.0 (±17.2) | 135.6 (±18.2) | 0.02 |
| DBP (mmHg), mean (SD) | 71.8 (±12.5) | 77.9 (±14.0) | 0.08 |
| SpO2 (%), mean (SD) | 97.3 (±2.10) | 97.02 (±1.66) | 0.67 |
Abbreviations: ARDS: Acute respiratory distress syndrome, mMRC: modified medical research council, HRQoL: Health Related Quality of life, SBP: Systolic blood pressure, DBP: Diastolic blood pressure. Highlights: statistically significant.
The results of the PFTs to evaluate pulmonary function are shown in Table 3 . We found differences in the predicted FVC. The mean predictive value was 83.3 (±15.8) % in the ARDS group vs. 95.8 (±15.8) % in the control group (p = 0.01); moreover, the FEV1 was 88.6 (±15.8) % vs. 99.5 (±14.8) % (p < 0.01). Nine participants in the ARDS group showed abnormal spirometry, and 12/34 (35.3%) ARDS survivors showed DLCO anomalies (<80% predicted). The average distance in the 6MWT was 539 (±87.2) meters in the control group vs. 509.5 (±121.9) meters in the ARDS group (p = 0.79).
Table 3.
Summary of Pulmonary function test and Chest CT findings.
| Control (n = 26) | ARDS (n = 34) | p-value | |
|---|---|---|---|
| Spirometry | |||
| FVC (%), mean (SD) | 95.8 (±15.79) | 83.32 (±15.89) | < 0.01 |
| FEV1 (%), mean (SD) | 99.5 (±14.81) | 88.67 (±15.85) | < 0.01 |
| FEV1/FVC <0.7, N (%) | 1 (3.8%) | 9 (26.4%) | 0.02 |
| DLCO | |||
| DLCOc (%), mean (SD) | 94.8 (18.7) | 85.5 (22.6) | 0.21 |
| AV (%), mean (SD) | 99.7 (13.7) | 87.7 (15.1) | 0.01 |
| DLCOc/AV, mean (SD) | 84.9 (15.5) | 83.5 (18.0) | 0.78 |
| DLCOc > 80%, N (%) | 23 (88.4) | 22 (64.7) | 0.03 |
| DLCOc <60–80%, N (%) | 3 (11.5) | 9 (26.5) | |
| DLCOc <60%, N (%) | 0 | 3 (8.8) | |
| Six minutes walking test | |||
| Distance (meters), mean (SD) | 539 (±87.2) | 509.5 (±121.9) | 0.79 |
| Distance (%), mean (SD) | 85.6 (±20.9) | 85.6 (±31.9) | 0.99 |
| Basal SpO2, mean (SD) | 98.5 (±1.36) | 97.2 (±1.65) | < 0.01 |
| Final SpO2, mean (SD) | 95.3 (±17.6) | 97.1 (±2.11) | < 0.01 |
| Drop down 1–3% SpO2, N (%) | 4 (15.3) | 8 (23.5) | 0.02 |
| Drop down >3% SpO2, N (%) | 0 | 3 (8.8) | |
| Chest CT finding | |||
| Normal, N (%) | 18 (69.2) | 11 (33) | < 0.01 |
| Ground glass opacities, N (%) | 4 (15.3) | 23 (67.6) | < 0.01 |
| Mixed ground-glass, N (%) | 0 (0) | 3 (8.8) | 0.17 |
| Consolidation, N (%) | 0 (0) | 1 (2.9) | 0.56 |
| Interlobular thickening, N (%) | 1 (3.8) | 13 (38.2) | < 0.01 |
| Bronchiectasis, N (%) | 0 (0) | 6 (27.6) | 0.02 |
| Atelectasis, N (%) | 1 (3.8) | 6 (27.6) | 0.10 |
| Solid nodule, N (%) | 1 (3.8) | 10 (29.4) | 0.01 |
| Nonsolid nodule, N (%) | 0 (0) | 9 (26.4) | < 0.01 |
| Reticular lesion, N (%) | 1 (3.8) | 3 (8.8) | 0.41 |
| Fibrotic lesions, N (%) | 0 (0) | 8 (23.5) | < 0.01 |
| Air trapping, N (%) | 4 (15.3) | 11 (32.3) | 0.11 |
| Number of lobes affected, mean (SD) | 0.57 (±0.9) | 2.0 (±1.4) | < 0.01 |
| TSS score, mean (SD) | 0.5 (±0.9) | 3.3 (±2.6) | < 0.01 |
Abbreviation: ARDS: Acute respiratory distress syndrome, SD: Standard deviation, FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 s, DLCOc: Diffusing capacity of the lungs for carbon monoxide corrected by hemoglobin. AV: Alveolar volume. CT: Computed tomography, TSS: Total severity score. Highlights: statistically significant.
Regarding chest CT at follow-up, we found a 67% persistence of CT alterations in the ARDS group and a 31% persistence of CT alterations in the control group (p < 0.01). Ground-glass opacities were found in 67.6% of patients, interlobular thickening in 38.2% of patients, gas trapping in 32.3% of patients, solid nodules in 29.4% of patients, and bronchiectasis and atelectasis in 27.6% of patients. The mean TSS score in this group was 3.3 (±2.6) points (Table 3).
3.3. Prevalence of sleep disordered breathing within groups
The HSAT showed an average RDI of 7.1 (±8.6)/h in the control group and 13.5 (±10.3)/h in the ARDS group. A total of 27/34 (79.4%) patients in the ARDS group reported SDB, and 38.2% of them reported moderate to severe SDB. Moreover, this group also showed increased markers of nocturnal hypoxemia (Table 4 ). Regarding sleep questionnaires, we found no differences regarding sleepiness within the groups according to the SATED, PSQI, or ESS questionnaires. The mean value of the STOP-BANG questionnaire was 2.25 (±1.6) points, compared to 3.88 (±1.7) points in the ARDS group.
Table 4.
Summary of Sleep assessment.
| Control (n = 26) | ARDS (n = 34) | p-value | |
|---|---|---|---|
| Sleep questionnaires | |||
| SATED, mean (SD) | 6.04 (±2.77) | 5.79 (±2.37) | 0.72 |
| PSQI, mean (SD) | 10.08 (±4.92) | 9.76 (±4.50) | 0.80 |
| ESS, mean (SD) | 8.80 (±5.37) | 7.97 (±5.22) | 0.55 |
| ESS ≥ 10 points | 12 (46.1) | 12 (35.2) | 0.27 |
| STOP-BANG, mean (SD) | 2.25 (±1.62) | 3.88 (±1.78) | < 0.01 |
| Home Sleep Apnea Test | |||
| RDI (ev/h), mean (SD) | 7.11 (±8.6) | 13.5 (±10.3) | < 0.01 |
| <5 ev/h, N (%) | 16 (61.5) | 7 (20.5) | |
| 5–15 ev/h, N (%) | 10 (38.4) | 27 (79.4) | < 0.01 |
| ≥15 ev/h, N (%) | 2 (7.6) | 13 (38.2) | < 0.01 |
| T90%, mean (SD) | 2.08 (±4.8) | 6.44 (±10.0) | < 0.01 |
| ODI-3%, mean (SD) | 6.44 (±10.0) | 15.9 (±18.4) | 0.01 |
| Mean SpO2, mean (SD) | 95.1 (±1.0) | 93.0 (±2.18) | < 0.01 |
| Lowest SpO2, mean (SD) | 86.2 (±6.6) | 82.1 (±6.26) | 0.02 |
Abbreviation: ARDS: Acute respiratory distress syndrome, SD: Standard deviation, SATED: Satisfaction, Alertness, Timing, Efficiency and Duration questionnaire, PSQI: Pittsburgh Sleep Quality Index, ESS: Epworth sleepiness scale, RDI: Respiratory disturbance index, T90%: Time with SpO2 under 90%, ODI-3%: Oxygen desaturation index ≥3%. Highlights: statistically significant.
3.4. Risk of undiagnosed SDB and ARDS during the acute phase
A summary of the unadjusted and adjusted analyses is shown in Table 5 . In univariate analysis, we found an independent association between undiagnosed SDB and the risk of ARDS. Unadjusted analysis showed an OR of 6.17 (CI, 1.96–9.43, p < 0.01). After multivariable analysis, the OR for this association was 6.72 (CI, 1.56–28.93, p < 0.01).
Table 5.
Summary of the results of the unadjusted and adjusted logistic regression cox proportional regression model assessing the association between undiagnosed sleep disorder breathing and COVID-19 outcomes.
| Variable | Unadjusted (95%, CI) | p-value | Adjusted (95%, CI) | p-value |
|---|---|---|---|---|
| Risk of ARDS | 6.17 (1.96–19.43)a | < 0.01 | 6.72 (1.56–28.93)ac | < 0.01 |
| Impairment Chest CT | 5.79 (2.07–16.17)b | < 0.01 | 17.29 (1.68–177.45)bd | 0.01 |
| Impairment DLCOc | 0.874 (0.31–2.46)b | 0.80 | 1.16 (0.27–4.95)bd | 0.84 |
Definition of abbreviations: ARDS: Acute respiratory distress syndrome; CT: Computed tomography; DLCO: Diffusing capacity of the lungs for carbon monoxide corrected by hemoglobin; CI: Confidence interval; Highlights: Statistically significance with a p-value<0.05.
Values expressed as Odds ratios.
values expressed as Hazard ratios.
Logistic regression model adjusted by age, sex, hypertension, BMI, smoking status, ferritin levels, and D-dimer levels at baseline.
Cox proportional hazard model adjusted by Age, gender, hypertension, BMI, smoking status, ARDS, awake vigil prone positioning, steroid usage, IMV, NMB, tracheotomy, and days on IMV), total time with oxyhemoglobin saturation below 90%, Oxygen desaturation index 3%, lowest SpO2.
3.5. Undiagnosed SDB and persistence of pulmonary impairment
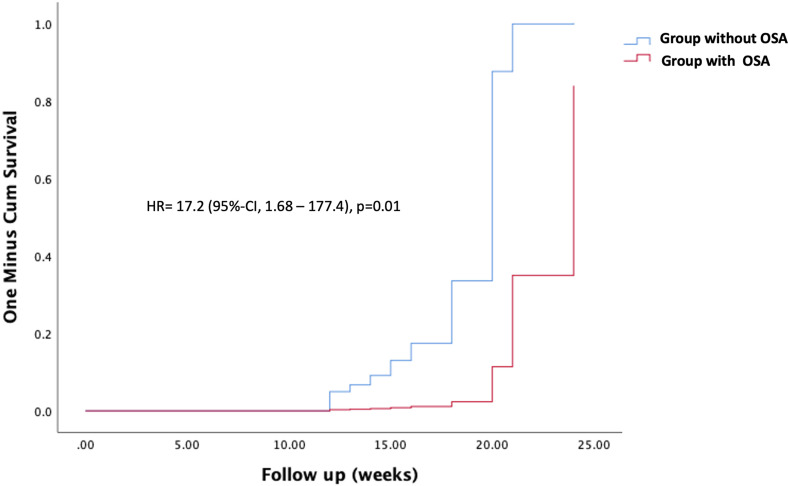
Regarding the association between undiagnosed SDB and pulmonary impairment during the follow-up within the groups, we found an independent association between untreated SDB and the risk of persistent CT alterations between 12 and 24 weeks after acute COVID-19. After multivariable analysis, the adjusted HR for this association was 17.2 (CI, 1.68–177.4, p = 0.01) (Fig. 2 ). Additionally, persistence of CT alteration was also associated with ARDS, HR 34.3 (CI, 3.9–297.7, p < 0.01); male gender, HR 4.7 (CI 1.2–18.9, p = 0.02); and days on IMV, HR 1.11 (CI, 1.0–1.2, p < 0.04). Finally, we found no association between DLCO anomalies and undiagnosed OSA; for this association, the use of HFNC therapy during the acute phase showed an increased risk, HR 6.64 (CI 1.7–25.2, p < 0.01).
Fig. 2.
Shows the cumulative hazard of normal Chest CT after acute COVID-19. Blueline: non-OSA group, Redline: OSA group.
4. Discussion
The main findings of this study were as follows: 1) in patient survivors with ARDS due to critical COVID-19, the prevalence of undiagnosed SDB was statistically significant compared with that in patients with mild/moderate COVID-19 illness; 2) after adjusting for other confounders linked to poor COVID-19 outcomes, SDB was independently associated with ARDS; and 3) in our cohort, undiagnosed SDB was independently associated with the persistence of CT alterations in the midterm, in addition to other variables such as male gender, ARDS, and total days on IMV.
According to the current literature, this is the first study aimed at evaluating the association between undiagnosed SDB and COVID-19 outcomes in a prospective cohort of patients. Our main results confirm our hypothesis that SDB is independently associated with worse COVID-19 prognosis, and therefore, undiagnosed SDB is associated with persistence of CT alterations in the midterm. The plausible mechanisms for these associations include systemic inflammation and chronic inflammation, which are commonly found in patients with untreated SDB [24]. Moreover, sleep fragmentation and chronic intermittent hypoxia can trigger the inflammatory response and sympathetic activation [25], and we hypothesize that this mechanism affects lung improvement in the recovery phase. Previous studies evaluating the association between SDB and COVID-19 also suggest this association. In the CORONADO observational study, untreated SDB was reported to be an independent risk of mortality after 7 days, with an OR of 2.8 (CI, 1.46–5.38) [8]. In another study including 46 patients hospitalized due to COVID-19, sleep apnea was diagnosed in 75% of the sample [26]. Finally, in an observational study in the United States, patients with SDB had an OR of 1.53 (CI, 1.09–2.15) for mortality and an OR of 1.29 (CI, 1.03–1.62) for ICU admission [26,27].
In our study, we found a high prevalence of current COVID-19 symptoms and an impact on HRQoL and fatigue in the midterm. Although we found no difference within groups, our findings were superior to those of previous reports. Using the same visual analog scale, Carfi et al. reported that 44% of patients had a worsened quality of life after acute COVID-19 [5]. For fatigue measurements, we used the Chalder fatigue scale to grade the severity of the symptoms; however, a prospective follow-up, including 6 or more months after a COVID-19 diagnosis, should explore the persistence of this symptom and the new onset of fatigue/chronic fatigue syndrome [10].
Regarding chest CT abnormalities, Xion et al. reported 74% of patients with radiological abnormalities 3 months after discharge [28]; in another study, 23% of patients had fibrosis, and the mean TSS was 8 points at 3 months after discharge [29]. In our cohort, the prevalence of persistent CT alterations was 67%, and after adjusting for several covariables, the probability of radiological regression was independently associated with the severity of ARDS and other variables, such as management in the ICU, male gender and undiagnosed SDB. We suggest that chronic intermittent hypoxia and nocturnal hypoxemia should contribute to the persistence of CT alterations. However, we explored the contribution of surrogate frequency-based markers of nocturnal hypoxemia (T90%, ODI-3%, and lowest SpO2), without significant associations.
Regarding the PFTs, although we found no differences in the average distance of the 6MWT, we found a 35% impairment in the DLCO between 3 and 6 months after discharge. In the study of Zhao et al. including data from SARS-CoV-2, 25% of patients had DLCO impairment, and 70.9% had chest CT abnormalities after 3 months [7]. Mo et al. including data achieved at the time of hospital discharge, showed DLCO impairment in 47% of patients [6]; however, both studies included small sample sizes and short-term follow-ups. Regarding midterm outcomes, previous data from patients with both SARS and ICU stays were similar to our findings, showing a prevalence of impaired DLCO ranging from 13.5% to 24% in the midterm [30,31]. Finally, data about DLCO impairment from the patients with MERS and ICU stays were similar to our study (35%) [32]. In our cohort, the use of HFNC therapy during the ICU stay was independently associated with DLCO impairment during our follow-up. However, in our study, the DLCO/AV ratio did not differ between groups; this is the most important result of the DLCO analysis, as the reduction in DLCO is probably related to the loss of alveolar space due to the fibrosis process. Further studies including additional populations are needed to validate this association.
The main limitations of this study are related to the small sample size; however, this study provides evidence about the association between undiagnosed SDB and the importance of acute COVID-19 and midterm outcomes. Second, this study has inclusion bias as a methodological limitation, and the conclusions obtained are restricted to the data obtained from the eligible population according to our selecting criteria. In addition, our study design included a population with different severities of COVID-19 illness, however we did not include a non-COVID-19 group. We were unable to enroll non-COVID-19 ARDS patients (or ARDS due to other viral pneumonias) for unpair comparison, and future research comparing these groups is necessary to evaluate the consequences of SARS-CoV-2 infection in the follow-up. Third, we included data between 12 and 24 weeks after a COVID-19 diagnosis, and future studies exploring this association and the impact on pulmonary function and quality of life in the long term are needed. Fourth, we were not able to check obstructive versus central events. Finally, we used a HSAT type III sleep test. This test is recommended in populations with a high pretest probability of SDB, and the gold standard remains polysomnography (PSG) [15,16]. However, we restricted our analysis to RDI, and further studies exploring the association between other sleep disorders (central apneas, restless leg syndrome, and others) and COVID-19 are needed.
5. Conclusion
Among patients with ARDS due to COVID-19, undiagnosed SDB is prevalent and is independently associated with ARDS, and undiagnosed SDB increases the risk of abnormal chest CT in the midterm, in addition to other confounders such as age, ARDS, gender and hospitalization in the ICU during the acute phase.
Funding/support
Supported by the Agencia Nacional de Investigacion y Desarrollo (ANID, COVID1005 and PAI79170073), Chilean Government and CIBERESUCICOVID Project (COV20/00110, ISCIII, Madrid, Spain).
Descriptor number
15.9 Sleep Disordered Breathing: Outcomes.
CRediT authorship contribution statement
Gonzalo Labarca: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. Mario Henriquez-Beltran: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Faryd Llerena: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – review & editing. Gustavo Erices: Data curation, Formal analysis, Investigation, Writing – review & editing. Jaime Lastra: Conceptualization, Data curation, Funding acquisition, Resources, Writing – review & editing. Daniel Enos: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Writing – review & editing. Daniela Castillo: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – review & editing. Marco Fraga: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – review & editing. Liliana Lamperti: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Writing – original draft, Writing – review & editing. Valeska Ormazabal: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Benilde Riffo: Conceptualization, Data curation, Funding acquisition, Writing – review & editing. Daniel Rubilar: Data curation, Formal analysis, Investigation, Writing – review & editing. Rocio Sanhueza: Data curation, Formal analysis, Investigation, Writing – review & editing. Jaime Vasquez: Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. Carolina Villanueva: Data curation, Investigation, Resources, Writing – review & editing. Gloria Horta: Data curation, Formal analysis, Investigation, Writing – review & editing. Felipe Sanhueza: Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. Pedro Melo: Data curation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing. Jorge Dreyse: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Jorge Jorquera: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Sebastian Fernandez-Bussy: Funding acquisition, Methodology, Writing – original draft. Jessica Gonzalez: Funding acquisition, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. Ferran Barbe: Funding acquisition, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. Estefania Nova-Lamperti: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2021.02.029.
Authors declare no conflict of interest.
Acknowledgment
We acknowledge Víctor Ríos Ruiz Hospital, Guillermo Grant Benavente Hospital, Los Andes Clinic, LABOCER laboratory, ALTASALUD laboratory and Sanatorio Aleman to provide the infrastructure to recruit patients, collect samples and perform pulmonary tests. We acknowledge the Vida Saludable Centre at University of Concepcion to provide the infrastructure to perform tests. Graphical Abstract was created with BioRender.com. We thank with gratitude to all our participants for their contribution with their samples and clinical data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sleep.2021.02.029.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 2.https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- 3.https://www.worldometers.info/coronavirus/.
- 4.Huang Y., Tan C., Wu J., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfi A., Bernabei R., Landi F., et al. Persistent symptoms in patients after acute COVID-19. J Am Med Assoc. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y.M., Shang Y.M., Song W.B., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller M.A., Cappuccio F.P. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med Rev. 2020;55:101382. doi: 10.1016/j.smrv.2020.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewlett S., Dures E., Almeida C. Measures of fatigue: bristol rheumatoid arthritis fatigue multi-dimensional questionnaire (BRAF MDQ), bristol rheumatoid arthritis fatigue numerical rating scales (BRAF NRS) for severity, effect, and coping, chalder fatigue questionnaire (CFQ), checklist individual strength (CIS20R and CIS8R), fatigue severity scale (FSS), functional assessment chronic illness therapy (fatigue) (FACIT-F), multi-dimensional assessment of fatigue (MAF), multi-dimensional fatigue inventory (MFI), pediatric quality of life (PedsQL) multi-dimensional fatigue scale, profile of fatigue (ProF), short form 36 vitality subscale (SF-36 VT), and visual analog scales (VAS) Arthritis Care Res. 2011;63(Suppl 11):S263–S286. doi: 10.1002/acr.20579. [DOI] [PubMed] [Google Scholar]
- 11.Beck Jackson-Koku G. Depression inventory. Occup Med (Lond) 2016;66(2):174–175. doi: 10.1093/occmed/kqv087. [DOI] [PubMed] [Google Scholar]
- 12.Bjelland I., Dahl A.A., Haug T.T., et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 13.Benitez I., Roure N., Pinilla L., et al. Validation of the satisfaction, alertness, timing, efficiency and duration (SATED) questionnaire for sleep health measurement. Ann Am Thorac Soc. 2020;17(3):338–343. doi: 10.1513/AnnalsATS.201908-628OC. [DOI] [PubMed] [Google Scholar]
- 14.Labarca G., Dreyse J., Salas C., et al. Performance of instruments aimed at detecting obstructive sleep apnea syndrome among individuals in Chile. J Bras Pneumol. 2019 Dec 13;46(1) doi: 10.1590/1806-3713/e20190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapur V.K., Auckley D.H., Chowdhuri S., et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qaseem A., Dallas P., Owens D.K., et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161(3):210–220. doi: 10.7326/M12-3187. [DOI] [PubMed] [Google Scholar]
- 17.Roca J., Burgos F., Sunyer J., et al. References values for forced spirometry. Group of the European community respiratory health survey. Eur Respir J. 1998;11(6):1354–1362. doi: 10.1183/09031936.98.11061354. [DOI] [PubMed] [Google Scholar]
- 18.Knudson R.J., Slatin R.C., Lebowitz M.D., et al. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 19.Roca J., Rodriguez-Roisin R., Cobo E., et al. Single-breath carbon monoxide diffusing capacity prediction equations from a Mediterranean population. Am Rev Respir Dis. 1990;141(4 Pt 1):1026–1032. doi: 10.1164/ajrccm/141.4_Pt_1.1026. [DOI] [PubMed] [Google Scholar]
- 20.Laboratories ATSCoPSfCPF ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Osses A.R., Yanez V.J., Barria P.P., et al. [Reference values for the 6-minutes walking test in healthy subjects 20-80 years old] Rev Med Chile. 2010;138(9):1124–1130. [PubMed] [Google Scholar]
- 22.Hansell D.M., Bankier A.A., MacMahon H., et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 23.Ooi G.C., Khong P.L., Muller N.L., et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230(3):836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 24.Labarca G., Cruz N.R., Descalzi F. [Multisystemic involvement in obstructive sleep apnea] Rev Med Chile. 2014;142(6):748–757. doi: 10.4067/S0034-98872014000600009. [DOI] [PubMed] [Google Scholar]
- 25.Labarca G., Gower J., Lamperti L., et al. Chronic intermittent hypoxia in obstructive sleep apnea: a narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath. 2020;24(2):751–760. doi: 10.1007/s11325-019-01967-4. [DOI] [PubMed] [Google Scholar]
- 26.Perger E., Soranna D., Pengo M., et al. Sleep-disordered breathing among hospitalized patients with COVID-19. Am J Respir Crit Care Med. 2021 Jan 15;203(2):239–241. doi: 10.1164/rccm.202010-3886LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cade B.E., Dashti H.S., Hassan S.M., et al. Sleep apnea and COVID-19 mortality and hospitalization. Am J Respir Crit Care Med. 2020 Nov 15;202(10):1462–1464. doi: 10.1164/rccm.202006-2252LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y., Sun D., Liu Y., et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. 2020;55(6):332–339. doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li K., Fang Y., Li W., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui D.S., Joynt G.M., Wong K.T., et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herridge M.S., Cheung A.M., Tansey C.M., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed H., Patel K., Greenwood D.C., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5) doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.