Abstract
Since the emergence of novel coronavirus pneumonia (NCP), a number of reports have pointed out an increased coagulation activity in these patients mostly during acute phase of the disease. We are reporting a case of acute superior mesenteric thrombosis in a 55-year-old man with NCP 1 week after hospital discharge. He returned to the emergency department 7 days later with severe acute abdominal pain and found to have superior mesenteric artery thrombosis. He subsequently underwent emergent exploratory laparotomy, superior mesenteric artery thrombectomy, and bowel resection. Acute arterial thrombosis may occur in the posthospitalization period in patients with NCP.
Keywords: Novel coronavirus pneumonia (NCP), COVID-19, Superior mesenteric artery thrombosis, Anticoagulation, d-Dimer
Since the emergence of novel coronavirus pneumonia (NCP) in December 2019, a number of reports have suggested that a large percentage of patients with novel coronavirus disease (COVID-19), particularly those who become critically ill, develop a prothrombotic state that places them at a significantly increased risk of thrombosis, especially during the acute phase that often requires intensive care unit stay with severe acute respiratory distress syndrome.1, 2, 3 Interestingly, a wide range of increases in d-dimer levels has also been documented in patients hospitalized with COVID-19 and there are early reports linking higher d-dimer levels to worse outcomes.4 Based on these reports, most centers used thrombosis prevention strategies ranging from prophylactic regimens to full anticoagulation during hospitalization for NCP with improved outcomes.5 The optimal potency and duration of antithrombotic management is not clear. In addition, there is little evidence on when the increased coagulation activity returns to normal levels after the onset of symptoms. We report a case of acute mesenteric ischemia due to superior mesenteric artery (SMA) thrombosis in a 55-year-old male patient with NCP 1 week after hospital discharge. Patient consent to publish the case report was obtained according to our institutional guidelines.
Case report
A 55-year-old African American man presented to the emergency department (ED) with complaints of a nonproductive cough, fatigue, myalgia, nausea, diarrhea, and abdominal pain for 4 days. His past medical history was significant for hypertension and Grave's disease. Medication history included atenolol 100 mg/d and methimazole 10 mg/d. The patient did not have a history of smoking or illicit drug abuse. He is a construction worker in the New York City area. On physical examination he had mild hypoxia and fever (87% on room air and 101°F). Laboratory tests revealed hyponatremia, hypokalemia, and elevated aspartate aminotransferase. A chest radiograph showed right basilar infiltrates. Influenza A/B was negative and a severe acute respiratory syndrome coronavirus-2 RNA test was positive. He was admitted and started on our hospital COVID-19 therapy regimen, including oral azithromycin 500 mg/d and hydroxychloroquine 400 mg/d for 5 days. During this time, he received prophylactic anticoagulation with heparin 5000 unit subcutaneously daily twice per day in the hospital.
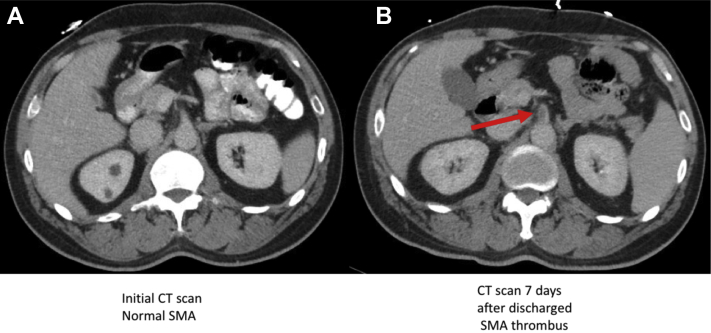
A computed tomography scan with intravenous and oral contrast showed bilateral lower lobe, right middle lobe, and lingula ground glass opacification with distribution suggestive of COVID-19 pneumonitis. The abdomen and pelvis were unremarkable with no evidence of mesenteric vessel disease (Fig 1). After treatment initiation, the patient's oxygenation and nausea improved, and he was discharged home after 5 days of hospitalization with a 5-day course of levofloxacin 500 mg orally.
Fig 1.
Comparison of superior mesenteric artery (SMA) in computed tomography (CT) imaging with intravenous contrast between two admissions. A, initial CT scan, normal SMA. B, CT scan 7 days after discharge showing the SMA thrombus.
The patient returned to the ED 7 days after discharge complaining of acute onset of severe abdominal pain. On physical examination, he was tachycardic but did not have hypoxia or fever. The abdomen was tender with guarding. Laboratory results are summarized in the Table . Lactic acid was 6.2 and d-dimer was 2400. A computed tomography scan of chest, abdomen, and pelvis with intravenous contrast showed interval development of a 1.6 cm long low-density thrombus in the proximal SMA causing a high-grade luminal stenosis (Fig 1). His electrocardiogram showed sinus rhythm with no evidence of myocardial ischemia. A transthoracic echocardiogram was performed and was within normal limits. He was immediately heparinized in the ED and was taken to the operating room emergently for exploratory laparotomy and SMA thrombectomy.
Institutional COVID-19 precautions and guidelines were implemented during intubation and operation with appropriate personal protective equipment and gear. A midline incision was made from the xiphoid to the pubic symphysis. The abdomen was entered and explored. Upon exploration, a short segment of distal ileum seemed to be necrotic and not viable. The rest of small bowel seemed to be viable. The SMA did not have a palpable pulse. The SMA was dissected at the root of mesentery. After proximal and distal control was obtained, a small transverse arteriotomy was made and thrombectomy was performed with multiple passes of a #3 Fogarty catheter. The proximal thrombus was removed, and pulsatile flow was established. The specimen was sent to pathology. Distal thrombectomy was performed with a #2 Fogarty catheter and good back bleeding verified. The arteriotomy was then closed with interrupted 7-0 Prolene sutures. After release of the clamps, there were palpable pulses on the SMA proximal and distal to the arteriotomy. Doppler signals were present on both the mesenteric and the antimesenteric border of duodenum, jejunum, and proximal ileum.
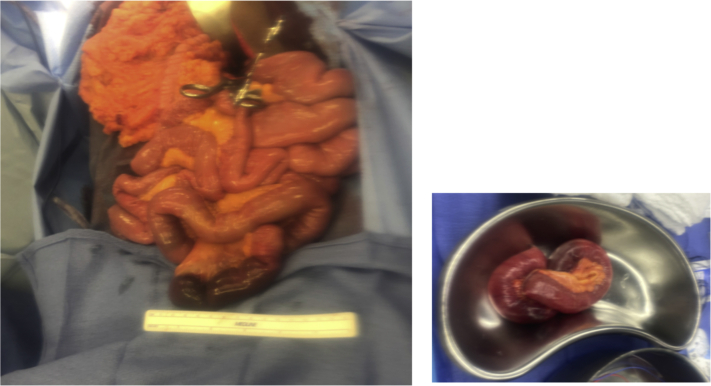
The distal ileum seemed to be necrotic and not viable (Fig 2); therefore, a small bowel resection was performed with primary end-to-end anastomosis. The abdomen was then closed. The patient tolerated the procedure well and was extubated at the end of the procedure. Postoperatively, the patient was continued on a heparin drip and then transitioned to therapeutic enoxaparin on postoperative day 3. The pathology report showed evidence of acute arterial thrombus. His respiratory status remained stable during the hospital course. Oral intake was initiated slowly after bowel function was restored on postoperative day 3. He continued to improve and was discharged home on anticoagulation with therapeutic enoxaparin for 3 months. After the patient was discharged, he was evaluated for hypercoagulability. The preliminary results have been negative to the time of this writing. The patient 3-month follow-up visit showed no major complications.
Fig 2.
A and B, Distal ilium necrosis requiring bowel resection.
Discussion
The NCP pandemic continues to spread worldwide with significant morbidity and mortality in patients with comorbodities.6,7 There have been several reports indicating increased coagulation in these patients.1, 2, 3 Thrombotic events include autopsy-proven microvascular thrombosis in a variety of vascular beds (pulmonary, hepatic, and renal),8,9 likely contributing to end-organ function deterioration, as well as large vessel thrombosis such as extensive deep vein thromboses (DVT) or even arterial thromboses resulting in stroke, myocardial infarction, or lower extremity ischemia in otherwise low-risk patients. In a recently published study from China, it was reported that 25% of all patients with COVID-19 infection admitted in the ICU developed an acute DVT and that was associated with poor prognosis.2 It has also been reported that patients with active COVID-19 infection can manifest antiphospholipid antibodies, which may also contribute to hypercoagulopathy and thrombotic microangiopathy.1 Elevated d-dimer levels has been documented in hospitalized patients with COVID-19 and there are early reports that have linked higher d-dimer levels to worse outcomes.4,10,11
Tang et al5 reported 449 patients with severe COVID-19; 99 were treated with low-molecular-weight heparin. They reported that anticoagulant therapy seems to be associated with better outcomes in severe COVID-19 with markedly elevated d-dimer. Whether the increase in d-dimer level reflects a more severe prothrombotic state or is the result of a more intense inflammatory response (likely both) is not clear at this point. Although this prothrombotic state in COVID-19 patients is documented and widely accepted, a consensus on if and when patients should receive anticoagulation, what type, and for how long has not been reached. It is not surprising, therefore, that across geographical areas and institutions a wide spectrum of approaches to this issue are reported, ranging from prophylactic DVT regimens for all hospital admitted COVID-19 patients to therapeutic anticoagulation for all. Moreover, we were not able to find any reports related to posthospitalization thrombotic events. After this patient, we identified at least four previously hospitalized COVID-19 patients who returned to the ED within 7 days of discharge with symptomatic acute lower extremity DVT. Although the patient we report was treated with prophylactic anticoagulation during his initial hospital stay, he developed acute SMA thrombus 1 week after discharge. This result suggests that the prothrombotic state in some patients with COVID-19 may continue well past the acute symptomatic phase.
In our institution, we have an anticoagulation protocol for the patient with COVID-19 infection based on d-dimer levels. Patients with high d-dimer levels or proven thrombotic complications are placed on therapeutic anticoagulation with heparin unless otherwise contraindicated. Therapeutic anticoagulation continues until the patient is discharged and is extended in the posthospitalization period. Patients, however, who are only placed on prophylactic doses of low-molecular-weight heparin during their hospitalization are discharged with no thromboprophylaxis. It is conceivable that some of these patients may benefit from an outpatient course of thromboprophylaxis, but there are no data to support that supposition.
Future research is needed to better understand the role of coagulopathy and anticoagulation treatment in the management of patient with COVID-19 infection.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and Antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18:786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 11.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]