Abstract
Autoimmunity may be generated by a variety of factors by creating a hyper-stimulated state of the immune system. It had been established long ago that viruses are a substantial component of environmental factors that contribute to the production of autoimmune antibodies, as well as autoimmune diseases. Epstein-Barr virus (EBV), cytomegalovirus (CMV) and human immunodeficiency virus (HIV) are viruses that withhold these autoimmune abilities. In a similar manner, SARS-CoV-2 may be counted to similar manifestations, as numerous records demonstrating the likelihood of COVID-19 patients to develop multiple types of autoantibodies and autoimmune diseases. In this review, we focused on the association between COVID-19 and the immune system concerning the tendency of patients to develop over 15 separate types of autoantibodies and above 10 distinct autoimmune diseases. An additional autoimmunity manifestation may be one of the common initial symptoms in COVID-19 patients, anosmia, the complete loss of the ability to sense smell, and other olfactory alterations. We summarize current knowledge on principal mechanisms that may contribute to the development of autoimmunity in the disease: the ability of SARS-CoV-2 to hyper-stimulate the immune system, induce excessive neutrophil extracellular traps formation with neutrophil-associated cytokine responses and the molecular resemblance between self-components of the host and the virus. Additionally, we will examine COVID-19 potential risk on the new-onsets of autoimmune diseases, such as antiphospholipid syndrome, Guillain-Barré syndrome, Kawasaki disease and numerous others. It is of great importance to recognize those autoimmune manifestations of COVID-19 in order to properly cope with their outcomes in the ongoing pandemic and the long-term post-pandemic period. Lastly, an effective vaccine against SARS-CoV-2 may be the best solution in dealing with the ongoing pandemic. We will discuss the new messenger RNA vaccination strategy with an emphasis on autoimmunity implications.
Keywords: COVID-19, SARS-CoV-2, Autoantibodies, Autoimmune diseases, NETosis, Molecular mimicry
Abbreviations
Auto-antibodies
- LAC
Lupus anticoagulant
- ANA
Anti-nuclear antibodies
- C-ANCA
Cytoplasmic anti neutrophil cytoplasmic antibodies
- P-ANCA
Perinuclear anti-neutrophil cytoplasmic antibodies
- Anti-ß2 GPI
Anti-ß2-glycoprotein I
- Anti-CASPR 2
Contactin-associated protein 2
- Anti-CCP
Anti-cyclic citrullinated peptide
- Anti-ACE-2
Anti-angiotensin-converting enzyme 2
- IFNs
Type I interferons
- Anti-MuSK
Anti-muscle-specific kinase.
Auto-immune diseases
- GD
Graves' disease
- AIHA
Autoimmune hemolytic anemia
- PNC
Polyneuritis cranialis
- POTS
post orthostatic tachycardia syndrome
- SLE
Systemic lupus erythematosus
- APS
Antiphospholipid syndrome
- GBS
Guillain-Barré syndrome
- VA
Viral arthritis
- ITP
Immune thrombocytopenic purpura
- MFS
Miller Fisher syndrome
- KD
Kawasaki disease
- MG
Myasthenia Gravis
1. Introduction
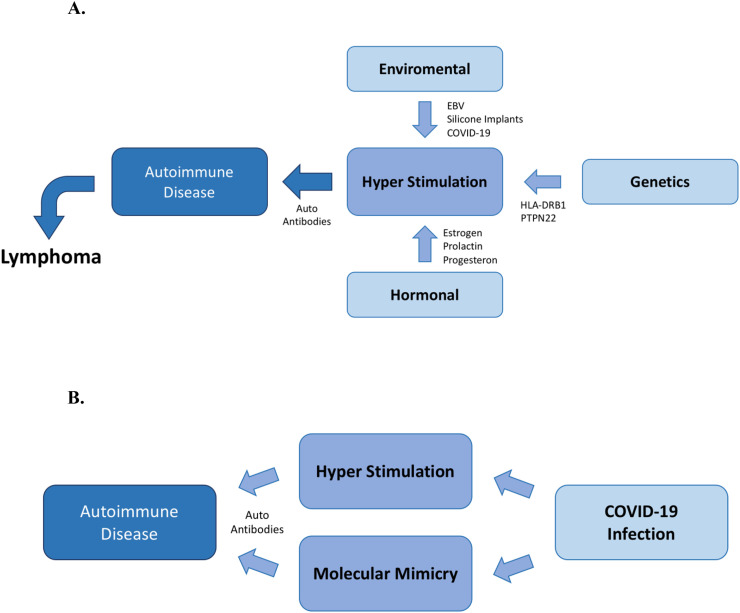
The onset of autoimmune diseases (AIDs) may be generated by a variety of factors through the creating a hyper-stimulated state of the immune system. It is accustomed to classifying factors that affect the immune system into three primary groups: genetical, environmental and hormonal [[1], [2], [3], [4]]. Viruses are a substantial component of the environmental factors that affect the immune system. Epstein-Barr virus (EBV), cytomegalovirus (CMV), human immunodeficiency virus (HIV) and human T lymphotropic virus 1 (HTLV-1) are examples of viruses with an established association to multiple AIDs [[5], [6], [7], [8], [9]]. The autoimmune influence of these viruses is not atypical, there are many other viruses that are also associated with AIDs [10]. The combination of a genetically predisposed individual with a hyper-stimulated state of the immune system may trigger an AID, and eventually lymphoma might develop as a consequence [4,11] (Fig. 1A).
Fig. 1.
A. Hyper-Stimulation of the immune system leading to autoimmune diseases and lymphoma. Three primary groups of factors, genetic, environmental and hormonal factors can lead to hyper-stimulation of the immune system when varying from their normal physiological effect. These factors may contribute to the development of autoantibodies, AIDs and even lymphoma. B. COVID-19 leading to Autoimmune Diseases. The SARS-CoV-2 may lead to AIDs though an additional mechanism, that of molecular mimicry with human self-components [[1], [2], [3], [4],12].
The ongoing pandemic of coronavirus disease 2019 (COVID-19) that first was identified in December 2019 in Wuhan, China, is induced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 had spread to numerous countries with roughly 107 million confirmed cases including 2.3 million deaths up to February 2021.
SARS-CoV-2 is using angiotensin-converting enzyme-2 (ACE-2) and the transmembrane serine protease-2 (TMPRSS2) as receptors, which are expressed on type 2 pneumocytes and many other cell types, in order to fuse the envelope with the cell membrane and penetrates the cells [12,13]. Thus ACE-2 and TMPRSS-2 are crucial viral fusion proteins of the SARS-CoV-2. ACE-2 is also widely expressed on endothelial cells and acts as a major constituent in the maintenance of vascular homeostasis [14]. Furthermore, SARS-CoV-2 downregulates ACE-2 in targeted cells, which leads to the excess generation of angiotensin II, an active metabolite that promotes inflammation, vasoconstriction, cell proliferation, and vascular leakage and eventually, pulmonary fibrosis [12]. These properties of SARS-CoV-2 contribute to the development of acute respiratory distress syndrome (ARDS) and as a result may lead to lung failure, as seen among many severely-ill patients [14].
Nowadays, cumulative evidence implicates that SARS-CoV-2 has the ability to induce hyper-stimulation of the immune system, therefore leading to the synthesis of multiple autoantibodies, with a trigger effect of, possibly pre-existing, AID [15]. These autoimmune responses may develop through two principal mechanisms known today: firstly, the ability of the virus to induce hyper-stimulation of the immune system, secondly, the molecular resemblance between the virus and self-components of the host. (Fig. 1B).
2. Hyper-stimulation of the immune system by the SARS-CoV-2
The ability of SARS-CoV-2 to induce a hyper-stimulated state of the immune system was acknowledged at the beginning of the pandemic [14,15]. COVID-19 is associated with changes in circulating leukocyte subsets and an extensive increase in the concentration of pro-inflammatory cytokines in sera that occurs in mild to severe form of the disease, particularly interleukin (IL) 6, IL-1β, IL-10, IL-17, TNF, GM-CSF, also referred to as ‘cytokine storm’ or ‘cytokine release syndrome’ [16]. Studies also show that COVID-19 non-survivors compared to survivors have higher levels of ferritin (hyperferritinemia) and pro-inflammatory cytokines [15,17,18]. Certain clinical manifestations of patients were identified by physicians in various locations worldwide that indicated a hyper-stimulation involvement of the immune system, such as ARDS and haemophagocytic lymphohistiocytosis (HLH) in severely-ill patients [19]. ARDS and HLH are clinical syndromes characterized by an aggressive immune response, creating severe inflammation and damage to vital organs. ARDS may lead directly to respiratory failure, which was found to be the cause of death in 70% of severely-ill COVID-19 patients [20]. ARDS also has an overall mortality assessment of 39% in COVID-19 patients, with the highest mortality valuation of 69% in China, whereas the lowest estimate, of 13%, was found in Germany [21]. The clinical conditions and laboratory tests described are confirming the speculation that the hyper-stimulated state of the immune system is a key element in the severity of illness and mortality of patients (Fig. 1B).
3. Molecular mimicry between SARS-CoV-2 and humans
In parallel to the ability of the virus to induce hyper-stimulation of the immune system, recent findings pointed out a homology of primary sequence between humans and components of SARS-CoV-2 [22]. In contrast, this homology was not found in mammals unaffected by SARS-CoV-2 [22]. As the acquired immune system produces antibodies cross-reacting with common molecules among pathogens and self-components, molecular mimicry readily contributes to the production of autoantibodies that possibly result in the new onset of an AID. In this regard, Table 1 documents a list of heptapeptides, the linear sequence of which is shared between SARS-CoV-2 and the human proteome with high pathological potential. Indeed, the viral versus human peptide overlaps involve human proteins that, if altered, mutated, deficient, or improperly functioning, can lead to severe pathologies. Examples are: cerebellum-2, alterations of which associate with MS [23]; follistatin-related protein 1 that protects against hypoxia-induced pulmonary hypertension [24]; and the protein solute carrier family 12 member 6, alterations of which may associate with areflexia and severe progressive neuropathy often accompanied by psychiatric symptoms and olfactory receptor 7D4, which is specific for smell [25,26]. These results correlate with the long-standing claim that identity of sequences between self- and viral proteins display a potential major role in the pathophysiology of AIDs [27]. In addition to the remarkable results shown in Table 1 identified by using linear sequences of 7 contiguous residues (7-mer), other possible identities may occur when the self- and viral proteins are folded in the secondary and tertiary structure. (See Table 1)
Table 1.
List and short description of 34 human proteins that share heptapeptides with SARS-CoV-2.
| Shared 7-mer | Human proteins sharing heptapeptides with SARS-CoV-2⁎ |
|---|---|
| SSRSSSR | Abl interactor 2 |
| ALALLLL | Insulin-like growth factor-binding protein complex acid labile subunit |
| ALALLLL | Cerebellin-2 |
| LLSAGIF | UPF0600 protein C5orf51 |
| SSRSSSR | CLK4-associating serine/arginine rich protein |
| RGQGVPI | Putative uncharacterized protein encoded by the long intergenic non-protein coding RNA 346 |
| ALALLLL | Cytochrome P450 2S1 |
| ALALLLL | Delta and Notch-like epidermal growth factor-related receptor |
| GLTVLPP | FH1/FH2 domain-containing protein 3 |
| LDKYFKN | Follistatin-related protein 1 |
| RQLLFVV | Guanosine triphosphate-binding protein 10 |
| IGAGICA | Hepatitis A virus cellular receptor 2 |
| SSRSSSR | Hornerin |
| LFAAETL | Tyrosine-protein kinase ITK/TSK |
| LASFSAS | Maltase-glucoamylase, intestinal |
| LIRAAEI | Unconventional myosin-XVIIIa |
| QRMLLEK | Unconventional myosin-Vc |
| TGRLQSL | Neuron navigator 3 |
| LIMLIIF | Sodium/potassium/calcium exchanger 2 |
| IIFWFSL | Olfactory receptor 7D4 |
| SLLSVLL | Orosomucoid 1-like protein 2 |
| SSRSSSR | Oxysterol-binding protein-related protein 10 |
| SSRSSSR | Pleckstrin homology domain-containing family G member 2 |
| SRGGSQA | Ras-associating and dilute domain-containing protein |
| SSRSSSR | Solute carrier family 12 member 6 |
| VLQLPQG | Prestin |
| AEGSRGG | snRNA-activating protein complex subunit 3 |
| ALALLLL | Translocon-associated protein subunit delta |
| IVDTVSA | Alanine-tRNA ligase, mitochondrial |
| NASVVNI | Thyroid adenoma-associated protein |
| ALALLLL | Thrombospondin-3 |
| LDDFVEI | Wolframin |
| SSRSSSR | Zinc finger CCCH domain-containing protein 18 |
| SSRSSSR | Zinc finger Ran-binding domain-containing protein 2 |
Human proteins sharing heptapeptides with SARS-CoV-2 are given by UniProt name. Details on function/ associated diseases, and references at www.uniprot.org.
4. Neutrophils extracellular traps and SARS-CoV-2 infection: another link with autoimmune responses
Neutrophil extracellular traps (NET) activation and release, or NETosis, is a dynamic process that plays a critical role in innate immunity. It represents a beneficial antimicrobial mechanism of neutrophils, which intervenes by trapping and killing invading pathogens while minimizing damage to the host cells. NETs are networks of extracellular fibers, primarily composed of DNA and chromatin that are expelled from neutrophils and bind pathogens. However, NETs can also serve a source of self-antigens resulting in autoimmune conditions. Thus, excessive NET formation has been involved in the autoinflammatory response in SLE, RA, myositis and MS, for example [[28], [29], [30]]. NET-derived neutrophil proteases, such as elastase, may cause the release of peptidylarginine deiminases (PADs) that enhance citrullination of self-proteins (e.g. histones, cartilage proteins, others), rendering them autoreactive and promoting pathogenic inflammatory cascade in these autoinflammatory diseases. NET formation has also been associated with thrombosis in antiphospholipid syndrome [31]. It is thus thought that excessive NETosis is implicated in early vascular ageing and increased risk of cardiovascular disease, a severe complication of SLE. Autoantibodies to NETs have been claimed to represent potential serological biomarkers in RA [32].
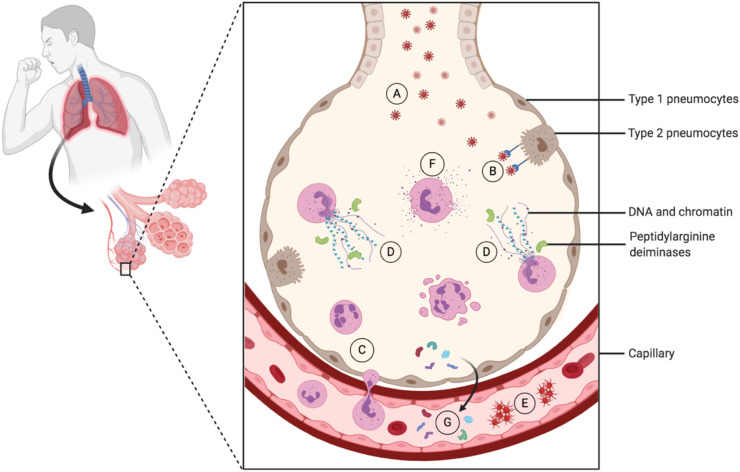
Excessive NET formation and neutrophil-associated cytokine responses have also been associated with SARS-CoV-2 pathogenesis [33]. Numerous clinical reports indicate a progressive rise in neutrophilia in SARS-CoV-2-infected non-survivors compared to survivors [34,35]. Activated neutrophils undergo degranulation and release NETs, which deliver their content in chromatin, DNA and histones, as well as toxic enzymes and proteases, which exacerbate lung tissue damage and may directly cause the lethal complications of COVID-19 (Fig. 2 ). Coagulation dysfunction and widespread thromboses have been observed in adverse outcomes of the SARS-CoV-2-infection [[36], [37], [38], [39], [40]] that resembles what has long-been revealed in lupus patients.
Fig. 2.
COVID-19 and NETosis. SARS-CoV-2 viral particles invade the alveoli in the lung where they bind type 2 pneumocytes via angiotensin-converting enzyme 2 (ACE2), which is also present on the surface of many other cell types. As a result of the infection, neutrophils transmigrate into the alveoli, where NETosis is activated leading to release of decondensed chromatin (and other nuclear, possibly modified, components) and granular contents to the extracellular space. This figure was created using BioRender (https://biorender.com/).
A – SARS-CoV-2 invading the alveoli.
B – SARS-CoV-2 binding to the angiotensin-converting enzyme 2 of the type 2 pneumocytes.
C – Neutrophil transmigrating to the alveoli.
D – Neutrophil extracellular traps activation and release (NETosis).
E – Enhancement of platelet aggregation induced by NETosis.
F – Neutrophil cytokines and proteases degranulation.
G – Modification of self-proteins in the citrullination induced by peptidylarginine deiminases.
These findings led to the conclusion that there is a crucial necessity to prevent excessive neutrophil recruitment, activation, degranulation and NET release, and control coagulation (i.e. “lupus” anticoagulant) in SARS-CoV-2-infected patients [40,41]. A few drugs might give some promise in this line of therapeutic development. It is the case, for example, of the autophagy regulator peptide P140, which inhibits NET formation [42] and also shows efficacy without toxicity among lupus patients [43].
5. Autoantibodies in COVID-19-infected patients
It had been established long ago that many viruses trigger an autoimmune response, a phenomenon that includes both the production of autoimmune antibodies, as well as AIDs. For instance, HIV, HTLV-I and hepatitis C virus infections contribute to the formation of IgG autoantibodies, such as anti-Ro52, anti-Ro60, anti-nuclear antibodies, anti-double-stranded DNA, synthetic peptides of ubiquitinated histone H2A and H4, anti-Sm-D and many more. The SARS-CoV-2 may be countable to similar manifestations, as numerous records demonstrating the tendency of COVID-19 patients to develop multiple types of autoantibodies.
An important group of antibodies are the three principle antiphospholipid antibodies (APLA) associated with anti-phospholipid syndrome (APS): anticardiolipin (aCL), lupus anticoagulant (LAC) and beta2 glycoprotein I (β2GPI) [44]. These antibodies bind to proteins on the cell membrane leading to coagulation dysfunction. As COVID-19 patients with severe illness are seen to produce blood clots that damage various organs, as mentioned earlier, it was found that many of them carry APLA [39,45]. It was found that 31 out of 66 (47%) severely-ill SARS-CoV-2-infected patients had produced β2GPI or/and aCL circulating autoantibodies [46]. Additionally, patients with severe COVID-19 had significantly higher aCL autoantibody levels than patients with moderate disease [47]. Evidence show also high concentrations of LAC among COVID-19 patients enduring coagulation disorders [48]. Though, there is a well-established link between LAC and common inflammation indices [49]. Due to the acute inflammation COVID-19 patients present, there is a possibility that a high concentration of LAC is caused by the inflammatory response, and not as a direct outcome of SARS-CoV-2. Phosphatidylserine/prothrombin (aPS/PT) autoantibodies are also associated with higher prevalence of thrombotic events, and usually found in some APLA carriers [50]. A study that included 172 hospitalized patients with SARS-CoV-2-infection reported that 24% carried aPS/PT IgG [51]. Additionally, anti-heparin-PF4 (aPF4), a platelet-activating antibody that is used as a marker for heparin-induced thrombocytopenia (HIT), were identified in severely-ill COVID-19 patients who ordeal HIT. In some patients aPF4 had been recognized without a pre-exposure to heparin, thus strengthening the hypothesis that SARS-CoV-2 has the ability cause coagulation disorders though an autoimmune mechanism, particularly in severely-ill patients [52,53].
A recent study showed that 101 of 987 patients (10.2%) with life-threatening COVID-19 pneumonia had neutralizing autoantibodies against type I interferons (IFNs), in contrast to individuals with asymptomatic or mild SARS-CoV-2 infection that these autoantibodies were absent [54]. IFNs are a large subtype of cytokines that are crucial for adequate regulation of the immune response, thus autoantibodies against them may, in some individuals, contribute to the development of severe COVID-19. Furthermore, out of the 101 patients that carried IFNs neutralizing autoantibodies 94% were men, providing an explanation for the higher prevalence of mortality and severe disease in men [54].
Noteworthy to point out a report that inspected the presents of multiple autoantibodies in 29 severely-ill COVID-19 patients, randomly chosen with no history of AID, that found antinuclear antibodies (ANA), antibodies to β2GPI, aCL, p-ANCA and to c-ANCA in 34.5%, 34.5%, 24.1%, 6.9% and 6.9% of the patients, respectively [55]. p-ANCA and c-ANCA autoantibodies are frequently detected among patients with autoimmune vasculitis, which is seen in severely-ill patients and will be discussed later.
Importantly, anti-Ro52 and anti-Ro60 antibodies were discovered in severely-ill COVID-19 patients, with the prevalence of 20% and 25% respectively [56]. These autoimmune antibodies are linked to certain autoimmune disorders, such as SLE, subacute cutaneous LE (SCLE), neonatal lupus and primary biliary cirrhosis [57].
Lastly, a study that categorized SARS-CoV-2-infected patients based on C-reactive protein (CRP) concentration, a general marker for inflammation, found that more patients with high CRP produced ANA in comparison to patients with low CRP. Moreover, the titer of the ANA was significantly higher in patients with high CRP, reaching up to 1:640, which is considered a significant titer. Rheumatoid factor (RF) autoantibody was also measured and was only detected only among patients with high CRP [58].
Although some of the studies presented include only a few dozen patients, their results may illustrate that the COVID-19 is not merely a reviler of pre-existing state, but a significant magnitude of autoimmunity. It is noteworthy that additional studies identified several more autoantibodies, such as contactin-associated protein 2 (anti-CASPR 2) [59], anti-cyclic citrullinated peptide (anti-CCP) [55] and anti-annexin-V [60] (See Table 2A ).
Table 2A.
List of the autoimmune antibodies described in the article with the relevant citation.
| Number | Antibody | Citation |
|---|---|---|
| 1 | LAC | 44, 48 |
| 2 | Anti-ß2 GPI | 44, 46, 55 |
| 3 | Anti-cardiolipin | 44, 46, 47, 55 |
| 4 | Anti-PS/PT | 51 |
| 5 | Anti-Heparin PF4 | 52, 53 |
| 6 | IFNs | 54 |
| 7 | ANA | 55, 58 |
| 8 | C-ANCA | 58 |
| 9 | P-ANCA | 58 |
| 10 | Anti-Ro60 | 56 |
| 11 | Anti-Ro52 | 56 |
| 12 | RF | 58 |
| 13 | Anti-CASPR 2 | 59 |
| 14 | Anti-CCP | 57 |
| 15 | Anti-Annexin V | 60 |
| 16 | Anti-ACE-2 | 74 |
| 17 | Anti-MuSK | 94 |
All the autoantibodies described were identified mostly in severely-ill patients in comparison with those with mild or moderate disease. These findings are consistent with the claim that SARS-CoV-2 has the ability to hyper-stimulate the immune system, as discussed above. The development of autoantibodies has a significant clinical importance considering that a substantial portion of patients displays pathogenic properties. Furthermore, systemic autoimmunity is known to arise from generalized polyclonal B cell activation, thus the existence of autoantibodies in patients may indicate a pre-AID [4,61,62].
6. Autoimmune diseases in COVID-19-infected patients
Alongside the evidence presented regarding the ability of SARS-CoV-2 to initiate a hyper-stimulated state of the immune system, leading to the synthesis of autoantibodies, there is also evidence for new-onsets of AIDs among patients with the infection.
It had been suggested that COVID-19 has an association with the immune-mediated neuropathy Gillian-Barré syndrome (GBS). In August 2020, about 31 documented cases of GBS that followed a SARS-CoV-2-infection were reported, since then, even more cases of the disease are disclosed [[63], [64], [65]]. GBS is characterized by damage to the myelin sheath of peripheral nerve cells. Multiple viruses are already known to be linked to the development of GBS, thus it may be less surprising that COVID-19 may be an additional origin [[63], [64], [65], [66]]. Likewise, acute onset of Miller Fisher syndrome (MFS) and Polyneuritis cranialis (PNC), rare variants of GBS, were also described in COVID-19 patients [67,68].
Autoimmune endocrine diseases had also been described, as evidence accumulates mostly regarding an autoimmune thyroiditis disorder. A recent study that included 191 individuals with COVID-19-infection had shown abnormalities in thyroid function of 13.1% [69]. Furthermore, case reports of Graves' disease after COVID-19 infection had been described, as well as atypical thyroiditis with characteristic features of autoimmune thyroiditis [70,71].
ACE-2, a crucial viral fusion protein of SARS-CoV-2 discussed earlier, is widely expressed by vascular endothelial cells [12,72]. Therefore, it had been proposed that SARS-CoV-2 invades the vascular endothelium, causing endothelial damage and vasculitis [73]. A recent study showed the presents of anti-ACE-2 IgM antibodies in 27% of severely-ill patients, in comparison with 3.8% among patients who were not ventilated, thus some argue that vascular damage may occur also as a result of T-independent immune response toward the antibodies in severely-ill patients [74].
COVID-19 often has a mild course among children in comparison to adults [75]. Nevertheless, recent evidence demonstrates autoimmune disorders triggered by COVID-19 in children as well. For instance, Kawasaki disease (KD) is an immunologic reaction that presents as an acute, self-limited vasculitis, that mainly occurs in children younger than 5 years of age [76]. Cases of SARS-CoV-2-infection followed by an acute onset of KD were documented worldwide, described in 36 different articles, reporting the sum of 320 children. [77]. Additionally, recent studies had shown an increment of new-onset diabetes type 1 in healthcare centers during the current pandemic, as well as case reports of SARS-CoV-2-infection followed by new-onset of diabetes type 1 in children [[78], [79], [80]].
Autoimmune hemolytic anemia (AIHA) is a relatively rare disease that is characterized by autoantibodies targeting erythrocytes causing hemolysis [81]. Articles had been published describing AIHA onset after SARS-CoV-2-infection, with both warm and cold IgG, thus enhancing the possibility that antibodies directed toward SARS-CoV-2 were acting also as AIHA autoantibodies to a specific protein on the surface of erythrocytes [[81], [82], [83], [84]]. As discussed, molecular mimicry might be at the root of severe COVID-19 and contribute specifically also to the onset of AIHA in those patients. In fact, it has to be underlined that the potential risk of cross-reactivity between SARS-CoV-2 and human proteins is much higher when considering that a pentapeptide represents the minimal immune determinant unit [85]. Therefore, if one analyzes the viral versus human commonalities at the 5-mer level, the extent of the peptide sharing would increase exponentially by two orders of magnitude and involve a highest number of human proteins. As regards AIHA, the Ankyrin-1 (ANK1) protein, which can be found on the erythrocyte membrane, has a putative 5-mer immunogenic epitope (amino acid residues LLLQY) in common with SARS-CoV-2 spike protein, thus supporting the possibility that molecular mimicry may influence AIHA onset in SARS-CoV-2-infected patients [86]. An additional humoral-related autoimmune response triggered by SARS-CoV-2-infection, which was reported in multiple case reports, is immune thrombocytopenic purpura (ITP) [87,88]. ITP is characterized by a reduction of platelets in the blood, leading to coagulation dysfunction. Other studies suggest additional autoimmune-related disorders that have an association with SARS-CoV-2 infection, such as SLE [89,90], post orthostatic tachycardia syndrome (POTS) [91], viral arthritis (VA) [92,93], myasthenia gravis [94] and others (See Table 2B ).
Table 2B.
List of the autoimmune diseases described in the article with the relevant citation.
| Number | Autoimmune Disease | Citation |
|---|---|---|
| 1 | Antiphospholipid syndrome | 44, 46, 47, 55 |
| 2 | Guillain-Barré syndrome | 63–65 |
| 3 | Miller Fisher syndrome | 67, 68 |
| 4 | Polyneuritis cranialis | 68 |
| 5 | Thyroid function | 69, 71 |
| 6 | Graves' disease | 70 |
| 7 | Vasculitis | 73 |
| 8 | Kawasaki disease | 77 |
| 9 | Type 1 Diabetes | 78–80 |
| 10 | Autoimmune hemolytic anemia | 81–84 |
| 11 | Immune thrombocytopenic purpura | 87, 88 |
| 12 | Systemic lupus erythematosus | 89, 90 |
| 13 | Post orthostatic tachycardia syndrome | 91 |
| 14 | Viral arthritis | 92, 93 |
| 15 | Myasthenia gravis | 94 |
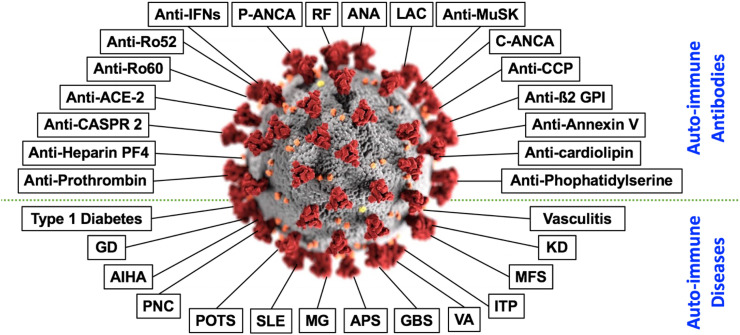
The autoimmune disorders discussed may occur as a result of an aberrant immune response toward SARS-CoV-2. Most of the findings were published in the literature only as case reports, and therefore it is necessary to further investigate the subject in order to assess the prevalence of the phenomenon and its implication. Nevertheless, we should take into consideration that many AIDs may break out only after years of the onset of autoantibody formation [95]. Hence, there is a possibility that the incidence of AIDs as a result of SARS-CoV-2-infection will significantly increase in the time to come (Fig 3 ).
Fig. 3.
In the center appears the SARS-CoV-2. Around it, at the upper part of the figure, appear autoantibodies linked to the SARS-CoV-2-infection. At the bottom part of the figure, appear autoimmune diseases linked to the SARS-CoV-2-infection [20,[22], [23], [24], [25],[27], [28], [29], [30], [31], [32], [33], [34], [35]].
7. Olfactory manifestations in COVID-19-infected patients
One of the common initial symptoms in COVID-19 patients is anosmia, the complete loss of the ability to sense smell, and other olfactory alterations [[96], [97], [98], [99], [100]]. These manifestations had been described in patients from the broad spectrum of mild to critically severe COVID-19 illness and surprisingly, even in individuals with no respiratory clinical presentation at all [101,102]. Early in the pandemic, a study performed in London reported 2428 patients with new-onset of anosmia, being at 17% an isolated symptom and in 51% related to other COVID-19 clinical manifestations, such as fever or cough [103]. In addition, almost 25% of 202 COVID-19 subjects of an Italian study reported olfactory changes as the first or only symptom during the disease course [104]. Indeed, in an American study, near 75% of 237 COVID-19-confirmed patients presented with anosmia, some of them even prior to diagnosis [98]. When comparing 60 COVID-19 patients to 60 matched for gender and age controls, by applying quantitative smell testing, a much higher incidence of olfactory dysfunction, 98% of the overall incidence, were upon the infected population, while more than 50% of them were classified with severe hyposmia or anosmia [105].
Olfactory symptoms following COVID-19 infection are already considered as a known symptom of the disease and in many countries as an indication for self-isolation, but the exact mechanism through which SARS-Co-2 leads to hyposmia/anosmia is still not well-defined. Different hypotheses had been raised [106].
The ACE-2 receptor, crucial viral fusion proteins of the SARS-CoV-2 and abundantly seen in the nasal mucosa, is known to take on a part in the inflammatory response in the respiratory system, such as partly controlling the bradykinin levels [107,108]. Since the olfactory symptoms of COVID-19 are usually not associated with rhinitis as in other respiratory virus infections, it is reasonable to conceive that the symptom is not induced by local inflammation and congestion, but instead by some level of damage of the olfactory pathways [96,97,109]. In fact, when infecting transgenic mice for the human ACE-2 receptor with the SARS-CoV-1, there was no local inflammation in the nasal tract that could explain the olfactory findings [110].
It has been indicated that neuronal death could be caused as a result of the increased pro-inflammatory cytokines, referred to as a cytokine storm, especially IL-6 [110,111]. On the other hand, the fact that COVID-19 patients usually regain the olfactory function after some weeks and that other neurologic symptoms are not common in the course of the disease, do not corroborate with the neuronal definitive damage hypothesis [[94], [95], [96], [97], [98],112,113].
Non-neural cells that have a role in the olfaction function and express ACE-2 receptors were also proposed to be responsible for the olfactory symptoms following the infection. Some of those cells include olfactory epithelium sustentacular cells, microvillar cells, Bowman's gland cells, horizontal basal cells and olfactory bulb pericytes [114]. Indeed, all those cell types express 2 genes that are essential for the SARS-CoV-2 entry and that are not found in olfactory sensorial neurons [114].
Moreover, the immune response was already associated with olfactory changes in other diseases, most of them being autoimmune diseases, such as SLE, Myasthenia Gravis and systemic sclerosis [[115], [116], [117], [118]]. For example, olfaction changes were shown to be more common in SLE patients than in control groups [119]. Moreover, olfaction manifestations had been linked to the disease activity level, with a higher incidence in active SLE patients, and, interestingly, in patients positive for anti-ribosomal P autoantibody, a specific marker of SLE [120,121].
In fact, the nose and the immune system share some mutual characteristics [122]: both have to differentiate the self to non-self-molecules and depend on the major histocompatibility complex (MHC). In animal models, olfactory bulbectomy led to an alteration in the cellular immunity, such as reduced neutrophil phagocytosis and lymphocyte mitogenesis, and increased leukocyte aggregation, monocyte phagocytosis and acute-phase-reaction proteins, suggesting a direct association between smell and immune-mediated process [123].
Inflammatory cytokines, such as IL-1, play a role both in the immune and in the nervous system. In animal models, receptors for this cytokine were shown to be moderately present in the primary olfactory cortex and highly seen in the olfactory bulb [124], indicating a role of IL-1 in the olfaction and possibly explaining why an immune imbalance could contribute to dysfunction in sensation.
COVID-19 had been described together with other autoimmune conditions, as the synthesis of various autoantibodies, Kawasaki disease, anti-phospholipid syndrome and Guillain-Barre syndrome [66,125,126]. Since smell loss has been described and linked to many autoimmune conditions [115], it is possible that hyposmia/anosmia in COVID-19 patients may be induced, at least partly, by autoimmune mechanisms.
8. Vaccination against SARS-CoV-2
An effective vaccine against SARS-CoV-2 may be the best solution in dealing with the ongoing pandemic. Moderna and Pfizer/BioNTech had developed two of the leading vaccines against the virus and conducted a successful phase 3 trial that demonstrated 1 to be safe and effective, thus the United States Food and Drug Administration (FDA) had recently approved their use. The phase 3 trials done by Moderna and Pfizer/BioNTech was performed as a randomized, placebo-controlled study that included 30,000 and 41,135 subjects and showed an efficacy rate of 94.1% and 95% (p < 0.0001), respectively. Furthermore, the studies had shown an instrumental reduction of vaccinated individuals illness severity in comparison to the control group. Following the encouraging results of the phase 3 trial and the approval of the FDA, major nations have started administrating massive vaccination of citizens, including the United Kingdom, the United States and Canada, with many more countries declaring their intentions to soon start massive vaccination as well.
Both vaccines of Moderna and Pfizer/BioNTech put to use a new messenger RNA (mRNA) strategy that had been studied for years. In this type of vaccination, artificial mRNA is injected into the deltoid muscle, in the upper part of the arm, thus generates the synthesis of specific viral proteins by health cells of the tissue. Importantly, these viral proteins do not cause COVID-19, for they are merely individual proteins identical to viral proteins and not complete SARS-CoV-2 viral particles. The concentration of viral protein reaches a peak in 24 to 48 h, then declines, as a result of the mRNA molecule breakdown [127]. Next, cells display synthesized viral protein fragments on its surface, introducing the viral components to the immune system. synthesized viral protein fragments are being through the inflammatory syndrome induced by adjuvants (ASIA) [128]. Positively, the mRNA vaccines of Moderna and Pfizer/BioNTech do not include adjuvants of any sort, thus decreasing the probability for any unwanted immune modulation.
9. Final comments
In a similar manner to many viruses, such as EBV, CMV, HIV and HTLV-1, SARS-CoV-2 may have the ability to contribute to autoimmunity. Numerous records demonstrate the likelihood of COVID-19 patients to develop over 15 separate types of autoantibodies along with above 10 distinct AIDs. The most probable mechanisms that we believe to have the capability of contributing to the development of autoimmunity in COVID-19 are the ability of SARS-CoV-2 to hyper-stimulate the immune system, induce excessive NETosis formation with neutrophil-associated cytokine responses and the molecular resemblance between self-components of the host and the virus. It is of great importance to recognize those autoimmune manifestations of COVID-19 in order to properly cope with their outcomes in the ongoing pandemic and the long-term post-pandemic period. Furthermore, understanding the underlying molecular mechanisms of other viruses that are known to stimulate an autoimmune reaction may be crucial for a better understanding of the COVID-19 pathophysiology.
Additionally, following the encouraging results of the phase 3 trial and the approval of the FDA of the newly developed Pfizer/BioNTech vaccine, massive vaccinations against the SARS-CoV-2 has already started in multiple regions in the world in order to cope with the ongoing pandemic. As hundreds of million individuals are expected to be vaccinated in 2021, we hope new side effects would not appear, nonetheless, extensive supervision must exist on the matter, in the same way, that is done to all newly administrated drugs. As previously discussed, molecular mimicry exists between SARS-CoV-2 and human components, therefore if autoimmune manifestations will start appearing in vaccinated individuals, we believe that mRNA sequences coding for peptides shared with humans should be removed from vaccines. Vaccination strategy should also be considered in order to decrease the danger of the COVID-19 infection in the most susceptible individuals, along with the autoimmune risks of severe illness.
Author contributions
Y. S. had conceived the idea for this manuscript. A.D. took the lead in writing the manuscript supported by S.M., D.K. and P·D that contributed critical sections to the manuscript. G.H., S.M, D.K. and Y.S. had provided critical feedback in the drafting process.
Declaration of Competing Interest
None.
Acknowledgements
This work is supported by the grant of the Government of the Russian Federation for the state support of scientific research carried out under the supervision of leading scientists, agreement 14.W03.31.0009.
References
- 1.Shoenfeld Y., Gilburd B., Abu-Shakra M., et al. The mosaic of autoimmunity: genetic factors involved in autoimmune diseases. Isr. Med. Assoc. J. 2008;10(1):3. [PubMed] [Google Scholar]
- 2.Shoenfeld Y., Zandman-Goddard G., Stojanovich, et al. The mosaic of autoimmunity: hormonal and environmental factors involved in autoimmune diseases. Isr. Med. Assoc. J. 2008;10(1):8. [PubMed] [Google Scholar]
- 3.Shoenfeld Y., Blank M., Abu-Shakra M., et al. The mosaic of autoimmunity: prediction, autoantibodies, and therapy in autoimmune diseases. Isr. Med. Assoc. J. 2008;10(1):13. [PubMed] [Google Scholar]
- 4.Watad A., Bragazzi L., Amital H., Shoenfeld Y. Hyperstimulation of adaptive immunity as the common pathway for silicone breast implants, autoimmunity, and lymphoma of the breast. Isr. Med. Assoc. J. 2019;21(8):517–519. [PubMed] [Google Scholar]
- 5.Váróczy L., Gergely L., Zeher M., Szegedi G., Illes A. Malignant lymphoma-associated autoimmune diseases–a descriptive epidemiological study. Rheumatol. Int. 2002;22(6):233–237. doi: 10.1007/s00296-002-0229-4. [DOI] [PubMed] [Google Scholar]
- 6.Toussirot E., Roudier J. Epstein–Barr virus in autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 2008;22(5):883–896. doi: 10.1016/j.berh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Muller S., Richalet P., Laurent-Crawford A., et al. Autoantibodies typical of non-organ-specific autoimmune diseases in HIV-seropositive patients. Aids. 1992;6(9):933–942. doi: 10.1097/00002030-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Muller S., Boire G., Ossondo M., Ricchiuti V., Smadja D., Vernant J.C., et al. IgG autoantibody response in HTLV-I-infected patients. Clin. Immunol. Immunopathol. 1995;77(3):282–290. doi: 10.1006/clin.1995.1154. [DOI] [PubMed] [Google Scholar]
- 9.Miller F., Afonso P.V., Gessain A., Ceccaldi P.E. Blood-brain barrier and retroviral infections. Virulence. 2012;3(2):222–229. doi: 10.4161/viru.19697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barzilai O., Sherer Y., Ram M., Izhaky D., Anaya M., Shoenfeld Y. Epstein–Barr virus and cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann. N. Y. Acad. Sci. 2007;1108(1):567–577. doi: 10.1196/annals.1422.059. [DOI] [PubMed] [Google Scholar]
- 11.Smatti K., Cyprian S., Nasrallah K., Thani A., Almishal O., Viruses Yassine M. Autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi G., Sanga V., Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9 doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovren F., Pan Y., Quan A., et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295(4):H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenfeld M., Tincani A., Andreoli L., et al. Covid-19 and autoimmunity. Autoimmun. Rev. 2020 Dec;19(12):102695. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020 Jul;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez Y., Novelli L., Rojas M., et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114 doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ragab D., Salah E., Taeimah H., Khattab R., Salem R. The covid-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P., McAuley F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay M., Poh C., Rénia L., MacAry P., Ng L. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–2. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasan S., Capstick T., Ahmed R., Kow C., Mazhar F., Merchant H., et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14(11):1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanduc D., Shoenfeld Y. Molecular mimicry between sars-cov-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol. Res. 2020 Oct;68(5):310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baranzini S., Wang J., Gibson R., et al. Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum. Mol. Genet. 2009;18(4):767–778. doi: 10.1093/hmg/ddn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W., Wang W., Liu J., et al. Follistatin-like 1 protects against hypoxia-induced pulmonary hypertension in mice. Sci. Rep. 2017;7(1):1–4. doi: 10.1038/srep45820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degerliyurt A., Akgumus G., Caglar C., Bilguvar K., Caglayan A. A new patient with Andermann syndrome: an underdiagnosed clinical genetics entity? Genet. Couns. 2013;24(3):283. [PubMed] [Google Scholar]
- 26.Keller A., Zhuang H., Chi Q., Vosshall L.B., Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449(7161):468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- 27.Oldstone M. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12(13):1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller S., Radic M. Oxidation and mitochondrial origin of NET DNA in the pathogenesis of lupus. Nat. Med. 2016;22(2):126–127. doi: 10.1038/nm.4044. [DOI] [PubMed] [Google Scholar]
- 29.Apel F., Zychlinsky A., Kenny E. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 2018;14(8):467–475. doi: 10.1038/s41584-018-0039-z. [DOI] [PubMed] [Google Scholar]
- 30.Wigerblad G., Kaplan M. NETs spread ever wider in rheumatic diseases. Nat. Rev. Rheumatol. 2020;16(2):73–74. doi: 10.1038/s41584-019-0352-1. [DOI] [PubMed] [Google Scholar]
- 31.Ali R., Gandhi A., Meng H., et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019;10(1):1–2. doi: 10.1038/s41467-019-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bont C., Stokman M., Faas P., Thurlings R., Boelens W., Wright H., et al. Autoantibodies to neutrophil extracellular traps represent a potential serological biomarker in rheumatoid arthritis. J. Autoimmun. 2020 Sep 1;113 doi: 10.1016/j.jaut.2020.102484. [DOI] [PubMed] [Google Scholar]
- 33.Narasaraju T., Tang B., Herrmann M., Muller S., Chow V., Radic M. Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients with COVID-19. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomar B., Anders H., Desai J., Mulay S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9(6):1383. doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes B., Adrover J., Baxter-Stoltzfus A., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6):1. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colling M., Kanthi Y. COVID–19-associated coagulopathy: an exploration of mechanisms. Vasc. Med. 2020;25(5):471–478. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merrill J., Erkan D., Winakur J., James J. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat. Rev. Rheumatol. 2020:1–9. doi: 10.1038/s41584-020-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bateman R., Barthélemy N., Horie K. Another step forward in blood-based diagnostics for Alzheimer’s disease. Nat. Med. 2020:1–2. doi: 10.1038/s41591-020-0797-4. [DOI] [PubMed] [Google Scholar]
- 39.Zuo Y., Estes S., Gandhi A., et al. medRxiv. 2020. Prothrombotic antiphospholipid antibodies in COVID-19. [Google Scholar]
- 40.Bowles L., Platton S., Yartey N., et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N. Engl. J. Med. 2020 Jul 16;383(3):288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemke G., Silverman G. Blood clots and TAM receptor signalling in COVID-19 pathogenesis. Nat. Rev. Immunol. 2020:1–2. doi: 10.1038/s41577-020-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bendorius M., Neeli I., Wang F., et al. The mitochondrion-lysosome axis in adaptive and innate immunity: effect of lupus regulator peptide P140 on mitochondria autophagy and NETosis. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02158. 2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmer R., Scherbarth H., Rillo O., Gomez J., Muller S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann. Rheum. Dis. 2013;72(11):1830–1835. doi: 10.1136/annrheumdis-2012-202460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cervera R. Antiphospholipid syndrome. Thromb. Res. 2017;151:S43–S47. doi: 10.1016/S0049-3848(17)30066-X. [DOI] [PubMed] [Google Scholar]
- 45.Tan W., Low H., Wong H., Chua Y., Goh L., Ng J. Critically-ill covid-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am. J. Hematol. 2020 Apr 8 doi: 10.1002/ajh.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.XiaoM Zhang Y., Zhang S., et al. Brief report: anti-phospholipid antibodies in critically-ill patients with coronavirus disease 2019 COVID-19. Arthritis Rheum. 2020 Dec;72(12):1998–2004. doi: 10.1002/art.41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertin D., Brodovitch A., Beziane A., Hug S., Bouamri A., Mege L., et al. Anti-cardiolipin IgG autoantibodies are an independent risk factor of COVID-19 severity. Arthritis Rheum. 2020 Nov;72(11):1953–1955. doi: 10.1002/art.41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sailer T., Vormittag R., Pabinger I., et al. Inflammation in patients with lupus anticoagulant and implications for thrombosis. J. Rheumatol. 2005;32(3):462–468. [PubMed] [Google Scholar]
- 49.Sidelmann J., Sjoland A., Gram J., et al. Lupus anticoagulant is significantly associated with inflammatory reactions in patients with suspected deep vein. Scand. J. Clin. Lab. Invest. 2007 Jan 1;67(3):270–279. doi: 10.1080/00365510601038992. [DOI] [PubMed] [Google Scholar]
- 50.Tonello M., Mattia E., Favaro M., et al. IgG phosphatidylserine/prothrombin antibodies as a risk factor of thrombosis in antiphospholipid antibody carriers. Thromb. Res. 2019;177:157–160. doi: 10.1016/j.thromres.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Zuo Y., Estes K., Gandhi A., et al. medRxiv. 2020. Prothrombotic antiphospholipid antibodies in COVID-19. [Google Scholar]
- 52.Liu X., Zhang X., Xiao Y., et al. MedRxiv. 2020. Heparin-induced thrombocytopenia is associated with a high risk of mortality in critical COVID-19 patients receiving heparin-involved treatment. [Google Scholar]
- 53.Riker R., May L., Fraser L., Gagnon J., Bandara M., Zemrak W., et al. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res. Pract. Thromb. Haemost. 2020 Jul;4(5):936–941. doi: 10.1002/rth2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vlachoyiannopoulos G., Magira E., Alexopoulos H., Jahaj E., Theophilopoulou K., Kotanidou A., et al. Autoantibodies related to systemic autoimmune rheumatic diseases in severely-ill patients with COVID-19. Ann. Rheum. Dis. 2020 Dec 1;79(12):1661–1663. doi: 10.1136/annrheumdis-2020-218009. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y., Han T., Chen J., et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020 Nov;13(6):1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franceschini F., Cavazzana I. Anti-Ro/SSA and La/SSB antibodies. Autoimmunity. 2005;38(1):55–63. doi: 10.1080/08916930400022954. [DOI] [PubMed] [Google Scholar]
- 58.Bertin D., Brodovitch A., Beziane A., Hug S., Bouamri A., Mege L., et al. Anticardiolipin IgG autoantibody level is an independent risk factor for COVID-19 severity. Arthritis Rheum. 2020;72(11):1953–1955. doi: 10.1002/art.41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guilmot A., Slootjes S.M., Sellimi A., et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2020;30:1–7. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cristiano A., Fortunati V., Cherubini F., Bernardini S., Nuccetelli M. Anti-phospholipids antibodies and immune complexes in COVID-19 patients: a putative role in disease course for anti-annexin-V antibodies. Res. Square. 2021 Jan 19:1–17. doi: 10.1007/s10067-021-05580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llorente L., Zou W., Levy Y., et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J. Exp. Med. 1995;181(3):839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klinman D. Polyclonal B cell activation in lupus-prone mice precedes and predicts the development of autoimmune disease. J. Clin. Invest. 1990;86(4):1249–1254. doi: 10.1172/JCI114831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toscano G., Palmerini F., Ravaglia S., et al. Guillain–Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020 Jun 25;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnaud S., Budowski C., Tin S., Degos B. Post SARS-CoV-2 Guillain-Barré syndrome. Clin. Neurophysiol. 2020;131(7):1652–1654. doi: 10.1016/j.clinph.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahimi K. Guillain-Barre syndrome during COVID-19 pandemic: an overview of the reports. Ital. J. Neurol. Sci. 2020;41(11):3149–3156. doi: 10.1007/s10072-020-04693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wijdicks F., Klein J. Guillain-Barré Syndrome. Mayo Clin. Proc. 2017;92(3):467–479. doi: 10.1016/j.mayocp.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Gutiérrez-Ortiz C., Méndez A., et al. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020 Aug 4;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 68.Lantos E., Strauss B., Lin E. COVID-19-associated Miller Fisher syndrome: MRI findings. Am. J. Neuroradiol. 2020;41(7):1184–1186. doi: 10.3174/ajnr.A6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lui D.T., Lee C.H., Chow W.S., et al. Thyroid dysfunction in relation to immune profile, disease status and outcome in 191 patients with COVID-19. J. Clin. Endocrinol. Metab. 2021 Feb;106(2):e926–e935. doi: 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mateu-Salat M., Urgell E., Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J. Endocrinol. Investig. 2020;43(10):1527–1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller I., Cannavaro D., Dazzi D., et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lovren F., Pan Y., Quan A., et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295(4):H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 73.Almashat A. Vasculitis in COVID-19: a literature review. J Vasc. 2020;6(1):1–5. [Google Scholar]
- 74.Rosen L., Thiemann R., et al. medRxiv. 2020. IgM autoantibodies recognizing ACE2 are associated with severe COVID-19. [Google Scholar]
- 75.Bialek S., Gierke R., Hughes M., McNamara L.A., Pilishvili T., Skoff T. Coronavirus Disease 2019 in Children—United States, February 12–April 2, 2020. Morb. Mortal. Wkly Rep. 2020;69(14):422. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newburger J.W., Takahashi M., Burns J.C. Kawasaki disease. J. Am Coll. 2016;67(14):1738–1749. doi: 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 77.Akca K., Kesici S., Ozsurekci Y., et al. Kawasaki-like disease in children with COVID-19. Rheumatol. Int. 2020;40(12):2105–2115. doi: 10.1007/s00296-020-04701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubino F., Amiel S.A., Zimmet P., et al. New-onset diabetes in Covid-19. N. Engl. J. Med. 2020 Aug 20;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Unsworth R., Wallace S., Oliver N.S., et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the UK. Diabetes Care. 2020;43(11):e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 80.Marchand L., Pecquet M., Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57(10):1265–1266. doi: 10.1007/s00592-020-01570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gehrs B., Friedberg R. Autoimmune hemolytic anemia. Am. J. Hematol. 2002;69(4):258–271. doi: 10.1002/ajh.10062. [DOI] [PubMed] [Google Scholar]
- 82.Lazarian G., Quinquenel A., Bellal M., et al. Autoimmune hemolytic anemia associated with Covid-19 infection. Br. J. Haematol. 2020 Jul;190(1):29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopez C., Kim J., Pandey A., Huang T., DeLoughery T. Simultaneous onset of COVID-19 and autoimmune Hemolytic Anemia. Br. J. Haematol. 2020 Jul;190(1):31–32. doi: 10.1111/bjh.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Capes A., Bailly S., Hantson P., Gerard L., Laterre P. COVID-19 infection associated with auto-immune Hemolytic Anemia. Res. Square. 2020 Jul;99(7):1679–1680. doi: 10.1007/s00277-020-04137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanduc D. Homology, similarity, and identity in peptide epitope immunodefinition. J. Pept. Sci. 2012;8:487–494. doi: 10.1002/psc.2419. [DOI] [PubMed] [Google Scholar]
- 86.Angileri F., Legare S., Marino Gammazza A., Conway de Macario E., Macario A., Cappello F. Is molecular mimicry the culprit in the autoimmune hemolytic anemia affecting COVID-19 patients? Br. J. Haematol. 2020 May 26 doi: 10.1111/bjh.16883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zulfiqar A., Villalba N., Hassler P., Andrès E. Immune thrombocytopenic purpura in a patient with Covid-19. N. Engl. J. Med. 2020;382(18):e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bomhof G., Mutsaers G., Leebeek W., Boekhorst A., Hofland J., Croles N., et al. COVID-19-associated immune thrombocytopenia. Br. J. Haematol. 2020 July;190(2):e61–e64. doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mantovani E., Hundal J., Feterman D., Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin. Rheumatol. 2020;39(9):2811–2815. doi: 10.1007/s10067-020-05310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonometti R., Sacchi C., Stobbione P., et al. The first case of systemic lupus erythematosus (sle) triggered by covid-19 infection. Eur. Rev. Med. Pharmacol. Sci. 2020;24(18):9695–9697. doi: 10.26355/eurrev_202009_23060. [DOI] [PubMed] [Google Scholar]
- 91.Miglis M.G., Prieto T., Shaik R., Muppidi S., Sinn D.I., Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 2020;30(5) doi: 10.1007/s10286-020-00727-9. 449–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Talarico R., Stagnaro C., Ferro F., Carli L., Mosca M. Symmetric peripheral polyarthritis developed during SARS-CoV-2 infection. Lancet Rheumatol. 2020;2(9):e518–e519. doi: 10.1016/S2665-9913(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parisi S., Borrelli R., Bianchi S., Fusaro E. Viral arthritis and COVID-19. Lancet Rheumatol. 2020;2(11):e655–e657. doi: 10.1016/S2665-9913(20)30348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muhammed L., Baheerathan A., Cao M., Leite M.I., Viegas S. MuSK antibody–associated myasthenia gravis with SARS-CoV-2 infection: a case report. Ann. Intern. Med. 2020 doi: 10.7326/L20-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suurmond J., Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 2015;125(6):2194–2202. doi: 10.1172/JCI78084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaira L.A., Salzano G., Deiana G., Anosmia De Riu G. Ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 July;130(7) doi: 10.1002/lary.28692. 1787-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lechien R., Chiesa-Estomba M., De Siati R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020 Aug;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaye R., Chang D., Kazahaya K., Brereton J., Denneny C. COVID-19 Anosmia reporting tool: initial findings. Otolaryngol. Head Neck Surg. 2020 Jul;163(1):132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 99.Gilani S., Roditi R., Naraghi M. COVID-19 and anosmia in Tehran. Iran Med Hypotheses. 2020;141 doi: 10.1016/j.mehy.2020.109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gane B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020 Apr 2;58(3):299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 101.Villalba L., Maouche Y., Ortiz M.B.A., et al. Anosmia and Dysgeusia in the absence of other respiratory diseases: should COVID-19 infection be considered? Eur J Case Rep. Intern Med. 2020;7(4):1641. doi: 10.12890/2020_001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan H., Faraji F., Prajapati P., Ostrander T., DeConde S. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. InInt. Forum Allergy Rhinol. 2020 Jul;10(7):821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hopkins C., Surda P., Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020 Apr 11;58(3):295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 104.Spinato G., Fabbris C., Polesel J., et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020 May 26;323(20):2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moein T., Hashemian R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020 Aug;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vaira L.A., Salzano G., Fois G., Piombino P., De G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int. Forum Allergy Rhinol. 2020 Sep;10(9):1103–1104. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ohkubo K., Lee H., Baraniuk N., Merida M., Hausfeld N., Kaliner A. Angiotensin-converting enzyme in the human nasal mucosa. Am. J. Respir. Cell Mol. Biol. 1994;11(2):173–180. doi: 10.1165/ajrcmb.11.2.8049077. [DOI] [PubMed] [Google Scholar]
- 109.Haro J., Roura J., Vizitiu A., Gonzalez A., Gonzalez A. Long term serious olfactory loss in colds and/or flu. Acta Otorrinolaringol. Esp. 2013;64(5):331–338. doi: 10.1016/j.otorri.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 110.Netland J., Meyerholz K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mullol J., Alobid I., Mariño-Sánchez F., et al. The loss of smell and taste in the COVID-19 outbreak: a tale of many countries. Curr Allergy Asthma Rep. 2020;20(10):61. doi: 10.1007/s11882-020-00961-1. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Jun 1;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klopfenstein T., Kadiane J., Toko L., et al. Features of anosmia in COVID-19. Med. Mal. Infect. 2020 Aug 1;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brann D., Tsukahara T., Weinreb C., Logan D., Datta S. 2020. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perricone C., Shoenfeld N., Agmon-Levin N., de Carolis C., Perricone R., Shoenfeld Y. Smell and autoimmunity: a comprehensive review. Clin. Rev. Allergy Immunol. 2013;45(1):87–96. doi: 10.1007/s12016-012-8343-x. [DOI] [PubMed] [Google Scholar]
- 116.Rattazzi L., Cariboni A., Poojara R., Shoenfeld Y., D’Acquisto F. Impaired sense of smell and altered olfactory system in RAG-1(−∕-) immunodeficient mice. Front. Neurosci. 2015;9:318. doi: 10.3389/fnins.2015.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Katzav A., Ben-Ziv T., Blank M., Pick C.G., Shoenfeld Y., Chapman J. Antibody-specific behavioral effects: intracerebroventricular injection of antiphospholipid antibodies induces hyperactive behavior while anti-ribosomal-P antibodies induces depression and smell deficits in mice. J. Neuroimmunol. 2014;272(1–2):10–15. doi: 10.1016/j.jneuroim.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 118.Shoenfeld Y. 2020. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning Autoimmunity reviews; p. 102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shoenfeld N., Agmon N., Flitman I., et al. The sense of smell in systemic lupus erythematosus. Arthritis Rheum. 2009;60(5):1484–1487. doi: 10.1002/art.24491. [DOI] [PubMed] [Google Scholar]
- 120.Chen Q., Qiu F., Liu H., Li X., Li J. Altered olfactory function in patients with systemic lupus erythematosus. Med. Sci. Monit. 2019;25:5929–5933. doi: 10.12659/MSM.915738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bombini F., Peres A., Lapa T., et al. Olfactory function in systemic lupus erythematosus and systemic sclerosis. A longitudinal study and review of the literature. Autoimmun. Rev. 2018;17(4):405–412. doi: 10.1016/j.autrev.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 122.Doherty C. On the nose: shared themes for the sensory and immune self. Nat. Immunol. 2003;4(11):1043–1045. doi: 10.1038/ni1103-1043. [DOI] [PubMed] [Google Scholar]
- 123.Song C., Leonard E. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 2005;29(4–5):627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 124.Song C. The effect of thymectomy and IL-1 on memory: implications for the relationship between immunity and depression. Brain Behav. Immun. 2002;16(5):557–568. doi: 10.1016/s0889-1591(02)00012-0. [DOI] [PubMed] [Google Scholar]
- 125.Jones G., Mills M., Suarez D., et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020 Jun 1;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Y., Xiao M., Zhang S., et al. Coagulopathy and Antiphospholipid antibodies in patients with COVID-19. N. Engl. J. Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28 [Google Scholar]
- 128.Shoenfeld Y., Agmon-Levin N. ASIA’–autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011;36(1):4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]