Abstract
Objective
To examine associations between Neisseria gonorrhoeae (NG) infection during pregnancy and the risk of preterm birth, spontaneous abortion, premature rupture of membranes, perinatal mortality, low birth weight and ophthalmia neonatorum.
Data sources
We searched Medline, EMBASE, the Cochrane Library and Cumulative Index to Nursing and Allied Health Literature for studies published between 1948 and 14 January 2020.
Methods
Studies were included if they reported testing for NG during pregnancy and compared pregnancy, perinatal and/or neonatal outcomes between women with and without NG. Two reviewers independently assessed papers for inclusion and extracted data. Risk of bias was assessed using established checklists for each study design. Summary ORs with 95% CIs were generated using random effects models for both crude and, where available, adjusted associations.
Results
We identified 2593 records and included 30 in meta-analyses. Women with NG were more likely to experience preterm birth (OR 1.55, 95% CI 1.21 to 1.99, n=18 studies); premature rupture of membranes (OR 1.41, 95% CI 1.02 to 1.92, n=9); perinatal mortality (OR 2.16, 95% CI 1.35 to 3.46, n=9); low birth weight (OR 1.66, 95% CI 1.12 to 2.48, n=8) and ophthalmia neonatorum (OR 4.21, 95% CI 1.36 to 13.04, n=6). Summary adjusted ORs were, for preterm birth 1.90 (95% CI 1.14 to 3.19, n=5) and for low birth weight 1.48 (95% CI 0.79 to 2.77, n=4). In studies with a multivariable analysis, age was the variable most commonly adjusted for. NG was more strongly associated with preterm birth in low-income and middle-income countries (OR 2.21, 95% CI 1.40 to 3.48, n=7) than in high-income countries (OR 1.38, 95% CI 1.04 to 1.83, n=11).
Conclusions
NG is associated with a number of adverse pregnancy and newborn outcomes. Further research should be done to determine the role of NG in different perinatal mortality outcomes because interventions that reduce mortality will have the greatest impact on reducing the burden of disease in low-income and middle-income countries.
PROSPERO registration number
CRD42016050962.
Keywords: Neisseria gonorrhoeae, premature birth, meta-analysis, pregnancy, systematic review
Introduction
Sexually transmitted infections (STIs) during pregnancy have been reported to be associated with poor pregnancy outcomes.1–3 Neisseria gonorrhoeae (NG) has been associated with premature rupture of membranes (PROM),1 preterm birth (PTB),1 4 5 low birth weight (LBW),1 4–6 neonatal and perinatal mortality7 8 as well as neonatal conjunctivitis.9 10
Preterm birth and its complications are a leading cause of perinatal mortality and the majority of perinatal and neonatal deaths occur in low-resource settings.11 12 Information about associations between NG during pregnancy and adverse pregnancy and birth outcomes is therefore necessary to improve our understanding of the evidence for causality, and to determine the potential impact of preventive interventions.1 13
To date, systematic reviews about adverse pregnancy and birth outcomes have examined, and found, associations with Chlamydia trachomatis (CT),14–16 Trichomonas vaginalis (TV)17 and Mycoplasma genitalium (MG).18 The objective of this study was to systematically review associations between NG infection during pregnancy and the risk of PTB, spontaneous abortion, PROM, perinatal mortality, LBW and ophthalmia neonatorum.
Methods
The protocol for this review has been published.19 We report our findings using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (online supplemental table S1).20
sextrans-2020-054653supp001.pdf (4.8MB, pdf)
Eligibility criteria
Studies reporting NG detected by culture and/or nucleic acid amplification test during pregnancy, labour or post partum were eligible for inclusion if they reported on one or more of the following outcomes: PTB, spontaneous abortion, PROM (preterm and term), LBW, perinatal or neonatal mortality, or ophthalmia neonatorum. We included clinical trials, cohort, case-control and cross-sectional studies but excluded individual case reports, case series, opinion articles and studies without a comparison group.
Information sources and search strategy
We searched Medline, Excerpta Medica database (EMBASE), the Cochrane Library and Cumulative Index to Nursing and Allied Health Literature (CINAHL) from 1948 to 14 January 2020. We examined reference lists of included studies or relevant reviews for additional articles. The searches did not apply language restrictions, but we included only articles published in English or German (languages spoken fluently by review team members). Details of the search strategy are listed in online supplemental text S1.
Study selection and data extraction
One reviewer (LV) screened titles and abstracts (online supplemental text S2) and two reviewers screened the full text of potentially relevant articles independently (LV, DE-G). Discrepancies were resolved by discussion or by the decision of a third reviewer (NL). Data were extracted into a standardised, piloted form in a Research Electronic Data Capture (REDCap) database (Vanderbilt University, Tennessee, USA) recording study design, participant characteristics, presence or absence of NG, pregnancy, perinatal or neonatal outcomes and other STI and genital infections. Standard definitions for outcomes were used,19 or as defined by the authors (online supplemental tables S2−S4).
Risk of bias in individual studies
Two reviewers assessed the risk of bias in each study independently (LV, DE-G), using checklists for cross-sectional,21 case-control and cohort studies,22 published by the UK National Institute for Health and Care Excellence (NICE). A third reviewer (NL) resolved discrepancies. Each study was assessed qualitatively overall as having all or most (++), some (+), or few or no checklist criteria fulfilled (−).
Data synthesis and analysis
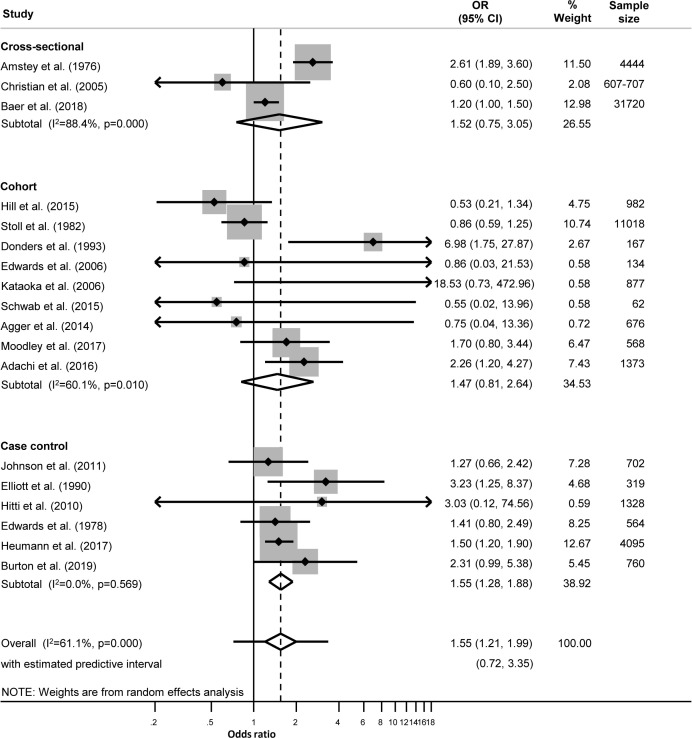
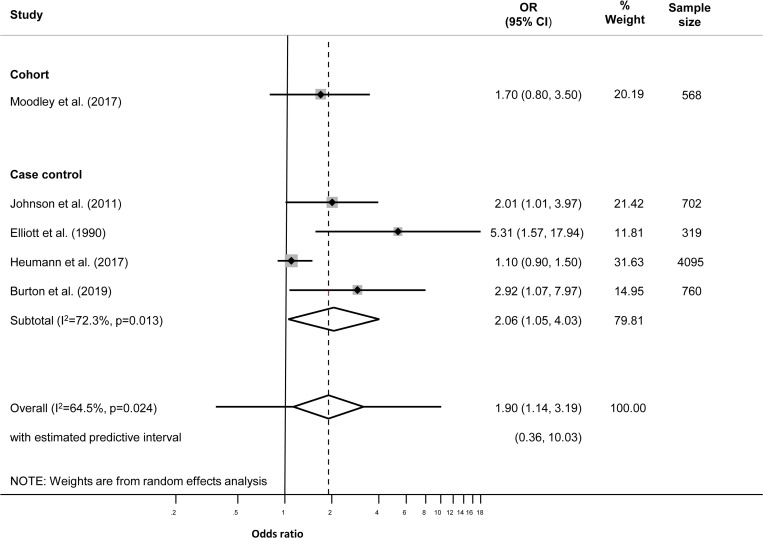
We used Stata V.14.0 (StataCorp, College Station, Texas, USA) for all analyses. Where possible, we used the odds ratio (OR) as the measure of association for all study designs, assuming that the relative risk (RR) and OR would be similar, as the outcomes of interest are rare events. We calculated the crude OR and its 95% CI using raw data from the paper, or extracted values provided by the authors if raw data were not available. Where authors reported a multivariable analysis, we extracted the adjusted OR (aOR, 95% CI) and recorded the variables included in the model. We examined forest plots for each outcome ((figures 1 and 2; online supplemental tables S3−S10), and used the I2 statistic to examine the level of between-study heterogeneity other than that due to chance.23
Figure 1.
Unadjusted effect sizes for Neisseria gonorrhoeae during pregnancy and preterm birth.
Figure 2.
Adjusted effect sizes for Neisseria gonorrhoeae during pregnancy and preterm birth.
For outcomes reported by two or more studies of the same design, we used a random effects model to estimate a summary OR (95% CI), which is the average effect across all included studies.24 We first stratified these estimates by study design because there are sources of bias that could result in overestimation or underestimation of an association and these biases differ according to the study design. If the stratified estimates were similar, as visualised in forest plots, we also reported the overall summary OR from meta-analysis, with its prediction interval for the estimated range of effect sizes across settings.24 Meta-analysis of the results of aORs used the same approach as for unadjusted estimates. For the outcome PTB, for which there were >10 included studies, we categorised studies as high-income and non-high-income (combining low-income and middle-income countries), based on the World Bank list.25 We repeated all meta-analyses using a fixed effects model as a sensitivity analysis.
Risk of bias across studies
Publication bias was examined by generating a funnel plot for outcomes that were reported by 10 or more studies.
Results
In total, 2914 records were identified and 2593 screened, after exclusion of duplicates. Eighty-five full-text articles were assessed and 33 studies were included, with 30 reporting data in a format suitable for meta-analysis (online supplemental figure S1). Three studies were excluded from meta-analyses because of zero counts or missing data,26–28 another three studies reported on more than one outcome but had sufficient data for only one outcome (online supplemental table S5).29–31 The 33 studies reported on 60 outcomes. Twenty-one studies reported PTB,4–8 27–29 31–43 3 reported spontaneous abortion,34 41 44 12 reported PROM,6 8 26 29–31 34 40 41 43 45 46 9 reported perinatal mortality outcomes,7 8 28 30 34 35 41 47 48 8 reported LBW4 6 7 35 37 40 42 49 and 7 reported ophthalmia neonatorum7 9 26 42 50–52 (online supplemental tables S2-S4).
We included 14 cohort, 11 case-control and 8 cross-sectional studies published between 1976 and January 2020. The number of outcomes reported varied from 6236 to 31 720.43 Two-thirds (22/33) of studies took place in high-income and upper middle-income settings (table 1; online supplemental tables S2-S4); most took place in health facilities (28/33) and more than half were in urban locations (19/33). Thirteen studies reported participant’s age, 23 ethnicity, 6 smoking status and 4 reported multiple pregnancies (full descriptive details are available in online supplemental tables S6-S8).
Table 1.
Summary, characteristics of included studies by country income group
| Study | Study design | Specimen collection timing and type |
Sample size for outcome of interest; number of adverse outcomes in women with gonorrhoea/total number of women with adverse outcome (%) |
NICE checklist criteria fulfilled, internal/external validity* | |||||
| PTB | Sp. ab. | PROM | PM | LBW | ON | ||||
| High-income group | |||||||||
| Agger WA, et al 32 | Cohort | First or second trimester; cervical | 676; 0/54 (0) |
+/+ | |||||
| Alger LS, et al 45 | Case-control | Second or third trimester; cervical | 129; 6/45 (13) |
+/+ | |||||
| Amstey MS, Steadman KT41 | Cross-sectional | First or third trimester or intrapartum; cervical | 4444; 56/613 (9) |
5065; 24/620 (4) |
4444; 52/851 (6) |
5065; 15/149 (10) |
−/− | ||
| Baer RJ, et al 43 | Cross-sectional | Second or third trimester; unclear | 31 720; NR |
31 720; 53/NR |
+/++ | ||||
| Burton AE, Thomas S39 | Case-control | First, second or third trimester; urine and vaginal | 760; 18/380 (5) |
+/− | |||||
| Charles et al 26 | Cohort | NR/unclear; cervical | NR; 10/NR |
2160*; 0/0 (0) |
−/− | ||||
| Choi SJ, et al 27 | Case-control | NR/unclear; vaginal | 217†; 0/100 (0) |
+/+ | |||||
| Edwards LE, et al 8 | Case-control | Unclear; NR/unclear | 564; 22/57 (39) |
564; 50/148 (34) |
564; 5/11 (45) |
+/+ | |||
| Edwards RK, et al33 | Cohort | NR/unclear; cervical | 134; 0/37 (0) |
+/+ | |||||
| Heumann CL, et al 40 | Case-control | NR/unclear; NR/unclear | 4095; 93/353 (26) |
4095; 90/416 (22) |
4095; 80/266 (30) |
+/++ | |||
| Hill MG, et al 29 | Cohort | First, second or third trimester; NR/unclear | 982; 5/171 (1) |
933†; 0/37 (0) |
+/++ | ||||
| Johnson HL, et al 4 | Case-control | NR/unclear; NR/unclear | 702; 13/135 (10) |
679; 8/112 (7) |
++/++ | ||||
| Kataoka S, et al 34 | Cohort | First trimester; vaginal | 877; 0/15 (0) |
877; 0/5 (0) |
877; 0/7 (0) |
877; 0/1 (0) |
+/− | ||
| Mann JR, et al 28 | Cross-sectional | Unclear; NR/unclear | 7931†; 749/7931 (9) |
+/− | |||||
| Maxwell GL, Watson WJ30 | Case-control | Second or third trimester; cervical | NR; 11/182 (6) |
182; 1/8 (13) |
−/− | ||||
| Stoll BJ, et al 37 | Cohort | First, second or third trimester; cervical | 11 018; 30/837 (4) |
11 018; 14/319 (4) |
11 018; 71/1754 (4) |
+/− | |||
| Upper middle-income group | |||||||||
| Adachi K, et al 7 | Cohort | Intrapartum or post partum; urine | 1373; 13/148 (9) |
1373; 4/41 (10) |
1373; 21/244 (9) |
1373; 0/2 (0) |
++/++ | ||
| Donders GG, et al 5 | Cohort | NR/unclear; cervical | 167; 5/29 (17) |
−/− | |||||
| Hitti J, et al 38 | Case-control | Post partum; cervical | 1328; 1/661 (<1) |
++/+ | |||||
| Moodley D, et al 35 | Cohort | First, second and third trimester and post partum; NR/unclear | 568; 13/157 (8) |
608; 9/77 (12) |
550; 3/54 (6) |
++/+ | |||
| Nasution TA, et al 31 | Cross-sectional | Intrapartum or post partum; vaginal, placental swab or blood | 60†; 0/30 (0) |
80; 0/40 (0) |
−/− | ||||
| Pourabbas B, et al 50 | Cross-sectional | Third trimester; cervical | 239; 1/29 (3) |
+/− | |||||
| Lower middle-income group | |||||||||
| Elliott B, et al 6 | Case-control | Post partum; cervical | 319; 18/160 (11) |
154; 4/46 (9) |
319; 18/160 (11) |
++/+ | |||
| Galega FP, et al 51 | Cross-sectional | Intrapartum; vaginal | 296; 12/12 (100) |
+/− | |||||
| Gichangi PB, et al 49 | Cohort | Post partum; cervical | 203; 11/51 (22) |
+/+ | |||||
| Gichuhi S, et al 52 | Case-control | Third trimester; cervical | 445; 1/99 (1) |
+/+ | |||||
| Laga M, et al 9 | Cohort | Post partum; cervical | 781; 28/181 (15) |
+/− | |||||
| Mason PR, et al 46 | Cross-sectional | Intrapartum; cervical | 105; 4/24 (17) |
+/+ | |||||
| Warr AJ, et al 47 | Cohort | Second and third trimester and post partum; vaginal | 1221; 1/19 (5) |
++/+ | |||||
| Schwab FD, et al 36 | Cohort | Second trimester; vaginal swab | 62; 0/23 (0) |
−/− | |||||
| Temmerman M, et al 44 | Case-control | First, second or third trimester; cervical | 387; 10/193 (5) |
+/+ | |||||
| Low-income group | |||||||||
| Christian P, et al 42 | Cross-sectional | Post partum; urine | 607–707; NR |
607–707; NR |
607–707; NR |
−/− | |||
| Kupka R, et al 48 | Cohort | First, second and third trimester; cervical or vaginal | 946; 1/21 (5) |
+/+ | |||||
*++, all or most checklist criteria fulfilled; +, some of checklist criteria fulfilled; −, few or no checklist criteria fulfilled.
†Study not included in meta-analysis.
LBW, low birth weight; NICE, National Institute of Health and Care Excellence; NR, not reported; ON, ophthalmia neonatorum; PM, perinatal mortality; PROM, premature rupture of membranes; PTB, preterm birth; Sp. ab., spontaneous abortion.
Characteristics of specimen collection, timing and laboratory tests are reported in online supplemental tables S2-S4. Briefly, 25 studies reported the timing of specimen collection, of which 12 obtained specimens during pregnancy; 29 30 32 34 36 37 43–45 48 50 52 2 collected specimens intrapartum;46 51 5 post partum;6 9 38 42 49 3 during both the antenatal and postpartum period;35 39 47 the remaining three studies tested during pregnancy or intrapartum,41 intrapartum or post partum7 and intrapartum and post partum.31 Most studies reported specimen type (26), with 24 collecting endocervical and/or vaginal swabs.5 6 9 26 27 30–34 36–39 41 44–52 Twenty-nine reported type of laboratory test.4–9 26 27 29–39 41 42 44–47 49–52
Twenty studies reported provision of treatment at the time of NG diagnosis: 8 treated all positive women4 9 30 35 45 47 51 52; 12 treated some women.5 8 26 32 34 37–39 41 42 44 49 Provision of treatment was unclear in 12 studies6 7 27–29 31 33 36 40 43 46 48 and 1 study did not provide treatment50 (online supplemental tables S9-S11).
Most studies (29) tested for other STI and genital infections: 6 tested for BV,4 6 33 36 47 52 25 tested for CT,4–7 9 27–36 38–40 42 43 45–47 50 52 9 tested for HIV,4 6 7 35 44 47–49 52 5 tested for MG,27 32–34 38 14 tested for syphilis4–7 26 33 39 40 43 44 47–49 52 and 10 tested for TV4 27 28 33 35 38 39 46 47 52 (online supplemental tables S9-S11).
Risk of bias
Based on the NICE checklists, of the 33 studies, 2 met all or most (++) checklist criteria,4 7 7 met all/ most or some (++/+),6 29 35 38 40 43 47 10 studies met some (+) criteria,8 27 32 33 44–46 48 49 52 7 met some or few/no criteria (+/−),9 28 34 37 39 50 51 and 7 met few or no checklist criteria (−)5 26 30 31 36 41 42 (table 1, online supplemental tables S12-S14). There was evidence of publication bias and other small study effects (Egger’s test, p=0.008) for the association between NG and preterm birth (online supplemental figure S2).
Preterm birth
Twenty-one studies reported on the association between NG in pregnancy and PTB. Eighteen studies involving at least 60 396 women were included in meta-analysis; nine cohort studies,5 7 29 32–37 six case-control4 6 8 38–40 and three cross-sectional studies.41–43 The overall unadjusted summary OR for NG and PTB was 1.55 (95% CI 1.21, 1.99; I2 61.1%; prediction interval 0.72, 3.35) (figure 1, table 2). Eleven studies were from high-income countries4 8 29 32–34 37 39 41 43 45 (table 1). NG was more strongly associated with PTB in non-high-income countries (OR 2.21, 95% CI 1.40 to 3.48; I2 14.7%) than in high-income countries (OR 1.38, 95% CI 1.04 to 1.83; I2 68.6%) (online supplemental figures S3, S4).
Table 2.
Summary estimates from random effects analyses
| Adverse outcome | Number of studies | Summary estimate OR (95% CI) |
I2 (%) | Prediction interval |
| Preterm birth | ||||
| Adjusted | 5 | 1.90 (1.14 to 3.19) | 64.5 | 0.36 to 10.03 |
| Unadjusted | 18 | 1.55 (1.21 to 1.99) | 61.1 | 0.72 to 3.35 |
| Low birth weight | ||||
| Adjusted | 4 | 1.48 (0.79 to 2.77) | 49.5 | 0.14 to 15.70 |
| Unadjusted | 8 | 1.66 (1.12 to 2.48) | 72.7 | 0.51 to 5.38 |
| Premature rupture of membrane | 9 | 1.41 (1.02 to 1.92) | 59.2 | 0.64 to 3.11 |
| Spontaneous abortion* | 3 | NA | NA | NA |
| Perinatal mortality | 9 | 2.16 (1.35 to 3.46) | 40.3 | 0.69 to 6.74 |
| Ophthalmia neonatorum | 6 | 4.21 (1.36 to 13.04) | 58.0 | 0.17 to 104.58 |
*Each study had a different design, therefore it was not appropriate to report a summary estimate for this outcome.
NA, not applicable.
In five studies, multivariable analysis was conducted. The variables adjusted for differed between studies (online supplemental table S15). The summary aOR for NG and PTB was 1.90 (95% CI 1.14 to 3.19; I2 64.5%; overall prediction interval 0.36 to 10.03) (figure 2, table 2).
Spontaneous abortion
Three studies involving 6329 women reported on spontaneous abortion.34 41 44 All three were different study designs, and none reported a multivariable analysis. There was insufficient information from these studies to determine the strength of association between NG and spontaneous abortion (online supplemental figure S5).
Premature rupture of membranes
Twelve studies reported on the association between NG in pregnancy and PROM; three cohort,26 29 34 five case-control6 8 30 40 45 and four cross-sectional studies.31 41 43 46 Nine studies involving 42 168 women were included in the meta-analysis.6 8 31 34 40 41 43 45 46 The unadjusted summary OR for NG and PROM was 1.41 (95% CI 1.02 to 1.92; I2 59.2%; prediction interval 0.64 to 3.11) (table 2, online supplemental figure S6). None of the included studies reported a multivariable analysis.
Perinatal mortality
Nine studies involving 21 854 women examined perinatal mortality outcomes: six cohort,7 34 35 37 47 48 two case-control8 30 and one cross-sectional study.41 Of these, two reported neonatal mortality,7 30 two perinatal mortality,8 41 three stillbirths34 35 48 and two reported on both stillbirths and neonatal mortality.37 47 The unadjusted summary OR for NG and any perinatal mortality outcome was 2.16 (95% CI 1.35 to 3.46; I2 40.3%, prediction interval 0.69 to 6.74) (table 2, online supplemental figure S7). Two studies conducted a multivariable analysis for stillbirth. Moodley et al adjusted for age, number of pregnancies, socioeconomic status, HIV-1, CT and TV infection.35 The aOR was the same as the unadjusted OR (2.2; 95% CI 1.0 to 4.9). Kupka et al adjusted for gestational age, maternal literacy, history of stillbirth, CD4 count and previous hospitalisation.48 They reported relative risks and found a stronger association in the adjusted than the unadjusted model (9.74, 95% CI 2.52 to 37.59 vs 7.58, 95% CI 1.33 to 43.28), but CIs were wide and overlapping.
Low birth weight
Eight studies involving at least 18 844 infants reported LBW: four cohort,7 35 37 49 three case-control4 6 40 and one cross-sectional study.42 The summary unadjusted OR for the association between NG and LBW was 1.66 (95% CI 1.12 to 2.48; I2 72.7%; prediction interval 0.51 to 5.38) (table 2, online supplemental figure S8). Five studies reported multivariable analyses.4 6 35 40 49 The studies adjusted for different variables, only four provided enough details to include in meta-analysis4 6 35 40 (online supplemental table S15). The summary aOR was 1.48 (95% CI 0.79 to 2.77; I2 64.5%; prediction interval 0.14 to 15.70) (table 2; online supplemental figure S9).
Ophthalmia neonatorum
Seven studies reported on the association between NG and ophthalmia neonatorum: three cohort,7 9 26 three cross-sectional42 50 51 and one case-control study.52 One was excluded from meta-analysis as there were no events. The six studies included in the meta-analysis involved at least 3741 infants. The unadjusted summary OR for NG and ophthalmia neonatorum was 4.21 (95% CI 1.36 to 13.04; I2 58%; prediction interval 0.17 to 104.58) (table 2, online supplemental figure S10). None of the included studies reported a multivariable analysis.
Sensitivity analysis
Sensitivity analysis was undertaken for all outcomes. Effect estimates from fixed effect meta-analyses were similar to those from random effects models but tended to be slightly lower (online supplemental table S16).
Discussion
This systematic review included 33 studies for the qualitative analysis and 30 studies for meta-analysis. In studies that controlled for potential confounding, NG during pregnancy was associated with an increase in the adjusted odds of PTB of 1.90 (95% CI 1.14 to 3.19, five studies) and in the adjusted odds of LBW of 1.48 (95% CI 0.79 to 2.77, four studies). The odds of PROM, perinatal mortality and ophthalmia neonatorum were also increased in women with NG, but most studies of these outcomes did not provide estimates that controlled for confounding. There was insufficient evidence from studies of spontaneous abortion. The association between NG in pregnancy and PTB was stronger in studies conducted in low-income and middle-income countries than in high-income countries.
The main strength of this review was the use of a protocol19 to define the outcomes and analyses in advance and independent work by two reviewers to reduce bias in study selection, data extraction and risk of bias assessment. An additional strength is the calculation of prediction intervals for the summary estimates.24 With random effects meta-analysis, the summary OR is an average of the effect estimate and its 95% CI. The prediction interval gives information about the range of effect sizes across the settings in which studies included in the review were conducted.24 We combined effect estimates from different study designs if the stratified summary estimates were similar. The biases affecting individual studies and each observational study design differ, with some likely to overestimate the strength of association and others likely to underestimate it. Triangulation of findings across study designs is a strength of this review. Consistency in the direction and strength of effects can increase confidence in a causal interpretation, if confounding is addressed adequately.53 There are also weaknesses in the review methods. Despite searching multiple databases, our search strategy might have missed relevant studies, for example, in languages other than English or German.
Our searches did not find any other systematic review of the association between NG and adverse pregnancy outcomes. In narrative reviews, the findings tend to group different adverse outcomes together and to cite those from studies that find the strongest associations.1 54 The advantage of this review is the systematic inclusion of all eligible studies and examination of evidence separately for each outcome.
Our findings suggest that NG in pregnancy increases the risk of PTB and LBW. The certainty of evidence for causal associations is challenged by confounding and bias.55 In all observational study designs, confounding is an issue so the confounder-adjusted effect estimates are of most interest.53 In this systematic review, PTB and LBW were the only outcomes for which there were enough included studies to estimate a confounder-adjusted summary OR. For PTB, the summary aOR (1.90, 95% CI 1.14 to 3.19) was higher than the unadjusted OR, but the wide CI included the unadjusted summary estimate. For LBW, the 95% CI for the summary aOR (1.48, 95% CI 0.79 to 2.77) was compatible with there being no increased risk of LBW, but might reflect the small number of studies. The presence of co-infections, especially HIV, could also confound these associations. All studies reporting the outcomes PTB and LBW reported testing for other genital infections, but only Moodley et al, reporting from a high-burden setting, adjusted for co-infections (CT, TV and HIV)35 for these two outcomes. In that study, adjustment did not change the effect size. Of the other 18 studies reporting PTB and/or LBW, 15 reported co-infection with CT, four with TV and four with HIV. Each of these infections has been reported to be associated with LBW14 15 56 and PTB.15 17 56 Measurement bias might also have resulted in underestimation of the strength of association because, in most studies, women with NG had received treatment. These studies are measuring the outcome of treated NG, when the causal association of interest is with untreated NG.55 In this situation, cross-sectional studies that measure the presence of NG at the time of delivery are assessing the association with untreated infection. Although the onset of infection is unknown, adjusted estimates from such studies might be less biased than some cohort and case-control studies.55
For spontaneous abortion there were only three studies, with insufficient evidence to determine whether there is an association with NG. The association with ophthalmia neonatorum was strong and is known to be causal because there is evidence from randomised controlled trials that effective antiseptic or antimicrobial treatment prevents the condition.57 The association with perinatal mortality outcomes deserves further investigation to determine whether it is a consequence of PTB and LBW, or whether NG is an independent risk factor. The potential mechanisms linking NG to adverse perinatal outcomes are not well understood but NG might cause low-grade inflammation of the placenta and fetal membranes, increasing the risk of chorioamnionitis and thus PROM and PTB.58 If placental inflammation or infection of the amniotic cavity are implicated in the pathogenesis of PTB, the timing of NG infection and treatment during pregnancy might modify the risk. There was insufficient data in the included studies to formally examine the effects of these factors in the meta-analyses.
Future studies to investigate the role of NG as a cause of adverse pregnancy outcomes should be designed to address the limitations of many of the studies in this review. First, observational studies should collect data about potential confounding factors and be large enough to conduct multivariable analyses. Second, if treatment is given, the timing should be recorded so that study findings can be interpreted with this information. Third, samples should be taken for other STIs and vaginal microbiota so that the role of co-infections can be better understood. Randomised controlled trials are one way to examine the causal role of NG. Several trials of screening and treatment interventions are underway.59 60 However, it will be difficult to determine the effect of NG alone, because the interventions often include treatment for multiple infections. These trials are taking place in low-income and middle-income countries where the burden of STIs and of adverse pregnancy outcomes is highest.11 12 Our review also found that the strength of association between NG and PTB was greater in low-income and middle-income than in high-income settings. In summary, this review suggests that NG is causally associated with PTB and LBW. Further research should be done to determine the role of NG in different perinatal mortality outcomes because interventions that reduce mortality will have the greatest impact on reducing the burden of disease in low-income and middle-income countries.
Key messages.
Women with Neisseria gonorrhoeae (NG) in pregnancy are more likely to experience adverse birth outcomes including preterm birth, premature rupture of membranes, low birth weight, perinatal mortality and ophthalmia neonatorum.
NG was more strongly associated with preterm birth in low-income and middle-income countries than in high-income countries.
Further studies are required to address the gap in evidence about the effects of testing and treatment of NG in pregnancy, particularly in low-income and middle-income settings.
Footnotes
Handling editor: Alec Miners
Twitter: @lisa_vallely, @nicolamlow
LMV and DE-G contributed equally.
Contributors: LV is the guarantor of the manuscript. AV, NL, JK and conceived the study. LV and DE-G led the study, including screening and data extraction; NL supported data extraction. HW undertook all data analysis, along with NL, DE-G. LV, DE-G and NL wrote the first draft of the manuscript, CSEH and AV reviewed the first draft. NL, AV, WSP, RG, HW, CSEH, BS, AR and JK contributed to various drafts of the manuscript. All authors read, provided feedback and approved the final manuscript.
Funding: LV receives salary support from the Australian National Health and Medical Research Council (NHMRC), through an early career fellowship. DE-G received salary support from r4d programme (Swiss Programme for Research on Global Issues for Development), grant number IZ07Z0-160909. AV receives salary support from the Australian NHMRC, through a Career Development Fellowship and CH through an NHMRC Research Fellowship.
Disclaimer: The funders played no role in the development of the protocol or the analyses conducted.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Not required.
References
- 1. Mullick S, Watson-Jones D, Beksinska M, et al. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect 2005;81:294–302. 10.1136/sti.2002.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blas MM, Canchihuaman FA, Alva IE, et al. Pregnancy outcomes in women infected with Chlamydia trachomatis: a population-based cohort study in Washington state. Sex Transm Infect 2007;83:314–8. 10.1136/sti.2006.022665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cotch MF, Pastorek JG, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and prematurity Study Group. Sex Transm Dis 1997;24:353–60. 10.1097/00007435-199707000-00008 [DOI] [PubMed] [Google Scholar]
- 4. Johnson HL, Ghanem KG, Zenilman JM, et al. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner City public sexually transmitted diseases clinics. Sex Transm Dis 2011;38:167–71. 10.1097/OLQ.0b013e3181f2e85f [DOI] [PubMed] [Google Scholar]
- 5. Donders GG, Desmyter J, De Wet DH, et al. The association of gonorrhoea and syphilis with premature birth and low birthweight. Genitourin Med 1993;69:98–101. 10.1136/sti.69.2.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis 1990;161:531–6. 10.1093/infdis/161.3.531 [DOI] [PubMed] [Google Scholar]
- 7. Adachi K, Klausner JD, Xu J, et al. Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-infected pregnant women and adverse infant outcomes. Pediatr Infect Dis J 2016;35:894–900. 10.1097/INF.0000000000001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards LE, Barrada MI, Hamann AA, et al. Gonorrhea in pregnancy. Am J Obstet Gynecol 1978;132:637–41. 10.1016/0002-9378(78)90856-6 [DOI] [PubMed] [Google Scholar]
- 9. Laga M, Plummer FA, Nzanze H, et al. Epidemiology of ophthalmia neonatorum in Kenya. Lancet 1986;2:1145–9. 10.1016/S0140-6736(86)90544-1 [DOI] [PubMed] [Google Scholar]
- 10. Mabey D, Hanlon P, Hanlon L, et al. Chlamydial and gonococcal ophthalmia neonatorum in the Gambia. Ann Trop Paediatr 1987;7:177–80. 10.1080/02724936.1987.11748502 [DOI] [PubMed] [Google Scholar]
- 11. Lee ACC, Katz J, Blencowe H, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health 2013;1:e26–36. 10.1016/S2214-109X(13)70006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reinebrant HE, Leisher SH, Coory M, et al. Making stillbirths visible: a systematic review of globally reported causes of stillbirth. BJOG 2018;125:212–24. 10.1111/1471-0528.14971 [DOI] [PubMed] [Google Scholar]
- 13. WHO Sexually transmitted infections fact sheet No. 110. Geneva: World Health Organization, 2013. [Google Scholar]
- 14. Silva MJPMdeA, Florêncio GLD, Gabiatti JRE, et al. Perinatal morbidity and mortality associated with chlamydial infection: a meta-analysis study. Braz J Infect Dis 2011;15:533–9. 10.1590/s1413-86702011000600006 [DOI] [PubMed] [Google Scholar]
- 15. Tang W, Mao J, Li KT, et al. Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: a global systematic review and meta-analysis. Sex Transm Infect 2020;96:322–9. 10.1136/sextrans-2019-053999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olson-Chen C, Balaram K, Hackney DN. Chlamydia trachomatis and adverse pregnancy outcomes: meta-analysis of patients with and without infection. Matern Child Health J 2018;22:812–21. 10.1007/s10995-018-2451-z [DOI] [PubMed] [Google Scholar]
- 17. Silver BJ, Guy RJ, Kaldor JM, et al. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis 2014;41:369–76. 10.1097/OLQ.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 18. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015;61:418–26. 10.1093/cid/civ312 [DOI] [PubMed] [Google Scholar]
- 19. Vallely LM, Egli-Gany D, Pomat W, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae, Mycoplasma genitalium, M. hominis, Ureaplasma urealyticum and U. parvum: a systematic review and meta-analysis protocol. BMJ Open 2018;8:e024175. 10.1136/bmjopen-2018-024175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 21. NICE Methods for the development of NICE public health guidance (third edition): process AMD methods. UK: National Institute for Health and care Excellence, 2012. [PubMed] [Google Scholar]
- 22. NICE The social care guidance manual: process and methods. UK: National Institute for Health and care Excellence, 2013. [PubMed] [Google Scholar]
- 23. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 25. Worldbank List of economies 2019. Available: https://databank.worldbank.org/data/download/site-content/CLASS.xls [Accessed 1 Aug 2019].
- 26. Charles AG, Cohen S, Kass MB, et al. Asymptomatic gonorrhea in prenatal patients. Am J Obstet Gynecol 1970;108:595–9. 10.1016/0002-9378(70)90238-3 [DOI] [PubMed] [Google Scholar]
- 27. Choi SJ, Park SD, Jang IH, et al. The prevalence of vaginal microorganisms in pregnant women with preterm labor and preterm birth. Ann Lab Med 2012;32:194–200. 10.3343/alm.2012.32.3.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mann JR, McDermott S, Gill T. Sexually transmitted infection is associated with increased risk of preterm birth in South Carolina women insured by Medicaid. J Matern Fetal Neonatal Med 2010;23:563–8. 10.3109/14767050903214574 [DOI] [PubMed] [Google Scholar]
- 29. Hill MG, Menon S, Smith S, et al. Screening for Chlamydia and gonorrhea cervicitis and implications for pregnancy outcome. are we testing and treating at the right time? J Reprod Med 2015;60:301–8. [PubMed] [Google Scholar]
- 30. Maxwell GL, Watson WJ. Preterm premature rupture of membranes: results of expectant management in patients with cervical cultures positive for group B Streptococcus or Neisseria gonorrhoeae. Am J Obstet Gynecol 1992;166:945–9. 10.1016/0002-9378(92)91369-L [DOI] [PubMed] [Google Scholar]
- 31. Nasution TA, Cheong SF, Lim CT, et al. Multiplex PCR for the detection of urogenital pathogens in mothers and newborns. Malays J Pathol 2007;29:19–24. [PubMed] [Google Scholar]
- 32. Agger WA, Siddiqui D, Lovrich SD, et al. Epidemiologic factors and urogenital infections associated with preterm birth in a midwestern U.S. population. Obstet Gynecol 2014;124:969–77. 10.1097/AOG.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Edwards RK, Ferguson RJ, Reyes L, et al. Assessing the relationship between preterm delivery and various microorganisms recovered from the lower genital tract. J Matern Fetal Neonatal Med 2006;19:357–63. 10.1080/00207170600712071 [DOI] [PubMed] [Google Scholar]
- 34. Kataoka S, Yamada T, Chou K, et al. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol 2006;44:51–5. 10.1128/JCM.44.1.51-55.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moodley D, Sartorius B, Madurai S, et al. Pregnancy outcomes in association with STDs including genital HSV-2 shedding in a South African cohort study. Sex Transm Infect 2017;93:460–6. 10.1136/sextrans-2017-053113 [DOI] [PubMed] [Google Scholar]
- 36. Schwab FD, Zettler EK, Moh A, et al. Predictive factors for preterm delivery under rural conditions in post-tsunami Banda Aceh. J Perinat Med 2016;44:511–5. 10.1515/jpm-2015-0004 [DOI] [PubMed] [Google Scholar]
- 37. Stoll BJ, Kanto WP, Glass RI, et al. Treated maternal gonorrhea without adverse effect on outcome of pregnancy. South Med J 1982;75:1236–8. 10.1097/00007611-198210000-00020 [DOI] [PubMed] [Google Scholar]
- 38. Hitti J, Garcia P, Totten P, et al. Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex Transm Dis 2010;37:81–5. 10.1097/OLQ.0b013e3181bf5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burton AE, Thomas S. Sexually transmitted infections and preterm birth among Indigenous women of the Northern Territory, Australia: a case-control study. Aust N Z J Obstet Gynaecol 2019;59:147-153. 10.1111/ajo.12850 [DOI] [PubMed] [Google Scholar]
- 40. Heumann CL, Quilter LAS, Eastment MC, et al. Adverse birth outcomes and maternal Neisseria gonorrhoeae infection: a population-based cohort study in Washington state. Sex Transm Dis 2017;44:266–71. 10.1097/OLQ.0000000000000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amstey MS, Steadman KT. Asymptomatic gonorrhea and pregnancy. J Am Vener Dis Assoc 1976;3:14–16. [PubMed] [Google Scholar]
- 42. Christian P, Khatry SK, LeClerq SC, et al. Prevalence and risk factors of Chlamydia and gonorrhea among rural Nepali women. Sex Transm Infect 2005;81:254–8. 10.1136/sti.2004.011817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baer RJ, Chambers CD, Ryckman KK, et al. An evaluation of sexually transmitted infection and odds of preterm or Early-Term birth using propensity score matching. Sex Transm Dis 2019;46:389–94. 10.1097/OLQ.0000000000000985 [DOI] [PubMed] [Google Scholar]
- 44. Temmerman M, Lopita MI, Sanghvi HC, et al. The role of maternal syphilis, gonorrhoea and HIV-1 infections in spontaneous abortion. Int J STD AIDS 1992;3:418–22. 10.1177/095646249200300603 [DOI] [PubMed] [Google Scholar]
- 45. Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol 1988;159:397–404. 10.1016/S0002-9378(88)80093-0 [DOI] [PubMed] [Google Scholar]
- 46. Mason PR, Katzenstein DA, Chimbira TH, et al. Microbial flora of the lower genital tract of women in labour at Harare maternity hospital. The puerperal sepsis Study Group. Cent Afr J Med 1989;35:337–44. [PubMed] [Google Scholar]
- 47. Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect 2019;95:60–6. 10.1136/sextrans-2018-053597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kupka R, Kassaye T, Saathoff E, et al. Predictors of stillbirth among HIV-infected Tanzanian women. Acta Obstet Gynecol Scand 2009;88:584–92. 10.1080/00016340902835901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gichangi PB, Ndinya-Achola JO, Ombete J, et al. Antimicrobial prophylaxis in pregnancy: a randomized, placebo-controlled trial with cefetamet-pivoxil in pregnant women with a poor obstetric history. Am J Obstet Gynecol 1997;177:680–4. 10.1016/S0002-9378(97)70164-9 [DOI] [PubMed] [Google Scholar]
- 50. Pourabbas B, Rezaei Z, Mardaneh J, et al. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections among pregnant women and eye colonization of their neonates at birth time, Shiraz, southern Iran. BMC Infect Dis 2018;18:477. 10.1186/s12879-018-3382-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galega FP, Heymann DL, Nasah BT. Gonococcal ophthalmia neonatorum: the case for prophylaxis in tropical Africa. Bull World Health Organ 1984;62:95–8. [PMC free article] [PubMed] [Google Scholar]
- 52. Gichuhi S, Bosire R, Mbori-Ngacha D, et al. Risk factors for neonatal conjunctivitis in babies of HIV-1 infected mothers. Ophthalmic Epidemiol 2009;16:337–45. 10.3109/09286580903144746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dekkers OM, Vandenbroucke JP, Cevallos M, et al. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med 2019;16:e1002742. 10.1371/journal.pmed.1002742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wynn A, Bristow CC, Cristillo AD, et al. Sexually transmitted infections in pregnancy and reproductive health. Sex Transm Dis 2020;47:5–11. 10.1097/OLQ.0000000000001075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Low N Chlamydia trachomatis and reproductive health: what can we learn from systematic reviews of observational studies? Sex Transm Infect 2020;96:315–7. 10.1136/sextrans-2019-054279 [DOI] [PubMed] [Google Scholar]
- 56. Xiao P-L, Zhou Y-B, Chen Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015;15:246. 10.1186/s12884-015-0684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. WHO WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
- 58. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113 Suppl 3:17–42. 10.1111/j.1471-0528.2006.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vallely AJ, Pomat WS, Homer C, et al. Point-Of-Care testing and treatment of sexually transmitted infections to improve birth outcomes in high-burden, low-income settings: study protocol for a cluster randomized crossover trial (the wantaim trial, Papua New Guinea). Wellcome Open Res 2019;4:53. 10.12688/wellcomeopenres.15173.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grant J, Chico M, ACC L. Sexually transmitted infections in pregnancy: global challenges and opportunities. Sex Transm Dis 2020;47:779–89. 10.1097/OLQ.0000000000001258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sextrans-2020-054653supp001.pdf (4.8MB, pdf)