Abstract
Background
With the recent advent of advanced technologies in the field, treatment of neurovascular diseases using endovascular techniques is rapidly evolving. Here we describe our experience with pre-surgical simulation using the Biomodex EVIAS patient-specific 3D-printed models to plan aneurysm treatment using endovascular robotics and novel flow diverter devices.
Methods
Pre-procedural rehearsals with 3D-printed patient-specific models of eight cases harboring brain aneurysms were performed before the first in-human experiences. To assess the reliability of the experimental model, the characteristics of the aneurysms were compared between the patient and 3D models. The rehearsals were used to define the patient treatment plan, including technique, device sizing, and operative working projections.
Results
The study included eight patients with their respective EVIAS 3D aneurysm models. Pre-operative simulation was performed for the first in-human robotic-assisted neurovascular interventions (n=2) and new generation flow-diverter stents (n=6). Aneurysms were located in both the anterior (n=5) and posterior (n=3) circulation and were on average 11.0±6.5 mm in size. We found reliable reproduction of the aneurysm features and similar dimensions of the parent vessel anatomy between the 3D models and patient anatomy. Information learned from pre-surgical in vitro simulation are described in detail, including an improved patient treatment plan, which contributed to successful first in-world procedures with no intraprocedural complications.
Conclusions
Pre-procedural rehearsal using patient-specific 3D models provides precise procedure planning, which can potentially lead to greater operator confidence, decreased radiation dose and improvements in patient safety, particularly in first in-human experiences.
Keywords: aneurysm, angiography, flow diverter, intervention, technology
Introduction
New technologies have been in constant development in endovascular neurosurgery since the first Guglielmi detachable coils were deployed in the early 1990s.1 New concepts in the treatment of intracranial aneurysms (IA) have brought new devices, such as new-generation flow-diverter stents (FDS), and new technologies, such as endovascular robotics, all of which come with their own unique learning curves.2 Thus, even for senior interventionalists, previous experience with novel technologies flattens this learning curve, facilitates ease of use, and reduces technical complications.
There is a strong body of evidence to support the use of simulator-based technology in surgical education and procedure planning.3 4 Standard training focuses on either cadaveric models or on simulator technology.5 Cadaveric models are expensive and in limited supply, and do not translate well to endovascular-based simulation. This has stimulated the development of 'in vitro' alternatives for endovascular training.6 Training models are available that can be generated based on medical imaging data.7 These aim to replicate the anatomy of an individual patient in a three-dimensional structured template. The most recent iterations of these simulators are also connected to a hydraulic system that reproduces patient blood flow for a more realistic experience.8–12 Pre-procedural training has been shown in various other fields of surgery to improve surgical skills and operational success rates, while decreasing operator time and technical errors, with a more pronounced benefit for early-career physicians.13
In this study, we describe our experience with pre-surgical simulation using patient-specific, 3D-printed models to plan aneurysm treatment for early in-human experiences of newer-generation FDS14 15 and robotic-assisted endovascular procedures.16
Methods
We included eight consecutive patients harboring intracranial aneurysms scheduled for treatment using new devices or techniques. Two cases included simulation of stent-assisted coiling treatments using the assistance of a robotic arm (CorPath GRX, Corindus, a Siemens Healthineers Company, Waltham, MA, USA), and six included rehearsal for first time use of new-generation FDS, including the Silk Vista Baby (SVB) (Balt, Montmorency, France) and Surpass Evolve (Stryker, Fremont, California). All rehearsals and procedures were performed by the same operator (VMP). We evaluated clinical and procedural factors as well as short-term patient outcomes.
Patient-specific 3-D printed aneurysm models
The training models used (Endovascular Intracranial Aneurysm Solution – EVIAS – Biomodex, Paris, France) were manufactured based on computed tomography angiography (CTA) scans acquired before the procedure. Images were segmented, post processed, and 3D-printed using the following specifications:
Image acquisition
CTA images acquired with a 0.5 mm3 isotropic resolution (Toshiba Aquilion One Scanner).
Segmentation
Biomodex uses a third-party segmentation service ISO 13485 certified and proprietary software suite CE- and FDA-cleared that accepts DICOM-compliant medical images acquired from a variety of imaging devices, including CT and MR. The segmentation method is based on an automated global hierarchical approach completed by an interactive process of local correction by anatomical experts using their proprietary software. The hierarchical approach consists of the sequential delineation of organs and pathologies. Each delineation is performed using density, morphological, and geometrical-based algorithms.
Post-processing
The 3D segmentation is edited for anatomical accuracy and verified by two radiological technicians specialized in anatomopathology and medical image processing. Then the 3D model is further edited by a Biomodex computer assisted design technician to adapt the segmented model to the Biomodex cartridge design. This is performed by the same operators each time to reduce variability.
3D-printing
The final step in 3D printing is performed by a company called Statasys, and has the following configuration:
Layer thickness: down to 27 microns
Accuracy of the printer accordingly: up to 200 microns
Materials: photopolymer acrylates
The aneurysm model is mounted on a standardized frame, which is then connected to the EVIAS station that includes a hydraulic system that replicates the blood flow by pumping a specific blood-analog fluid through the vessels (online supplementary figure 1A). Models were printed using a proprietary algorithm called INVIVOTECH. It combines soft and rigid materials at the microscopic level to produce a more realistic mechanical behavior of the 3D-printed models. Unlike single-material models such as silicone, 3D-printed anatomies are made with various flexible photopolymers which allow control of the pliability of the target area, providing critical tactile feedback. In this way, the internal structure of the vascular model simulates the friction against the arterial wall and hemodynamic forces experienced during catheter navigation. The hemodynamics of the model system are as follows.
neurintsurg-2020-015990supp001.pdf (26MB, pdf)
Blood mimicking fluid
The proprietary 'BloodSim' formula contains the following properties: viscosity: μ=(4.3554±0.0001).10–3 Pa.s (the viscosity of blood at 37°C is normally 3×10–3 to 4×10–3); density: P=1.030+/−0.005 g.cm-3 (average density around 1060 kg/m3).
Flow rates
The pump is set to 180–200 mL/min. Actual flow rate fluctuates between 2–4 mL/s depending on the anatomy of the loaded cartridge.
The EVIAS system was set up in the angiosuite using the typical clinical set-up, including flushes and automatic injector pump, to simulate the real patient procedure (online supplementary figure 1B).
In order to assess the reliability of the experimental model, we analyzed the patient’s anatomy (size of proximal and distal parent vessel: the presence of side branch vessel) and the characteristics of the intracranial aneurysm (aneurysm type and shape; aneurysms sizing – neck, dome, diameter, irregularities in the aneurysm sac) from the intra-operative 3D-RA images and compared these measurements to the measurements made on the 3D-RA from the 3D models. Stent sizes were estimated based on the 3Ds acquired from the models during the simulations. Similarly, achievable biplane working angles were also planned using the 3D models. In the case of the robotic-assisted procedures, device compatibility with the robotic system (guide-/intermediate-/micro-catheter combinations+stent & coils) were tested.
Study population and outcomes
During rehearsal in the 3D models, we evaluated if the device that was chosen was appropriate and also practiced techniques for deployment and assessed the feasibility of access, support, and procedure planning. Furthermore, any technical issues were recorded during the training and the clinical use, such as: recapture and repositioning; poor opening of the proximal or distal ends of the stent; oversized device; need for second device; need for removal of the device; and incorrect placement with partial aneurysm coverage. Additionally, we subjectively evaluated the relevance of the patient-specific 3D models in terms of anatomic details and determination of a treatment strategy for each individual case, and we present the highlights of this evaluation in a case report style.
Statistical analysis
Continuous variables were compared using the student t-test. Statistical analysis was conducted with Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, version 24.0. Armonk, NY: IBM Corp.). Statistical significance was assumed for P<0.05.
Results
We included eight patient-specific simulation models used for training before the patient’s treatment: six before first time novel FDS treatments and two before the first in-world robotic-assisted intracranial endovascular neurointerventional procedures. The mean age was 60.3±9.0 years. All aneurysms were diagnosed as incidental findings (n=8; 100%). Aneurysms treated with the Surpass Evolve arose from either the paraophthalmic (n=4; 50%) or cavernous segment (n=1; 12.5%) of the internal carotid artery, while the patient treated with the SVB had a dissecting aneurysm of the posterior inferior cerebellar artery (PICA) (n=1; 12.5%). The two patients treated with robotic assistance had aneurysms arising from the basilar artery (n=2; 25%) (table 1). Patients possessed various aneurysm risk factors including hypertension (n=3; 37.5%), family history of aneurysms (n=2; 25%), smoking (n=1; 12.5%), multiple aneurysms (n=1; 12.5%), or dyslipidemia (n=1; 12.5%).
Table 1.
Patients' aneurysms characteristics vs patient-specific models
| Patients (n%) | 3D models (n%) | P-value | |
| Location | |||
| Paraophthalmic | 4 (50%) | – | |
| Cavernous | 1 (12.5%) | – | |
| PICA | 1 (12.5%) | – | |
| Basilar | 2 (25%) | ||
| Aneurysm type | |||
| Saccular | 7 (87.5%) | – | |
| Dissecting | 1 (12.5%) | – | |
| Irregular shape (lobulation) | 1 (11.1%) | 3 (37.5%) | – |
| Dome (mm) | 6.7±2.1 | 5.9±2.6 | 0.74 |
| Neck (mm) | 5.9±2.6 | 6.0±1.6 | 0.28 |
| Dome:neck ratio | 1.19±0.2 | 1.21±0.2 | 0.50 |
| Parent vessel (mm) | |||
| Proximal | 3.5±0.4 | 3.2±0.6 | 0.17 |
| Distal | 4.0±0.8 | 3.2±0.5 | 0.19 |
| Aneurysm size | 11.0±6.5 | 11.2±8.5 | 0.58 |
| Side branch vessel | 1 (11.1%) | 1 (11.1%) | – |
PICA, posterior inferior cerebellar artery.
For the flow divertor series (n=6), the Surpass Evolve stent was used in five patients and the SVB in one patient. The stent sizes ranged from 3.25×25 mm (SVB) to 5.0×40 mm (Evolve). In four out of the six (66.7%) flow divertor cases, the same stent size was deployed in the patient as was chosen during the rehearsal, with the stent size being underestimated in the model in one (16.7%) case and oversized in another (16.7%) case. For the robotic-assisted stent assisted coiling procedures, an Atlas stent (4.25×21 mm; Stryker, Freemont, California) was placed followed by various Axium coils (eV3, Medtronic, Irvine, California). There were no intraprocedural complications.
Comparing the patient-specific 3D models and the patient’s anatomy, we found a very reliable reproduction of the aneurysm features and similar dimensions of the parent vessel anatomy. There were no statistical differences in sizes of the IAs and parent vessels between the simulation models and patients (table 1). The biplane working projections for stent deployment that were previously rehearsed by using the 3D models were all successfully replicated during the patient’s treatment. The main concerns about the 3D models were related to the aneurysm shapes and the vessel tortuosity: it was felt that ‘in vivo’ the arteries were smoother and more tortuous than in the ‘in vitro’ models. Each of the rehearsals provided additional value by allowing the operator to practice challenging maneuvers, such as how to open FDS in tortuous anatomies or how to safely cross through the Atlas stent using the robotic arm to coil the aneurysm. The following are a selection of representative case illustrations to describe these case-specific learnings in greater detail.
Subjective assessment of 3D models: representative case illustrations
Case 1– First in-human Surpass Evolve: maneuvers to improve apposition of flow diverter stent in tortuous anatomy
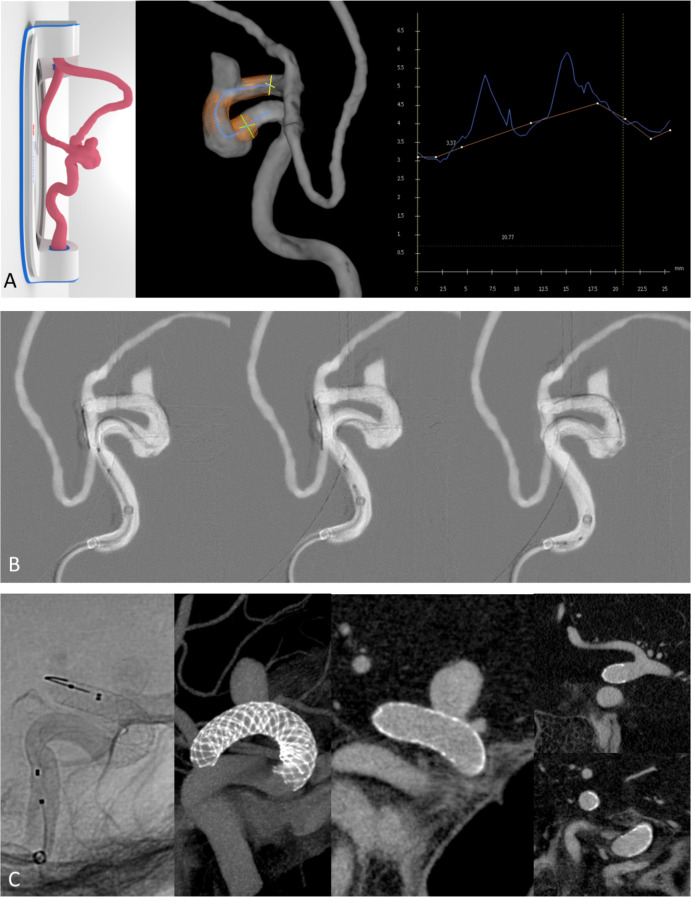
A patient with hypertension and family history of subarachnoid hemorrhage presented with an 8 mm left-sided paraophthalmic aneurysm. This case was challenging, as the carotid cave segment was tortuous and dysplastic. A 3D model was made based on the CTA for pre-treatment simulation. We used the opportunity of the simulation to see how the stent would behave in the curve and understand the degree of stent foreshortening in the dysplastic segment. The working projections were defined and measurements were made to plan the stent size. Although the model measured 3.4 mm distally and 4.0 mm proximally with a 21 mm predicted stent length (figure 1A, right), we only had a 4.5×25 mm stent available for experimentation purposes. Despite being longer than desired, we learned that by alternating between pushing the stent and putting forward slack on the system we were able to open the stent in the curve. We also learned that the proximal end of the stent was more difficult to open after this curve (figure 1B, middle), so this is why we decided to use a 4.0×20 mm stent for the patient procedure. This resulted in a precise proximal landing with excellent stent opening and apposition along the parent artery wall (figure 1C). Three months later, the aneurysm was completely occluded on imaging follow-up.
Figure 1.
(A) 3D-printed model demonstrated (left) with 3DRA (middle) and endoluminal cross-section analysis (right). Model measures 3.4 mm distally (yellow) and 4.0 mm proximally (green). Centerline length from planned proximal to distal landing zone approximately 20.8 mm. (B) processed fluoroscopy runs show kink of the stent in the model at the level of the dysplastic carotid cave segment (left). the middle panel shows the difficultly in opening the stent (4.5×25 mm Surpass Evolve) proximal to this curve. The stent was deployed and then the microcatheter was used to re-enter the stent over the stent pusher wire in order to open the stent. (C) Unsubtracted DSA (left) and VasoCT images from the patient procedure demonstrate excellent stent (4.0×20 mm Surpass Evolve) apposition over the neck (middle) and distal (right, top) and proximal (right, bottom) landing zones.
Case 2 – Treatment strategy and working projections for a giant aneurysm
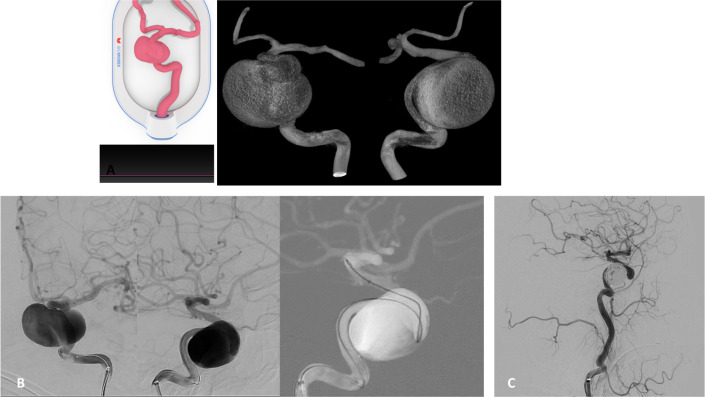
A patient had an incidental finding of a 20 mm aneurysm in the cavernous segment of the right internal carotid artery (ICA). We planned a flow diverter for patient’s treatment using a 3D-printed model. During the rehearsal, the main challenge was to define an ideal working projection for microcatheter navigation and placement of the stent since the aneurysm sac imaging on the digital subtraction angiography (DSA) would always overlap the parent vessel image on any single projection. Using the working angles selected during the model simulation, the microcatheter was efficiently navigated across the aneurysm neck and the stent was placed with excellent wall apposition during the patient procedure. A microcatheter was jailed in the aneurysm between the stent, and coils were placed inside the aneurysm sac. At 4-months' follow-up, the aneurysm was completely occluded (figure 2).
Figure 2.
(A) Cartridge (left) and surface rendered 3D-rotational angiogram (3DRA)(right) demonstrate 3D-printed model and working projects for navigation of right-sided giant cavernous IA. (B) DSA (left) and fluoroscopy roadmap image (right) demonstrate mimicked anteroposterior (AP) and lateral working projection views selected from the pre-procedural simulation that allowed for successful catheterization of the distal ICA. (C) DSA control run after coiling the aneurysm sac with jailed microcatheter, demonstrating total occlusion – Raymond–Roy scale I.20
Case 3 – Simulating the world’s first robotic-assisted intracranial neurointervention
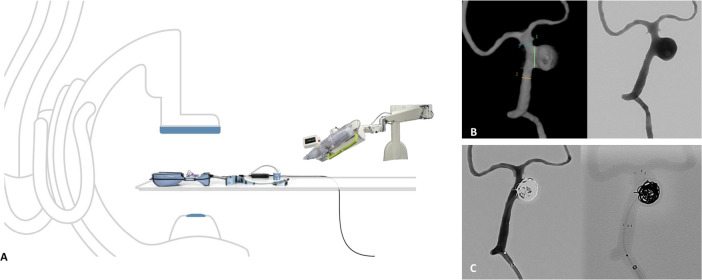
A patient presented to the ER with severe vertigo. The CTA revealed an 11 mm wide-necked saccular basilar aneurysm arising at the left lateral aspect of the basilar artery between the origins of the anterior inferior cerebellar artery (AICA) and superior cerebellar artery (SCA). The patient was followed-up in clinic and consented to robotic stent-assisted coiling using the GRX neuro system from Corindus (Walthan, MA, USA). Two case simulations using 3D-printed models of the patient’s specific anatomy were performed the day before the procedure. During the simulation, access equipment for the triaxial treatment approach were fine-tuned for optimal robotic compatibility, and stent and coil sizing were planned. During the simulation, after the Neuroform Atlas (Stryker, Freemont, California) stent was placed, the robotic operator encountered some difficulty crossing through the stent with the microcatheter to proceed with aneurysm coiling. Interestingly, this procedural challenge was precisely replicated during the patient procedure as well. The operator said they felt more confident handling this challenge since they had already practiced it during the simulation. The robotic-assisted treatment was a success, with the stent being placed with millimetric precision and 14 coils densely packing the aneurysm sac (figure 3), and no intraprocedural complications.
Figure 3.
(A) Angiosuite room with aneurysm model set-up and robotic arm. (B) surface rendered 3D DSA (left) demonstrating a 12 mm lA in simulated model (1–7.6 mm; 2–3.32 mm; 3–3.22 mm); DSA AP view (right) demonstrating working projection for treatment of the saccular basilar Ia. (C) DSA control run (left) demonstrating total aneurysm occlusion and unsubtracted left vertebral run (right) showing precise stent position and coil placement using the robotic system in the simulated model. Corindus Inc. used with permission.
Discussion
The use of simulation in surgical training is well established. It has been shown to improve trainee confidence, decrease errors, improve procedural success, and, more importantly, has improved patient safety.17 Endovascular simulation in particular has been shown to decrease procedure time, fluoroscopy time, and contrast volume administered.2 18 19 Most endovascular simulators are based on a combination of computer hardware and software. These simulators offer many advantages, however, there are some significant limitations, such as the lack of haptic feedback, limited applicability to novel devices, as well as the tactile ‘feel’ of such novel devices inside a given patient anatomy. Most simulators also lack accurate simulacra of patient hemodynamics.
The 3D-printed patient-specific models we used allowed us to simulate our imaging working angles as well as mimic patient hemodynamics, providing a high-fidelity replica of the patient’s anatomy. Performing the simulation using these models on the same fluoroscopy equipment as the actual ‘in vivo’ case allowed the team to better delineate the patient's anatomy, to plan working angles, and to assess procedural feasibility and technical strategies using these novel stents or robotic equipment. Furthermore, simulation using the 3D-printed patient-specific models to rehearse for novel/first-in-man procedures, such as the robotic-assisted cases or Evolve FDS cases, provided the operator with improved confidence through learned experience when treating the actual patient.
An ideal 3D 'in vitro' model requires a patient-specific reconstruction, spatial accuracy with a uniform scaling compared with a patient, and allow a degree of tactile feedback.9 Many of the previously described simulation models are limited in one or more of these regards.6 The Biomodex EVIAS model we used provided several advantages in this respect. Our confidence in the spatial accuracy of the model allowed us to predict and plan the landing zones for the FDS deployment and the stent sizes we would require, as well as be confident regarding the biplane angiographic working angles we could use.
One of the main downsides of these simulation models are their relatively high cost.10 It must be considered, though, that for complex giant aneurysms or when using novel devices or robotic equipment for the first time, the added patient safety gained by use of the pre-surgical simulation might be worth this cost. Less expensive 3D models can potentially be created in-house as an alternative to the expensive manufactured templates,9 10 however, these models may be constructed from silicone which may be flimsy and may not provide the same realistic feedback or vessel wall response to devices as was seen with the models used in this study. In addition, over time it is likely the 3D-printing methods will become more affordable, allowing more widespread use of these pre-surgical simulation tools in the neurovascular practice.
Limitations
Our study has some limitations. This is a single center, single-arm study with a relatively small sample size. At the present time, we believe that the pre-surgical rehearsal models should ideally be used for selected complex cases where the benefits of pre-procedural rehearsal are clear. There were some concerns regarding vessel tortuosity and the irregularity of the aneurysm sac in the models. The aneurysm sac was more irregular in the flow models than they appeared from the formal angiogram and the parent artery diameters were between 10%–20% smaller, which could have been a result of the spatial resolution of the CTA or 'smoothing' that happened during conversion of the CTA into a 3D- model. Ideally 3DRA imaging, which has a higher spatial resolution, is recommended as an input for 3D-modeling, however, diagnostic angiograms are not standard of care at our institution and we were limited to the imaging resolution of CTA. This being said, this slight difference in the appearances of the aneurysm wall did not influence the procedure rehearsal since the FDS consist of an extrasaccular reconstructive technique.
Conclusions
Pre-procedural use of 3D-printed patient-specific simulation models provides enormous training and rehearsal advantages for the endovascular treatment of intracranial aneurysms. We believe that for complex anatomies, such as giant aneurysms, or in those cases where novel devices or equipment are being used, the benefits of a pre-procedural experience in a patient-specific model might allow more precise procedure planning, decrease procedure time and radiation exposure, and, above all, improve patient safety. This technology is likely to play a growing role in the field of endovascular neurointervention in the future.
Footnotes
VNY and NMC contributed equally.
Contributors: NMC and VMP contributed to the design of the study. All authors contributed to the acquisition and/or analysis of the data, and the drafting and/or editing of the manuscript. All authors approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Research ethics board approval was not required as the study did not involve human participants.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request.
References
- 1. Guglielmi G, Viñuela F, Sepetka I, et al. . Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1–7. 10.3171/jns.1991.75.1.0001 [DOI] [PubMed] [Google Scholar]
- 2. Sullivan S, Aguilar-Salinas P, Santos R, et al. . Three-dimensional printing and neuroendovascular simulation for the treatment of a pediatric intracranial aneurysm: case report. J Neurosurg Pediatr 2018;22:672–7. 10.3171/2018.6.PEDS17696 [DOI] [PubMed] [Google Scholar]
- 3. Stefanidis D, Sevdalis N, Paige J, et al. . Simulation in surgery: what's needed next? Ann Surg 2015;261:846–53. 10.1097/SLA.0000000000000826 [DOI] [PubMed] [Google Scholar]
- 4. Nawka MT, Spallek J, Kuhl J, et al. . Evaluation of a modular in vitro neurovascular procedure simulation for intracranial aneurysm embolization. J Neurointerv Surg 2020;12:214–9. 10.1136/neurintsurg-2019-015073 [DOI] [PubMed] [Google Scholar]
- 5. Wang J-L, Yuan Z-G, Qian G-L, et al. . 3D printing of intracranial aneurysm based on intracranial digital subtraction angiography and its clinical application. Medicine 2018;97:e11103. 10.1097/MD.0000000000011103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torres I, De Luccia N. Artificial vascular models for endovascular training (3D printing). Innov Surg Sci 2018;3:225–34. 10.1515/iss-2018-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chueh JY, Wakhloo AK, Gounis MJ. Neurovascular modeling: small-batch manufacturing of silicone vascular replicas. AJNR Am J Neuroradiol 2009;30:1159–64. 10.3174/ajnr.A1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan IS, Kelly PD, Singer RJ. Prototyping of cerebral vasculature physical models. Surg Neurol Int 2014;5:11. 10.4103/2152-7806.125858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chivukula VK, Levitt MR, Clark A, et al. . Reconstructing patient-specific cerebral aneurysm vasculature for in vitro investigations and treatment efficacy assessments. J Clin Neurosci 2019;61:153–9. 10.1016/j.jocn.2018.10.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson JR, Thompson WL, Alkattan AK, et al. . Three-dimensional printing of anatomically accurate, patient specific intracranial aneurysm models. J Neurointerv Surg 2016;8:517–20. 10.1136/neurintsurg-2015-011686 [DOI] [PubMed] [Google Scholar]
- 11. Cancelliere NM, Nicholson P, Radovanovic I, et al. . Comparison of intra-aneurysmal flow modification using optical flow imaging to evaluate the performance of Evolve and Pipeline flow diverting stents. J Neurointerv Surg 2020. 10.1136/neurintsurg-2019-015696. [Epub ahead of print: 21 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaneko N, Mashiko T, Namba K, et al. . A patient-specific intracranial aneurysm model with endothelial lining: a novel in vitro approach to bridge the gap between biology and flow dynamics. J Neurointerv Surg 2018;10:306–9. 10.1136/neurintsurg-2017-013087 [DOI] [PubMed] [Google Scholar]
- 13. Marconi S, Pugliese L, Botti M, et al. . Value of 3D printing for the comprehension of surgical anatomy. Surg Endosc 2017;31:4102–10. 10.1007/s00464-017-5457-5 [DOI] [PubMed] [Google Scholar]
- 14. Orru E, Rice H, De Villiers L, et al. . First clinical experience with the new Surpass Evolve flow diverter: technical and clinical considerations. J Neurointerv Surg 2020. 10.1136/neurintsurg-2019-015734. [Epub ahead of print: 12 Feb 2020]. [DOI] [PubMed] [Google Scholar]
- 15. Martínez-Galdámez M, Biondi A, Kalousek V, et al. . Periprocedural safety and technical outcomes of the new Silk Vista Baby flow diverter for the treatment of intracranial aneurysms: results from a multicenter experience. J Neurointerv Surg 2019;11:723–7. 10.1136/neurintsurg-2019-014770 [DOI] [PubMed] [Google Scholar]
- 16. Mendes Pereira V, Cancelliere NM, Nicholson P, et al. . First-in-human, robotic-assisted neuroendovascular intervention. J Neurointerv Surg 2020;12:338–40. 10.1136/neurintsurg-2019-015671.rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cook DA, Hatala R, Brydges R, et al. . Technology-enhanced simulation for health professions education: a systematic review and meta-analysis. JAMA 2011;306:978–88. 10.1001/jama.2011.1234 [DOI] [PubMed] [Google Scholar]
- 18. Miranpuri AS, Nickele CM, Akture E, et al. . Neuroangiography simulation using a silicone model in the angiography suite improves trainee skills. J Neurointerv Surg 2014;6:561–4. 10.1136/neurintsurg-2013-010826 [DOI] [PubMed] [Google Scholar]
- 19. See KWM, Chui KH, Chan WH, et al. . Evidence for endovascular simulation training: a systematic review. Eur J Vasc Endovasc Surg 2016;51:441–51. 10.1016/j.ejvs.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 20. Mascitelli JR, Moyle H, Oermann EK, et al. . An update to the Raymond–Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 2015;7:496–502. 10.1136/neurintsurg-2014-011258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2020-015990supp001.pdf (26MB, pdf)