Abstract
Objective
To generate a score which clinically identifies surface-directed autoantibodies in adults with new-onset focal epilepsy, and evaluate the value of immunotherapy in this clinical setting.
Methods
Prospective clinical and autoantibody evaluations in a cohort of 219 consecutive patients with new-onset focal epilepsy.
Results
10.5% (23/219) of people with new-onset focal epilepsy had detectable serum autoantibodies to known or novel cell surface antigenic targets. 9/23 with autoantibodies were diagnosed with encephalitis, by contrast to 0/196 without autoantibodies (p<0.0001). Multivariate analysis identified six features which predicted autoantibody positivity (area under the curve=0.83): age ≥54 years, ictal piloerection, lowered self-reported mood, reduced attention, MRI limbic system changes and the absence of conventional epilepsy risk factors. 11/14 (79%) patients with detectable autoantibodies, but without encephalitis, showed excellent long-term outcomes (modified Rankin Score=0) despite no immunotherapy. These outcomes were superior to those of immunotherapy-treated patients with confirmed autoantibody-mediated encephalitis (p<0.05).
Conclusions
Seizure semiology, cognitive and mood phenotypes, alongside inflammatory investigation findings, aid the identification of surface autoantibodies among unselected people with new-onset focal epilepsy. The excellent immunotherapy-independent outcomes of autoantibody-positive patients without encephalitis suggests immunotherapy administration should be guided by clinical features of encephalitis, rather than autoantibody positivity. Our findings suggest that, in this cohort, immunotherapy-responsive seizure syndromes with autoantibodies largely fall under the umbrella of autoimmune encephalitis.
Keywords: autoimmune encephalitis, epilepsy, neuroimmunology
Introduction
Neuronal surface-directed antibodies (NSAbs) are considered pathogenic in patients with autoimmune encephalitis (AE). AE commonly presents with prominent seizures and neuropsychiatric features and shows a preferential response to immunotherapies versus anti-seizure medications (ASMs).1–4 This has prompted the introduction of ‘epilepsy of immune aetiology’ within the International League Against Epilepsy (ILAE) 2017 classification.5 The same NSAbs, as well as high levels of antibodies to intraneuronal glutamic acid decarboxylase-65 (GAD65), are also described in the serum of people with more isolated forms of epilepsy, without core features of encephalitis.6–8 In this context, their clinical, aetiological and therapeutic relevance is unclear, but of major potential importance to all neurologists who manage new-onset epilepsy. In our large, prospective, real-world study of new-onset focal epilepsy, we predicted that formes frustes of AE would help identify clinical features suggesting the presence of NSAbs and asked whether detection of these NSAbs should alter patient management.
Materials and methods
Between 9 December 2011 and 4 November 2015, consecutive adult patients (≥18 years) with a diagnosis of new-onset focal epilepsy and their first seizure within the previous 12 months were prospectively recruited from the routine practice of two epileptologists at the Oxford University Hospitals NHS Foundation Trust. Written informed consent and sera were obtained (Ethical approvals: Oxfordshire RECA 07/Q160X/28 and REC16/YH/0013). Clinical data gathered at onset (online supplemental table 1) included detailed phenotype and investigation results, Quality of Life in Epilepsy-31, Hospital Anxiety and Depression Score, Addenbrooke’s Cognitive Examination (ACE) and modified Rankin Score (mRS); as well as information to inform the Antibody Prevalence in Epilepsy and Encephalopathy (APE2) score (online supplemental table 2)9 10 and diagnostic criteria for possible or definite AE.11 Subsequently, 1-year and 3-year mRS were ascertained from patients with NSAbs.
jnnp-2020-325011supp001.pdf (83.6KB, pdf)
For NSAbs, sera were tested against autoantigen-expressing live HEK293 cells (live cell-based assay; online supplemental table 3), and for reactivity with the surface of live cultured hippocampal neurons, using sensitive protocols.12 13 Autoantibodies to GAD65 were determined using a commercial radioimmunoprecipitation assay.
Statistical analysis was conducted in R (V.3.6.1). Dimensionality reduction was performed using Multiple Factor Analysis in ‘FactoMineR’ with up to 10% missing data imputed using missForest. Stepwise Bayesian general linear modelling analysis was undertaken using ‘arm’. Wilson 95% CIs with continuity correction were calculated using ‘DescTools’.
Results
NSAb findings
Of 241 recruited patients, 22 were excluded (online supplemental table 4). Of the remaining 219, median age was 49 years (range 16–91) and 109 (49.8%) were female. In 23/219 (10.5%) patients, serum NSAbs were detected across candidate and novel autoantigens (table 1) including roughly equal frequencies against leucine-rich glioma inactivated-1 (LGI1), contactin-associated protein-like 2 (CASPR2), plus the N-methyl-d-aspartate receptor (NMDAR) and γ-aminobutyric acid A/B receptors (GABAAR and GABABR). An additional five patients had antibodies to the surface of live neurons, without an established autoantigen. Autoantibodies to contactin-2, the glycine receptor and the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) were each found in one patient. No dipeptidyl-peptidase-like protein 6 (DPPX) or high-titre GAD65 antibodies were detected. Overall, from the 23 people with NSAbs, 9 had a clinical diagnosis of AE (7/9 fulfilling published criteria).11 By contrast, none of the 196 without NSAbs had a clinical diagnosis of AE (p<0.0001; Fisher’s exact test).
Table 1.
Clinical and laboratory features of patients with epilepsy and positive neuronal surface autoantibodies
| Age | Sex | Autoantibody | Live hippocampal neuron binding | APE2 score | Criteria AE | Clinical AE | Pilo | Epil RF | Lowered Mood | ACE Att | MRI Δlimbic | Number of ASMs | mRS 1 year |
mRS 3 years | Immunotherapy |
| 79 | M | CASPR2 | + | 3 | Definite | Yes | No | None | No | 14 | Yes | 2 | 3 | 2 | IVMP, Pred, IVIg, PLEX |
| 55 | F | CASPR2 | 6 | Definite | Yes | No | None | Yes | 18 | Yes | 3 | 2 | 2 | IVMP, Pred, IVIg, PLEX | |
| 55 | F | GABABR | + | 9 | Definite | Yes | No | None | No | NA | Yes | 6 | 6 | NA | IVMP, Pred, PLEX |
| 18 | F | NMDAR | + | 8 | Definite | Yes | No | None | Yes | 15 | Yes | 3 | 2 | 2 | IVMP, Pred, PLEX, Aza, MMF |
| 60 | F | NMDAR | 4 | Definite | Yes | No | None | Yes | 13 | No | 3 | 0 | 0 | Pred | |
| 77 | F | GABAAR, GABABR | + | 8 | Possible | Yes | No | None | Yes | 18 | Yes | 4 | 1 | 0 | Pred |
| 54 | M | LGI1 | + | 6 | Possible | Yes | Yes | None | Yes | 18 | Yes | 2 | 0 | 0 | None |
| 59 | F | LGI1 | + | 4 | No | Yes | No | None | Yes | 18 | No | 1 | 1 | 0 | None |
| 69 | M | Unknown | + | 4 | No | Yes | No | None | No | 13 | No | 1 | 1 | 2 | Pred, Aza |
| 63 | M | AMPAR | + | 2 | No | No | No | None | No | NA | No | 1 | 0 | 0 | None |
| 28 | M | CASPR2 | 2 | No | No | No | FHx | Yes | 17 | No | 1 | 0 | 0 | None | |
| 41 | F | CASPR2 | 2 | No | No | No | None | Yes | 18 | No | 0 | 0 | 0 | None | |
| 75 | F | Contactin-2 | 1 | No | No | No | None | No | NA | No | 1 | 0 | 0 | None | |
| 37 | F | GABAAR | 2 | No | No | No | None | Yes | 18 | No | 2 | 0 | 0 | None | |
| 36 | F | GABABR | 2 | No | No | No | None | No | 18 | No | 1 | 1 | 0 | None | |
| 69 | F | GlyR | 4 | No | No | No | None | Yes | 15 | No | 4 | 1 | 1 | None | |
| 61 | M | LGI1 | + | 2 | No | No | Yes | None | Yes | 18 | No | 1 | 0 | 0 | None |
| 36 | F | NMDAR | 2 | No | No | No | None | Yes | 18 | No | 1 | 2 | 2 | None | |
| 68 | F | NMDAR, GABAAR | 1 | No | No | No | None | No | 18 | No | 1 | 0 | 0 | None | |
| 77 | F | Unknown | + | 2 | No | No | No | None | Yes | NA | No | 0 | 0 | NA | None |
| 55 | F | Unknown | + | 4 | No | No | No | None | Yes | 18 | No | 5 | 2 | 2 | None |
| 91 | M | Unknown | + | 1 | No | No | No | None | No | 9 | No | 1 | 6 | NA | None |
| 37 | M | Unknown | + | 2 | No | No | No | None | Yes | 18 | No | 2 | 0 | 0 | None |
APE2 score of 4 or more is considered positive. Diagnosis of encephalitis based on clinical impression (‘Clinical AE’; corresponding rows shaded in grey) or published criteria (‘Criteria AE’), as per Graus et al.11
‘Unknown’ autoantibody specificities reflect IgG binding to the surface of live hippocampal neurons, without a known antigenic target.
ACE Att, Addenbrooke’s Cognitive Examination attention domain score; AE, autoimmune encephalitis; APE2, Antibody Prevalence in Epilepsy and Encephalopathy Score; ASM, anti-seizure medication; Aza, azathioprine; Epil RF, epilepsy risk factors (neonatal, perinatal, focal neurological insults, family history of epilepsy or Alzheimer’s disease); FHx, family history; IVIg, intravenous immunoglobulin; IVMP, intravenous methylprednisolone; mRS, modified Rankin Score; Myco, mycophenolate; NA, not available; PLEX, plasma exchange; Pred, prednisolone.
Factors associated with the presence of NSAbs and AE
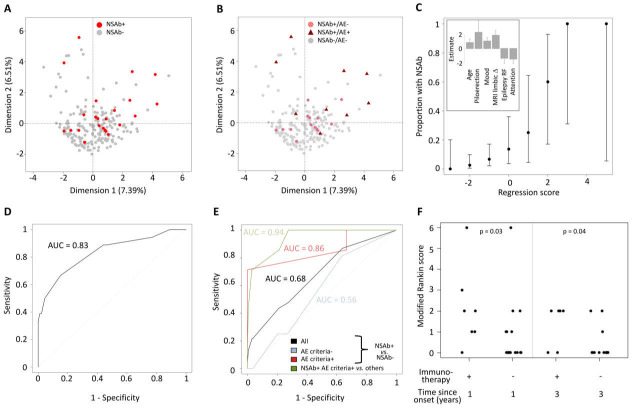
Dimensionality reduction with multiple factor analysis showed that patients were highly heterogeneous and the modest clustering of those with NSAbs was largely driven by a clinical diagnosis of AE (figure 1A, B). Univariate analysis identified 11 clinical parameters that differed significantly between patients with and without NSAbs: age (p=0.04), ictal piloerection (p=0.02), lesional MRI (p=0.04), self-reported mood disturbance (p=0.007), ACE attention domain (p=0.01), ACE total score (p=0.04), QOLIE-31 score (p=0.02), self-reported neuropsychiatric features (p=0.03), epilepsy risk factors (p=0.05), inflammatory cerebrospinal fluid (CSF; p=0.004) and limbic system lesions on MRI (p=0.0002). A multivariate stepwise regression model allocated weighted scores to six of these: age ≥54 years=+1, self-reported mood disturbance=+1, limbic system lesions on MRI=+2, ictal piloerection=+2.5, ACE attention score ≥16=−1.5 and epilepsy risk factors=−1.5 (figure 1C). The probability of NSAb positivity increased with higher scores (Spearman’s ρ=0.99, p<0.0001; figure 1C) and receiver operating characteristic (ROC) analysis confirmed these features strongly predicted NSAb status (area under the curve (AUC)=0.83; total score ≥0; sensitivity=66.7%, specificity=84.9%; figure 1D). By contrast, the APE2 score performed less well in predicting NSAb status (sensitivity 43.5%, specificity 79.1%, AUC=0.68) and more accurately predicted criteria-defined AE, particularly if associated with NSAbs (sensitivity 85.7%, specificity 78.8%, AUC=0.94; figure 1E).
Figure 1.
Clinical phenotypes associated with NSAb status in new-onset focal epilepsy. The first two dimensions are shown, highlighting: (A) NSAb-positive (red) or NSAb-negative (grey) status and (B) NSAb-positive (pale red) or NSAb-negative (grey) without encephalitis (dots), or NSAb-positive (dark red) with clinically diagnosed autoimmune encephalitis (triangles). (C) The proportion of patients by total model score. Error bars show 95% CIs. The inset shows the weighting and SE of each factor within the regression model. (D) Receiver operator characteristic (ROC) curve of the total model score for predicting NSAb status across all patients. (E) ROC curve of the APE2 score for predicting NSAb status across all patients (black), patients not meeting the criteria for autoimmune encephalitis (blue), patients meeting the criteria for autoimmune encephalitis (red) and predicting NSAb-positive criteria-confirmed autoimmune encephalitis across all patients. (F) Scatter plot of modified Rankin score in NSAb-positive patients by immunotherapy status over time (Mann-Whitney U test p values<0.05). AE, autoimmune encephalitis; APE2, Antibody Prevalence in Epilepsy and Encephalopathy; epilepsy RF, epilepsy risk factors; MRI limbic Δ, changes within the limbic system on MRI.
Comparisons of those with and without AE
From 23 patients with NSAbs (table 1), a comparison of those with (n=9) and without (n=14) a clinical diagnosis of AE revealed several differences in the AE cohort: more ASMs (median of 3 vs 1; p=0.0073), more frequent immunotherapies (7/9 vs 0/14, p=0.0001), higher APE2 scores (median of 6 vs 2; p<0.0001), more frequent MRI limbic inflammation (6/9 vs 0/14; p=0.0008) and a trend towards greater positivity of serum IgGs targeting the surface of live neurons (7/9 vs 5/14, p=0.09). Compared with the seven patients administered immunotherapy, those with NSAbs who were not administered immunotherapy showed lower disability after 1 and 3 years (both p<0.05), and 11/16 (68.8%) were asymptomatic at 3-year follow-up (mRS=0; figure 1F). Hence, despite no immunotherapy, patients with NSAbs, but without AE, generally showed good outcomes.
Discussion
In this prospective study of 219 consecutive adults with new-onset focal epilepsy, NSAb status was best predicted by a combination of clinical parameters which closely resemble features observed in AE. Almost half of our patients with NSAbs were diagnosed with AE, and ~30% fulfilled stringent criteria for AE.11 Of those with NSAbs and more isolated forms of epilepsy, without individual features of AE, almost all were treated with ASMs alone and typically remained asymptomatic at long-term follow-up. Overall, these findings suggest that detection of NSAbs in patients with new-onset seizures, but without features of AE, should not alter current clinical management. Our observations should help guide the frequent clinical dilemma of which patients with new-onset seizures to test for autoantibodies and subsequently treat with immunotherapy. Taken together, our data suggest the clinical phenotype is paramount in guiding the relevance of autoantibody results, and provide data to address an outstanding question from a recent ILAE consensus statement.7
This ILAE statement also highlighted controversy over the term ‘autoimmune epilepsy’.7 In routine clinical practice, this nomenclature acts as a valuable signpost and aide memoire when seeing patients with seizures.2 14 However, ‘epilepsy’ carries several social stigmata and is defined by an enduring tendency to seizures. In AE, this lifelong risk is refuted by a recent study,4 despite several forms of AE commonly leading to hippocampal atrophy.2–4 7 10 The alternative concept of acute symptomatic seizures may more accurately capture the nature of seizures in patients with AE. Data-driven modifications to nomenclature will benefit from longer-term follow-up studies.
Ictal piloerection, low mood and attention and MRI limbic system changes are recognised features of late-onset AE, particularly in association with LGI1 antibodies.2 4 14 15 The absence of movement disorders or more diffuse cognitive impairment as predictive factors in our model suggests the overall syndrome may reflect a formes frustes of AE. This contrasts with APE2 score parameters,9 which appear to largely reflect more florid features seen in classical AE.
Our observational study has several limitations. These include limited CSF autoantibody measurements, which reflected UK practice particularly at the start of the study period. Yet, w ithout this valuable parameter, a diagnosis of NMDAR-antibody encephalitis is still possible.11 Yet, two of our four patients with serum NMDAR antibodies did not have features consistent with encephalitis, likely suggesting detection of clinically unrelated serum antibodies in these cases. In addition, our series in total only identified nine AE cases, although this may be considered substantial given the largely outpatient-based recruitment. This, and the high (~10%) seroprevalence rate, may reflect a referral bias given Oxford’s interest in AE, but is well aligned with other available estimates.6 9 10 Our serological data identified some samples with NSAbs proven by live cell-based assays, but without concomitant cell surface neuronal reactivities. This was especially evident in the cohort without a clinical diagnosis of AE, and perhaps these antibodies reflect low-affinity or low-titre autoantibodies which are not disease relevant. Their specificity, however, remains reassuring given their typical selectivity for just one of eight surface-expressed autoantigens.
In the future, our prediction model will benefit from validation in independent, larger studies which may compare the risk of enduring seizures in the NSAb-positive versus NSAb-negative populations, with and without AE, something which we did not survey at follow-up. Hence, we cannot comment on long-term seizure status in the 5/16 patients (31%) who had NSAbs, no diagnosis of AE and 3-year mRS >0. In these patients, it remains possible that immunotherapy would have led to a greater benefit. However, in our view, this finding is more likely to be consistent with the predicted ~30% of all people with epilepsy who are known to become ASM resistant: this provides a testable hypothesis for a future randomised controlled trial.
Overall, our observations support the concept that, in patients who present with new-onset focal seizures, clinical features which are consistent with a ‘mild encephalitis’ helps identify those with NSAbs which should alter patient management. This clinico-serological syndrome appeared characteristic and its recognition will improve detection and treatment of these patients. These findings should discourage widespread screening strategies to identify patients with autoantibodies among unselected seizure cohorts.
Footnotes
Twitter: @ANG_Oxford
Correction notice: This article has been corrected since it appeared Online First. Author initial and ORCID have been added for Ronan N McGinty.
Contributors: Conception and design: BL, JA, AS, SRI. Acquisition of data: all authors. Analysis or interpretation of data: RMcG, AH, AR, SRI. Drafting of the manuscript: RMcG, AH, SRI. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: AH, AR, RMcG.
Funding: NIHR Clinical Lectureship to AH. SRI is supported by Epilepsy Research UK (P1201), the Wellcome Trust (104079/Z/14/Z), the UCB–Oxford Alliance, BMA Research Grants—Vera Down grant (2013) and Margaret Temple (2017), the Fulbright UK–US commission (MS-Society Research Award) and by the NIHR Oxford Biomedical Research Centre.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: SRI and PW are coapplicants and receive royalties on patent application WO/210/046716 (UK patent no. PCT/GB2009/051441) entitled ‘Neurological Autoimmune Disorders’. The patent has been licensed for the development of assays for LGI1 and other VGKC-complex antibodies.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Irani SR, Michell AW, Lang B, et al. . Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011;69:892–900. 10.1002/ana.22307 [DOI] [PubMed] [Google Scholar]
- 2. Thompson J, Bi M, Murchison AG, et al. . The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 2018;141:348–56. 10.1093/brain/awx323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geis C, Planagumà J, Carreño M, et al. . Autoimmune seizures and epilepsy. J Clin Invest 2019;129:926–40. 10.1172/JCI125178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Bruijn MAAM, van Sonderen A, van Coevorden-Hameete MH, et al. . Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology 2019;92:e2185–96. 10.1212/WNL.0000000000007475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheffer IE, Berkovic S, Capovilla G, et al. . ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:512–21. 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brenner T, Sills GJ, Hart Y, et al. . Prevalence of neurologic autoantibodies in cohorts of patients with new and established epilepsy. Epilepsia 2013;54:1028–35. 10.1111/epi.12127 [DOI] [PubMed] [Google Scholar]
- 7. Steriade C, Britton J, Dale RC, et al. . Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia 2020;61:1341–51. 10.1111/epi.16571 [DOI] [PubMed] [Google Scholar]
- 8. von Podewils F, Suesse M, Geithner J, et al. . Prevalence and outcome of late-onset seizures due to autoimmune etiology: a prospective observational population-based cohort study. Epilepsia 2017;58:1542–50. 10.1111/epi.13834 [DOI] [PubMed] [Google Scholar]
- 9. Dubey D, Alqallaf A, Hays R, et al. . Neurological autoantibody prevalence in epilepsy of unknown etiology. JAMA Neurol 2017;74:397–402. 10.1001/jamaneurol.2016.5429 [DOI] [PubMed] [Google Scholar]
- 10. Dubey D, Kothapalli N, McKeon A, et al. . Predictors of neural-specific autoantibodies and immunotherapy response in patients with cognitive dysfunction. J Neuroimmunol 2018;323:62–72. 10.1016/j.jneuroim.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 11. Graus F, Titulaer MJ, Balu R, et al. . A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makuch M, Wilson R, Al-Diwani A, et al. . N-Methyl-D-aspartate receptor antibody production from germinal center reactions: therapeutic implications. Ann Neurol 2018;83:553–61. 10.1002/ana.25173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramberger M, Berretta A, Tan JMM, et al. . Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain 2020;143:1731–45. 10.1093/brain/awaa104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quek AML, Britton JW, McKeon A, et al. . Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol 2012;69:582–93. 10.1001/archneurol.2011.2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rocamora R, Becerra JL, Fossas P, et al. . Pilomotor seizures: an autonomic semiology of limbic encephalitis? Seizure 2014;23:670–3. 10.1016/j.seizure.2014.04.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2020-325011supp001.pdf (83.6KB, pdf)