Abstract
Objectives
To evaluate efficacy and safety of abatacept in adults with active primary Sjögren’s syndrome (pSS) in a phase III, randomised, double-blind, placebo-controlled trial.
Methods
Eligible patients (moderate-to-severe pSS [2016 ACR/European League Against Rheumatism (EULAR) criteria], EULAR Sjögren’s Syndrome Disease Activity Index [ESSDAI] ≥5, anti-SS-related antigen A/anti-Ro antibody positive) received weekly subcutaneous abatacept 125 mg or placebo for 169 days followed by an open-label extension to day 365. Primary endpoint was mean change from baseline in ESSDAI at day 169. Key secondary endpoints were mean change from baseline in EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI) and stimulated whole salivary flow (SWSF) at day 169. Other secondary clinical endpoints included glandular functions and patient-reported outcomes. Selected biomarkers and immune cell phenotypes were examined. Safety was monitored.
Results
Of 187 patients randomised, 168 completed double-blind period and 165 continued into open-label period. Mean (SD) baseline ESSDAI and ESSPRI total scores were 9.4 (4.3) and 6.5 (2.0), respectively. Statistical significance was not reached for primary (ESSDAI −3.2 abatacept vs −3.7 placebo, p=0.442) or key secondary endpoints (ESSPRI, p=0.337; SWSF, p=0.584). No clinical benefit of abatacept over placebo at day 169 was seen with other clinical and PRO endpoints. Relative to baseline, abatacept was associated with significant differences vs placebo in some disease-relevant biomarkers (including IgG, IgA, IgM-rheumatoid factor) and pathogenic cell subpopulations (post hoc analyses). No new safety signals were identified.
Conclusions
Abatacept treatment did not result in significant clinical efficacy compared with placebo in patients with moderate-to-severe pSS, despite evidence of biological activity.
Keywords: autoimmune diseases, Sjogren's syndrome, therapeutics
Key messages.
What is already known about this subject?
In patients with primary Sjögren’s syndrome (pSS), open-label uncontrolled studies of various therapeutic agents with efficacy in other autoimmune diseases have shown some promising results based on different outcome measures, but large controlled studies have so far been unable to demonstrate a meaningful treatment benefit.
What does this study add?
Abatacept treatment did not result in significant clinical efficacy versus placebo in this randomised controlled trial, but it showed evidence of disease-relevant biological activity.
The lack of clinical benefit of abatacept treatment for patients with pSS in the face of an apparent biological effect is not understood.
How might this impact on clinical practice or future developments?
Although this study does not support the use of abatacept in pSS, further studies would be needed to assess the impact of factors such as the heterogeneity of pSS.
In highlighting the clinical heterogeneity of pSS and the major challenges in designing efficacy studies of novel therapies targeting systemic disease, the results from this study can be used to inform the development of new composite endpoints—which are sensitive to change and reflect clinical and biological effects—to aid future clinical development in pSS.
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic, systemic autoimmune disease typically affecting the salivary and lacrimal glands and producing symptoms of dry mouth, dry eyes, fatigue and pain.1 The estimated prevalence of pSS in the general population is 0.01%–0.1%; pSS is associated with a high burden of disease and diminished quality of life.2 3
Treatment recommendations for patients with pSS focus mainly on symptomatic agents.4 Available symptomatic therapies include artificial tears and saliva, cholinergic agonists such as pilocarpine5 and cevimeline,6 cyclosporine7 and lifitegrast eye drops.8 There are currently no approved disease-modifying treatments for pSS. Small, open-label, uncontrolled and controlled clinical efficacy studies of methotrexate,9 leflunomide,10 hydroxychloroquine,11 rituximab,12–14 epratuzumab (B-cell-targeted agents),15 belimumab (B-cell-activating factor-blocking agent)16 and infliximab (tumour necrosis factor-α-blocking agent)17 have shown mixed results using a variety of outcome measures. Additionally, randomised placebo-controlled trials of hydroxychloroquine18 and rituximab12 13 for pSS have been negative.
Abatacept is a selective costimulation modulator that blocks the interaction between CD80/CD86 on antigen-presenting cells and CD28 on T cells,19 20 thereby disrupting T-cell activation, a likely key step in pSS pathogenesis.21 Proven efficacy of abatacept for treatment of patients with rheumatoid arthritis (RA),22 23 a T-cell-driven systemic autoimmune disease,19 24–27 supports the rationale that blocking this co-stimulatory pathway can produce clinical efficacy in an autoimmune disease.
Early studies of abatacept in pSS showed promising results. In two small, open-label pilot studies with pSS, a 24-week course of intravenous abatacept treatment was associated with a beneficial effect on disease activity and an acceptable safety profile.28 29 Additionally, a small, prospective observational study of 11 patients with pSS from Brazil recently reported a significant reduction in European League Against Rheumatism (EULAR) Sjögren’s Syndrome Disease Activity Index (ESSDAI) and improved salivary flow following treatment with intravenous abatacept.30 An open-label study of secondary SS (associated with RA) in Japanese patients demonstrated efficacy of intravenous abatacept for RA-related and SS-related manifestations.31
Here, we present the results of a double-blind (day 169) phase III, randomised, placebo-controlled trial with an extended open-label (day 365) treatment period to assess efficacy and safety of subcutaneous (SC) abatacept in patients with moderate-to-severe pSS.
Methods
Study design
In this phase III, double-blind, placebo-controlled trial (ClinicalTrials.gov: NCT02915159), eligible patients with active pSS were randomised 1:1 to receive either weekly SC abatacept 125 mg or SC matching placebo for 169 days. A subsequent 197-day (365-day in Japan) open-label extension followed the initial double-blind period, when all eligible patients received SC abatacept 125 mg/week (those receiving placebo switched to abatacept). Post-treatment safety follow-up lasted an additional 168 days.
Patients were recruited from December 2016 to January 2018 from 60 centres in 13 countries. Random assignment of study treatment was performed by a central system. Randomisation schedules were generated by the Randomisation Group within Drug Supply Management of Bristol Myers Squibb Company. Randomisation was stratified globally by current corticosteroid use, current hydroxychloroquine use, enrolment in Japan (yes/no) and level of stimulated whole salivary flow (SWSF; </≥0.1 mL/min). A block size of 2 was applied.
This study was conducted in accordance with the Declaration of Helsinki32 and the International Conference on Harmonisation Good Clinical Practice guidelines.33 All patients enrolled provided written informed consent in accordance with local laws.
Patients
Patients aged ≥18 years with pSS defined by 2016 American College of Rheumatology/EULAR criteria34 and moderate-to-severe disease activity with an ESSDAI score ≥5,35 who were refractory to symptomatic or local therapy (eg, non-steroidal anti-inflammatory drugs) and anti-SS-related antigen A/anti-Ro antibody positive, were included. Patients were excluded if: they had another systemic autoimmune disease, inflammatory conditions, severe fibromyalgia or other medical conditions associated with clinical features of pSS that could interfere with assessment of treatment response; or they had received intravenous, intramuscular, SC or intra-articular corticosteroids within 4 weeks prior to randomisation, rituximab within 12 months or belimumab, other biological therapy or methotrexate within 12 weeks. Additional information regarding exclusion criteria can be found in the online supplemental appendix.
annrheumdis-2020-218599supp001.pdf (186.8KB, pdf)
Primary and key secondary endpoints
The primary endpoint was mean change from baseline (day 1) in ESSDAI at day 169 for abatacept versus placebo. The two key secondary endpoints were mean changes from baseline in EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI) and in SWSF (among patients with SWSF ≥0.1 mL/min at screening and baseline) of abatacept versus placebo at day 169. ESSDAI includes 12 domains (cutaneous, respiratory, renal, articular, muscular, peripheral nervous system, central nervous system, haematological, glandular, constitutional, lymphadenopathy and lymphoma, and biological)36 37 and ESSPRI is a patient-reported symptom index for dryness, fatigue and limb (joint/muscular) pain.38 SWSF was determined by vigorously chewing (one chew/second) a piece of preweighed sterile gauze for 2 min and determining difference in weight.
Other efficacy and exploratory endpoints
Other secondary clinical endpoints included mean change from baseline in 28-joint Disease Activity Score based on C reactive protein (DAS28 [CRP]) at day 169, ESSDAI score according to hydroxychloroquine and corticosteroid use (both were stratification variables for randomisation), Physician Global Assessment score and proportion of patients with minimally clinically important improvement in both ESSDAI score (decrease ≥3)35 and in ESSPRI score (decrease ≥1).35 Secondary patient-reported outcome endpoints included mean changes from baseline in Patient Global Assessment score, Patient-Reported Outcomes Measurement Information System fatigue score and Female Sexual Function Index score, which measures sexual function in six subdomains. Other glandular function endpoints were also evaluated and included mean changes from baseline in SWSF, unstimulated WSF (UWSF; expectorated unstimulated saliva for 15 min), numeric rating scale for eye and mouth dryness, Schirmer’s test (measure of aqueous tear production over 5 min), tear break-up time (TBUT; seconds between patient’s last blink and first appearance of a random dry spot on the cornea) and ocular staining scores39 (OSS; cornea and conjunctiva staining pattern). All ocular assessments were performed by a trained ophthalmologist. Exploratory endpoints included mean changes from baseline in biomarkers of B-cell hyperactivity and immune cell phenotypes.
Assessments
Patient demographics and disease characteristics were assessed at baseline; clinical disease activity and safety were assessed regularly during the double-blind and open-label periods. The endpoint assessments were conducted at various intervals throughout the study along with tender and swollen joint counts.
For the post hoc analysis of biomarkers and laboratory parameters, changes from baseline were determined for erythrocyte sedimentation rate, high-sensitivity CRP, CH50 complement, C3 complement, C4 complement, IgG, IgA, IgM, IgM-rheumatoid factor (RF), kappa light chain, lambda light chain and beta-2 microglobulin. Serum biomarker chemokine ligand 13 (CXCL13) analyte was measured using the SIMOA assay from Myriad RBM. Immune cell phenotyping of whole blood samples was assessed in a subpopulation of patients from sites that participated in the flow cytometry analysis with data analysed using BD FACSDiva software.
Adverse events (AEs) were monitored throughout the study.
Statistical analysis
A hierarchical testing procedure was applied to the primary and key secondary endpoints to preserve the overall type I error of 5%. The first key secondary endpoint would only be tested (at significance level 5%) if the test for the primary endpoint was statistically significant (significance level 5%). If both the test for the primary endpoint and the first key secondary endpoint were statistically significant (both at significance level 5%), the second key secondary endpoint would be tested (at significance level 5%). The primary and key secondary endpoints, along with selected biomarkers, were analysed by a longitudinal repeated measures model. Power and sample size calculations are included in the online supplemental appendix.
Baseline demographics and disease characteristics of the study population were summarised descriptively. All efficacy analyses used the modified intent-to-treat (mITT) population, which comprised all randomised patients who received ≥1 dose of study medication. Missing data for responders were imputed as non-responders. Estimates of adjusted mean change were derived from a repeated measures mixed model; model analysis details are included in the online supplemental appendix.
Safety was summarised descriptively throughout the trial up to 56 days after last study drug dose.
Patient and public involvement
In addition to implementation of the intervention, patient-reported outcomes were key components of the study clinical efficacy outcomes. Independently of the study and through a patient engagement network, patients with pSS provided input towards key aspects of the final study design (such as the desired concomitant use of stable-dose hydroxychloroquine). Patients and patient advocacy groups were not involved in the data interpretation, writing or editing of this manuscript.
Results
Patient disposition and baseline characteristics
Of 187 patients randomised (abatacept n=92; placebo n=95), 168 completed the double-blind period and 165 continued into the open-label period (online supplemental figure S1). A total of 19 patients discontinued treatment during the double-blind period; reasons for discontinuation were generally balanced between treatment arms (online supplemental figure S1). Patient baseline characteristics were similar between treatment groups (table 1). For the overall study population, mean age was 52 years, 95% of patients were female and 64% were white; mean disease duration was 5 years. Mean baseline ESSDAI and ESSPRI total scores were 9.4 and 6.5 and were similar between treatment groups. At baseline, 39% of patients received concomitant stable-dose hydroxychloroquine and 24% received oral corticosteroids (≤10 mg/day prednisone equivalent). Mean baseline SWSF (mL/min) was 1.0 and similar between treatment groups.
Table 1.
Baseline patient demographics and disease characteristics
| Characteristic | Abatacept (n=92) |
Placebo (n=95) |
Total (n=187) |
| Age, years | 51.2 (12.3) | 52.9 (13.5) | 52.0 (12.9) |
| Weight, kg | 71.4 (18.6) | 67.5 (17.3) | 69.4 (18.0) |
| Female, n (%) | 85 (92.4) | 92 (96.8) | 177 (94.7) |
| Race, white, n (%) | 60 (65.2) | 60 (63.2) | 120 (64.2) |
| Disease duration, years | 5.0 (5.0) | 5.1 (5.3) | 5.0 (5.2) |
| ESSDAI total score | 8.7 (3.4) | 10.1 (5.0) | 9.4 (4.3) |
| ESSPRI total score | 6.6 (2.1) | 6.5 (1.9) | 6.5 (2.0) |
| SWSF, mL/min | 1.1 (0.9) | 0.9 (0.9) | 1.0 (0.9) |
| SWSF ≥0.1 mL/min, n (%) | 84 (91.3) | 86 (90.5) | 170 (90.9) |
| Concomitant treatment at day 1, n (%) | |||
| Non-steroidal anti-inflammatory drugs | 44 (47.8) | 31 (32.6) | 75 (40.1) |
| Topical eye preparation | 13 (14.1) | 14 (14.7) | 27 (14.4) |
| Parasympathomimetics | 15 (16.3) | 21 (22.1) | 36 (19.3) |
| Hydroxychloroquine | 37 (40.2) | 36 (37.9) | 73 (39.0) |
| Oral corticosteroids* | 22 (23.9) | 22 (23.2) | 44 (23.5) |
| Concomitant treatment prior to day 1, n (%) | |||
| Non-steroidal anti-inflammatory drugs | 49 (53.3) | 37 (38.9) | 86 (46.0) |
| Topical eye preparation | 17 (18.5) | 16 (16.8) | 33 (17.6) |
| Parasympathomimetics | 17 (18.5) | 22 (23.2) | 39 (20.9) |
| Hydroxychloroquine | 48 (52.2) | 45 (47.4) | 93 (49.7) |
| Methotrexate | 20 (21.7) | 15 (15.8) | 35 (18.7) |
| Oral corticosteroids | 32 (34.8) | 27 (28.4) | 59 (31.6) |
Data are mean (SD) unless otherwise stated.
*≤10mg/day prednisone equivalent.
ESSDAI, EULAR Sjögren’s Syndrome Disease Activity Index; ESSPRI, EULAR Sjögren’s Syndrome Patient Reported Index; SWSF, stimulated whole salivary flow.
Primary and key secondary endpoints
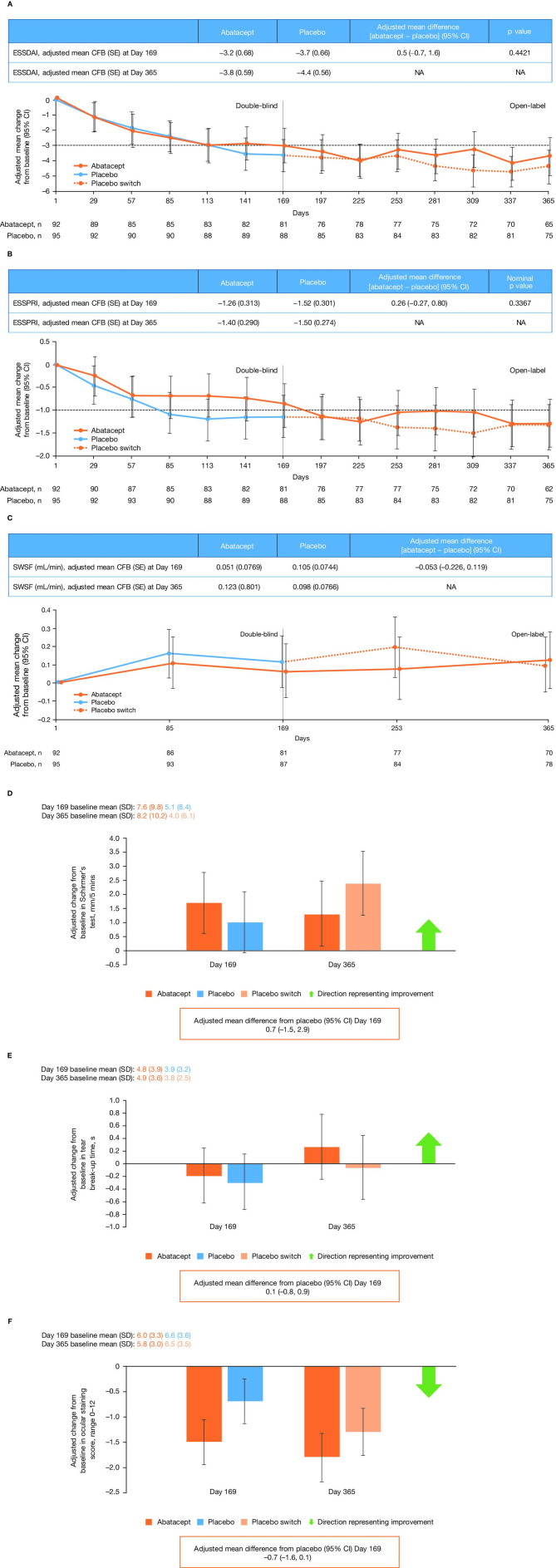
At day 169, adjusted mean change from baseline in ESSDAI score (primary endpoint) was not statistically different between treatment groups: −3.2 for abatacept vs −3.7 for placebo (p=0.442; figure 1A). At day 365 (end of open-label period), adjusted mean change from baseline in ESSDAI score was −3.8 for abatacept vs −4.4 for placebo (switched to abatacept at day 169; figure 1A). At days 169 and 365, proportions of patients with minimally clinically important improvements from baseline in ESSDAI total score (decrease ≥3) were 55% and 48% for abatacept, and 58% and 56% for placebo (switched to abatacept at day 169), respectively. In the stratified subgroups, patients not receiving corticosteroids or hydroxychloroquine at baseline had similar mean changes in ESSDAI score in both treatment groups (adjusted mean differences from placebo [95% CI] 0.1 [−1.3 to 1.4] and 0.4 [−1.1 to 1.9], respectively). In patients who received concomitant stable-dose oral corticosteroids during the double-blind period, adjusted mean difference from placebo (95% CI) in ESSDAI at day 169 was 2.7 (0.2 to 5.1).
Figure 1.
Adjusted mean changes from baseline in clinical efficacy outcomes over time for (A) total ESSDAI score, (B) total ESSPRI score, (C) SWSF (mITT population), (D) Schirmer’s test, (E) tear break-up time and (F) ocular staining scores. (A–C) The results for day 169 in the table are from the primary analysis and the data in the plot are based on the 1-year analysis. (D–F) The adjusted mean differences from placebo (95% CI) at day 169 in the text boxes are from the primary analysis and the data in the plot are based on the 1-year analysis. Study eye is defined as the eye with the higher total score for ocular surface staining at baseline. If both eyes have the same total score for ocular surface staining at baseline, the eye with the lower Schirmer’s test time (STT) at baseline will be selected. If both eyes have equal STT at baseline, then the eye with the lower tear break-up time will be selected. If all of the parameters above are equal, then the right eye will be selected as the study eye. CFB, change from baseline; ESSDAI, EULAR Sjögren’s Syndrome Disease Activity Index; ESSPRI, EULAR Sjögren’s Syndrome Patient Reported Index; mITT, modified intent to treat; NA, not applicable; SWSF, stimulated whole salivary flow.
Due to non-statistically significant primary endpoint results, the two key secondary endpoints, ESSPRI and SWSF, could not be tested for significance; nominal p values are presented. For ESSPRI score, adjusted mean changes from baseline at day 169 were −1.3 and −1.5 in the abatacept and placebo groups, respectively (nominal p=0.337; figure 1B). At day 365, the adjusted mean change from baseline in ESSPRI score was −1.4 and −1.5 with abatacept and placebo (switched to abatacept at day 169), respectively (figure 1B). Proportions of patients with minimally clinically important improvement from baseline in ESSPRI total score (≥1) at days 169 and 365 were 41% and 41% for abatacept, and 53% and 51% for placebo (switched to abatacept at day 169), respectively. Among patients with SWSF ≥0.1 mL/min at screening and baseline, adjusted mean change from baseline at day 169 was 0.06 for abatacept vs 0.11 for placebo (p=0.5841).
The study was terminated prematurely by the sponsor after the primary analysis failed to show a statistically significant difference in the primary endpoint, and analyses of secondary endpoints failed to demonstrate clinically meaningful differences between abatacept and placebo groups.
Other efficacy endpoints
For SWSF score, adjusted mean changes from baseline at days 169 and 365 were 0.05 and 0.12 for abatacept vs 0.11 and 0.10 for placebo (switched to abatacept at day 169), respectively (figure 1C) in the overall mITT population. The adjusted mean treatment difference (95% CI) for UWSF at day 169 was –0.004 (−0.03, 0.03) (table 2). We observed no significant differences between treatment groups in mean change in DAS28 (CRP) from baseline (table 2). Mean changes from baseline in Schirmer’s test, TBUT and OSS were all similar between treatment groups (figure 1D–F).
Table 2.
Summary of change from baseline in primary and secondary clinical, glandular and patient-reported outcome measures at days 169 and 365
| Day 1 (baseline scores) | Day 169 (adjusted mean change from baseline [SE] scores) | Day 365 (adjusted mean change from baseline [SE] scores) | |||||
| Abatacept | Placebo | Abatacept | Placebo | Adjusted mean treatment difference for abatacept versus placebo (95% CI) |
Abatacept | Placebo | |
| Disease activity | |||||||
| ESSDAI score | 8.7 (3.4) | 10.1 (5.0) | –3.2 (0.7) | –3.7 (0.7) | 0.5 (–0.7 to 1.6) | –3.8 (0.6) | –4.4 (0.6) |
| ESSDAI responders*, n/N (%) | NA | NA | 51/92 (55.4) | 55/95 (57.9) | –2.7 (–17.2 to 11.7)** | 44/92 (47.8) | 53/95 (55.8) |
| DAS28 (CRP) | 3.5 (1.3) | 3.6 (1.3) | –0.9 (0.1) | –1.1 (0.1) | 0.3 (0.0 to 0.5) | –0.9 (0.1) | –1.1 (0.1) |
| Physician GDA | 47.8 (17.3) | 47.8 (19.3) | –23.0 (2.4) | –23.7 (2.4) | 0.6 (–4.3 to 5.6) | ND | ND |
| Patient-reported outcomes | |||||||
| ESSPRI score | |||||||
| Total | 6.6 (2.1) | 6.5 (1.9) | –1.3 (0.3) | –1.5 (0.3) | 0.3 (–0.3 to 0.8) | –1.4 (0.3) | –1.5 (0.3) |
| Dryness | 7.0 (2.4) | 7.0 (2.3) | –0.8 (0.3) | –1.0 (0.3) | 0.2 (–0.5 to 0.8) | –1.2 (0.3) | –1.4 (0.3) |
| Fatigue | 6.6 (2.4) | 6.6 (2.5) | –1.3 (0.3) | –1.6 (0.3) | 0.3 (–0.4 to 0.9) | –1.9 (0.4) | –2.0 (0.3) |
| Pain | 6.1 (2.7) | 6.0 (2.7) | –1.1 (0.3) | –1.5 (0.3) | 0.3 (–0.3 to 1.0) | –1.3 (0.4) | –1.4 (0.3) |
| ESSPRI responders, n/N (%)‡ | NA | NA | 38/92 (41.3) | 50/95 (52.6) | –11.2 (–25.6 to 3.2)** | 38/92 (41.3) | 48/95 (50.5) |
| Ocular dryness, NRS§ | 6.8 (2.4) | 6.6 (2.5) | –0.9 (0.3) | –1.0 (0.3) | ND | –1.3 (0.4) | –1.4 (0.3) |
| Oral dryness, NRS§ | 7.3 (2.3) | 6.9 (2.5) | –1.3 (0.3) | –1.2 (0.3) | ND | –1.7 (0.3) | –1.6 (0.3) |
| Patient GDA | 58.6 (22.4) | 58.0 (21.1) | –10.1 (3.1) | –9.0 (3.0) | –1.1 (–7.4 to 5.1) | –12.9 (3.4) | –12.6 (3.2) |
| PROMIS-Fatigue | 61.2 (8.8) | 59.5 (8.6) | –5.6 (1.2) | –5.6 (1.1) | 0.04 (–2.3 to 2.4) | –6.5 (1.2) | –6.3 (1.2) |
| FSFI | 13.9 (8.7)†† | 17.3 (9.7)†† | –2.3 (1.7) | –1.9 (1.8) | –0.5 (–3.5 to 2.6) | –0.3 (1.0) | 2.3 (1.0) |
| Glandular function | |||||||
| Schirmer’s test, mm | 7.4 (9.4)†† | 5.0 (8.0)†† | 1.7 (1.1) | 1.0 (1.1) | 0.7 (–1.5 to 2.9) | 1.3 (1.2) | 2.4 (1.1) |
| TBUT, s | 4.7 (3.8)†† | 3.7 (3.1)†† | –0.2 (0.4) | –0.3 (0.4) | 0.1 (–0.8 to 0.9) | 0.3 (0.5) | –0.1 (0.5) |
| OSS | 6.1 (3.2)†† | 6.5 (3.5)†† | –1.5 (0.4) | –0.7 (0.4) | –0.7 (–1.6 to 0.1) | –1.8 (0.5) | –1.3 (0.2) |
| SWSF, mL/min | 1.1 (0.9) | 0.9 (0.8) | 0.1 (0.1) | 0.1 (0.1) | –0.1 (–0.2 to 0.1) | 0.1 (0.1) | 0.1 (0.1) |
| UWSF, mL/min | 0.1 (0.1)†† | 0.1 (0.1)†† | 0.02 (0.01) | 0.03 (0.01) | –0.004 (–0.03 to 0.03) | 0.02 (0.01) | 0.03 (0.01) |
Values are mean (SD) unless otherwise noted. Ocular assessments are for study eye. The primary and key secondary endpoints (except those marked §) were analysed by a longitudinal repeated measures model, which included randomisation stratification factors of current corticosteroid use (yes/no), current hydroxychloroquine use (yes/no), enrolment in Japan (yes/no) and SWSF </≥0.1 mL/min. Data at day 169, including adjusted mean treatment differences, are based on the primary analysis, while data at day 365 are based on the 1-year analysis. The change in outcome measures was equal to the difference between the values at baseline (day 1) and day 169 or day 365, as shown. The adjusted mean treatment difference was equal to the adjusted change in the abatacept group minus the adjusted change in the placebo group. Baseline data are for all randomised patients, except where marked with †, which were based on those patients included at day 29 or ††, which were based on day 85 (earliest post-baseline analysis) of the primary analysis. SWSF data at baseline and day 169 are for patients in the mITT population with SWSF of at least 0.1 mL/min at baseline and data at day 365 are for the overall mITT population; baseline measurements for this endpoint were from those patients included at day 169.
*Patients with minimally clinically important improvement from baseline (≥3 points) in ESSDAI total score.
**Estimate of difference (rather than adjusted mean treatment difference).
‡Patients with minimally clinically important improvement from baseline (≥1 point) in ESSPRI total score.
DAS28 (CRP), 28-joint Disease Activity Score based on C reactive protein; ESSDAI, EULAR Sjögren’s Syndrome Disease Activity Index; ESSPRI, EULAR Sjögren’s Syndrome Patient Reported Index; FSFI, Female Sexual Function Index; GDA, global disease assessment; NA, not available; ND, not determined; NRS, numeric rating scale; OSS, ocular staining scores; PROMIS-Fatigue, Patient-Reported Outcomes Measurement Information System Fatigue Score; SWSF, stimulated whole salivary flow; TBUT, tear break-up time; UWSF, unstimulated whole salivary flow.
Changes from baseline in other clinical, glandular and patient-reported outcome measures at days 169 and 365 are summarised in table 2.
Post hoc analyses
Numerical differences between ESSDAI domains were observed. For example, in patients with an ESSDAI biological domain involvement at baseline, the proportion of those with improvements (moderate to low/no activity and low to no activity) in this domain was higher (statistical significance was not tested) with abatacept (12/40; 30%) vs placebo (6/41; 15%) at day 169; this was maintained up to day 365 (data not shown). Additionally, proportions of patients with improvements in haematological and pulmonary domains of ESSDAI were numerically higher with abatacept (7/16; 44% and 3/6; 50%) vs placebo (6/29; 21% and 1/14; 7%) at day 169, respectively, among those with involvement of the corresponding domain at baseline (data not shown). Proportions of patients by ESSDAI domain activity at baseline and day 169 are shown (online supplemental figure S2). A high placebo effect was seen in several ESSDAI domains such as lymphadenopathy and articular.
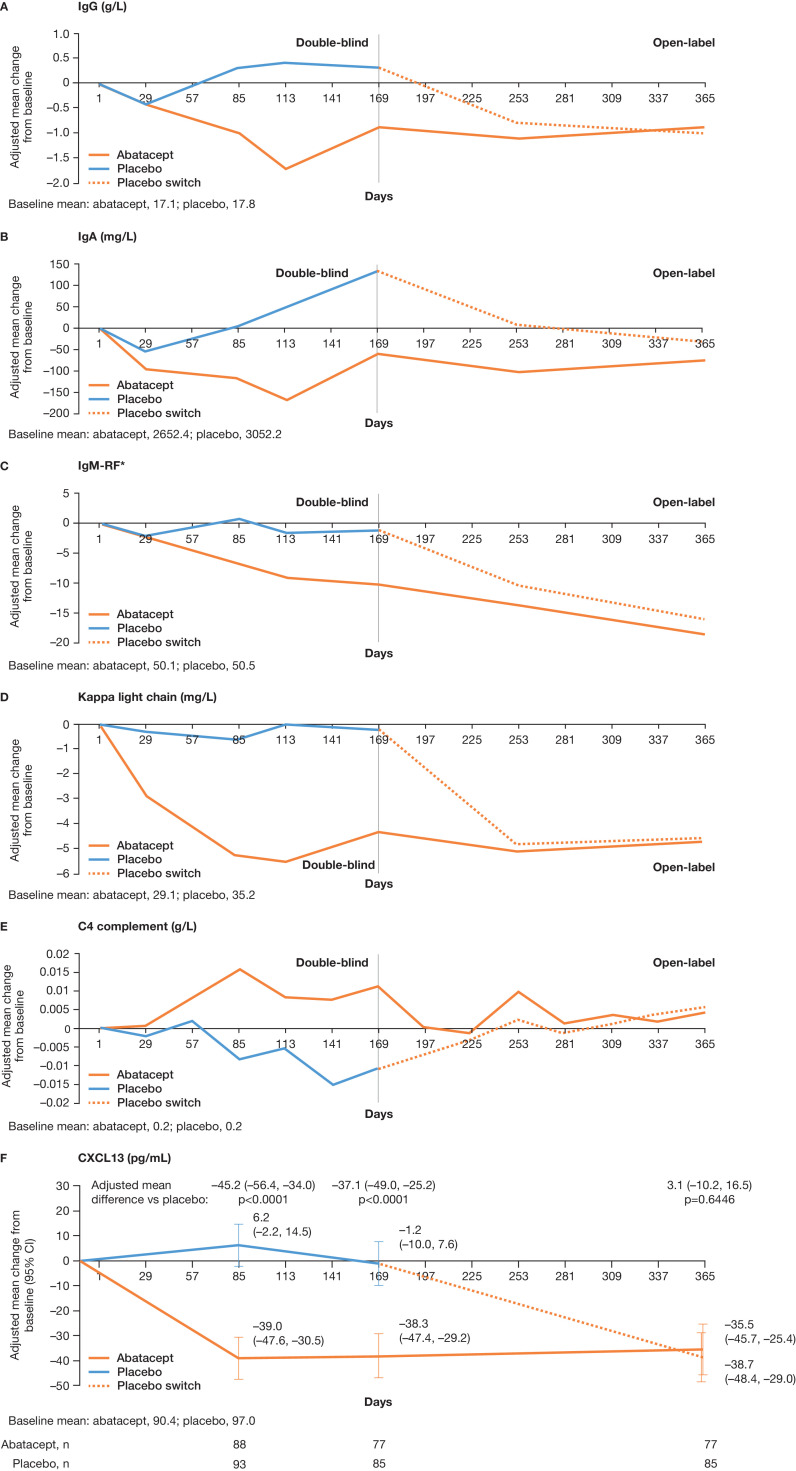
Of 12 selected disease-relevant laboratory parameters and biomarkers, mean change in IgG, IgA, IgM-RF, kappa light chain and C4 complement serum levels was significantly different between the abatacept and placebo treatment groups at day 169 (figure 2A–E; Benjamini–Hochberg procedure). At baseline, based on patients with data available at day 85, mean serum CXCL13 levels were similar between treatment arms (abatacept 90.4; placebo 97.0); however, by day 169, these levels were significantly reduced in the abatacept vs placebo group (nominal p<0.0001; figure 2F). At day 365, adjusted mean changes from baseline in IgG, IgA, IgM-RF, kappa light chain, C4 complement and CXCL13 serum levels were similar for the abatacept and placebo (switched to abatacept at day 169) treatment groups (figure 2). The numbers (%) of patients at baseline and day 169 with abnormally elevated IgG levels were 38 (41.3) and 31 (33.7) with abatacept, and 45 (47.4) and 50 (52.6) with placebo; those with elevated kappa light chains were 54 (58.7) and 41 (44.6) with abatacept, and 68 (71.6) and 61 (64.2) with placebo, respectively.
Figure 2.
Adjusted mean change from baseline over time for selected biomarkers to day 365: (A) IgG, (B) IgA, (C) IgM-RF, (D) kappa light chain, (E) C4 complement and (F) CXCL13. P values were nominal. Adjusted mean differences at day 365 are versus the placebo arm switched to abatacept (rather than vs placebo). Biomarker assessments up to 56 days post-dose are included. Estimates of adjusted mean change are from a repeated measure mixed model that includes baseline biomarker result, treatment group, randomisation stratification factors (baseline oral corticosteroid use [yes/no], baseline hydroxychloroquine use [yes/no]), time, time-by-treatment group interaction and time-by-baseline biomarker result interaction. Baseline values were based on those patients included at day 29 (day 85 for CXCL13). *Units are calibrated against standard curves derived from a WHO international reference. CXCL13, chemokine ligand 13; RF, rheumatoid factor.
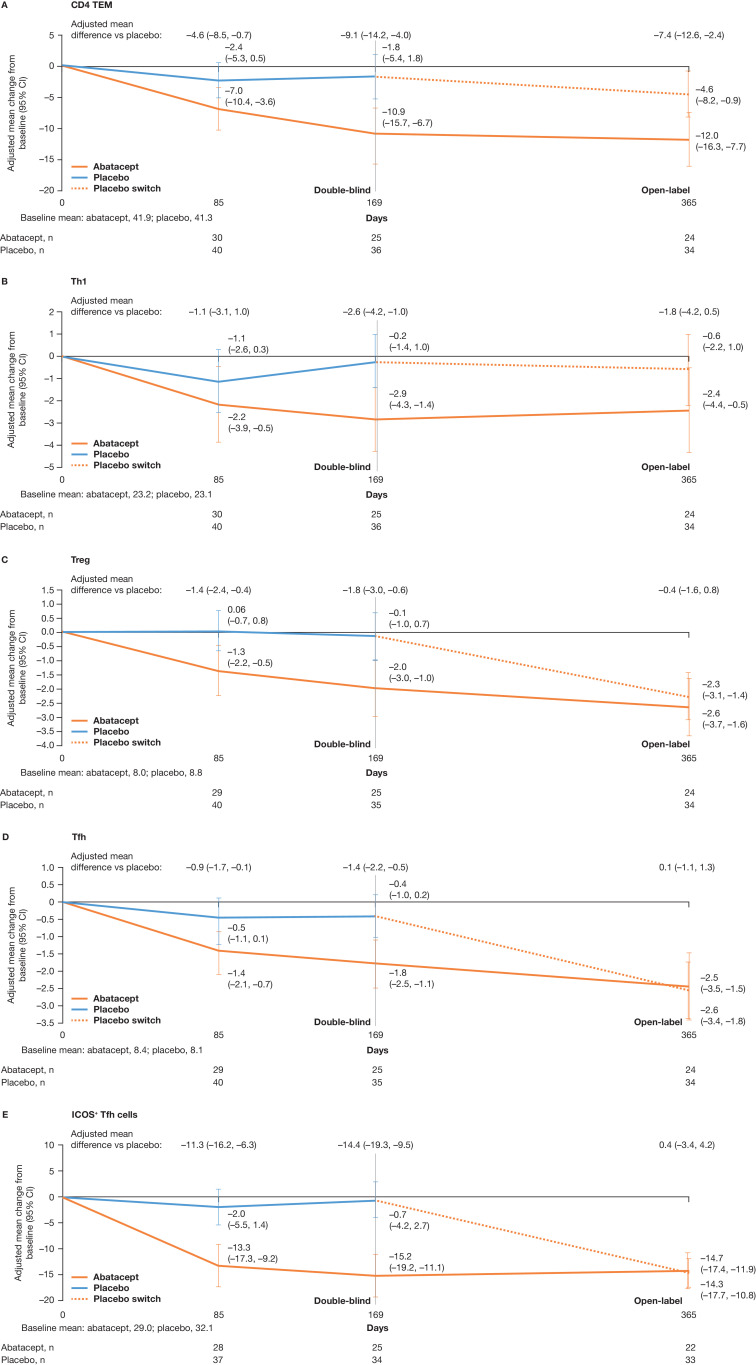
The subset of patients included for the immune cell phenotyping analysis (n=78) had similar baseline demographics and disease characteristics to the study population. In this subset, abatacept-treated patients had numerically greater decreases at day 169 (year 1 analysis) in the proportions of blood CD4+ effector memory T cells (TEM) (adjusted mean difference [95% CI] –9.1 [–14.2 to –4.0]), T helper type 1 cells (Th1) (adjusted mean difference [95% CI]–2.6 [–4.2 to –1.0]), regulatory T cells (Treg) (adjusted mean difference [95% CI] –1.8 [–3.0 to –0.6]), T follicular helper cells (Tfh) (adjusted mean difference [95% CI] –1.4 [–2.2 to –0.5]) and ICOS-positive Tfh (ICOS+ Tfh) (adjusted mean difference [95% CI] –14.4 [–19.3 to –9.5]) cells vs placebo (figure 3A–E). After switch to abatacept at day 169, mean changes from baseline in Treg, Tfh and ICOS+ Tfh cellular subsets were similar for abatacept and placebo treatment groups (figure 3C–E); for CD4+TEM and Th1, mean differences seen at day 169 were less pronounced by day 365 (figure 3A, B).
Figure 3.
Adjusted mean change over time from baseline to day 365 in circulating T-cell subtypes: (A) CD4 TEM, (B) Th1, (C) Treg, (D) Tfh and (E) ICOS+ Tfh cells. Adjusted mean differences at day 365 are versus the placebo arm switched to abatacept (rather than vs placebo). (A) CD4+TEM expressed as a percentage of CD4+ cells. Markers for CD4 TEM cells=CD3+CD4+CD45RA–CCR7–. (B) Markers for Th1 cells=CD3+CD4+CXCR3+CCR6–. (C) Treg expressed as a percentage of CD4 T cells. Markers for Treg cells=CD3+CD4+CD25+CD127–/LO. (D) Tfh is expressed as a percentage of CD4 +T cells (CXCR5+PD1+). Markers for Tfh cells=CD3+CD4+CD185+CD279+. (E) ICOS+ Tfh expressed as a percentage of Tfh cells. Markers for ICOS+ Tfh cells=CD3+CD4+CD185+CD279+CD278+. ICOS+ Tfh, ICOS-positive Tfh costimulator; TEM, effector memory T cells; Tfh, T follicular helper cells; Th1, T helper type 1 cells; Treg, regulatory T cells.
Safety
A summary of AEs in the double-blind and open-label treatment periods is shown in table 3. In the double-blind period, serious AEs (SAEs) were reported in 12 patients. Among patients treated with abatacept, 20 had SAEs: 9 in the double-blind period and 11 in the open-label period with follow-up to 56 days after the last treatment. Reported SAEs included two deaths (one placebo-treated patient [septic shock] and one abatacept-treated patient [cardiac event; patient had a history of pulmonary embolism]) and one neoplasm (plasma cell myeloma) in one abatacept-treated patient. SAEs related to study drug occurred during the double-blind treatment period in 3% (pneumonia bacterial, anaphylactoid reaction and drug hypersensitivity) of abatacept-treated and 1% (septic shock) of placebo-treated patients. Related AEs occurred during the double-blind treatment period in 46% and 25% of abatacept-treated and placebo-treated patients, respectively, but this difference was not driven by any specific AE. No new safety signals were identified compared with the known abatacept safety profile.
Table 3.
Summary of patients with adverse events* reported in the double-blind period and in the cumulative abatacept-treated population
| Double-blind treatment period | Cumulative abatacept-treated population† (n=178) |
||
| Abatacept (n=92) |
Placebo (n=95) |
||
| Deaths | 0 (0) | 1 (1.1) | 1 (0.6) |
| Serious adverse events | 9 (9.8) | 3 (3.2) | 20 (11.2) |
| Cardiac disorders | 1 (1.1) | 1 (1.1) | 2 (1.1) |
| Gastrointestinal disorders | 1 (1.1) | 1 (1.1) | 1 (0.6) |
| Immune system disorders | 2 (2.2) | 0 | 2 (1.1) |
| Infections and infestations | 1 (1.1) | 1 (1.1) | 3 (1.7) |
| Musculoskeletal and connective tissue disorders | 2 (2.2) | 0 | 4 (2.2) |
| Hepatobiliary disorders | 1 (1.1) | 0 | 2 (1.1) |
| Neoplasms | 1 (1.1) | 0 | 3 (1.7) |
| General disorders | 0 | 0 | 2 (1.1) |
| Blood and lymphatic system disorders | 0 | 0 | 1 (0.6) |
| Product issues | 0 | 0 | 1 (0.6) |
| Respiratory, thoracic and mediastinal disorders | 0 | 0 | 1 (0.6) |
| Study drug-related serious adverse events | 3 (3.3) | 1 (1.1) | 6 (3.4) |
| Discontinuations due to serious adverse events | 2 (2.2) | 1 (1.1) | 4 (2.2) |
| Adverse events | 79 (85.9) | 68 (71.6) | 127 (71.3) |
| Study drug-related adverse events‡ | 42 (45.7) | 24 (25.3) | 67 (37.6) |
| Discontinuations due to adverse events | 3 (3.3) | 2 (2.1) | 5 (2.8) |
Data are n, %.
*Adverse events reported up to 56 days post-last abatacept dose. Serious adverse events include hospitalisations for elective surgical procedures. Study drug-related adverse event or serious adverse event is defined as an adverse event or serious adverse event with a related or missing relationship to study medication.
†The cumulative abatacept-treated population were followed from the first day of abatacept treatment in the study up to 56 days after the last abatacept treatment in the study.
‡Adverse events related to abatacept were not driven by any specific system organ class.
Discussion
This large, randomised, double-blind study evaluated efficacy and safety of treatment with abatacept versus placebo in patients with active, moderate-to-severe pSS. The treatment effect did not reach statistical significance for the primary or two key secondary endpoints, and showed no clinical benefit of abatacept over placebo in other clinical efficacy and patient-reported outcome endpoints at the end of the double-blind period at day 169, or at the end of the open-label extended follow-up period at day 365. The safety profile of abatacept in patients with pSS was similar to that in other diseases treated with abatacept.40 Notably, abatacept therapy did have a clear impact on selected disease-relevant markers of biological activity likely related to central mechanisms of pSS pathogenesis.
In a recent randomised, placebo-controlled, investigator-initiated, single-centre study of SC abatacept in patients with early active pSS in the Netherlands (ASAP III NCT02067910; n=80), results for the primary endpoint (ESSDAI at 24 weeks) were similar to the present study.41 In contrast to the current study, the secondary ESSPRI endpoint (ESSPRI responders at weeks 12 and 24) was significantly different, in favour of abatacept versus placebo. Differences in results between ASAP III and the current study may be due to variations between study populations and designs, including the single-centre versus multiple-centre nature of the two studies. For instance, in ASAP III the use of hydroxychloroquine was not allowed and corticosteroids were used by fewer patients than in the current study. Additionally, at study entry all patients in ASAP III had positive biopsies, a ≤7 year disease duration and higher baseline activity (mean ESSDAI baseline score 13.5) than the current study.
Despite no detectable clinical effect in the current study, favourable improvements were observed in disease-relevant laboratory parameters and biomarkers. Some of these findings suggested an effect of therapy on T-cell-induced, B-cell hyperactivity. For example, CXCL13, the serum levels of which were significantly reduced by abatacept treatment,21 is a chemokine secreted by Tfh cells, which play a pivotal role in the migration and activation of B cells in salivary gland ectopic lymphoid structures42 43; in pSS, its serum levels correlate with disease activity and histomorphological parameters.21 44 45 In previous open-label pilot studies,28 29 24 week intravenous abatacept treatment reduced glandular inflammation, induced cellular changes (lymphocytic foci and B and T cell subtypes) and increased salivary production in 11 patients with pSS.29 Additionally, a study of 15 patients with early pSS found that 24-week intravenous abatacept treatment significantly reduced ESSDAI, ESSPRI, RF and IgG at 24 weeks.28 More recently, it has been reported that 24-week intravenous abatacept treatment decreased the number of germinal centres in parotid glands of patients with pSS.46 While abatacept has been proven effective for treatment of RA, polyarticular juvenile idiopathic arthritis and psoriatic arthritis, it has not shown significant therapeutic efficacy in systemic lupus erythematosus and multiple sclerosis.47 48 The mechanistic underpinnings across the autoimmune spectrum are complex and incompletely understood. A partial overlap in the clinical and serological features of different autoimmune diseases does not necessarily extrapolate to mutually shared treatment efficacy. Further explanation for why a detectable clinical effect was not observed with abatacept in this study, despite evidence of biological activity, may be due to limitations in the design of pSS studies. The variable characteristics and heterogeneity seen within the pSS patient population raise major challenges for study design.49 In addition, some pSS outcome measures can be subjective or difficult to standardise (eg, salivary flow has high intervariability and intravariability). Furthermore, there is a need for the development of composite study endpoints with improved cut-off and assessment time points. For example, although ESSDAI score reflects all domains of disease activity, its value in detecting small changes has been debated; as a result, there is a minimum ESSDAI score threshold required for trial entry, effectively excluding a large proportion of patients.13 50
The current trial did not confirm the promising early results from open-label studies of abatacept in pSS; this disparity has also been seen in the development of other biologics for treatment of pSS.12 13 In the TEARS13 and TRACTISS12 randomised controlled trials, rituximab demonstrated no significant improvement in ESSDAI score,12 13 despite promising early results from a previous smaller study.14 Potential explanations for the disparate findings in these rituximab trials include lack of patient stratification, insufficient tissue depletion of B cells, and the choice and timing of primary outcome evaluation.50 A study of leniolisib (a P13Kδ inhibitor), which had outcome measures and a patient population similar to the current study, showed no significant improvement in clinical outcome measures despite a significant decrease in CXCL13 serum levels, similar to our study.51 Other randomised controlled trials in pSS, like the current study, show evidence of a strong placebo effect.12 13 Considering the large placebo effect seen in this study, a reduction of at least –6.7 in ESSDAI score (placebo effect +≥3) from a baseline value of 8.7 would have been required to demonstrate therapeutic benefit over placebo.
Conclusion
No significant clinical effect was seen with abatacept versus placebo in this randomised controlled trial in patients with active, moderate-to-severe pSS. However, abatacept therapy had a positive effect on disease-relevant biomarkers, providing evidence of biological activity. No new safety signals were identified for abatacept.
Acknowledgments
These data were presented in part at EULAR 2019 (OP0039) and ACR 2019 (1907). We thank the patients and all the investigators who participated in the study. We thank the contributions of Marianne Peluso as protocol manager of this study. Professional medical writing and editorial assistance was provided by Fiona Boswell, PhD, at Caudex, and was funded by Bristol Myers Squibb Company.
Footnotes
Handling editor: Josef S Smolen
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors are accountable for all aspects of the work and will ensure questions related to the accuracy or integrity of any part of the work will be appropriately investigated and resolved. Study conception and design: ANB, J-EG, EWSC, TT, GF, MN, RW and NR. Acquisition of data: EWSC, TS, RS, GF, SM, RW and NR. Analysis and interpretation: ANB, J-EG, EWSC, TT, RS, GF, MN, SM, RW, NR and HB.
Funding: This study was sponsored by Bristol Myers Squibb Company.
Competing interests: ANB: Consultant: Bristol Myers Squibb Company, Sanofi, VielaBio; Fees: UpToDate; Clinical trials: VielaBio, Novartis. J-EG: Grant/research support: Bristol Myers Squibb Company; Consultant: Bristol Myers Squibb Company, Lilly, UCB, Sanofi-Genzyme, Pfizer. EWSC: Consulting fees and grant/research support: Bristol Myers Squibb Company; Consulting fees: AbbVie, VielaBio. TS: Grant/research support and Speakers’ bureau: Bristol Myers Squibb Company. TT: Grant/Research: AbbVie, Asahi Kasei, Astellas, AYUMI, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Nipponkayaku, Novartis, Pfizer Japan, Takeda; Consultant: AbbVie, Astellas, Astra Zeneca, Chugai, Eli Lilly Japan, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Nipponkayaku, Novartis, Taiho, Taisho Toyama, UCB Japan; Speakers’ bureau: AbbVie, Astellas, Bristol Myers Squibb Company, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Novartis, Pfizer Japan, Sanofi, Takeda, Teijin. RS: Grant/research support: Pfizer; Consultant: Amgen, Bristol Myers Squibb Company, Celgene, GlaxoSmithKline, Lilly, Pfizer, Roche. GF: Consultant: Aldeyra, Allysta, Aurinia, Bristol Myers Squibb Company, Clemencia, Hovione, Kala, Lexitas PharmaServices, Nicox, Noveome, Sight Sciences, Tarsus, Tear Solutions; Stock: TearLab. MN: Employee: Bristol Myers Squibb Company; Shareholder: Bristol Myers Squibb Company. SM: Employee: Bristol Myers Squibb Company; Shareholder: Bristol Myers Squibb Company. RW: Employee: Bristol Myers Squibb Company; Shareholder: Bristol Myers Squibb Company. NR: Employee: Bristol Myers Squibb Company; Shareholder: Bristol Myers Squibb Company. HB: Unrestricted grant: Bristol Myers Squibb Company, Roche; Consultant: Speakers bureau: Bristol Myers Squibb Company, Novartis.
Patient consent for publication: Not required.
Ethics approval: This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The study protocol and patient enrolment materials were approved by local ethics committees and institutional review boards prior to study initiation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplemental information. Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Vogelsang P, Jonsson MV, Dalvin ST, et al. . Role of dendritic cells in Sjögren's syndrome. Scand J Immunol 2006;64:219–26. 10.1111/j.1365-3083.2006.01811.x [DOI] [PubMed] [Google Scholar]
- 2. Fogel O, Rivière E, Seror R, et al. . Role of the IL-12/IL-35 balance in patients with Sjögren syndrome. J Allergy Clin Immunol 2018;142:258–68. 10.1016/j.jaci.2017.07.041 [DOI] [PubMed] [Google Scholar]
- 3. Maldini C, Seror R, Fain O, et al. . Epidemiology of primary Sjögren's syndrome in a French multiracial/multiethnic area. Arthritis Care Res 2014;66:454–63. 10.1002/acr.22115 [DOI] [PubMed] [Google Scholar]
- 4. Ramos-Casals M, Brito-Zerón P, Bombardieri S, et al. . EULAR recommendations for the management of Sjögren's syndrome with topical and systemic therapies. Ann Rheum Dis 2020;79:3–18. 10.1136/annrheumdis-2019-216114 [DOI] [PubMed] [Google Scholar]
- 5. Peluso G, De Santis M, Inzitari R, et al. . Proteomic study of salivary peptides and proteins in patients with Sjögren's syndrome before and after pilocarpine treatment. Arthritis Rheum 2007;56:2216–22. 10.1002/art.22738 [DOI] [PubMed] [Google Scholar]
- 6. Petrone D, Condemi JJ, Fife R, et al. . A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren's syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum 2002;46:748–54. 10.1002/art.510 [DOI] [PubMed] [Google Scholar]
- 7. Devecı H, Kobak S. The efficacy of topical 0.05 % cyclosporine A in patients with dry eye disease associated with Sjögren's syndrome. Int Ophthalmol 2014;34:1043–8. 10.1007/s10792-014-9901-4 [DOI] [PubMed] [Google Scholar]
- 8. Donnenfeld ED, Karpecki PM, Majmudar PA, et al. . Safety of lifitegrast ophthalmic solution 5.0% in patients with dry eye disease: A 1-year, multicenter, randomized, placebo-controlled study. Cornea 2016;35:741–8. 10.1097/ICO.0000000000000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skopouli FN, Jagiello P, Tsifetaki N, et al. . Methotrexate in primary Sjögren's syndrome. Clin Exp Rheumatol 1996;14:555–8. [PubMed] [Google Scholar]
- 10. van der Heijden EHM, Blokland SLM, Hillen MR, et al. . Leflunomide–hydroxychloroquine combination therapy in patients with primary Sjögren's syndrome (RepurpSS-I): a placebo-controlled, double-blinded, randomised clinical trial. Lancet Rheumatol 2020;2:e260–9. 10.1016/S2665-9913(20)30057-6 [DOI] [PubMed] [Google Scholar]
- 11. Demarchi J, Papasidero S, Medina MA, et al. . Primary Sjögren's syndrome: extraglandular manifestations and hydroxychloroquine therapy. Clin Rheumatol 2017;36:2455–60. 10.1007/s10067-017-3822-3 [DOI] [PubMed] [Google Scholar]
- 12. Bowman SJ, Everett CC, O'Dwyer JL, et al. . Randomized controlled trial of rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjögren's syndrome. Arthritis Rheumatol 2017;69:1440–50. 10.1002/art.40093 [DOI] [PubMed] [Google Scholar]
- 13. Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, et al. . Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med 2014;160:233–42. 10.7326/M13-1085 [DOI] [PubMed] [Google Scholar]
- 14. Meijer JM, Meiners PM, Vissink A, et al. . Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010;62:960–8. 10.1002/art.27314 [DOI] [PubMed] [Google Scholar]
- 15. Steinfeld SD, Tant L, Burmester GR, et al. . Epratuzumab (humanised anti-CD22 antibody) in primary Sjögren's syndrome: an open-label phase I/II study. Arthritis Res Ther 2006;8:R129. 10.1186/ar2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Vita S, Quartuccio L, Seror R, et al. . Efficacy and safety of belimumab given for 12 months in primary Sjögren's syndrome: the BELISS open-label phase II study. Rheumatology 2015;54:2249–56. 10.1093/rheumatology/kev257 [DOI] [PubMed] [Google Scholar]
- 17. Mariette X, Ravaud P, Steinfeld S, et al. . Inefficacy of infliximab in primary Sjögren's syndrome: results of the randomized, controlled trial of remicade in primary Sjögren's syndrome (TRIPSS). Arthritis Rheum 2004;50:1270–6. 10.1002/art.20146 [DOI] [PubMed] [Google Scholar]
- 18. Joubert J-M, Gottenberg J-E, Paintaud G, et al. . Recherche translationnelle dans les pathologies immuno-inflammatoires : quels défis, quels progrès attendre, pour quelles innovations thérapeutiques? Therapies 2014;69:291–6. 10.2515/therapie/2014049 [DOI] [PubMed] [Google Scholar]
- 19. Malmström V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol 2017;17:60–75. 10.1038/nri.2016.124 [DOI] [PubMed] [Google Scholar]
- 20. Westhovens R Abatacept: the first-in-class costimulation blocker for the treatment of rheumatoid arthritis. Fut Rheumatol 2006;1:15–22. 10.2217/17460816.1.1.15 [DOI] [Google Scholar]
- 21. Verstappen GM, Meiners PM, Corneth OBJ, et al. . Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjögren's syndrome. Arthritis Rheumatol 2017;69:1850–61. 10.1002/art.40165 [DOI] [PubMed] [Google Scholar]
- 22. Genovese MC, Becker J-C, Schiff M, et al. . Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353:1114–23. 10.1056/NEJMoa050524 [DOI] [PubMed] [Google Scholar]
- 23. Genovese MC, Covarrubias A, Leon G, et al. . Subcutaneous abatacept versus intravenous abatacept: a phase IIIB noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum 2011;63:2854–64. 10.1002/art.30463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambrus J, Suresh L, Peck A. Multiple roles for B-lymphocytes in Sjogren’s syndrome. J Clin Med 2016;5:87 10.3390/jcm5100087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen L, Suresh L. Autoantibodies, detection methods and panels for diagnosis of Sjögren's syndrome. Clin Immunol 2017;182:24–9. 10.1016/j.clim.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 26. Platt AM, Gibson VB, Patakas A, et al. . Abatacept limits breach of self-tolerance in a murine model of arthritis via effects on the generation of T follicular helper cells. J Immunol 2010;185:1558–67. 10.4049/jimmunol.1001311 [DOI] [PubMed] [Google Scholar]
- 27. Glatigny S, Höllbacher B, Motley SJ, et al. . Abatacept targets T follicular helper and regulatory T cells, disrupting molecular pathways that regulate their proliferation and maintenance. J Immunol 2019;202:1373–82. 10.4049/jimmunol.1801425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meiners PM, Vissink A, Kroese FGM, et al. . Abatacept treatment reduces disease activity in early primary Sjögren's syndrome (open-label proof of concept ASAP study). Ann Rheum Dis 2014;73:1393–6. 10.1136/annrheumdis-2013-204653 [DOI] [PubMed] [Google Scholar]
- 29. Adler S, Körner M, Förger F, et al. . Evaluation of histologic, serologic, and clinical changes in response to abatacept treatment of primary Sjögren's syndrome: a pilot study. Arthritis Care Res 2013;65:1862–8. 10.1002/acr.22052 [DOI] [PubMed] [Google Scholar]
- 30. Machado AC, Dos Santos LC, Fidelix T, et al. . Effectiveness and safety of abatacept for the treatment of patients with primary Sjögren's syndrome. Clin Rheumatol 2020;39:243–8. 10.1007/s10067-019-04724-w [DOI] [PubMed] [Google Scholar]
- 31. Tsuboi H, Matsumoto I, Hagiwara S, et al. . Effectiveness of abatacept for patients with Sjögren's syndrome associated with rheumatoid arthritis. An open label, multicenter, one-year, prospective study: ROSE (Rheumatoid arthritis with Orencia trial toward Sjögren's syndrome Endocrinopathy) trial. Mod Rheumatol 2016;26:891–9. 10.3109/14397595.2016.1158773 [DOI] [PubMed] [Google Scholar]
- 32. World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–6. [PubMed] [Google Scholar]
- 33. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med 2001;47:45-50. [PubMed] [Google Scholar]
- 34. Shiboski CH, Shiboski SC, Seror R, et al. . 2016 American College of Rheumatology/European League against rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. 10.1136/annrheumdis-2016-210571 [DOI] [PubMed] [Google Scholar]
- 35. Seror R, Bootsma H, Saraux A, et al. . Defining disease activity states and clinically meaningful improvement in primary Sjögren's syndrome with EULAR primary Sjögren's syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann Rheum Dis 2016;75:382–9. 10.1136/annrheumdis-2014-206008 [DOI] [PubMed] [Google Scholar]
- 36. Seror R, Theander E, Brun JG, et al. . Validation of EULAR primary Sjögren's syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann Rheum Dis 2015;74:859–66. 10.1136/annrheumdis-2013-204615 [DOI] [PubMed] [Google Scholar]
- 37. Seror R, Bowman SJ, Brito-Zeron P, et al. . EULAR Sjögren's syndrome disease activity index (ESSDAI): a user guide. RMD Open 2015;1:e000022–e. 10.1136/rmdopen-2014-000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seror R, Ravaud P, Mariette X, et al. . EULAR Sjogren's syndrome patient reported index (ESSPRI): development of a consensus patient index for primary Sjogren's syndrome. Ann Rheum Dis 2011;70:968–72. 10.1136/ard.2010.143743 [DOI] [PubMed] [Google Scholar]
- 39. Whitcher JP, Shiboski CH, Shiboski SC, et al. . A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren's Syndrome International Registry. Am J Ophthalmol 2010;149:405–15. 10.1016/j.ajo.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orencia prescribing information, 2017. Available: http://packageinserts.bms.com/pi/pi_orencia.pdf
- 41. van Nimwegen JF, Mossel E, van Zuiden GS, et al. . Abatacept treatment for patients with early active primary Sjögren's syndrome: a single-centre, randomised, double-blind, placebo-controlled, phase 3 trial (ASAP-III study). Lancet Rheumatol 2020;2:e153–63. 10.1016/S2665-9913(19)30160-2 [DOI] [PubMed] [Google Scholar]
- 42. Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat Rev Rheumatol 2013;9:544–56. 10.1038/nrrheum.2013.110 [DOI] [PubMed] [Google Scholar]
- 43. Amft N, Curnow SJ, Scheel-Toellner D, et al. . Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjögren's syndrome. Arthritis Rheum 2001;44:2633–41. [DOI] [PubMed] [Google Scholar]
- 44. Nocturne G, Seror R, Fogel O, et al. . CXCL13 and CCL11 serum levels and lymphoma and disease activity in primary Sjögren's syndrome. Arthritis Rheumatol 2015;67:3226–33. 10.1002/art.39315 [DOI] [PubMed] [Google Scholar]
- 45. Colafrancesco S, Priori R, Smith CG, et al. . CXCL13 as biomarker for histological involvement in Sjögren's syndrome. Rheumatology 2020;59:165–70. 10.1093/rheumatology/kez255 [DOI] [PubMed] [Google Scholar]
- 46. Lenora CU, Carniato F, Shen Y, et al. . Structural features of europium(II)-containing cryptates that influence relaxivity. Chemistry 2017;23:15404–14. 10.1002/chem.201702158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khoury SJ, Rochon J, Ding L, et al. . ACCLAIM: a randomized trial of abatacept (CTLA4-Ig) for relapsing-remitting multiple sclerosis. Mult Scler 2017;23:686–95. 10.1177/1352458516662727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Merrill JT, Burgos-Vargas R, Westhovens R, et al. . The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010;62:3077–87. 10.1002/art.27601 [DOI] [PubMed] [Google Scholar]
- 49. Devauchelle-Pensec V, Gottenberg J-E, Jousse-Joulin S, et al. . Which and how many patients should be included in randomised controlled trials to demonstrate the efficacy of biologics in primary Sjögren's syndrome? PLoS One 2015;10:e0133907. 10.1371/journal.pone.0133907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fisher BA, Everett CC, Rout J, et al. . Effect of rituximab on a salivary gland ultrasound score in primary Sjögren's syndrome: results of the TRACTISS randomised double-blind multicentre substudy. Ann Rheum Dis 2018;77:412–6. 10.1136/annrheumdis-2017-212268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dörner T, Zeher M, Laessing U, et al. . A randomised, double-blind study to assess the safety, tolerability and preliminary efficacy of leniolisib (CDZ173) in patients with primary Sjogren’s syndrome. Ann Rheu Dis 2018;77:A174. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-218599supp001.pdf (186.8KB, pdf)