Abstract
Anterograde interference emerges when two differing tasks are learned in close temporal proximity, an effect repeatedly attributed to a competition between differing task memories. However, recent development alternatively suggests that initial learning may trigger a refractory period that occludes neuroplasticity and impairs subsequent learning, consequently mediating interference independently of memory competition. Accordingly, this study tested the hypothesis that interference can emerge when the same motor task is being learned twice, that is when competition between memories is prevented. In a first experiment, the inter-session interval (ISI) between two identical motor learning sessions was manipulated to be 2 min, 1 h or 24 h. Results revealed that retention of the second session was impaired as compared to the first one when the ISI was 2 min but not when it was 1 h or 24 h, indicating a time-dependent process. Results from a second experiment replicated those of the first one and revealed that adding a third motor learning session with a 2 min ISI further impaired retention, indicating a dose-dependent process. Results from a third experiment revealed that the retention impairments did not take place when a learning session was preceded by simple rehearsal of the motor task without concurrent learning, thus ruling out fatigue and confirming that retention is impaired specifically when preceded by a learning session. Altogether, the present results suggest that competing memories is not the sole mechanism mediating anterograde interference and introduce the possibility that a time- and dose-dependent refractory period—independent of fatigue—also contributes to its emergence. One possibility is that learning transiently perturbs the homeostasis of learning-related neuronal substrates. Introducing additional learning when homeostasis is still perturbed may not only impair performance improvements, but also memory formation.
Keywords: anterograde interference, motor learning, motor memories, retention, visuomotor adaptation
1. Introduction
Extensively studied over the last two decades, anterograde interference is the phenomenon whereby initial learning of motor task A interferes with subsequent learning and retention of a differing motor task B [1–6]. This effect has been documented to be temporally graded, meaning that interference does not occur if a sufficiently long time interval (approx. 4–6 h) elapses between the two learning sessions [7,8].
The most common explanation for this phenomenon is that anterograde interference emerges because of competition between two differing task memories for storage in overlapping brain areas [9–11]. However, this notion of competition in the strict sense can be hard to reconcile with other lines of behavioural evidence. Namely, it has been shown that motor tasks A and B can both be acquired and retained if their learning occurs in different contexts [12–18], even if their learning involves common motor cortical areas [19]. Moreover, acquiring differing motor tasks in an interleaved manner in the same context facilitates their long-term storage, a well-documented effect referred to as contextual interference [20]. Altogether, this evidence raises the possibility that mechanisms other than competing memories could also contribute to the emergence of anterograde interference. Along these lines, neurobiological work and human transcranial magnetic stimulation (TMS) studies have shown that neuroplastic induction capabilities are limited over a short period of time [21–24], such that a learning event can transiently occlude subsequent capabilities to induce neuroplasticity in motor areas [7,25]. In this light, an interesting possibility is that anterograde interference does not only stem from competing motor memories, but could also be attributed to transiently occluded neuroplastic capabilities, where an initial learning session triggers a refractory period impairing any kind of subsequent learning-induced neuroplasticity in the same network [26]. So far, this possibility has been difficult to isolate because paradigms used to study interference have systematically used tasks of differing nature (A → B). A more direct and compelling test would be to observe anterograde interference of A over A, that is when competition between differing memories does not occur.
The objective of the present study was to investigate the possibility that anterograde interference emerges when learning the same motor task twice (A → A). To do so, participants acquired the same gradually introduced clockwise 21° visual deviation twice over two separate, but identical, sessions. Immediately after acquisition, retention was assessed through persisting reach biases (i.e. after-effects; [12,16,27]) as a means to indirectly quantify the extent of learning-induced neuroplastic changes that occurred during acquisition [28–30]. Specifically, it was hypothesized that anterograde interference between A → A would be temporally graded. To address this possibility, a first experiment manipulated the inter-session interval (ISI) to be either 2 min (n = 20), 1 h (n = 20), or 24 h (n = 20). Based on the results of this first experiment, a dose-dependent relationship between the number of sessions and the extent of interference was further hypothesized. To test this possibility, a second experiment had participants (n = 20) take part in three sessions each separated by a 2 min ISI. A third experiment (n = 20) was conducted a posteriori to rule out the possibility that fatigue could mediate the posited anterograde interference.
2. Methods
(a). Participants
A total of 100 right-handed human participants took part in this study (57 females; 23.2 ± 0.7 years old; all reported values represent means ± 95% confidence intervals). Specifically, three groups of 20 individuals and one group of 20 individuals took part in experiment no. 1 and no. 2, respectively. An additional group of 20 individuals took part in experiment no. 3. Participants were self-reported neurologically healthy with normal or corrected-to-normal vision. Informed consent forms approved by the ethical committee of the Centre intégré universitaire de santé et services sociaux de l'Estrie were signed prior to the start of the experiment. The experiment conformed to the standards set by the Declaration of Helsinki.
The sample size was determined based on an a priori sample size analysis conducted with G*Power 3 (version 3.1.9.2; [31]). The analysis revealed that 20 participants per group would be needed to achieve sufficient power in the present experimental design (see Statistical analyses).
(b). Gradual visuomotor adaptation paradigm
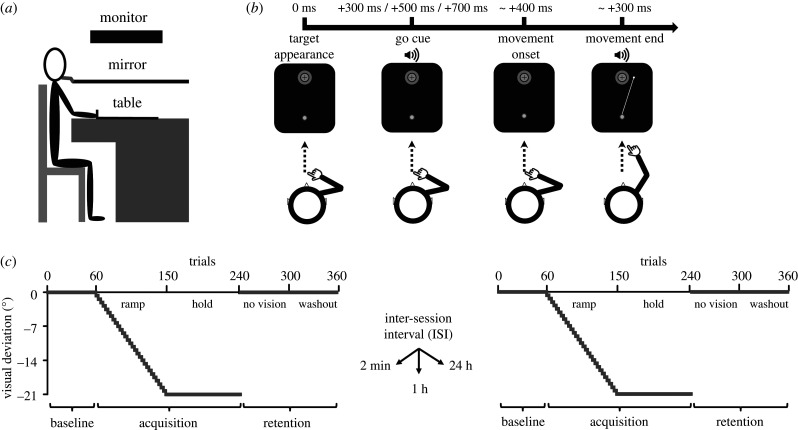
In a virtual environment (figure 1a and electronic supplementary material), participants had to perform centre-out reaching movements with their right hand (figure 1b) towards visual targets while adapting to a visuomotor deviation. The time-course of visuomotor adaptation sessions is shown in figures 1c, 3a and 4a. For both experiments, all sessions were identical. Prior to the start of the experiment, a practice phase of 120 trials preceded the first session to allow participants to familiarize themselves with the task requirements (not shown in figures). Participants first executed a ‘baseline’ phase (60 trials) with veridical visual feedback. Then, during ‘acquisition’, participants adapted to a gradually introduced visual deviation that steadily increased by −0.7° per 3-trial bins over 90 trials (ramp phase) until it reached −21°. Then, the −21° visual deviation was maintained constant for 90 trials (hold phase). Immediately upon completion of the hold phase, ‘retention’ was assessed with an initial phase in which vision of the cursor was occluded for 60 trials (no vision phase). This phase allowed us to evaluate the persistence of the adapted reaching behaviours in the absence of corrective visual feedback. Subsequently, participants executed 60 trials with veridical visual feedback (washout phase). Importantly, participants were never informed that a visual deviation had been introduced. Verbal reports confirmed that none of the 100 participants noticed the visual deviation at any moment.
Figure 1.
Apparatus and procedures of experiment no. 1. (a) Side view of the virtual environment. (b) Chronology of a typical trial. (c) Overview of the gradual visuomotor adaptation sessions. The two sessions were identical. The average of each phase (baseline, 60 trials; acquisition, 180 trials; retention, 120 trials) was calculated for the statistical analyses.
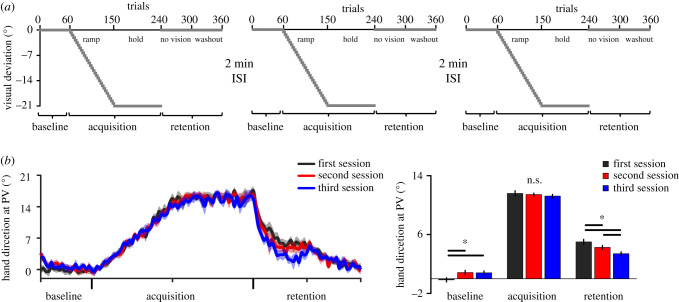
Figure 3.
Procedures and reaching performance of experiment no. 2. (a) Overview of the three identical sessions. The sessions were identical to those of experiment no. 1. Each session was separated by 2 min ISIs. (b) Left panel: hand direction at PV for the three sessions. Right panel: average hand direction at PV, shown separately for each phase of each session. Breakdown of a two-way interaction revealed that, in addition to replicating the results of the 2 min group of experiment no. 1, retention was further impaired in the third session as compared to the second one (all Cohen's dz > 0.616; medium to large effect sizes). For (b), error bars represent within-subject 95% CIs. Asterisks (*) indicate significant differences (p < 0.05). (Online version in colour.)
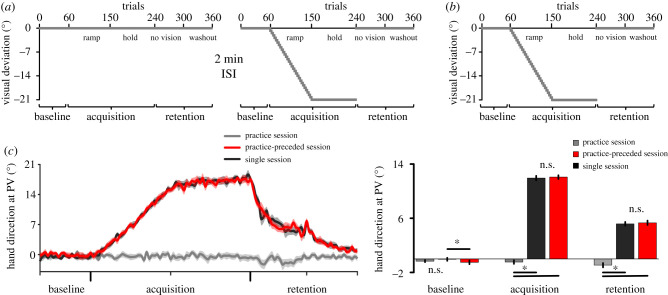
Figure 4.
Procedures and reaching performance of experiment no. 3. (a) Overview of the practice (left panel) and practice-preceded sessions (right panel). The sessions were identical to those of the previous experiments, with the exception that no visual deviation was introduced in the practice session to cause the accumulation of fatigue without concurrent learning. These sessions were separated by a 2 min ISI. (b) Overview of the single session. This session is also identical to those used in previous experiments. (c) Left panel: hand direction at PV for the three sessions. Right panel: averaged hand direction at PV, shown separately for each phase of each session. Breakdown of a two-way interaction revealed that neither acquisition nor retention differed between the practice-preceded and single sessions (both Cohen's dz < 0.140; negligible effect sizes). For (c), error bars represent within-subject 95% CIs. Asterisks (*) indicate significant differences (p < 0.05). (Online version in colour.)
(c). Manipulation of the inter-session intervals
Regarding experiment no. 1, the rationale behind the 2 min and the 24 h ISIs was to prevent and allow, respectively, a return to baseline of neuroplastic—and learning—capabilities. The 1 h interval was chosen as an intermediate ISI to inquire about the time-course of anterograde interference. This interval was selected on the basis of animal, cellular and molecular studies showing that 1 h should be sufficient to restore neuroplastic capabilities [26]. During their 1 h ISI, participants of the 1 h group watched a documentary (Planet Earth (2006); British Broadcasting Corporation) in order to experimentally control their behaviour during the ISI. Regarding experiments no. 2 and no. 3, ISIs of 2 min were selected upon the results of experiment no. 1.
(d). Kinematic data reduction
A custom-made MATLAB script was used to display and acquire kinematic data during the experiment. The primary variable of interest was hand direction at peak tangential velocity (PV), which was used to evaluate performance. This early kinematic marker was chosen because it is considered a reflection of the movement planning process. Additionally, reaction time (RT; defined as the temporal difference in milliseconds between the auditory go cue and movement onset), movement time (MT; defined as the temporal difference in milliseconds between movement onset and movement end) and accuracy at movement endpoint (the absolute distance in centimetres between the cursor and target centroids at movement end) were also analysed. Data were averaged across phases (baseline, acquisition, retention) to perform subsequent analyses.
(e). Statistical analyses
The a priori analysis assumed that two-tailed dependent t-tests would be conducted, Cohen's dz values of 0.8 (large effect size), deemed as the smallest effect size of interest in the context of this research [32], a power of 80% and an α-value of 0.017. Namely, rather than using an α-value of 0.05, 0.017 was used in anticipation that the significance threshold would decrease upon correction for multiple comparisons (0.05/3 comparisons = ∼0.017). This was to ensure adequate power even when correcting for multiple comparisons.
To analyse data, mixed or repeated measures factorial analyses of variance (ANOVAs) were separately conducted on hand direction at PV, RT, MT and accuracy at movement endpoint. For experiment no. 1, 3 groups (2 min, 1 h, 24 h) × 2 sessions (first, second) × 3 phases (baseline, acquisition, retention) ANOVAs were conducted, where groups was the sole between-subject factor. For experiment no. 2, 3 sessions (first, second, third) × 3 phases (baseline, acquisition, retention) ANOVAs were conducted (no between-subject factor). For experiment no. 3, 3 sessions (practice, practice-preceded, single) × 3 phases (baseline, acquisition, retention) ANOVAs were conducted (no between-subject factor). ANOVAs were used over their equivalent non-parametric tests because they can handle multifactorial designs, but also because they are robust to deviations from normality or variance homogeneity [33]. Posthoc pairwise tests were conducted to break down significant two-way interactions and main effects [34]. If data were abnormally distributed (Shapiro–Wilk test; p < 0.05), Wilcoxon's and U Mann–Whitney signed-rank tests were used over dependent and independent t-tests, respectively. Alpha values below 0.05 were deemed statistically significant. The Benjamini–Hochberg procedure [35] was used to correct for multiple comparisons during posthoc pairwise tests.
(f). Additional methods
Additional methodological details concerning the procedures, delivery of performance-contingent feedback, and outlying data rejection are reported as electronic supplementary material.
3. Results of experiment no. 1
(a). A 2 min inter-session interval selectively impaired retention during the second session
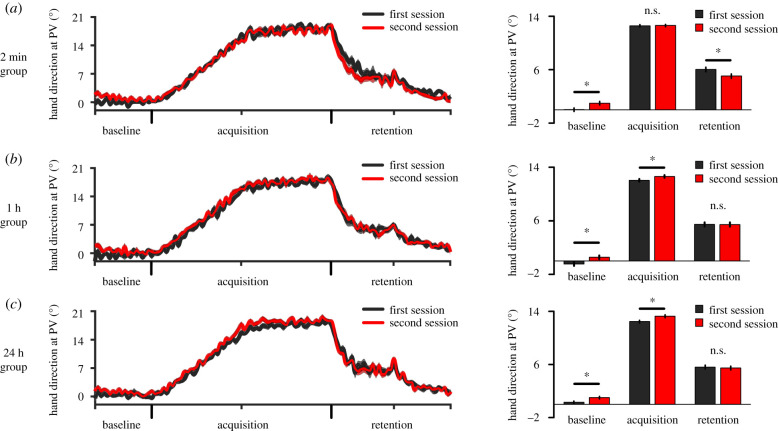
Behavioural data from experiment no. 1 are presented in figure 2. The descriptive statistics of the data used in the following analyses are reported in the electronic supplementary material, table S1. A three-way ANOVA conducted on hand direction at PV revealed a three-way interaction (F4,110 = 2.652, p = 0.050, ), which was broken down by conducting two-way ANOVAs on each level of the moderating group factor. Namely, subsequent 2 sessions × 3 phases ANOVAs revealed two-way interactions for each level of the group factor (all F > 7.414, all p < 0.002, all ). Subsequent dependent pairwise comparisons revealed that, for all groups, reaching performance was systematically greater at baseline during the second session as compared to the first one (all t > 3.692, all p < 0.002; all Cohen's dz > 0.847). For the 2 min group (figure 2a), results revealed that acquisition was similar across the two sessions (t18 = 0.225, p = 0.825, Cohen's dz = 0.052) but that retention was impaired in the second session as compared to the first one (t18 = 2.976, p = 0.024, Cohen's dz = 0.683). These results indicate that learning twice the same task in close temporal proximity impaired its retention. A different pattern emerged for the 1 h and 24 h groups (figure 2b,c); namely, acquisition was enhanced in the second session as compared to the first one (both t > 2.845, both p < 0.017, both Cohen's dz > 0.653), but retention was similar across the two sessions (both t < 0.498, both p > 0.936, both Cohen's dz < 0.114). Thus, when using ISIs of 1 h or 24 h, there was no evidence of interference. Hence, when taken together, the pattern of results for acquisition (i.e. enhanced in the 1 h and 24 h groups, but not the 2 min group) is fully compatible with that for retention (i.e. impaired in the 2 min group, but not the 1 h and 24 h groups), in that they both point towards the notion that the capacity for new learning was reduced in the 2 min group. For additional results, see the electronic supplementary material.
Figure 2.
Reaching performance for experiment no. 1. Left panels: hand direction at PV for the first and second sessions for the 2 min (a), 1 h (b) and 24 h groups (c). Right panels: average hand direction at PV for each phase of each session, shown separately for the 2 min (a), 1 h (b) and 24 h groups (c). Breakdown of a three-way interaction revealed that retention was impaired in the second session as compared to the first one only in the 2 min group (Cohen's dz = 0.683; medium to large effect size). For (a–c), error bars represent within-subject 95% confidence intervals (CIs). Asterisks (*) indicate significant differences (p < 0.05). (Online version in colour.)
4. Results of experiment no. 2
(a). Adding a third session further impaired retention
Behavioural data from experiment no. 2 are presented in figure 3b. The descriptive statistics of the data used in the following analyses are reported in the electronic supplementary material, table S2). A two-way ANOVA conducted on hand direction at PV revealed an interaction (F4,76 = 19.203, p < 0.001, ). Subsequent dependent pairwise comparisons revealed that reaching performance at baseline was greater during both the second and third sessions as compared to the first one (both t19 > 3.640, both p < 0.003, both Cohen's dz > 0.814), but did not differ from each other (t19 = 0.118, p = 0.907, Cohen's dz = 0.026). Acquisition was similar across the three sessions (all t19 < 1.445, all p > 0.341, all Cohen's dz < 0.323), but retention was found to decrease across all three sessions (all t19 > 2.754, all p < 0.013, all Cohen's dz > 0.616). In addition to replicating the findings from experiment no. 1, these results indicate that adding a third session further impaired retention as compared to the second one. For additional results, see the electronic supplementary material.
5. Rationale and results of experiment no. 3
An additional experiment was conducted in order to verify that the accumulation of fatigue could not account for the retention impairments observed when the ISI was of 2 min. For that purpose, an additional group of 20 participants took part in two distinct experimental visits that were separated by a 7-day interval and occurred in a counter-balanced order to minimize carry-over effects. For each participant, visits occurred at the same time of day. One of the experimental visits consisted of an initial practice session (hereafter referred to as practice session) that preceded a single adaptation session (hereafter referred to as practice-preceded session). A 2 min ISI separated these two sessions. Both of these sessions were identical to the ones used in the previous experiments, except that no visual deviation was introduced during the practice session (figure 4a). The other experimental visit consisted of a single adaptation session that was identical to the ones used in the previous experiments (hereafter referred to as single session; figure 4b). The same procedures as reported in the Methods section apply for this experiment and for data analysis.
It was expected that if fatigue—as putatively accumulating during the practice session—mediated the previously observed retention impairments, then the practice-preceded session should show impaired retention as compared to the single session where no fatigue would have previously accumulated. In the event that no retention difference would be observed between the practice-preceded and single sessions, the results would indicate that the retention impairments previously reported could not be accounted for by fatigue. More importantly, they would also indicate that a prior learning session is required to interfere with subsequent retention capabilities.
Behavioural data from experiment no. 3 are presented in figure 4c. Additional results and the descriptive statistics of the data (electronic supplementary material, table S3) are reported as electronic supplementary material. Briefly, concerning hand direction at PV, breakdown of a sessions × phases interaction (F4,76 = 521.500, p < 0.001, = 0.965) revealed that reaching performance did not differ between the practice-preceded and single sessions during both acquisition (t19 = 0.625, p = 0.539, Cohen's dz = 0.140) and retention (t19 = 0.486, p = 0.633, Cohen's dz = 0.109). These results indicate that the practice session did not interfere with subsequent acquisition and retention capabilities, showing that the accumulation of fatigue cannot account for the previously observed retention impairments.
6. Discussion
This study investigated the possibility that anterograde interference is not a phenomenon exclusive to differing tasks (A → B) by testing the hypothesis that anterograde interference can also emerge when acquiring the same task twice (A → A). The present study provides evidence in support of this hypothesis. On the one hand, in the 2 min group of experiment no. 1, acquisition was found to be similar in the two sessions, whereas retention was lower in the second session as compared to the first one. On the other hand, the 1 h and 24 h groups both showed enhanced acquisition but similar retention. Globally, the results of experiment no. 1 suggest that learning the same task twice can induce temporally graded anterograde interference. Results of experiment no. 2 replicated the results of the 2 min group but also showed that adding a third session further impaired retention when compared to the second session. These results hint towards a dose-dependent relationship between the number of learning sessions occurring in close temporal succession and the extent of retention. Results of experiment no. 3 revealed that practicing baseline reaches without concurrent learning did not impair subsequent retention capabilities, thus ruling out fatigue as a confounding factor for the previously observed retention impairments. These results further indicate that subsequent retention capabilities are impaired only when preceded by learning. Altogether, these results suggest that learning transiently perturbs the homeostasis of learning-related neuronal substrates and that introducing additional learning while homeostasis remains perturbed is detrimental to memory formation.
(a). Anterograde interference may not be exclusive to differing memories
One novelty of this work is to use A → A to study anterograde interference, which allowed us to determine if anterograde interference necessarily requires competition between differing memories as in A → B paradigms. Results indicate that competition between differing memories is not mandatory for anterograde interference to emerge. Specifically, performance levels were greater at baseline of the second session across every group and condition, but ensuing performance during acquisition and retention differed depending on the ISI and the number of sessions experienced. Namely, ISIs of 2 min did not enhance subsequent acquisition and impaired subsequent retention, whereas ISIs of 1 h and 24 h enhanced subsequent acquisition but did not enhance subsequent retention, indicating that acquiring the same motor task twice can also lead to temporally graded anterograde interference.
First, although the impairments were only observed during retention, the lack of acquisition enhancements when ISIs were of 2 min may constitute the flip side of the retention impairments, thus also suggestive of anterograde interference. Recently, Lerner and co-workers [6] showed that the initial acquisition of A may interfere with the subsequent acquisition of B by transiently decreasing error sensitivity (less than 1 h), thus temporarily reducing the brain's subsequent learning capabilities. Moreover, human TMS studies have related such acquisition impairments of A over B to transiently (less than 6 h) occluded neuroplastic capabilities [7,25]. Hence, an interesting contention is that the present lack of acquisition enhancements of A → A when the ISIs were of 2 min could be imputed to occluded neuroplastic capabilities. However, the notion of occlusion is unlikely to solely account for the present retention impairments, which indicate a reversal (forgetting)—rather than an occlusion (saturation)—of the induced neuroplastic changes during learning. Hence the current results extend these previous lines of work by showing that initial learning does not only occlude subsequent acquisition capabilities but also impairs the mechanisms of memory formation (see below for mechanistic explanations).
Second, despite controlling for acquisition rates with the gradual introduction of the deviation, the 1 h and 24 h groups showed enhancements of subsequent acquisition (see [6] for similar results). Such a result is reminiscent of classic savings (defined as a faster relearning upon re-exposure to a perturbation; [36]). However, because savings are thought to stem from explicit learning processes [37] and that none of the 100 participants consciously perceived the visual deviation—suggesting that the task was predominantly implicit—the similarity of the mechanisms that underlie the current results versus those that mediate classic savings remains an open question. Interestingly, the enhanced acquisition for both the 1 h and 24 h ISIs is not entirely incompatible with results from Hotermans et al. [38], who revealed a short-lived performance boost—akin to savings—emerging after a break of 5 min, 30 min and 24 h—but not of 4 h—following the explicit learning of a finger sequence press task. It should be noted, however, that because the mechanisms of memory formation between explicit sequence learning and implicit motor adaptation probably differ [39], the ISIs used by Hotermans et al. [38] may not directly map onto the present ones. Furthermore, the occurrence of such boost has not been corroborated by others [40] and has yet to be replicated. Nevertheless, this evidence suggests that a sufficiently long ISI—which duration may depend on the task demand (task complexity and requirements, number of muscles involved, extent and overlap of the neural structures involved, the presence or the absence of overlearning, etc.)—is crucial for performance to improve during a subsequent learning session.
Of interest, the present results echo well with studies that investigated the influence of massed versus spaced distribution of practice sessions on acquisition and retention [41]. For instance, Shea et al. [41] found that an ISI of 24 h between two acquisition sessions of a balance task enhanced long-term (24 h) retention as compared to a 20 min ISI. Furthermore, in a second experiment, the authors added a third acquisition session of the same task and found that an ISI of 24 h enhanced long-term (24 h) retention, whereas a 10 min ISI impaired it. Although the present investigation only probed short-term retention (immediately after acquisition), results from Shea et al. [41] suggest that long-term retention could also be negatively influenced by short ISIs and by the number of sessions during the acquisition phase of learning. One important implication of these results is that the learning history, even if it involves identical tasks, may not only prevent performance improvements but also impair the retention of memories. These results indicate that cumulating multiple learning sessions in a short time period may become counter-productive for memory formation.
(b). Evaluation of alternative interpretations
An alternative interpretation of the present results could be that the non-rotated trials between the two sessions (washout and baseline phases of the first and second sessions, respectively) acted as a competing memory causing the emergence of anterograde interference (as in A → B paradigms; [5,8]). However, because this feature of the protocol was constant across all groups of experiment no. 1, it would be expected to affect them all similarly and is thus unlikely to account for the retention impairment selectively observed for the 2 min group. Although not impossible in other contexts, the present results thus do not support the possibility that non-rotated trials acted as a competing memory that triggered anterograde interference. Moreover, in all three experiments, none of the participants consciously perceived the gradually introduced visual deviation and the retention impairments were apparent in the no vision phase, that is when participants were unaware of their performance. This makes it unlikely that participants used explicit strategies to revert to baseline levels of performance quicker during the second session as compared to the first one.
The possibility that the accumulation of fatigue—whether physical or attentional—could also account for the present results was examined by a series of additional analyses (see the electronic supplementary material) and by a third experiment. In the three experiments, the additional analyses revealed no evidence of fatigue in RT, MT, and accuracy at movement endpoint, as these variables either remained stable or improved across sessions. Moreover, the additional results also revealed that similar levels of adaptation were reached by the end of the acquisition phase in all experiments, which further suggests that fatigue did not emerge. More importantly, the results of the third experiment revealed that practising in the absence of concurrent learning—with the intent of causing fatigue accumulation—did not impair subsequent retention capabilities. While these results suggest that fatigue can be ruled out as an alternative interpretation, they also indicate that the retention impairments are learning-specific. This implies that the mechanisms mediating these impairments must interact with—or oppose—those involved in learning (see below).
(c). Learning may trigger transient refractoriness to subsequent learning and promote forgetting
One interesting result of the present study is that retention, assessed through the persistence of reach biases, was impaired when learning sessions were separated by 2 min ISIs. Reasonably assuming that the assessment of retention represents the extent of neuroplastic changes that occurred during acquisition [28–30], one possibility is that neuroplastic capabilities were temporarily constrained following the first learning session. Such a phenomenon has been previously referred to as metaplasticity [21,22] and emphasizes that learning can transiently perturb the homeostasis of learning-related neuronal substrates [42]. A perturbation to homeostasis may induce a refractory period during which subsequent learning capabilities are impaired [26] or even reversed [21,22], that is until homeostasis is restored [26]. One possibility is that anterograde interference is mediated, at least in part, by such a phenomenon.
Although the mechanisms involved in the emergence of a refractory period remain to be fully uncovered [43], several mechanisms have been documented to fit the present time-course of anterograde interference (less than 1 h; for a review, see [26]). For instance, approximately 45–60 min are required to stabilize the reorganization of dendritic spines as well as to allow sufficient time for intracellular signalling pathways to induce gene expression and de novo protein synthesis (for a review, see [26]). A subsequent learning episode occurring within this posited 45–60 min interval may impair subsequent learning by activating enzymes that perturb and oppose the signalling pathways involved in synaptic potentiation (protein phosphatases; [44,45]). Namely, by maintaining intracellular calcium levels to moderate concentrations [46], continuous (massed) learning would favour the activation of protein phosphatases [44,45] which oppose learning by promoting synaptic depression and forgetting (for a review, see [47]). In this light, one possibility is that cumulating a large amount of learning in a short time-period becomes counter-productive to learning by promoting forgetting [48]. The dose-dependent effect found in experiment no. 2 dovetails this possibility; accumulating a third learning session further impaired retention, thus suggesting that the larger the amount of learning, the greater the opposition to subsequent learning. In the light of all this evidence, one possibility is that the 2 min ISIs were too brief to allow recovery from these molecular constraints and restore neuroplastic capabilities upon the second session, thus leading to impaired subsequent retention capabilities. Globally, the above evidence indicates that the learning history can homeostatically constrain subsequent learning capabilities.
It is worth pointing out that the above mechanisms implicitly lead to infer that these molecular constraints are specific to the neurons involved in both (or multiple) learning sessions, suggesting that such homeostatic constraints should not emerge when consecutive learning sessions recruit non-overlapping neuronal substrates. The required degree of overlap between learning-related neuronal substrates to observe the emergence of homeostatic constraints remains a query for future studies.
(d). Implicitly acquired memories may be stabilized faster than explicitly acquired ones
At odds with previous studies showing that 4–6 h is required for memories to become resistant to interference [49,50], results from experiment no. 1 rather revealed that 1 h may have been sufficient for memories to become resistant to interference in a manner similar to if 24 h had elapsed. One possibility is that the present use of a gradual rather than sudden sensorimotor perturbation favoured the formation of implicit rather than explicit memories, leading us to speculate that implicitly acquired memories may consolidate faster (approx. 1 h) than explicitly acquired ones (approx. 4–6 h; [51]). In line with previous behavioural studies [52], this possibility finds support in functional magnetic resonance imaging studies [53–55] showing that explicit and implicit learning activate overlapping brain networks over time, but that the recruitment of sensorimotor areas occurs sooner after learning under implicit as compared to explicit conditions. Specifically, Sami et al. [55] showed that motor sequence learning under explicit conditions necessitated 6 h to enhance functional connectivity in a sensorimotor cortical network, whereas this enhancement was seen immediately after acquisition under implicit conditions. These results suggest that memories acquired implicitly may require less time to consolidate than those formed under explicit conditions in cortical motor areas. Given that considerable efforts have been devoted to investigating the interaction between explicit and implicit learning processes during acquisition [56–58], future studies may benefit from extending knowledge on the potentially differing time-course of consolidation between implicitly and explicitly acquired memories. Doing so may hold the promise to speed up consolidation by favouring the contribution of implicit processes during the acquisition phase of learning.
Supplementary Material
Ethics
This project has been approved by the local Institutional Research Board (project number 2019-3106).
Data accessibility
All of the data of the present experiments are freely available at the following: https://drive.google.com/file/d/10iED2IYK1gfsM0YdHAJaAUo2O3FjUgJK/view?usp=sharing.
Authors' contributions
R.H. designed the experiment, collected the data, performed the analyses and wrote the manuscript. L.D.-J. and E.D.L.F. collected the data and helped to perform the analyses. J.F.L. and P.M.B. helped to design the experiment and reviewed the manuscript.
Competing interests
The authors declare no competing financial interests.
Funding
This work was funded by the Natural Sciences and Engineering Research Council of Canada (grant nos 418589 and 05510).
References
- 1.Miall RC, Jenkinson N, Kulkarni K. 2004. Adaptation to rotated visual feedback: a re-examination of motor interference. Exp. Brain Res. 154, 201–210. ( 10.1007/s00221-003-1630-2) [DOI] [PubMed] [Google Scholar]
- 2.Overduin SA, Richardson AG, Lane CE, Bizzi E, Press DZ. 2006. Intermittent practice facilitates stable motor memories. J. Neurosci. Off. J. Soc. Neurosci. 26, 11 888–11 892. ( 10.1523/JNEUROSCI.1320-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigmore V, Tong C, Flanagan JR. 2002. Visuomotor rotations of varying size and direction compete for a single internal model in motor working memory. J. Exp. Psychol. Hum. Percept. Perform. 28, 447–457. ( 10.1037/0096-1523.28.2.447) [DOI] [PubMed] [Google Scholar]
- 4.Sing GC, Smith MA. 2010. Reduction in learning rates associated with anterograde interference results from interactions between different timescales in motor adaptation. PLoS Comput. Biol. 6, e1000893 ( 10.1371/journal.pcbi.1000893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinder MR, Walk L, Woolley DG, Riek S, Carson RG. 2007. The interference effects of non-rotated versus counter-rotated trials in visuomotor adaptation. Exp. Brain Res. 180, 629–640. ( 10.1007/s00221-007-0888-1) [DOI] [PubMed] [Google Scholar]
- 6.Lerner G, Albert S, Caffaro PA, Villalta JI, Jacobacci F, Shadmehr R, Della-Maggiore V. 2020. The origins of anterograde interference in visuomotor adaptation. Cereb. Cortex 30, 4000–4010. ( 10.1093/cercor/bhaa016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantarero G, Tang B, O'Malley R, Salas R, Celnik P. 2013. Motor learning interference is proportional to occlusion of LTP-like plasticity. J. Neurosci. Off. J. Soc. Neurosci. 33, 4634–4641. ( 10.1523/JNEUROSCI.4706-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villalta JI, Landi SM, Fló A, Della-Maggiore V. 2015. Extinction interferes with the retrieval of visuomotor memories through a mechanism involving the sensorimotor cortex. Cereb. Cortex 25, 1535–1543. ( 10.1093/cercor/bht346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krakauer JW 2009. Motor learning and consolidation: the case of visuomotor rotation. In Progress in motor control [internet] (ed. Sternad D), pp. 405–421. Boston, MA: Springer US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krakauer JW, Shadmehr R. 2006. Consolidation of motor memory. Trends Neurosci. 29, 58–64. ( 10.1016/j.tins.2005.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson EM 2012. New insights in human memory interference and consolidation. Curr. Biol. 22, R66–R71. ( 10.1016/j.cub.2011.11.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirashima M, Nozaki D. 2012. Distinct motor plans form and retrieve distinct motor memories for physically identical movements. Curr. Biol. 22, 432–436. ( 10.1016/j.cub.2012.01.042) [DOI] [PubMed] [Google Scholar]
- 13.Imamizu H, Sugimoto N, Osu R, Tsutsui K, Sugiyama K, Wada Y, Kawato M. 2007. Explicit contextual information selectively contributes to predictive switching of internal models. Exp. Brain Res. 181, 395–408. ( 10.1007/s00221-007-0940-1) [DOI] [PubMed] [Google Scholar]
- 14.Lee J-Y, Schweighofer N. 2009. Dual adaptation supports a parallel architecture of motor memory. J. Neurosci. Off. J. Soc. Neurosci. 29,10 396–10 404. ( 10.1523/JNEUROSCI.1294-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistry S, Contreras-Vidal JL. 2004. Learning multiple visuomotor transformations: adaptation and context-dependent recall. Motor Control 8, 534–546. ( 10.1123/mcj.8.4.534) [DOI] [PubMed] [Google Scholar]
- 16.Nozaki D, Yokoi A, Kimura T, Hirashima M, Orban de Xivry J-J. 2016. Tagging motor memories with transcranial direct current stimulation allows later artificially-controlled retrieval. eLife 5, 15378 ( 10.7554/eLife.15378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osu R, Hirai S, Yoshioka T, Kawato M. 2004. Random presentation enables subjects to adapt to two opposing forces on the hand. Nat. Neurosci. 7, 111–112. ( 10.1038/nn1184) [DOI] [PubMed] [Google Scholar]
- 18.Wada Y, Kawabata Y, Kotosaka S, Yamamoto K, Kitazawa S, Kawato M. 2003. Acquisition and contextual switching of multiple internal models for different viscous force fields. Neurosci. Res. 46, 319–331. ( 10.1016/S0168-0102(03)00094-4) [DOI] [PubMed] [Google Scholar]
- 19.Lage GM, Ugrinowitsch H, Apolinário-Souza T, Vieira MM, Albuquerque MR, Benda RN. 2015. Repetition and variation in motor practice: a review of neural correlates. Neurosci. Biobehav. Rev. 57, 132–141. ( 10.1016/j.neubiorev.2015.08.012) [DOI] [PubMed] [Google Scholar]
- 20.Wright D, Verwey W, Buchanen J, Chen J, Rhee J, Immink M. 2016. Consolidating behavioral and neurophysiologic findings to explain the influence of contextual interference during motor sequence learning. Psychon. Bull. Rev. 23, 1–21. ( 10.3758/s13423-015-0887-3) [DOI] [PubMed] [Google Scholar]
- 21.Abraham WC 2008. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9, 387 ( 10.1038/nrn2356) [DOI] [PubMed] [Google Scholar]
- 22.Keck T, Hübener M, Bonhoeffer T. 2017. Interactions between synaptic homeostatic mechanisms: an attempt to reconcile BCM theory, synaptic scaling, and changing excitation/inhibition balance. Curr. Opin Neurobiol. 43, 87–93. ( 10.1016/j.conb.2017.02.003) [DOI] [PubMed] [Google Scholar]
- 23.Rioult-Pedotti M-S, Donoghue JP, Dunaevsky A. 2007. Plasticity of the synaptic modification range. J. Neurophysiol. 98, 3688–3695. ( 10.1152/jn.00164.2007) [DOI] [PubMed] [Google Scholar]
- 24.Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. 1998. Strengthening of horizontal cortical connections following skill learning. Nat. Neurosci. 1, 230–234. ( 10.1038/678) [DOI] [PubMed] [Google Scholar]
- 25.Cantarero G, Lloyd A, Celnik P. 2013. Reversal of long-term potentiation-like plasticity processes after motor learning disrupts skill retention. J. Neurosci. Off. J. Soc. Neurosci. 33, 12 862–12 869. ( 10.1523/JNEUROSCI.1399-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smolen P, Zhang Y, Byrne JH. 2016. The right time to learn: mechanisms and optimization of spaced learning. Nat. Rev. Neurosci. 17, 77–88. ( 10.1038/nrn.2015.18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galea JM, Mallia E, Rothwell J, Diedrichsen J. 2015. The dissociable effects of punishment and reward on motor learning. Nat. Neurosci. 18, 597–602. ( 10.1038/nn.3956) [DOI] [PubMed] [Google Scholar]
- 28.Della-Maggiore V, Landi SM, Villalta JI. 2015. Sensorimotor adaptation: multiple forms of plasticity in motor circuits. Neuroscientist 21, 109–125. ( 10.1177/1073858414545228) [DOI] [PubMed] [Google Scholar]
- 29.Lai CSW, Adler A, Gan W-B. 2018. Fear extinction reverses dendritic spine formation induced by fear conditioning in the mouse auditory cortex. Proc. Natl Acad. Sci. USA 115, 9306–9311. ( 10.1073/pnas.1801504115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai CSW, Franke TF, Gan W-B. 2012. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 483, 87–91. ( 10.1038/nature10792) [DOI] [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Lang A-G, Buchner A. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. ( 10.3758/BF03193146) [DOI] [PubMed] [Google Scholar]
- 32.Algermissen J, Mehler DMA. 2018. May the power be with you: are there highly powered studies in neuroscience, and how can we get more of them? J. Neurophysiol. 119, 2114–2117. ( 10.1152/jn.00765.2017) [DOI] [PubMed] [Google Scholar]
- 33.Blanca MJ, Alarcón R, Arnau J, Bono R, Bendayan R. 2017. Non-normal data: is ANOVA still a valid option? Psicothema 29, 552–557. [DOI] [PubMed] [Google Scholar]
- 34.Sawyer SF 2009. Analysis of variance: the fundamental concepts. J. Man. Manip. Ther. 17, 27E–38E. ( 10.1179/jmt.2009.17.2.27E) [DOI] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300. [Google Scholar]
- 36.Huang VS, Haith A, Mazzoni P, Krakauer JW. 2011. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70, 787–801. ( 10.1016/j.neuron.2011.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morehead JR, Qasim SE, Crossley MJ, Ivry R. 2015. Savings upon re-aiming in visuomotor adaptation. J. Neurosci. Off. J. Soc. Neurosci. 35,14 386–14 396. ( 10.1523/JNEUROSCI.1046-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P. 2006. Early boost and slow consolidation in motor skill learning. Learn. Mem. 13, 580–583. ( 10.1101/lm.239406) [DOI] [PubMed] [Google Scholar]
- 39.Debas K, et al. 2010. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc. Natl Acad. Sci. USA 107, 17 839–17 844. ( 10.1073/pnas.1013176107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Press DZ, Casement MD, Pascual-Leone A, Robertson EM. 2005. The time course of off-line motor sequence learning. Brain Res. Cogn. Brain Res. 25, 375–378. ( 10.1016/j.cogbrainres.2005.05.010) [DOI] [PubMed] [Google Scholar]
- 41.Shea CH, Lai Q, Black C, Park J-H. 2000. Spacing practice sessions across days benefits the learning of motor skills. Hum. Mov. Sci. 19, 737–760. ( 10.1016/S0167-9457(00)00021-X) [DOI] [Google Scholar]
- 42.Kukushkin NV, Carew TJ. 2017. Memory takes time. Neuron 95, 259–279. ( 10.1016/j.neuron.2017.05.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zenke F, Gerstner W. 2017. Hebbian plasticity requires compensatory processes on multiple timescales. Phil. Trans. R. Soc. B 372, 20160259 ( 10.1098/rstb.2016.0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. 2002. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature 418, 970–975. ( 10.1038/nature00928) [DOI] [PubMed] [Google Scholar]
- 45.Malleret G, et al. 2001. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104, 675–686. ( 10.1016/S0092-8674(01)00264-1) [DOI] [PubMed] [Google Scholar]
- 46.Muzzio IA, Ramirez RR, Talk AC, Matzel LD. 1999. Interactive contributions of intracellular calcium and protein phosphatases to massed-trials learning deficits in Hermissenda. Behav. Neurosci. 113, 103–117. ( 10.1037/0735-7044.113.1.103) [DOI] [PubMed] [Google Scholar]
- 47.Moreno A 2020. Molecular mechanisms of forgetting. Eur J Neurosci. See https://onlinelibrary.wiley.com/doi/abs/10.1111/ejn.14839.
- 48.Waddell S 2003. Protein phosphatase 1 and memory: practice makes PP1 imperfect? Trends Neurosci. 26, 117–119. ( 10.1016/S0166-2236(03)00029-8) [DOI] [PubMed] [Google Scholar]
- 49.Brashers-Krug T, Shadmehr R, Bizzi E. 1996. Consolidation in human motor memory. Nature 382, 252–255. ( 10.1038/382252a0) [DOI] [PubMed] [Google Scholar]
- 50.Shadmehr R, Brashers-Krug T. 1997. Functional stages in the formation of human long-term motor memory. J. Neurosci. Off. J. Soc. Neurosci. 17, 409–419. ( 10.1523/JNEUROSCI.17-01-00409.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shelhamer M, Aboukhalil A, Clendaniel R. 2005. Context-specific adaptation of saccade gain is enhanced with rest intervals between changes in context state. Ann. NY Acad. Sci. 1039, 166–175. ( 10.1196/annals.1325.016) [DOI] [PubMed] [Google Scholar]
- 52.Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW. 2009. Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. J. Neurophysiol. 101, 2218–2229. ( 10.1152/jn.01138.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schendan HE, Searl MM, Melrose RJ, Stern CE. 2003. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37, 1013–1025. ( 10.1016/S0896-6273(03)00123-5) [DOI] [PubMed] [Google Scholar]
- 54.Willingham DB, Salidis J, Gabrieli JDE. 2002. Direct comparison of neural systems mediating conscious and unconscious skill learning. J. Neurophysiol. 88, 1451–1460. ( 10.1152/jn.2002.88.3.1451) [DOI] [PubMed] [Google Scholar]
- 55.Sami S, Robertson EM, Miall RC. 2014. The time course of task-specific memory consolidation effects in resting state networks. J. Neurosci. Off. J. Soc. Neurosci. 34, 3982–3992. ( 10.1523/JNEUROSCI.4341-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor JA, Krakauer JW, Ivry RB. 2014. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J. Neurosci. Off. J. Soc. Neurosci. 34, 3023–3032. ( 10.1523/JNEUROSCI.3619-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDougle SD, Bond KM, Taylor JA. 2015. Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. J. Neurosci. Off. J. Soc. Neurosci. 35, 9568–9579. ( 10.1523/JNEUROSCI.5061-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schween R, Taylor JA, Hegele M. 2018. Plan-based generalization shapes local implicit adaptation to opposing visuomotor transformations. J. Neurophysiol. 120, 2775–2787. ( 10.1152/jn.00451.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data of the present experiments are freely available at the following: https://drive.google.com/file/d/10iED2IYK1gfsM0YdHAJaAUo2O3FjUgJK/view?usp=sharing.