Abstract
Many tropical mammals are vulnerable to heat because their water budget limits the use of evaporative cooling for heat compensation. Further increasing temperatures and aridity might consequently exceed their thermoregulatory capacities. Here, we describe two novel modes of torpor, a response usually associated with cold or resource bottlenecks, as efficient mechanisms to counter heat. We conducted a field study on the Malagasy bat Macronycteris commersoni resting in foliage during the hot season, unprotected from environmental extremes. On warm days, the bats alternated between remarkably short micro-torpor bouts and normal resting metabolism within a few minutes. On hot days, the bats extended their torpor bouts over the hottest time of the day while tolerating body temperatures up to 42.9°C. Adaptive hyperthermia combined with lowered metabolic heat production from torpor allows higher heat storage from the environment, negates the need for evaporative cooling and thus increases heat tolerance. However, it is a high-risk response as the torpid bats cannot defend body temperature if ambient temperature increases above a critical/lethal threshold. Torpor coupled with hyperthermia and micro-torpor bouts broaden our understanding of the basic principles of thermal physiology and demonstrate how mammals can perform near their upper thermal limits in an increasingly warmer world.
Keywords: heat tolerance, heterothermy, hyperthermia, thermal limits, bats, tropics
1. Background
The safety margin between euthermia and lethally high body temperatures in mammals is very narrow and even minor changes can be life threatening, particularly in the tropics. Mammals obtain their body heat mainly from metabolic activities and usually regulate their body temperature within a set range of a few degrees [1,2]. They reach their thermal limits when body temperature approaches 41–44°C due to overheating or dehydration [3,4]. The maximum temperatures experienced by mammals are increasing because of extensive habitat modification [5] or more frequent and more intense heatwaves associated with global climate change [6], and these maxima can be fatal [7–11]. Indeed, heatwaves have led to several recent mass mortalities of flying foxes (Pteropus spp.) in Australia [10,12], with ambient temperatures (Ta) of 42°C or higher representing a critical threshold [8].
Efficient thermoregulation is essential for survival, but there are few options for downregulating body temperature near the upper limit. Radiation, convection and conduction are passive mechanisms of heat loss only used when Ta is lower than the desired body temperature [13]. When Ta exceeds body temperature, the only options are evaporative cooling or tolerating short-term hyperthermia [14]. In warm and dry environments with unpredictable or limited water availability, evaporation might be constrained by the risk of dehydration. By accumulating heat instead of dumping it and thereby tolerating an increase in body temperature, the need for evaporative cooling can be postponed or even avoided, and considerable amounts of water can be conserved [15,16]. The classic example of this adaptive hyperthermia comes from dromedary camels (Camelus dromedarius), which regularly cycle between 41°C in daytime and 34–35°C at night when dehydrated [17], but other mammals also allow hyperthermia during hot phases (llamas [18], elephants [19], large treeshrews [20], ringtail possums [11], bats [7,16,21]).
The most dramatic thermoregulatory response of mammals is the controlled downregulation of virtually all metabolic processes during torpor (hypometabolism) [22,23]. This is widely believed to be an adaptive response of endotherms to cold stress or food limitation during seasonal periods of scarcity. However, the last two decades have shown that torpor is also common in the tropics [24] and could negate some effects of heat [25]. A reduction of metabolic rate (MR) is accompanied by reduced water consumption through respiration, defecation, urine formation and metabolic heat dissipation [26–28], which could permit torpid animals to tolerate greater heat loads than euthermic ones. Nonetheless, how mammals perform and thermoregulate at high temperatures remains poorly understood [14,25,29–31], yet is essential to predict their responses to rising temperatures. Global warming confronts many small mammals more regularly with fatal mismatches between environmental conditions and their physiological limitations. To this end, we studied how a small tropical bat withstands heat in its natural environment and found that novel modes of torpor were a critical part of their response.
2. Short methods
We worked with the insectivorous bat Macronycteris commersoni (mean body mass: females = 46.3 ± 6.8 g; males = 79.6 ± 8.0 g) in a tropical dry forest in western Madagascar (Kirindy Forest/CNFEREF; S 20.06714°/E 44.65745°, 40 m) during the hottest season of the year. The bats rest alone in vegetation during the day exposed to temperature extremes and cannot necessarily expend the water required to cool their bodies below ambient temperatures. On sunny days, maximum Ta can reach over 41°C in Kirindy Forest/CNFEREF whereas relative humidity is comparatively low at 44.2 ±6.6% (29.0–53.6%). We monitored the physiological responses to regular daytime heat (73% sunny days) by measuring mass-specific MR as rate of oxygen consumption (O2 ml h−1 g−1) and skin temperature (Tskin) in 16 adult bats in their natural environment (nine females, seven males; see electronic supplementary material for a detailed description of procedures). Our methods complied with the current ethical regulations and laws of Madagascar (see ‘Ethics’).
3. Two novel modes of torpor
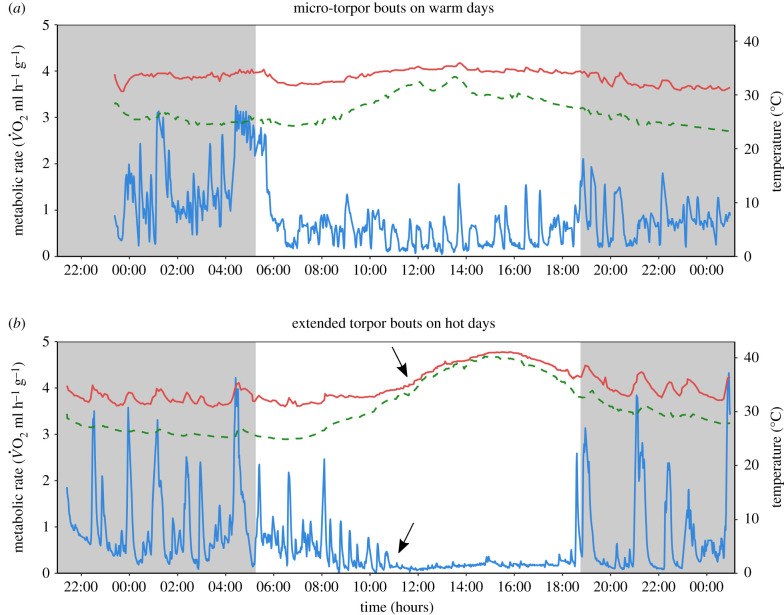
Unexpectedly, all bats entered torpor on all days and we observed two unique, novel modes of torpor, regardless of sex. On warm, rainy or cloudy, days (mean max Ta = 33.9 ± 1.98°C), most bats (67%) alternated between short micro-torpor bouts lasting between 3 and 53 min (mean = 12.4 ± 10.5 min; n = 462 bouts) and resting MR (figure 1a; electronic supplementary material, figure S1A). This pattern occurred throughout their resting phase from sunrise at approximately 05:30 to sunset at approximately 18:30 before nightly activity. Interestingly, micro-torpor did not lead to notable changes in Tskin compared to euthermia (figure 1a; electronic supplementary material, figure S1A). The pattern of recurring micro-torpor bouts is likely a fine balance: Individuals can save energy [32] and water [27] on the one hand, but simultaneously are still alert and responsive to any threats given the exposed roosting conditions.
Figure 1.
Two new modes of torpor in a tropical bat species. Metabolic rate as O2 (MR, ml h−1 g−1; blue; lower line) and skin temperature (Tskin,°C; red; upper line) of two male individuals of the bat Macronycteris commersoni and the bats' ambient temperature (Ta,°C; green; dashed line). Grey shaded blocks indicate the dark phase. (a) The typical response on regular warm (rainy or cloudy) days. Under these conditions, bats alternated between regular resting MR and micro-torpor bouts (12.4 ± 10.5 min) over the course of their usual inactive phase (approx. 05:30–18:30 h). (b) The response of bats on hot days. When Ta exceeded euthermic body temperature, the bats extended their torpor bouts, which lasted until the beginning of their active phase in the late afternoon when Ta had decreased again (293.6 ± 101.2 min). The arrows highlight the decline in MR and almost simultaneous increase in Tskin, which passively followed Ta, during extended torpor up to 42.9°C indicating that bats tolerated hyperthermia while torpid (see electronic supplementary material, figure S1 for two examples of female bats showing the same patterns). (Online version in colour.)
On hotter, days (mean max Ta = 37.8 ± 1.85°C), the bats used micro-torpor bouts only during the cooler, early morning hours (figure 1b; electronic supplementary material, figure S1B). However, when Ta exceeded normal body temperature, most bats (94%) stopped switching between micro-torpor and resting MR and extended their torpor bouts. This usually led to a significant increase in Tskin because active regulation of body temperature was suspended and thus Tskin approximated Ta. This pattern is contrary to the traditional view of torpor as cold response and we refer to it as ‘hot torpor'. Therewith we want to stress that the physiological underpinnings seem to be the same as in arctic species entering torpor, only the environmental conditions lead to different patterns. Individuals were torpid over the hottest time of the day and aroused at the beginning of their active phase when Ta and Tskin had decreased again to 33.5 ± 2.3°C and 36.0 ± 1.8°C, respectively. The extended torpor bouts lasted between 78 and 436 min (mean = 293.6 ± 101.2 min; n = 27 bouts) and Tskin passively increased with Ta up to a maximum value of 42.9°C. Thus, the bats applied a well-known response to cold conditions, i.e. entering torpor, while tolerating hyperthermia (figure 1b; electronic supplementary material, figure S1B). In contrast to adaptive hyperthermia used by e.g. camels, the bats actively depressed metabolism and bodily functions to a minimum during hottest body temperatures. This allows for negligible internal heat production and thus substantial water savings, making hot torpor an efficient option to withstand tropical heat. When small mammals are euthermic and confronted with Ta near the upper critical temperature, excess heat generated by metabolism or activity and the absorption of heat from the environment can quickly exceed their thermoregulatory capacities. Other tropical bats enter extended torpor, but only in the cooler morning hours and always accompanied by a reduction in body temperature, probably to save water in anticipation of responding to afternoon heat [33,34]. By contrast, M. commersoni entered hot torpor only under heat stress and maintained micro-bouts during cooler daytimes. This flexible response to high Ta might allow M. commersoni to thrive in the dry tropics in areas where buffered diurnal roosts, such as caves, are absent.
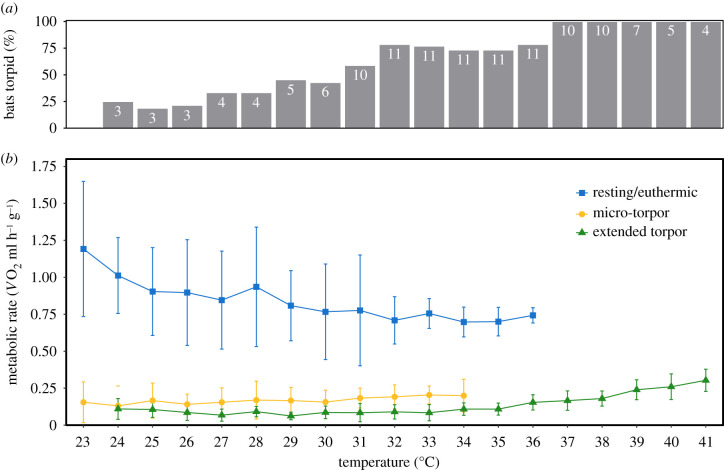
Over the inactive phase, we found that resting MR decreased with increasing Ta and plateaued between 31.9°C and 36°C (figure 2b). We could not determine the upper end of this plateau, because all individuals entered extended torpor above 36°C, but it is very likely to represent the thermal neutral zone of this population during the hot season (i.e. the range in which heat production and loss to the environment are balanced and no energy is needed to actively thermoregulate). In general, the warmer it became, the more individuals entered torpor (figure 2a). Above 36°C, thermoregulation at euthermia required excessive water consumption (see electronic supplementary material, video S1 for a thermal imaging video of a bat salivating its forearms extensively for evaporative cooling at Ta = 36.9°C) and we found bats to be torpid even at Ta of 41°C (figure 2b). Clearly, torpor was more beneficial than defending euthermia, suggesting the existence of an upper limit of tolerable euthermia.
Figure 2.
More bats enter torpor as ambient temperature rises. (a) The proportion of torpid bats at different ambient temperatures (Ta; integers indicate number of bats measured per interval). When Ta increased, more bats entered torpor and all bats were torpid above 36°C. (b) The mass-specific metabolic rate as O2 (ml h−1 g−1) of bats in the three different physiological states: resting (blue; squares), micro-torpor bouts (yellow; circles) and extended torpor bouts (green; triangles) at each temperature interval. Error bars represent standard deviation. (Online version in colour.)
4. High body temperature masks torpor
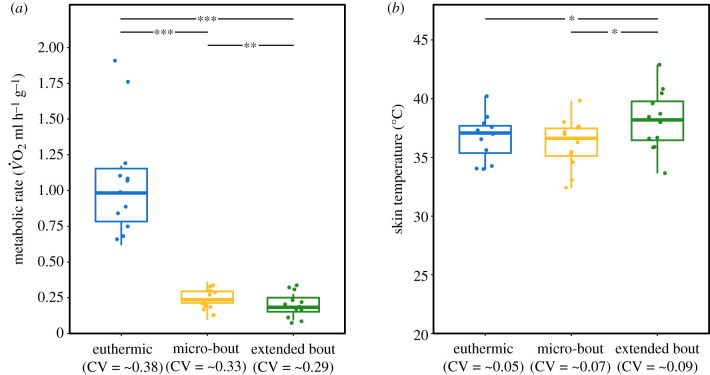
The magnitude of metabolic depression during torpor was high and the MR for both modes of torpor was significantly lower than resting MR, despite similar daytime temperatures (resting MR versus extended torpor: t11 = 7.737, p < 0.001; resting MR versus micro-torpor: t11 = 7.434, p < 0.001; table 1 and figure 3a). Although MR during extended torpor bouts was significantly lower than during micro-torpor bouts (t11 = −3.427, p = 0.006; table 1 and figure 3a), both modes of torpor resulted in a similar level of metabolic depression of 82.2 and 77.6%, respectively. This is close to the highest metabolic reductions seen during more continuous torpor in warm environments (25–84% [35–38]). By contrast, variation in corresponding Tskin values of these three different physiological states was less clear-cut (maximum mean difference 1.9°C; figure 3b). Tskin during micro-torpor bouts was similar to that of euthermia (t11 = 1.052, p = 0.316), but both were significantly lower than Tskin during extended hot torpor bouts (extended versus micro-torpor: t11 = 2.511, p = 0.029; extended torpor versus euthermia: t11 = −2.425, p = 0.034; table 1 and figure 3b). Consequently, although the reduction in metabolism during torpor was substantial, the magnitude of the MR decline did not dictate Tskin, regardless of torpor length.
Table 1.
Physiological key variables of the metabolic states. Mass-specific metabolic rate as O2 (ml h−1 g−1) and mean maximum skin temperature (°C) of bats (n = 12) when resting and euthermic, during micro-torpor and during extended hot torpor.
| euthermia | micro-torpor | extended torpor | |
|---|---|---|---|
| mean metabolic rate (ml O2 h−1 g−1) | 1.07 ± 0.41 | 0.24 ± 0.08 | 0.19 ± 0.06 |
| mean skin temperature (°C) | 36.7 ± 1.9 | 36.2 ± 2.1 | 38.1 ± 2.6 |
Figure 3.
Depressed metabolism does not dictate skin temperature. Mass-specific metabolic rate as O2 (MR, ml h−1 g−1; (a); n = 12) and skin temperature (Tskin,°C; (b); n = 12) of animals in three different physiological states: resting metabolism/euthermia (blue), micro-torpor bouts (yellow) and extended torpor bouts (green; centre line, median; box limits, upper and lower quartiles; whiskers, 1.5× interquartile range; points, all data included). The coefficient of variation (CV) is given in parentheses; significant differences are marked with asterisks (*p ≤ 0.05; **p < 0.01; ***p < 0.001). (Online version in colour.)
Both modes of torpor could not have been detected with the classical temperature recordings often used in eco-physiological research because their impact on body temperature was marginal; the mean deviation of Tskin from euthermia was less than 2°C. Our study has identified two hitherto unknown modes of torpor, which suggest that the traditional concept of torpor needs to be re-considered. Torpor was originally defined as a substantial decline in MR and body temperature, mainly in response to low Ta [22,23]. However, a decline in body temperature is clearly not a necessity and we have shown that it may even increase when an animal is entering torpor. Thus, while a controlled depression in MR has always been a central part of torpor, a more universal definition should also specify that body temperature during torpor can be variable: it may approximate Ta in either direction (as per the traditional definition and as seen during hot torpor [24,37,39–41] or may remain stable as seen during micro-torpor bouts. Different environmental conditions can result in different patterns of torpor and study methods should recognize this. Researchers should not rely solely on traditional indicators of torpor such as body temperature and should strive to include other proxies for torpor, such as heart rate or oxygen consumption, particularly when studying endotherms in hot environments.
5. Conclusion
Our study dramatically broadens our knowledge on the fundamental concepts of thermal physiology and describes newly discovered options for mammals to cope with heat based on data from a free-living endotherm. We found that a tropical bat coped with daytime heat by using torpor (a response classically still associated with cold conditions), in novel ways: micro-torpor bouts at regular warm temperatures and hot torpor coupled with body temperature above euthermia during hot afternoon hours. This demonstrates the capacity of small mammals to survive rising ambient temperatures. Hot torpor allows M. commersoni to exist in habitats lacking well-buffered diurnal roosts and in regions that are even more arid. However, this response is not without risk: these bats could face lethal temperatures while in a torpid state if temperature increases too much, which would necessitate a premature re-arousal including evaporative cooling as a last resort. The inevitable upregulation of metabolic heat production during emergency arousals could easily push the individual beyond tolerable temperature maxima. Hot torpor is thus a high-risk response that relies on ambient temperature to not exceed certain maxima and cool again. Whether hot torpor occurs more widely in the dry tropics remains to be determined. Our discovery of variations of the classical pattern of torpor should spur studies of the responses of other species living near their thermal limits in an era of global warming and is also relevant for the potential use of induced torpor for medical purposes and possible future space travel near human euthermic levels.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Bill Foley and the Functional Ecology working group for comments on earlier drafts of the manuscript as well as Hajatiana Rabarison, Robert Jean Niry and Arne Wulff for their constant help during fieldwork. We are grateful to Peter Kappeler and Claudia Fichtel for allowing us to operate and use the facilities from the field station of the German Primate Center (DPZ) and the Centre National de Formation d'Etude et de Recherche en Environment et Foresterie (CNFEREF) for support in the field. In addition, Jacques Rakotondranary's help with the organization of logistics and authorizations is highly appreciated.
Ethics
This study has been conducted under the ‘Accord de Collaboration’ between the Université d'Antananarivo (Département de Biologie Animale), Madagascar National Parks and the Universität Hamburg. We thank these authorities and the Ministère de l'Environnement, de l'Ecologie et des Forêts for support and project authorization. The research was approved by the Directeur du Système des Aires Protégées, Ministère de l'Environnement, Antananarivo (permit no. 296/17/MEEF/SG/DGF/DSAP/SCB.Re) and all described procedures comply with the current ethical regulations and laws of Madagascar.
Data accessibility
The data used for the analysis underlying the study are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.80gb5mkpk [65].
Authors' contributions
K.H.D. and S.R. designed the study. S.R. carried out fieldwork, data analysis and wrote the first draft. K.H.D. commented, critically revised and edited the manuscript. S.R. and K.H.D. read and approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The German Research Foundation (DA 1013/7-1) and IDEA WILD (REHEMADA1116) supported this work financially.
References
- 1.McKechnie AE, Wolf BO. 2019. The physiology of heat tolerance in small endotherms. Physiology 34, 302–313. ( 10.1152/physiol.00011.2019) [DOI] [PubMed] [Google Scholar]
- 2.Clarke A, Rothery P. 2008. Scaling of body temperature in mammals and birds. Funct. Ecol. 22, 58–67. ( 10.1111/j.1365-2435.2007.01341.x) [DOI] [Google Scholar]
- 3.Lepock JR 2003. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int. J. Hyperthermia 19, 252–266. ( 10.1080/0265673031000065042) [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Nielsen K 1997. Animal physiology: adaptation and environment. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Tuff KT, Tuff T, Davies KF. 2016. A framework for integrating thermal biology into fragmentation research. Ecol. Lett. 19, 361–374. ( 10.1111/ele.12579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IPCC. 2014. Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, Pachauri RK, Meyer LA). Geneva, Switzerland: Intergovernmental Panel on Climate Change. [Google Scholar]
- 7.Czenze ZJ, Naidoo S, Kotze A, McKechnie AE. 2020. Bat thermoregulation in the heat: limits to evaporative cooling capacity in three southern African bats. J. Therm. Biol. 89, 102542 ( 10.1016/j.jtherbio.2020.102542) [DOI] [PubMed] [Google Scholar]
- 8.Ratnayake HU, Kearney MR, Govekar P, Karoly D, Welbergen JA. 2019. Forecasting wildlife die-offs from extreme heat events. Anim. Conserv. 22, 386–395. ( 10.1111/acv.12476) [DOI] [Google Scholar]
- 9.Meade J, VanDerWal J, Storlie C, Williams S, Gourret A, Krockenberger A, Welbergen JA. 2018. Substantial reduction in thermo-suitable microhabitat for a rainforest marsupial under climate change. Biol. Lett. 14, 20180189 ( 10.1098/rsbl.2018.0189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welbergen JA, Klose SM, Markus N, Eby P. 2008. Climate change and the effects of temperature extremes on Australian flying-foxes. Proc. R. Soc. B 275, 419–425. ( 10.1098/rspb.2007.1385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner JM 2020. Facultative hyperthermia during a heatwave delays injurious dehydration of an arboreal marsupial. J. Exp. Biol. 223, jeb219378 ( 10.1242/jeb.219378) [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Stephen A. 2018. Extreme heat wipes out almost one third of Australia's spectacled flying fox population. See www.abc.net.au/news/2018-12-19/heat-wipes-out-one-third-of-flying-fox-species/10632940.
- 13.Hill R, Wyse GA, Anderson M. 2016. Animal physiology. Sunderland, MA: Sinauer. [Google Scholar]
- 14.Mitchell D, Snelling EP, Hetem RS, Maloney SK, Strauss WM, Fuller A. 2018. Revisiting concepts of thermal physiology: predicting responses of mammals to climate change. J. Anim. Ecol. 87, 956–973. ( 10.1111/1365-2656.12818) [DOI] [PubMed] [Google Scholar]
- 15.Licht P, Leitner P. 1967. Physiological responses to high environmental temperatures in three species of microchiropteran bats. Comp. Biochem. Physiol. 22, 371–387. ( 10.1016/0010-406X(67)90601-9) [DOI] [Google Scholar]
- 16.Maloney SK, Bronner GN, Buffenstein R. 1999. Thermoregulation in the Angolan free-tailed bat Mops condylurus: a small mammal that uses hot roosts. Physiol. Biochem. Zool. 72, 385–396. ( 10.1086/316677) [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Nielsen K, Schmidt-Nielsen B, Jarnum SA, Houpt TR. 1956. Body temperature of the camel and its relation to water economy. Am. J.Physiol. Legacy Content 188, 103–112. ( 10.1152/ajplegacy.1956.188.1.103) [DOI] [PubMed] [Google Scholar]
- 18.Riek A, Brinkmann L, Gauly M, Perica J, Ruf T, Arnold W, Hambly C, Speakman JR, Gerken M. 2017. Seasonal changes in energy expenditure, body temperature and activity patterns in llamas (Lama glama). Sci. Rep. 7, 1–12. ( 10.1038/s41598-017-07946-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissenböck NM, Arnold W, Ruf T. 2012. Taking the heat: thermoregulation in Asian elephants under different climatic conditions. J. Comp. Physiol. B 182, 311–319. ( 10.1007/s00360-011-0609-8) [DOI] [PubMed] [Google Scholar]
- 20.Levesque DL, Tuen AA, Lovegrove BG. 2018. Staying hot to fight the heat-high body temperatures accompany a diurnal endothermic lifestyle in the tropics. J. Comp. Physiol. B 188, 707–716. ( 10.1007/s00360-018-1160-7) [DOI] [PubMed] [Google Scholar]
- 21.Bondarenco A, Körtner G, Geiser F. 2014. Hot bats: extreme thermal tolerance in a desert heat wave. Naturwissenschaften 101, 679–685. ( 10.1007/s00114-014-1202-2) [DOI] [PubMed] [Google Scholar]
- 22.Geiser F 2004. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274. ( 10.1146/annurev.physiol.66.032102.115105) [DOI] [PubMed] [Google Scholar]
- 23.Heldmaier G, Ortmann S, Elvert R. 2004. Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317–329. ( 10.1016/j.resp.2004.03.014) [DOI] [PubMed] [Google Scholar]
- 24.Nowack J, Levesque DL, Reher S, Dausmann KH. 2020. Variable climates lead to varying phenotypes: ‘weird’ mammalian torpor and lessons from non-Holarctic species. Front. Ecol. Evol. 8, 60 ( 10.3389/fevo.2020.00060) [DOI] [Google Scholar]
- 25.Lovegrove BG, Canale C, Levesque D, Fluch G, Řeháková-Petrů M, Ruf T. 2014. Are tropical small mammals physiologically vulnerable to Arrhenius effects and climate change? Physiol. Biochem. Zool. 87, 30–45. ( 10.1086/673313) [DOI] [PubMed] [Google Scholar]
- 26.Levin E, Plotnik B, Amichai E, Braulke LJ, Landau S, Yom-Tov Y, Kronfeld-Schor N. 2015. Subtropical mouse-tailed bats use geothermally heated caves for winter hibernation. Proc. R. Soc. B 282, 20142781 ( 10.1098/rspb.2014.2781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper CE, McAllan BM, Geiser F. 2005. Effect of torpor on the water economy of an arid-zone marsupial, the stripe-faced dunnart (Sminthopsis macroura). J. Comp. Physiol. B 175, 323–328. ( 10.1007/s00360-005-0488-y) [DOI] [PubMed] [Google Scholar]
- 28.Herreid CF, Schmidt-Nielsen K. 1966. Oxygen consumption, temperature, and water loss in bats from different environments. Am. J. Physiol. Legacy Content 211, 1108–1112. ( 10.1152/ajplegacy.1966.211.5.1108) [DOI] [PubMed] [Google Scholar]
- 29.Levesque DL, Nowack J, Stawski C. 2016. Modelling mammalian energetics: the heterothermy problem. Clim. Change Responses 3, 7 ( 10.1186/s40665-016-0022-3) [DOI] [Google Scholar]
- 30.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welman S, Tuen AA, Lovegrove BG. 2017. Using thermoregulatory profiles to assess climate change vulnerability in an arboreal tropical bat: heterothermy may be a pre-adaptive advantage. Clim. Res. 74, 161–170. ( 10.3354/cr01496) [DOI] [Google Scholar]
- 32.O'Mara MT, Wikelski M, Voigt CC, Ter Maat A, Pollock HS, Burness G, Desantis LM, Dechmann DK. 2017. Cyclic bouts of extreme bradycardia counteract the high metabolism of frugivorous bats. eLife 6, e26686 ( 10.7554/eLife.26686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bondarenco A, Körtner G, Geiser F. 2016. How to keep cool in a hot desert: torpor in two species of free-ranging bats in summer. Temperature 3, 476–483. ( 10.1080/23328940.2016.1214334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiser F, Holloway JC, Körtner G, Maddocks TA, Turbill C, Brigham RM. 2000. Do patterns of torpor differ between free-ranging and captive mammals and birds? In Life in the cold (eds Heldmaier G, Klingenspor M), pp. 95–102. Berlin, Germany: Springer. [Google Scholar]
- 35.Kobbe S, Nowack J, Dausmann KH. 2014. Torpor is not the only option: seasonal variations of the thermoneutral zone in a small primate. J. Comp. Physiol. B 184, 789–797. ( 10.1007/s00360-014-0834-z) [DOI] [PubMed] [Google Scholar]
- 36.Grimpo K, Legler K, Heldmaier G, Exner C. 2013. That's hot: golden spiny mice display torpor even at high ambient temperatures. J. Comp. Physiol. B 183, 567–581. ( 10.1007/s00360-012-0721-4) [DOI] [PubMed] [Google Scholar]
- 37.Dausmann KH, Glos J, Heldmaier G. 2009. Energetics of tropical hibernation. J. Comp. Physiol. B 179, 345–357. ( 10.1007/s00360-008-0318-0) [DOI] [PubMed] [Google Scholar]
- 38.Song X, Körtner G, Geiser F. 1997. Thermal relations of metabolic rate reduction in a hibernating marsupial. Am. J. Physiol. Regul., Integr. Comp. Physiol. 273, R2097–R2104. ( 10.1152/ajpregu.1997.273.6.R2097) [DOI] [PubMed] [Google Scholar]
- 39.Treat MD, et al. 2018. Extreme physiological plasticity in a hibernating basoendothermic mammal, Tenrec ecaudatus. J. Exp. Biol. 221, jeb185900 ( 10.1242/jeb.185900) [DOI] [PubMed] [Google Scholar]
- 40.Canale CI, Levesque DL, Lovegrove BG. 2012. Tropical heterothermy: does the exception prove the rule or force a re-definition? In Living in a seasonal world: thermoregulatory and metabolic adaptations (eds Ruf T, Bieber C, Arnold W, Millesi E), pp. 29–40. Berlin, Germany: Springer. [Google Scholar]
- 41.Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. 2004. Hibernation in a tropical primate. Nature 429, 825–826. ( 10.1038/429825a) [DOI] [PubMed] [Google Scholar]
- 42.Goodman S, Raherilalao M, Wohlhauser S. 2018. Les aires protégées terrestres de Madagascar: leur histoire, description et biote/The terrestrial protected areas of Madagascar: their history, description, and biota. Antananarivo, Madagascar: Association Vahatra. [Google Scholar]
- 43.Kappeler PM, Fichtel C. 2012. A 15-year perspective on the social organization and life history of sifaka in Kirindy Forest. In Long-term field studies of primates (eds Kappeler PM, Watts DP), pp. 101–122. Berlin, Germany: Springer. [Google Scholar]
- 44.Goodman SM 2011. Les chauves-souris de Madagascar: guide de leur distribution, biologie et identification. Antananarivo, Madagascar: Association Vahatra. [Google Scholar]
- 45.Reher S, Ehlers J, Rabarison H, Dausmann KH. 2018. Short and hyperthermic torpor responses in the Malagasy bat Macronycteris commersoni reveal a broader hypometabolic scope in heterotherms. J. Comp. Physiol. B 188, 1015–1027. ( 10.1007/s00360-018-1171-4) [DOI] [PubMed] [Google Scholar]
- 46.Aldridge HDJN, Brigham RM. 1988. Load carrying and maneuverability in an insectivorous bat: a test of the 5% ‘rule’ of radio-telemetry. J. Mammalogy 69, 379–382. ( 10.2307/1381393) [DOI] [Google Scholar]
- 47.Langer F, Fietz J. 2014. Ways to measure body temperature in the field. J. Therm. Biol. 42, 46–51. ( 10.1016/j.jtherbio.2014.03.002) [DOI] [PubMed] [Google Scholar]
- 48.Dausmann KH 2005. Measuring body temperature in the field—evaluation of external vs. implanted transmitters in a small mammal. J. Therm. Biol. 30, 195–202. ( 10.1016/j.jtherbio.2004.11.003) [DOI] [Google Scholar]
- 49.Willis CKR, Brigham RM. 2003. Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J. Comp. Physiol. B 173, 379–389. ( 10.1007/s00360-003-0343-y) [DOI] [PubMed] [Google Scholar]
- 50.Audet D, Thomas DW. 1996. Evaluation of the accuracy of body temperature measurement using external radio transmitters. Can. J. Zool. 74, 1778–1781. ( 10.1139/z96-196) [DOI] [Google Scholar]
- 51.Lighton JRB 2008. Measuring metabolic rates: a manual for scientists. Oxford, UK: Oxford University Press. [Google Scholar]
- 52.Muggeo VMR 2008. Modeling temperature effects on mortality: multiple segmented relationships with common break points. Biostatistics 9, 613–620. ( 10.1093/biostatistics/kxm057) [DOI] [PubMed] [Google Scholar]
- 53.Wood SN 2001. Minimizing model fitting objectives that contain spurious local minima by bootstrap restarting. Biometrics 57, 240–244. ( 10.1111/j.0006-341X.2001.00240.x) [DOI] [PubMed] [Google Scholar]
- 54.R Development Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 55.RStudio Team. 2016. RStudio: integrated development for R. Boston, MA: RStudio; See http://www.rstudio.com/ [Google Scholar]
- 56.Grolemund G, Wickham H. 2011. Dates and times made easy with lubridate. J. Stat. Softw. 40, 3 ( 10.18637/jss.v040.i03) [DOI] [Google Scholar]
- 57.Wickham H. 2011. The split-apply-combine strategy for data analysis. J. Stat. Softw. 40, 1 ( 10.18637/jss.v040.i01) [DOI] [Google Scholar]
- 58.Wickham H, Bryan J. 2019. readxl: Read excel files. R package version 1.3.1. See https://CRAN.R-project.org/package=readxl.
- 59.Muggeo VMR 2008. Segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25. [Google Scholar]
- 60.Fox J, Weisberg S. 2019. An {R} companion to applied regression, 3rd edn Thousand Oaks, CA: Sage. [Google Scholar]
- 61.Wickham H 2016. Ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 62.Kassambara A2019. ggpubr: ‘ggplot2’ based publication ready plots. R package version 0.2.4. See https://CRAN.R-project.org/package=ggpubr .
- 63.Wilke CO2019. cowplot: streamlined plot theme and plot annotations for 'ggplot2'. R package version 1.0.0. See https://CRAN.R-project.org/package=cowplot .
- 64.Wickham H, Seidel D. 2019. scales: scale functions for visualization. R package version 1.1.0. See https://CRAN.R-project.org/package=scales.
- 65.Reher S, Dausmann KH. 2021. Data from: Tropical bats counter heat by combining torpor with adaptive hyperthermia. Dryad Digital Repository ( 10.5061/dryad.80gb5mkpk) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Reher S, Dausmann KH. 2021. Data from: Tropical bats counter heat by combining torpor with adaptive hyperthermia. Dryad Digital Repository ( 10.5061/dryad.80gb5mkpk) [DOI]
Supplementary Materials
Data Availability Statement
The data used for the analysis underlying the study are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.80gb5mkpk [65].