Abstract
Like many animals, adult male chimpanzees often compete for a limited number of mates. They fight other males as they strive for status that confers reproductive benefits and use aggression to coerce females to mate with them. Nevertheless, small-bodied, socially immature adolescent male chimpanzees, who cannot compete with older males for status nor intimidate females, father offspring. We investigated how they do so through a study of adolescent and young adult males at Ngogo in Kibale National Park, Uganda. Adolescent males mated with nulliparous females and reproduced primarily with these first-time mothers, who are not preferred as mating partners by older males. Two other factors, affiliation and aggression, also influenced mating success. Specifically, the strength of affiliative bonds that males formed with females and the amount of aggression males directed toward females predicted male mating success. The effect of male aggression toward females on mating success increased as males aged, especially when they directed it toward females with whom they shared affiliative bonds. These results mirror sexual coercion in humans, which occurs most often between males and females involved in close, affiliative relationships.
Keywords: chimpanzee, Pan troglodytes, alternative mating tactics, sexual coercion, development
1. Introduction
Throughout the animal kingdom, males use their strength, size and status to compete for mates [1–3]. Winners in direct combat are typically the largest, strongest, most-experienced males (e.g. African elephants (Loxodonta africana): [4]; migratory terns (Sterna hirundo): [5] and fallow deer (Dama dama): [6]. Because of their superior size and strength, males can also injure females and coerce them to mate (e.g. elephant seals (Mirounga angustirostris): [7]; multiple species of waterfowl: [8]; bighorn sheep (Ovis canadensis): [9], dolphins (Tursiops spp): [10]; orangutans (Pongo pygmaeus): [11] and reviewed in: [12–14]). Young, small males, however, are unable to coerce females sexually and instead adopt alternative mating tactics [15]. For example, small, subordinate males mate quickly (e.g. marine iguanas, Amblyrhynchus cristatus, [16]) or furtively (e.g. Sika deer, Cervus nippon, [17]). For animals that develop slowly, reproductive tactics may shift during development. This applies to young male bushbucks (Tragelaphus scriptus), who sneak copulations with females, but later grow up to defend groups of females and territories [18].
Our closest living relatives, chimpanzees (Pan troglodytes), develop very slowly. They live in relatively stable groups or communities, whose members form temporary subgroups that change in size and composition [19,20]. Because single females mate with multiple males, sperm competition ensues [21–23]. In addition, adult males employ several behavioural tactics that increase their chances of mating and reproducing. First, they compete with others for status, and high dominance rank yields mating opportunities [20,21,24,25]. To reduce levels of male–male competition, males also form mate-guarding coalitions with each other and concede mating opportunities to their coaltionary partners [26,27]. Third, adult males use aggression to intimidate females and coerce them to mate with them and not with others, sometimes leading females away from other males on extended ‘consortships’ [12,20,21,28–31], but see [32]. Consequently, high-ranking males typically sire more offspring than low-ranking males [27,33–37], and males are more likely to father offspring with females to whom they direct aggression [30].
Adolescent male chimpanzees between 8 and 15 years old are still physically and socially immature and therefore cannot compete effectively with larger, stronger adult males or serve as effective coalitionary partners [38]. Their small size and reduced strength may also prevent adolescent males from sexually coercing females. By age 16, males have usually finished growing and can dominate females, but as young adults (16–20 years), they still occupy low ranks in the male dominance hierarchy [20,39,40]. Despite these disadvantages, adolescent and young adult male chimpanzees sire a non-trivial number of offspring [33–37,41,42]. How they do so is not entirely clear.
One tactic young males adopt is to selectively pursue specific females as mating partners. In this context, nulliparous, adolescent female chimpanzees cycle for many years without conceiving and often lose their first offspring [43–46]. Adult males typically show little sexual interest in these females [42,47]. Adolescent male chimpanzees consequently target nulliparous females as mating partners and primarily reproduce with these first-time mothers [42,48]. Nevertheless, this strategy cannot explain adolescent and young adult male mating and reproductive success completely because young males also father the offspring of older, parous females [35,37,42].
A second, non-mutually exclusive possibility is that young male chimpanzees mate by forming affiliative bonds with females. Males living at Ngogo in Kibale National Park, Uganda, forge affiliative bonds with females during adolescence and young adulthood [49]. These affiliative bonds manifest when females are cycling as well as when they are pregnant and lactating, and males and females who form bonds display relatively equitable grooming relationships, keep track of one another during travel and reassure each other [49]. Males in both age groups, however, selectively target their female partners for aggression irrespective of the latter's reproductive state [49]. It is currently unclear whether the affiliative or aggressive aspects of these relationships contribute to the mating success of young male chimpanzees. Prior research suggests this as a possibility as adult male and female chimpanzees who frequently associate and range in the same areas of the Ngogo communal territory often produce offspring together [37].
The Ngogo chimpanzee community is extremely large, including many adolescent and young adult males. This creates an ideal opportunity to investigate the reproductive strategies employed by young male chimpanzees. In this paper, we start by examining adolescent and young adult male chimpanzee mating behaviour and reproduction to determine whether they favour nulliparous instead of parous females as mating partners. Specifically, we investigate whether adolescent males father the offspring of first-time mothers and whether this changes as males grow older and higher ranking. We then proceed to examine how affiliation and coercive aggression influence male mating success from adolescence through adulthood.
2. Methods
All research reported here was approved by the Institutional Animal Care and Use Committee at the University of Michigan, the Uganda National Council for Science and Technology, and the Uganda Wildlife Authority.
(a). Study site
Research took place at Ngogo in Kibale National Park, Uganda, a mid-altitude rainforest [50]. Members of the Ngogo chimpanzee community occupy a territory of approximately 35 km2 [51]. Male chimpanzees have been followed continuously since 1995, and females identified and regularly followed since 2004. The community size has ranged between 140 and 219 chimpanzees [45]; Ngogo Chimpanzee Project, unpublished data. The ages of natal individuals younger than 20 years old are known within 1 day to 1 year, while the ages of older individuals are estimated based on their genetic relationships to other individuals, physical appearance and behaviour [45]. Immigrant females are estimated to be 13 years old when they enter the community [45].
After the completion of this study in 2018, the Ngogo chimpanzee community fissioned [52,53]. The split occurred gradually over time, starting in July 2015 at the inception of observations made here. Because males and females from the entire community continued to associate and mate throughout the study period, we treat all members of the Ngogo chimpanzee community together in the following analyses. Nevertheless, males and females, who became members of the same group after the completion of the split in 2018, tended to associate more frequently with each other during the observations reported here (Reddy, unpublished data). We accounted for this by including association as a predictor of mating frequency (see below).
(b). Behavioural observations
Behavioural data on the affiliative and aggressive social interactions between male chimpanzees and females and their influence on mating success were derived from focal observations made by R.R. over 17 months during June–August 2015 and June 2016–August 2017. Twenty adolescent (9–15 years old) and 10 young adult male chimpanzees (16–20 years old) were focal subjects. Males were considered adolescent if their testes were visible and enlarged [54]. Each male was followed for at least 25 h (mean ± s.d. = 47.6 ± 6.6 h per focal; range: 25–61 h) and on at least 19 different days (mean ± s.d. = 33.8 ± 6.2 days per focal; range: 19–47 days). Focal observation sessions typically lasted 3 h. If after 3 h no other focal subjects were present, R.R. remained with her current subject for up to 5 h before leaving to search for a new one. In rare instances when there were no other focal subjects available, she followed her current focal individual for more than 5 h. During focal observation sessions, R.R. recorded behavioural interactions between adolescent and young adult males and other community members, including 78 mature females who were not their mothers or maternal sisters (n = 2,276 total dyads). Females were considered mature if they had begun to exhibit sexual swellings, which was around 11 years old. Sixty-two of these mature females were parous at the start of observations or gave birth for the first time during the study, while 16 were nulliparas.
During focal following sessions, we recorded all chimpanzees who were within visual range of the focal male during the hour-long focal following episodes. These individuals were defined to be in association, or in the same subgroup with focal subjects ([55–57]). The amount of time focal subjects gave and received grooming was noted to the nearest second. We recorded all observed instances of aggression directed by focal males to mature females. We quantified male aggression as the number of times males threatened, displayed, charged, chased, hit, kicked and bit females [28,58]. We also recorded mating and noted daily whether females in association with focal subjects had full sexual swellings.
(c). Analyses
(i). Male dominance rank
Data on rank relationships between adult males come from focal observations made by J.M. between 2003 and 2018 on 62 adult males. He assigned yearly ordinal ranks to males if they had reached adulthood (i.e. 16 years old) and were observed making pant grunts, calls given by subordinate individuals to higher ranking individuals [24]. To account for the number of males in the hierarchy each year, we standardized males' yearly ordinal ranks by subtracting the male's ordinal rank (r) from the total number of adult males present in the community that year (nM) and dividing this number by one less than the total number of males in the community that year [47]:
During years where a conception occurred but a male's rank was missing due to incomplete observations but his subsequent year's rank was available, we averaged the previous and subsequent year's standardized ranks. When a subsequent year's rank was unavailable (e.g. the male had died), we used the previous years' standardized rank. We replaced missing ranks for five males in the preceding manner in nine instances, representing 0.19% of 4744 total siring opportunities.
(ii). Paternity success
We assigned paternity to 105 offspring born since 2003 after all possible sires were genotyped. We conducted likelihood-based parentage analyses [59] using 19–44 autosomal microsatellites typed from faecal samples collected non-invasively from chimpanzees. Paternities were determined in earlier research (N = 91: [37,60–62]) or newly generated for this study (N = 14) following previously published methods. Most offspring (77% = 81/105) had a parous mother at the time of their conception. Twenty-four had mothers known or assumed to be nulliparous based on behavioural observations, the female's estimated age and the median age at first birth reported for East African chimpanzees who emigrate (16 years; [46]).
We determined how often adolescent and adult males of varying ages and ranks sired offspring with nulliparous females (i.e. first-time mothers) and parous females. To do so, we conducted two mixed effects logistic regression analyses where the outcome variable was whether a male was the father of an infant he had the opportunity to sire. We considered males who were at least 9 years old at the time of the infant's conception as potential fathers because this is the age of the youngest known sire in chimpanzees [41,42]. We also excluded males as potential sires of infants born to their mothers or maternal sisters, as this type of close inbreeding has not been documented at Ngogo and occurs rarely in chimpanzees elsewhere [63]. We were interested in both age and rank as indicators of a male's ability to win in direct male–male competition, but male rank and age were correlated (Spearman's ρ = 0.77, p ≪ 0.001). We therefore conducted two analyses, one that included only age at the time an infant was conceived as a predictor, and one that included only rank as a predictor. For the purposes of the latter analysis, we set the ranks of adolescent males and young adults who had not entered the hierarchy to zero because adolescent males almost never receive pant grunts from adult males and rarely engage in dominance interactions between themselves [64,65].
(iii). Factors affecting male mating success
We employed a model comparison approach to investigate the effects of several factors predicted to influence male mating success. We assayed mating success by the number of times males and females mated. In our analyses, we included only females who exhibited full oestrous swellings and were able to mate (N = 57 females, 14 nulliparas, 43 parous females, 1,671 pairs) and constructed 15 biologically plausible candidate models for comparison. These models comprised combinations of the following seven variables: (i) male age, calculated as his median age (in years) across the study period; (ii) female parity, with females classified as nulliparous or parous; (iii) association, measured by how often we observed males and females together in the same party; (iv) male aggression defined as the number of times a male-directed aggression toward a particular female; (v) affiliative bond strength, assayed by the amount of time males and females spent grooming measured in minutes; (vi) an observation effort offset, defined as the number of hours we observed a focal male while a particular female was alive and present in the community and (vii) oestrus time, defined as the number of days each female had a full oestrous swelling and was able to mate. Grooming was highly correlated with a composite affiliation index, which combines grooming and closes spatial proximity that we used in a previous study [49]. We chose to use absolute grooming time rather than the composite affiliation index as a predictor in this analysis of mating success because maintaining close spatial proximity might reflect mate-guarding by males instead of affiliation per se.
We also included interactions between five of the preceding seven variables in some of our models. To investigate developmental changes in mating strategies, we included interactions between male age and female parity, association, male aggression and grooming time in models where these variables appeared together. Because we hypothesized that aggression might work differently for females with whom males have strong compared to weak affiliative bonds [49], we included an interaction between grooming time and male aggression in half of the models where both variables appeared and excluded it in others. All models assumed negative binomial distributions and included male and female identity as random intercepts. The constrained list of 15 candidate models permitted us to evaluate the factors that influence male chimpanzee mating success across adolescence and early adulthood [66]. We provide a detailed description and justification for the inclusion of each candidate model in the electronic supplementary material.
We used Akaike's information criterion, corrected for small sample size (AICc), to determine the best-approximating model(s). We considered the model with the lowest AICc value and those within two AICc values of it to have strong explanatory power. We evaluated the impact of predictor variables that appeared in the explanatory models by reporting their coefficient estimates, standard errors and incidence rate ratios.
All analyses were conducted in R using the packages lme4 and MuMIn [67–69]. To account for the varying scales of predictor variables in our multivariate analyses, we centred and standardized all predictors by using their z-scores, subtracting each data point from the mean and dividing this value by two times the standard deviation [70]. We also determined that the fixed effects in our models were not highly correlated using the function glmer. We include raw data files and R code used to conduct all analyses and correlation checks in the electronic supplementary material.
3. Results
(a). Relationships between male age and rank and paternity
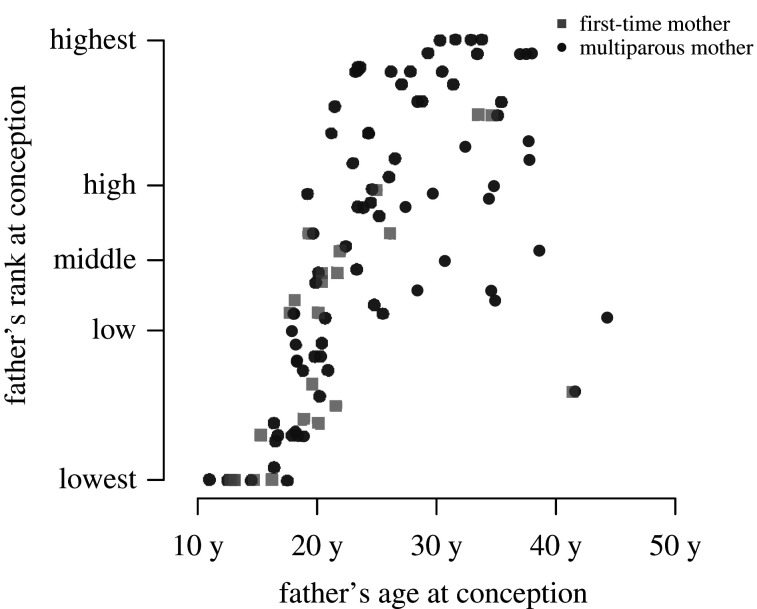
Adolescent male chimpanzees fathered 9% (9/105) of the infants in our sample, a considerably smaller proportion than would be expected by chance (32% = 1531/4744 of siring opportunities that were available to adolescent males). Adolescent males primarily fathered the infants of first-time mothers (6/9 = 67%), who conceived about a quarter of all offspring in our sample (23% = 24/105). As males transitioned to adulthood and grew increasingly high-ranking, they sired proportionately fewer offspring with first-time mothers than they did with parous females. Only 29% (10/34) of the offspring fathered by young adult males between 16 and 20 years old had nulliparous mothers, while older adult males (greater than 20 years) produced even fewer of their offspring with first-time mothers (13% = 8/62). These proportions were considerably lower for high-ranking adult males defined as those in the upper third of the dominance hierarchy with standardized ranks greater than or equal to 0.67 ([47]). All of these high-ranking males were older than 20 years and rarely fathered offspring of first-time mothers (6% = 2/35 offspring; figure 1).
Figure 1.
Father's rank and age at time of conception for offspring born to first-time mothers (squares) and multiparous mothers (circles) (N = 105 offspring). Adolescent males (less than 16 years) who have not entered the male hierarchy are those in the ‘lowest' rank category, while low-ranking males are those with a standardized rank less than 0.34, high-ranking males are those with standardized ranks greater than 0.67 and middle-ranking males are those in-between [47]. (Online version in colour.)
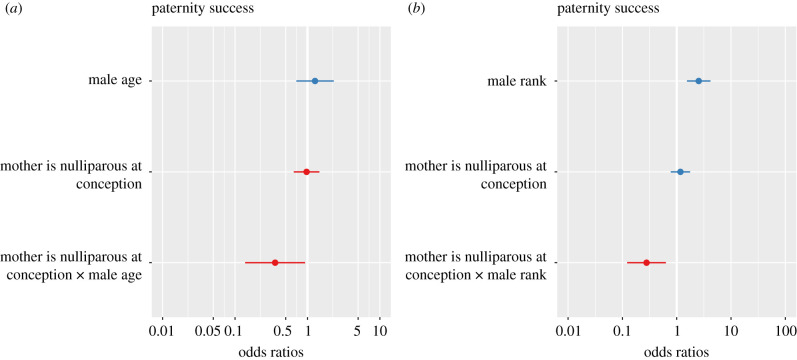
Two analyses indicated that both age and rank influenced the probability that males fathered infants of first-time mothers. A mixed effects logistic regression analysis that accounted for the number of opportunities males had to sire offspring indicated that a 1-year increase in a male's age decreased his probability of fathering an infant born to a first-time mother by a factor of 1.03 (figures 1 and 2, electronic supplementary material, table S1). An additional analysis indicated that an increase in a male's rank decreased the probability that he fathered an infant born to a first-time mother by a factor of 1.28 (figures 1 and 2, electronic supplementary material, table S1).
Figure 2.
Odds ratio plots showing main effect predictors for mixed effects logistic regression models where the outcome variable was whether a male sired a particular infant he had the opportunity to father and predictors included: (a) male age or (b) male rank. (N = 4744 fathering opportunities, 76 males, 58 mothers, 105 offspring, 24 born to first-time mothers, electronic supplementary material, table S1). (Online version in colour.)
(b). Relationships between male age, female parity, male aggression and affiliation on mating success
Mating occurred 339 times in 12% (197/1671) of potential pairs formed between 20 adolescent and 10 young adult males and 57 reproductively active females (mean = 0.20 times per pair, s.d. = 0.74). Pairs that mated groomed more frequently (mean = 4.3 min, s.d. = 9.3) than pairs that did not mate (mean = 0.6 min, s.d. = 3.7). In addition, males behaved aggressively more often to females with whom they mated than to other females. On average, male aggression occurred 0.73 times (s.d. = 1.23) per pair in which mating occurred and 0.15 times (s.d. = 0.73) in pairs that never mated. Younger males mated more frequently with nulliparous females than did older males. Mating occurred on average 0.50 times (s.d. = 1.3) in adolescent male-nulliparous female pairs compared to 0.17 times (s.d. = 0.65) in young adult male-nulliparous female pairs.
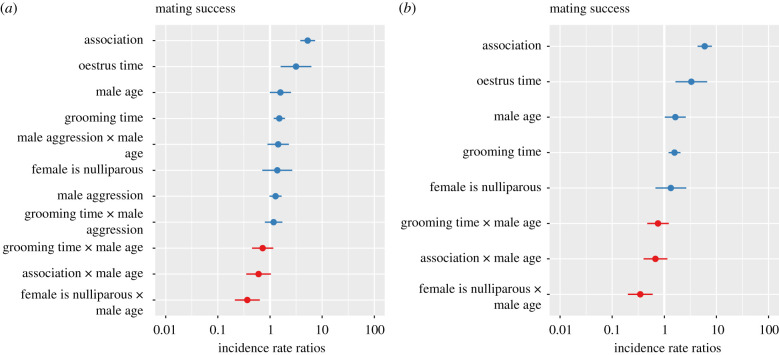
A model comparison analysis indicated that the best-approximating model with the lowest AICc value included six variables: oestrus time, male age, female parity, male aggression, grooming time and association. Interactions between male age and four other factors, female parity, male aggression, grooming time and association, were also included in the best-approximating model (electronic supplementary material, tables S2 and S3). This model's Akaike weight (57%) was considerably higher than that of any of the other 14 models (electronic supplementary material, tables S2 and S3, figure 3a). Only one other candidate model, with an Akaike model weight of 29% and Δ AICc of 1.38 from the best-approximating model, displayed some explanatory power (electronic supplementary material, tables S2 and S3, figure 3b). This model consisted of all of the variables in the best-approximating model plus an interaction between grooming time and male aggression.
Figure 3.
Incidence rate ratio plots showing main effect predictors for the generalized linear mixed models that had the: (a) lowest AICc value and composed 57% of the model weight and (b) second lowest AICc value (ΔAICc = 1.38) and composed 29% of the model weight, in a comparison of 15 models (electronic supplementary material, table S2). In these models, the outcome variable was the number of times males and females mated (n = 1671 male–female pairs including 20 adolescent males, 10 young adult males, 57 reproductively available females, 14 of whom were nulliparas). (Online version in colour.)
Closer examination of the best-approximating model revealed how male age, female parity, grooming time, male aggression and association influenced male mating success. First, consistent with results of the long-term paternity analysis, males mated less often with nulliparous females as they entered adulthood. For each unit increase in male age, the frequency with which males mated nulliparous females decreased by a factor of 0.4 (electronic supplementary material, tables S2 and S4, figures 3 and 4).
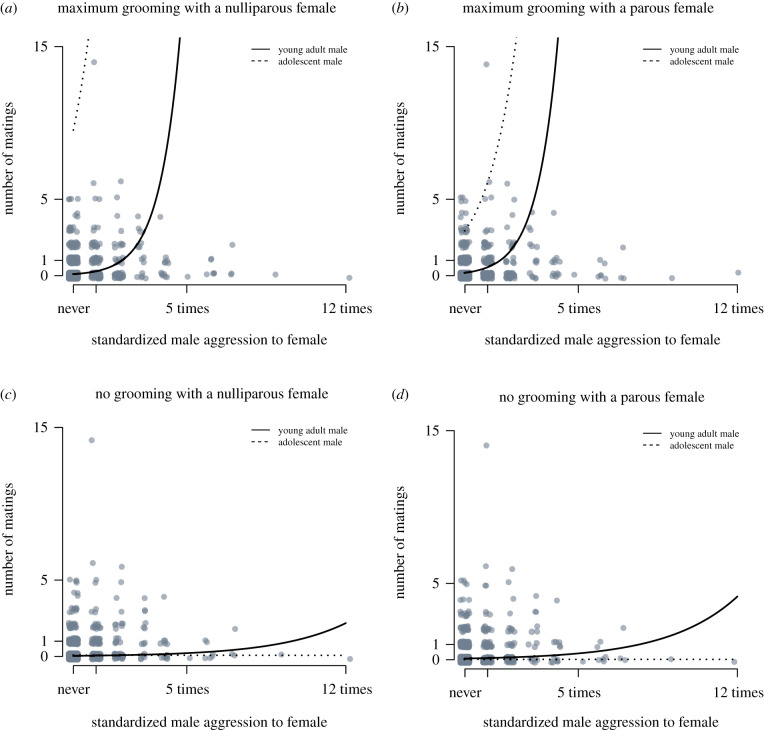
Figure 4.
Mating frequency of adolescent and young adult males and nulliparous and parous females, plotted as a function of the number of times the male in a pair directed aggression to the female. Lines illustrate predictions of mating frequency based on coefficient estimates from the best-approximating models (figure 3, electronic supplementary material, tables S2 and S3) for an adolescent (set to 9 years) and a young adult (set to 20 years) with: (a) a nulliparous female with whom he had a ‘strong bond’, set as grooming for the maximum amount of time in this dataset; (b) parous female with whom he had a ‘strong bond’, defined as in (a); (c) nulliparous female with whom he never groomed and (d) a parous female with whom he never groomed. Association values, oestrous time and observation effort are set to the mean values in the dataset for all prediction lines. Male age and grooming time were continuous variables.
Second, affiliative bond strength, assayed by grooming, was positively associated with the number of times male chimpanzees mated, and its effect on mating success changed as males transitioned from adolescence to adulthood (electronic supplementary material, tables S2 and S4, figures 3 and 4). A unit increase in a male's grooming time with a particular female increased the number of times he mated with her by a factor of 1.5. The relationship between grooming time and mating success, however, weakened as males grew older, decreasing by a factor of 0.73 with each unit increase in male age.
Third, aggression also had a positive relationship with male mating success. Mating success increased by a factor of 1.3 with each unit increase in male aggression. Male aggression toward females became more effective as males grew older. The predicted impact of aggression on mating success increased by a factor of 1.6 with each unit increase in male age (electronic supplementary material, tables S2–S4, figures 3 and 4).
Results from an analysis of the only other model which displayed some explanatory power did not differ appreciably from the findings presented above. This model, however, suggested that aggression interacted with grooming time to influence male mating success. Here aggression by males toward females increased their predicted frequency of mating by a factor of 1.2 with every unit increase in a pair's grooming time (electronic supplementary material, tables S2 and S4, figures 3 and 4).
4. Discussion
The results presented here suggest that adolescent male chimpanzees, who cannot effectively compete with older males nor sexually coerce adult females, employ at least two behavioural tactics to mate and reproduce. First, as reported in previous research, adolescent males appear to target adolescent, nulliparous females as mating partners; they mate with nulliparous females frequently and father their first offspring more often in adolescence than they do in adulthood [42,48]. Our findings also corroborate past research indicating that nulliparous female chimpanzees are less preferred as mating partners than are parous females. Specifically, as male chimpanzees transition from adolescence to adulthood and rise in dominance rank, they show less sexual interest in nulliparous females and target them for aggression infrequently [47]. High-ranking males also rarely father the first offspring of these females [33–37,42]. Second, mating success for adolescent and young adult males was predicted by the strength of affiliative bonds that males formed with females. Male aggression, by contrast, had a relatively weak relationship with mating success, but one that strengthened as males grew older and increasingly dominant to females.
These findings increase our understanding of the nature of sexual coercion in chimpanzees. We have recently shown that adolescent and young adult males selectively direct aggression toward females with whom they form strong affiliative bonds [49]. Here, we demonstrate that aggression has a reduced effect on mating success outside of these bonds for young, adolescent males who are not yet physically mature and unlikely to dominate females [49,54,71]. Instead, mating success increases when an older adolescent or young adult male directs aggression to a female with whom he frequently affiliates and can dominate.
These results complement prior research that indicates aggression, mating and reproduction are linked in chimpanzees [28,30,31] and clarify the role that affiliation plays in creating those linkages. Specifically, sexual coercion is more effective when adolescent and young adult males have affiliative bonds with the females they attack. One reason may be that females suffer higher costs if they refuse to mate with males with whom they frequently affiliate compared to males with whom they rarely affiliate [12]. The nature of these costs remains to be explored. Nor is it clear whether and how affiliative relationships with males benefit female chimpanzees. It is important to note that our findings are consistent with patterns of intersexual aggression in other species where males are highly aggressive to females with whom they share bonds. This includes hamadryas baboons (Papio hamadryas), where females live in one-male groups, and most social activity is directed by the single males in these groups, i.e. ‘leader males’. After being attacked by their leader male, hamadryas females appear fearful and follow him even more closely than they had previously [72]. In our own species too, many women are subject to frequent sexual coercion by their male partners, but often remain in such relationships for reasons that vary widely [73].
Scant data exist about the proximate psychological mechanisms that underlie male aggression and female compliance in chimpanzees. However, investigating these proximate mechanisms may provide information about how bonds that affect paternity in chimpanzees might lead to a human-like social system [74]. One interpretation consistent with our preliminary observations is that male aggression toward their social partners is motivated by sexual possessiveness (e.g. [73]), and that females have a psychologically distinct experience when attacked by a male with whom they have an affiliative bond. For example, adolescent and young adult males make direct attacks on male peers infrequently, but when they do so, it is when another male mates or attempts to mate with one of their female social partners [49]. Anecdotally, when female chimpanzees received aggression from an adult male who did not have a strong affiliative bond with them, they often just screamed and ran away. Females receiving similar aggression from males with whom they shared strong affiliative relationships, however, react in an entirely different way. When attacked, these females remain in place, lunge toward their male partners while clutching their arms, rocking back and forth, and screaming repeatedly until making choking sounds.
Our study has several limitations. First, we cannot evaluate the relative impacts of affiliation and aggression on adolescent male paternity success. Only seven males in this study have reproduced thus far, siring 15 offspring, creating a small sample to make strong inferences. Our preliminary findings based on this small sample suggest that males who affiliate with and direct aggression to specific females gain a reproductive advantage with those females, but additional data are clearly needed. As these data accumulate, evaluating the effects of affiliation and aggression on male reproduction will be complicated because additional factors that we have not considered will require examination. For instance, we are likely to have underestimated the importance of sexual coercion, as it may act to ensure mating exclusivity as well as increasing a male's ability to mate with a specific female [12]. In this context, aggression is often used to initiate consortships in chimpanzees, where males lead females away from other community members and mate with them exclusively for several days (e.g. [21]). The challenge of maintaining exclusivity is not uniform. It may be relatively easy for high-ranking males because fewer males will challenge them to mate, or easier to accomplish with nulliparas, who are not preferred mating partners [47]. Second, we conducted this study over a relatively short period spanning two years, which covered only a single reproductive cycle for most females. Additional research is required to determine whether affiliative bonds between males and females endure and whether the patterns of aggressive and affiliative behaviour between bonded pairs persist and impact male reproduction over the long term (e.g. [30,37]). Determining whether such long-term relationships exist and how they impact male mating and reproductive success will improve our understanding of male chimpanzee development and the functional consequences of their behaviour.
Our findings also provide insights into the evolution of human pair bonds. Although the mechanisms that ensure paternity certainty in our species are diverse, including intimate partner violence [73–75] and larger cultural structures (e.g. religion: [76,77]), our finding that affiliative bonds between males and females appear entwined with sexual coercion in one of our two closest living relatives suggests that this aspect of intersexual relationships may be embedded deeply in our past.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
This project would have been impossible without help, company, and guidance from many people who have worked for and been part of the Ngogo Chimpanzee Project, including Samuel Angedakin, Chris Aliganyira, Charles Birungi, Charles Businge, Jeremy Clift, Rebecca Davenport, Bethany Hansen, Brian Kamugyisha, Sarah Dunphy-Leili, Jeremiah Lwanga, Godfrey Mbabazi, Braise Mugyisha, Lawrence Ndangizi, Jacob Negrey, Carolyn Rowney, Thomas Struhsaker, William Sunday, Ambrose Twineomujuni, Alfred Tumusiime, James Tibisimwa and David Watts. For their perspectives on the findings and development of this study, we thank Jacinta Beehner, Andrew Marshall, Alexandra Rosati, Barbara Smuts and David Watts. Laboratory work was conducted by Anette Nicklisch, Amy Heilman, Sebastian Ramirez Amaya and Veronika Staedele. Statistical advice was provided by Andrew Marshall, Chris Andrews, Josh Errickson and others at the Center for Statistics, Computing, and Analytics Research at the University of Michigan. We thank two anonymous reviewers for their thoughtful feedback on this manuscript.
Ethics
All research protocols reported in this manuscript were approved by the Institutional Animal Care and Use Committee at the University of Michigan. Work conducted in Uganda was approved by the Uganda National Council for Science and Technology (NS488) and the Uganda Wildlife Authority (EDO-35-01).
Data accessibility
All data presented in this manuscript are available in the electronic supplementary material.
Authors' contributions
R.R. planned the study, collected behavioural data, conducted the analyses and wrote the manuscript. K.L. contributed to the development of the study, conducted the paternity analyses and wrote the manuscript. A.S. contributed to the development of the study and wrote the manuscript. L.V. supervised the genetic analyses. J.M. planned the study, collected behavioural data and wrote the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This project was supported by grants to R.R. from the National Geographic Society (9742-15), the United States National Science Foundation (BCS-1613392, DGE-1256260), the Nacey-Maggioncalda Foundation, the L.S.B. Leakey Foundation, the African Studies Center, International Institute, Rackham Graduate School and Department of Anthropology at the University of Michigan. Additional support was provided by the Max Planck Society and by a grant from the NIH (R01AG049395) to J.M. and others.
References
- 1.Darwin C 1871. Sexual selection and the descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 2.Trivers R 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B), pp. 136–179. Chicago, IL: Aldine Publishing Company. [Google Scholar]
- 3.Clutton-Brock TH (ed.). 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 4.Hollister-Smith JA, Poole JH, Archie EA, Vance EA, Georgiadis NJ, Moss CJ, Alberts SC. 2007. Age, musth and paternity success in wild male African elephants, Loxodonta africana. Anim. Behav. 74, 287–296. ( 10.1016/j.anbehav.2006.12.008) [DOI] [Google Scholar]
- 5.Becker PH, Dittmann T, Ludwigs JD, Limmer B, Ludwig SC, Bauch C, Braasch A, Wendeln H. 2008. Timing of initial arrival at the breeding site predicts age at first reproduction in a long-lived migratory bird. Proc. Natl Acad. Sci. USA 105, 12 349–12 352. ( 10.1073/pnas.0804179105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komers PE, Pélabon C, Stenström D. 1997. Age at first reproduction in male fallow deer: age-specific versus dominance-specific behaviors. Behav. Ecol. 8, 456–462. ( 10.1093/beheco/8.4.456) [DOI] [Google Scholar]
- 7.Le Boeuf BJ, Mesnick S.. 1991. Sexual behavior of male northern elephant seals: I. Lethal injuries to adult females. Behaviour 116, 143–162. ( 10.1163/156853990X00400) [DOI] [Google Scholar]
- 8.Mineau P, McKinney F, Derrickson SR. 1983. Forced copulation in waterfowl. Behaviour 86, 250–293. ( 10.1163/156853983X00390) [DOI] [Google Scholar]
- 9.Hogg JT 1984. Mating in bighorn sheep: multiple creative male strategies. Science 225, 526–529. ( 10.1126/science.6539948) [DOI] [PubMed] [Google Scholar]
- 10.Mann J, Richards AF, Smolker RA, Connor RC. 1996. Patterns of female attractiveness in Indian Ocean bottlenose dolphins. Behaviour 133, 37–69. ( 10.1163/156853996X00026) [DOI] [Google Scholar]
- 11.Mitani JC 1985. Mating behaviour of male orangutans in the Kutai Game Reserve, Indonesia. Anim. Behav. 33, 392–402. ( 10.1016/S0003-3472(85)80063-4) [DOI] [Google Scholar]
- 12.Smuts BB, Smuts RW. 1993. Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv. Stud. Behav. 22, 1–63. ( 10.1016/S0065-3454(08)60404-0) [DOI] [Google Scholar]
- 13.Clutton-Brock TH, Parker GA. 1995. Sexual coercion in animal societies. Anim. Behav. 49, 1345–1365. ( 10.1006/anbe.1995.0166) [DOI] [Google Scholar]
- 14.Palombit RA 2014. Sexual conflict in nonhuman primates. Adv. Stud. Behav. 46, 191–280. ( 10.1016/B978-0-12-800286-5.00005-5) [DOI] [Google Scholar]
- 15.Gross MR 1996. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 11, 92–98. ( 10.1016/0169-5347(96)81050-0) [DOI] [PubMed] [Google Scholar]
- 16.Wikelski M, Bäurle S. 1996. Pre-copulatory ejaculation solves time constraints during copulations in marine iguanas. Proc. R. Soc. Lond. B 263, 439–444. ( 10.1098/rspb.1996.0066) [DOI] [Google Scholar]
- 17.Endo A, Doi T. 2002. Multiple copulations and post-copulatory guarding in a free-living population of sika deer (Cervus nippon). Ethology 108, 739–747. ( 10.1046/j.1439-0310.2002.00803.x) [DOI] [Google Scholar]
- 18.Apio A, Plath M, Tiedemann R, Wronski T. 2007. Age-dependent mating tactics in male bushbuck (Tragelaphus scriptus). Behaviour 144, 585–610. [Google Scholar]
- 19.Nishida T 1968. The social group of wild chimpanzees in the Mahali Mountains. Primates 9, 167–224. ( 10.1007/BF01730971) [DOI] [Google Scholar]
- 20.Goodall J 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Harvard University Press. [Google Scholar]
- 21.Tutin CE 1979. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 6, 29–38. ( 10.1007/BF00293242) [DOI] [Google Scholar]
- 22.Harcourt A, Harvey P, Larson S, Short RV. 1981. Testis weight, body weight and breeding system in primates. Nature 293, 55–57. ( 10.1038/293055a0) [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa T, Hiraiwa-Hasegawa M. 1983. Opportunistic and restrictive matings among wild chimpanzees in the Mahale Mountains, Tanzania. J. Ethol. 1, 75–85. ( 10.1007/BF02347833) [DOI] [Google Scholar]
- 24.Bygott JD 1974. Agonistic behaviour and dominance in wild chimpanzees. PhD thesis, University of Cambridge, Cambridge, UK. [Google Scholar]
- 25.Muller MN 2002. Agonistic relations among Kanyawara chimpanzees. In Behavioral diversity in chimpanzees and bonobos (eds Boesch C, Hohmann G, Marchant LF), pp. 112–124. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Watts DP 1998. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 44, 43–55. ( 10.1007/s002650050513) [DOI] [Google Scholar]
- 27.Bray J, Pusey AE, Gilby IC. 2016. Incomplete control and concessions explain mating skew in male chimpanzees. Proc. R. Soc. B 283, 20162071 ( 10.1098/rspb.2016.2071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW. 2007. Male coercion and the costs of promiscuous mating for female chimpanzees. Proc. R. Soc. B 274, 1009–1014. ( 10.1098/rspb.2006.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller MN, Emery Thompson M, Kahlenberg SM, Wrangham RW. 2011. Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behav. Ecol. Sociobiol. 65, 921–933. ( 10.1007/s00265-010-1093-y) [DOI] [Google Scholar]
- 30.Feldblum JT, Wroblewski EE, Rudicell RS, Hahn BH, Paiva T, Cetinkaya-Rundel M, Pusey AE, Gilby IC. 2014. Sexually coercive male chimpanzees sire more offspring. Curr. Biol. 24, 2855–2860. ( 10.1016/j.cub.2014.10.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaburu SS, Newton-Fisher NE. 2015. Trading or coercion? Variation in male mating strategies between two communities of East African chimpanzees. Behav. Ecol. Sociobiol. 69, 1039–1052. ( 10.1007/s00265-015-1917-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stumpf RM, Boesch C. 2010. Male aggression and sexual coercion in wild West African chimpanzees, Pan troglodytes verus. Anim. Behav. 79, 333–342. ( 10.1016/j.anbehav.2009.11.008) [DOI] [Google Scholar]
- 33.Constable JL, Ashley MV, Goodall J, Pusey AE. 2001. Noninvasive paternity assignment in Gombe chimpanzees. Mol. Ecol. 10, 1279–1300. ( 10.1046/j.1365-294X.2001.01262.x) [DOI] [PubMed] [Google Scholar]
- 34.Boesch C, Kohou G, Nene H, Vigilant L. 2006. Male competition and paternity in wild chimpanzees of the Taï forest. Am. J. Phys. Anthropol. 130, 103–115. ( 10.1002/ajpa.20341) [DOI] [PubMed] [Google Scholar]
- 35.Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885. ( 10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newton-Fisher NE, Emery Thompson M, Reynolds V, Boesch C, Vigilant L. 2010. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am. J. Phys. Anthropol. 142, 417–428. ( 10.1002/ajpa.21241) [DOI] [PubMed] [Google Scholar]
- 37.Langergraber KE, Mitani JC, Watts DP, Vigilant L. 2013. Male–female socio-spatial relationships and reproduction in wild chimpanzees. Behav. Ecol. Sociobiol. 67, 861–873. ( 10.1002/ajp.20711) [DOI] [Google Scholar]
- 38.Enigk DK, Thompson ME, Machanda ZP, Wrangham RW, Muller MN. 2020. Competitive ability determines coalition participation and partner selection during maturation in wild male chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 74, 89 ( 10.1007/s00265-020-02872-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawanaka K 1993. Age differences in spatial positioning of males in a chimpanzee unit-group at the Mahale Mountains National Park, Tanzania. Primates 34, 255–270. ( 10.1007/BF02382620) [DOI] [Google Scholar]
- 40.Watts DP 2018. Male dominance relationships in an extremely large chimpanzee community at Ngogo, Kibale National Park, Uganda. Behaviour 155, 969–1009. ( 10.1163/1568539X-00003517) [DOI] [Google Scholar]
- 41.Langergraber KE, et al. 2012. Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proc. Natl Acad. Sci. USA 109, 15 716–15 721. ( 10.1073/pnas.1211740109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller MN, et al. 2020. Sexual dimorphism in chimpanzee (Pan troglodytes schweinfurthii) and human age-specific fertility. J. Hum. Evol. 144, 102795 ( 10.1016/j.jhevol.2020.102795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pusey A, Williams J, Goodall J. 1997. The influence of dominance rank on the reproductive success of female chimpanzees. Science 277, 828–831. ( 10.1126/science.277.5327.828) [DOI] [PubMed] [Google Scholar]
- 44.Emery Thompson M 2013. Reproductive ecology of female chimpanzees. Am. J. Primatol. 75, 222–237. ( 10.1002/ajp.22084) [DOI] [PubMed] [Google Scholar]
- 45.Wood BM, Watts DP, Mitani JC, Langergraber KE. 2017. Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter–gatherers. J. Hum. Evol. 105, 41–56. ( 10.1016/j.jhevol.2017.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker KK, Walker CS, Goodall J, Pusey AE. 2018. Maturation is prolonged and variable in female chimpanzees. J. Hum. Evol. 114, 131–140. ( 10.1016/j.jhevol.2017.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller MN, Thompson ME, Wrangham RW. 2006. Male chimpanzees prefer mating with old females. Curr. Biol. 16, 2234–2238. ( 10.1016/j.cub.2006.09.042) [DOI] [PubMed] [Google Scholar]
- 48.Watts DP 2015. Mating behavior of adolescent male chimpanzees (Pan troglodytes) at Ngogo, Kibale National Park, Uganda. Primates 56, 163–172. ( 10.1007/s10329-014-0453-z) [DOI] [PubMed] [Google Scholar]
- 49.Reddy RB, Mitani JC. 2020. Adolescent and young adult male chimpanzees form affiliative, yet aggressive, relationships with females. J. Hum. Evol., 144, 102813 ( 10.1016/j.jhevol.2020.102813) [DOI] [PubMed] [Google Scholar]
- 50.Struhsaker TT 1997. Ecology of an african rain forest: logging in Kibale and the conflict between conservation and exploitation. Gainesville, FL: University Press of Florida. [Google Scholar]
- 51.Mitani JC, Watts DP, Amsler SJ. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507–R508. ( 10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 52.Mitani J.C 2020. My life among the apes. Am. J. Primatol. e23107 ( 10.1002/ajp.23107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandel AA, Watts D. In press Lethal coalitionary aggression associated with a community fission in chimpanzees (Pan troglodytes) at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pusey AE 1990. Behavioural changes at adolescence in chimpanzees. Behaviour 115, 203–246. ( 10.1163/156853990X00581) [DOI] [Google Scholar]
- 55.Chapman CA, White FJ, Wrangham RW. 1994. Party size in chimpanzees and bonobos: a reevaluation of theory based on two similarly forested sites. In Chimpanzee cultures (eds Wrangham RW, McGrew WC, Heltne PG), pp. 41–58. Cambridge, MA: Harvard University Press; ( 10.1002/ajpa.1330980216) [DOI] [Google Scholar]
- 56.Mitani J, Watts D, Pepper J, Merriwether D. 2002. Demographic and social constraints on male chimpanzee behaviour. Anim. Behav. 64, 727–737. ( 10.1006/anbe.2002.4014) [DOI] [Google Scholar]
- 57.Sandel AA, Langergraber KE, Mitani JC. 2020. Adolescent male chimpanzees (Pan troglodytes) form social bonds with their brothers and others during the transition to adulthood. Am. J. Primatol. 82, e23091 ( 10.1002/ajp.23091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Lawick-Goodall J. 1968. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim. Behav. Monogr. 1, 161 ( 10.1016/S0066-1856(68)80003-2) [DOI] [Google Scholar]
- 59.Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. ( 10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 60.Langergraber KE, Mitani JC, Vigilant L. 2007. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 7786–7790. ( 10.1073/pnas.0611449104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langergraber K, Mitani J, Vigilant L. 2009. Kinship and social bonds in female chimpanzees (Pan troglodytes). Am. J. Primatol. 71, 840–851. ( 10.1002/ajp.20711) [DOI] [PubMed] [Google Scholar]
- 62.Langergraber KE, Watts DP, Vigilant L, Mitani JC. 2017. Group augmentation, collective action, and territorial boundary patrols by male chimpanzees. Proc. Natl Acad. Sci. USA 114, 7337–7342. ( 10.1073/pnas.1701582114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker KK, Rudicell RS, Li Y, Hahn BH, Wroblewski E, Pusey AE. 2017. Chimpanzees breed with genetically dissimilar mates. R. Soc. Open Sci. 4, 160422 ( 10.1098/rsos.160422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayaki H 1988. Association partners of young chimpanzees in the Mahale Mountains National Park, Tanzania. Primates 29, 147–161. ( 10.1007/BF02381119) [DOI] [Google Scholar]
- 65.Sandel AA, Reddy RB, Mitani JC. 2017. Adolescent male chimpanzees do not form a dominance hierarchy with their peers. Primates 58, 39–49. ( 10.1007/s10329-016-0553-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–23 ( 10.1007/s00265-010-1029-6) [DOI] [Google Scholar]
- 67.Barton K 2009. Mu-MIn: Multi-model inference. R package version 0.12.2/r18. See http://R-Forge.R-project.org/projects/mumin/.
- 68.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 69.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 70.Gelman A, Hill J. 2007. Data analysis using regression and multilevel/hierarchical models (vol. 1). New York, NY: Cambridge University Press. [Google Scholar]
- 71.Nishida T 2003. Harassment of mature female chimpanzees by young males in the Mahale Mountains. Int. J. Primatol. 24, 503–514. ( 10.1023/A:1023870229247) [DOI] [Google Scholar]
- 72.Swedell L, Schreier A. 2009. Male aggression towards females in hamadryas baboons: conditioning, coercion, and control. In Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females (eds Muller M, Wrangham RW), pp. 244–268. Cambridge, UK: Harvard University Press. [Google Scholar]
- 73.Wilson M, Daly M. 2009. Coercive violence by human males against their female partners. In Sexual coercion in primates and humans (eds Muller M, Wrangham RW), pp. 271-291. Cambridge, MA: Harvard University Press. [Google Scholar]
- 74.Smuts BB 1995. The evolutionary origins of patriarchy. Hum. Nat. 6, 1–32. ( 10.1007/BF0273413) [DOI] [PubMed] [Google Scholar]
- 75.Devries KM, et al. 2013. The global prevalence of intimate partner violence against women. Science 340, 1527–1528. ( 10.1126/science.1240937) [DOI] [PubMed] [Google Scholar]
- 76.Bamshad MJ, Watkins WS, Dixon ME, Jorde LB, Rao BB, Naidu JM, Ravi Prasad BV, Rasanayagam A, Hammer MF. 1998. Female gene flow stratifies Hindu castes. Nature, 395, 651–652. ( 10.1038/27103) [DOI] [PubMed] [Google Scholar]
- 77.Strassmann BI, Kurapati NT, Hug BF, Burke EE, Gillespie BW, Karafet TM, Hammer MF. 2012. Religion as a means to assure paternity. Proc. Natl Acad. Sci. USA 109, 9781–9785. ( 10.1073/pnas.1110442109) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this manuscript are available in the electronic supplementary material.