Abstract
Background: Congestion is one of the main predictors of poor outcome in patients with heart failure (HF); thus, a simple tool to evaluate plasma volume (PV), which can be used for risk stratification of HF patients, is necessary. We sought to compare the prognostic values of commonly used formulas for the estimation of PV and relative PV status (PVS) in patients admitted with acute decompensated HF (ADHF).
Methods and Results: We analyzed 384 consecutive ADHF patients who survived to discharge. The PV was calculated by 3 commonly used formulas (Strauss, Kaplan, and Hakim), and the relative PVS was calculated using the Hakim formula at both admission and discharge. The primary endpoint was a composite of all-cause mortality and hospitalization for worsening HF. The secondary endpoints were pump failure death (PFD) and sudden cardiac death (SCD). During a median follow-up of 743 days, 175 patients reached the primary endpoint, 28 patients had PFD, and 20 patients had SCD. Multivariate Cox analysis revealed that among the PV indices, only the PVS values at admission and discharge were independent predictors of the primary endpoint. In addition, the PVS values at admission and discharge were independent predictors of PFD and SCD in the multivariate analysis.
Conclusions: Among the indices of PV, the calculated PVS may be the most useful for predicting prognosis in ADHF patients.
Key Words: Acute decompensated heart failure, Congestion, Plasma volume
Despite acute decompensated heart failure (ADHF) being a major health problem worldwide, there has been no improvement in postdischarge mortality or readmission rates.1 The main reason for hospitalization for ADHF is signs and symptoms of congestion.2,3 Because both congestion on admission and residual congestion at discharge are associated with poor clinical outcomes in patients admitted for ADHF,4,5 the detection and quantification of congestion has paramount importance in the management of patients with ADHF. The assessment of congestion is often difficult, especially when symptoms are mild.6 However, it has been reported that patients are still at risk of a poor clinical outcome even when the signs and symptom of congestion are absent or minimal.5 Therefore, tools and methods for the detection of subclinical hemodynamic congestion are required. Although several biomarkers, biological parameters, clinical congestion scores, imaging tools, and pressure or impedance-based tools are available, right heart catheterization remains the gold standard for the evaluation of hemodynamic congestion in patients with heart failure (HF).6,7 However, right heart catheterization is invasive and can be harmful when used routinely.8
Plasma volume (PV) expansion plays an essential role in HF; PV and the change in PV can be estimated by several formulas based on readily available data such as hematocrit (Hct), hemoglobin (Hb), and body weight (BW).9–11 In addition, it has recently been reported that PV status (PVS), which represents the deviation from a patient’s ideal PV, can be calculated using a simple formula.12,13 These PV indices are inexpensive, easy-to-use, and non-invasive methods for the assessment of congestion, and they can be used repeatedly during a single hospital stay. Although several studies have assessed the prognostic impact of these PV indices in patients with ADHF,14–17 little comparative information is available, or the clinical significance of these PV indices, in ADHF patients. Moreover, it is unknown whether these PV indices are useful for the prediction of the mode of death, which may assist in decision making with regards to specific medications or devices. Accordingly, we aimed to evaluate and compare the prognostic value of these PV indices and their association with the mode of death in ADHF patients.
Methods
Subjects
We analyzed patients who were enrolled in our ongoing single-center, prospective cohort registry, “Osaka Prefectural trial: Acute heart failure syndrome Registry (OPAR)” (clinical registration with the University hospital Medical Information Network: UMIN000015246). The registry included consecutive patients who were admitted with a diagnosis of ADHF according to the Framingham criteria18 and who survived to discharge.19 Only the first admission of each patient during the study period was registered. Patients with acute coronary syndrome and active malignancy were not registered. The exclusion criteria were as follows: missing Hct, Hb, or BW data at admission or discharge; chronic hemodialysis; severe valvular or coronary artery disease that required surgical treatment during hospitalization or just after discharge; and withdrawal of informed consent. Patients were also excluded if they were judged inappropriate for this study by their primary physicians because of difficulty with follow-up and predicted poor adherence. Enrollment was performed from October 2011 to April 2016. The study was carried out in accordance with the principles outlined in the Declaration of Helsinki, and the institutional ethics committee approved the study protocol. Written informed consent was provided by all participants prior to their enrollment.
Data Collection
All patients underwent venous blood sampling and BW measurement at admission and discharge. Blood samples were drawn from an IV cannula after the patient had rested supine for >30 min. The estimated PV (ePV) was calculated with the Kaplan formula as follows: ePV (L)=(0.065×BW (kg))×(1−Hct).10 The ePV was also calculated with the Hakim formula as follows: ePV (mL)=(1−Hct)×[a+(b×lean BW (kg))], where a=1,530 in males and 864 in females, and b=41.0 in males and 47.2 in females.11 The lean BW was calculated from the height and BW as previously reported.20 The percent change in PV between admission and discharge (%∆PV) was calculated using the ePV values from the Kaplan and Hakim formulas: %∆PV=[(ePV (at discharge)−ePV (on admission))/ePV (on admission)]×100 (%). With the Strauss formula, %∆PV was calculated as %∆PV=[((Hb1/Hb2)×((1−Hct2)/(1−Hct1)))−1]×100 (%), where 1=admission value and 2=discharge value.9 The PVS was calculated with the Hakim formula as follows: PVS=[(ePV−ideal PV)/ideal PV]×100 (%), where ideal PV=c×BW (c=39 in males and 40 in females).21
We collected clinical variables, including age, sex, history of hypertension, presence of coronary artery disease (CAD), diabetes mellitus (DM), AF, chronic obstructive pulmonary disease (COPD), anemia, and prior HF admissions. Anemia was defined as an admission Hb level <13.0 g/dL for males and <12.0 g/dL for females, according to the World Health Organization criteria.14,17 The left ventricular ejection fraction (LVEF) was measured by echocardiography using a standard technique.19 Patients were classified according to the Clinical Scenario (CS) classification, based on systolic blood pressure at admission and other symptoms as previously reported.22 We also collected laboratory data, such as blood urea nitrogen (BUN) levels, serum levels of sodium and creatinine, plasma levels of B-type natriuretic peptide (BNP), and information on discharge prescriptions and device therapy. The estimated glomerular filtration rate (eGFR) was calculated using the modified isotope dilution mass spectrometry traceable modification of diet in renal disease (IDMS-MDRD) study equation with a Japanese coefficient.23
Endpoints
After discharge, all patients were followed-up in the HF unit of our center at least once every 1 or 2 months. The primary endpoint was a composite of all-cause death and unplanned hospitalization for worsening HF. The principal secondary endpoints were pump failure death (PFD) and sudden cardiac death (SCD). PFD was defined as death resulting from a deterioration of congestive HF with progression of congestive symptoms. SCD was defined as witnessed cardiac arrest or death within 1 h of the onset of acute symptoms, or unexpected, unwitnessed death in a patient known to have been well within the previous 24 h.24 Cardiac death, including PFD and SCD, non-cardiac death, and unplanned hospitalization for worsening HF were additional secondary endpoints. The data on these events were obtained by physicians from direct contact with their patients at the hospital in an outpatient setting, or by mail or a telephone interview of patients or their families by dedicated coordinators and investigators.
Statistical Analysis
Results are reported as the median (25–75th percentiles) for continuous data and percentages for categorical data. The Mann-Whitney U-test and chi-square test were used to compare differences in continuous and categorical variables, respectively. The prognostic value of the baseline characteristics was assessed with a Cox proportional hazards regression analysis. A multivariate Cox model for the primary endpoint in the total patients was adjusted for a total of 17 admission and discharge characteristics (age, sex, hypertension, CAD, DM, AF, anemia, LVEF at admission, systolic blood pressure at admission, New York Heart Association functional class III or IV at discharge, BUN at discharge, serum sodium at admission, eGFR at admission, plasma BNP level at discharge, loop diuretic received at discharge, angiotensin-converting enzyme inhibitor or angiotensin II type 1 receptor blocker received at discharge, and β-blocker received at discharge). These variables are thought to be clinically important or have been previously demonstrated to have prognostic significance.25,26 A multivariate Cox analysis for the principal secondary endpoints was performed using forced inclusion models involving age and pertinent covariates, taking into consideration a relatively small number of events. Multivariate Cox models for the primary endpoint in subgroups (patients with LVEF <45% and those with LVEF ≥45%, and patients in CS1 and those not in CS1) and for additional secondary endpoints were adjusted for a total of 5 admission and discharge characteristics (age, LVEF at admission, eGFR at admission, and plasma BNP level at discharge). The plasma BNP level was log10 transformed prior to inclusion in the Cox models. The event-free survival rates were calculated using the Kaplan-Meier method, and the differences in survival rates were compared between groups with the log-rank test. The predictive value of the indices of PV for the primary endpoint was compared using receiver-operating characteristic (ROC) curve analysis, and results are expressed in terms of the area under the curve (AUC) and 95% confidence interval (CI) for this area. A P-value <0.05 was considered significant. Statistical analysis was performed with a standard statistical program package (MedCalc Statistical Software version 18.9, MedCalc Software bvba, Ostend, Belgium).
Results
During the enrollment period, 432 patients were registered. After excluding 48 patients who met the exclusion criteria (missing Hct, Hb or BW data, n=6; chronic hemodialysis, n=19; severe valvular disease or CAD that required surgical treatment during hospitalization or just after discharge, n=6; withdrawal of informed consent, n=1; judged inappropriate as subjects for this study by primary physicians, n=16), the final cohort for analysis consisted of 384 patients. During a median follow-up of 743 days, 175 patients reached the primary endpoint of all-cause death or unplanned hospitalization for worsening HF. Cardiac death occurred in 48 patients: 28 patients had PFD and 20 patients had SCD. Non-cardiac death occurred in 71 patients (pneumonia, n=19; cancer, n=14; infection/sepsis, n=9; old age, n=6; stroke, n=5; renal failure, n=3; gastrointestinal bleeding, n=2; other causes of death, n=13), and unplanned hospitalization for worsening HF occurred for 110 patients.
Comparison of Baseline Characteristics
The baseline characteristics are summarized in Table 1. There was a significant difference in the proportion of HF etiology and CS classification between the patients with and without the primary endpoint. The patients who reached the primary endpoint were older and had a higher prevalence of CAD and anemia, and were more likely to have a history of prior HF admission. In addition, the NYHA class at discharge, BUN and serum creatinine levels at admission and discharge, plasma BNP level at discharge, and usage of an aldosterone antagonist and device therapy at discharge were higher in patients who reached the primary endpoint. The systolic and diastolic blood pressures and heart rates at admission, BW at admission and discharge, weight loss during hospital stay, serum sodium and eGFR at admission and discharge, and the usage of angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers and β-blockers at discharge were lower in patients who reached the primary endpoint.
Table 1.
Baseline Characteristics of the Study Patients
| Characteristics | Total (n=384) |
Patients with events (n=175) |
Patients without events (n=209) |
P value* |
|---|---|---|---|---|
| Age (years) | 76 (67~84) | 79 (72~86) | 73 (64~82) | <0.0001 |
| Male sex | 57% | 57% | 58% | 0.7943 |
| Etiology | 0.0005 | |||
| DCM | 25% | 18% | 30% | |
| ICM | 24% | 30% | 20% | |
| VHD | 9% | 13% | 5% | |
| Other | 42% | 39% | 45% | |
| Hypertension | 81% | 83% | 78% | 0.2204 |
| CAD | 37% | 45% | 31% | 0.0049 |
| Diabetes mellitus | 43% | 45% | 41% | 0.4316 |
| Atrial fibrillation | 48% | 50% | 46% | 0.4498 |
| COPD | 23% | 22% | 23% | 0.6870 |
| Anemia | 62% | 75% | 51% | <0.0001 |
| Prior HF hospitalization | 19% | 31% | 10% | <0.0001 |
| LVEF at admission (%) | 39 (30~53) | 41 (30~57) | 39 (30~50) | 0.1371 |
| LVEF <45% | 61% | 57% | 64% | |
| LVEF ≥45% | 39% | 43% | 36% | |
| Category of HF | 0.1045 | |||
| HFrEF | 52% | 48% | 55% | |
| HFmrEF | 17% | 16% | 19% | |
| HFpEF | 31% | 36% | 26% | |
| CS classification | 0.0305 | |||
| CS1/CS2/CS3/CS5 | 61/34/4/1% | 56/37/6/1% | 65/32/2/1% | |
| SBP at admission (mmHg) | 148 (130~174) | 144 (125~169) | 150 (133~177) | 0.0167 |
| DBP at admission (mmHg) | 86 (72~102) | 78 (67~91) | 93 (79~108) | <0.0001 |
| Heart rate at admission (beats/min) | 99 (82~122) | 90 (77~111) | 112 (92~130) | <0.0001 |
| BW | ||||
| At admission (kg) | 57 (49~67) | 53 (47~62) | 61 (51~71) | <0.0001 |
| At discharge (kg) | 51 (43~61) | 48 (42~55) | 54 (45~62) | 0.0001 |
| ΔBW (kg) | −6.0 (−8.9~−3.6) | −5.5 (−8.1~−3.3) | −6.2 (−9.2~−4.0) | 0.0293 |
| NYHA class III/IV at discharge | 28% | 41% | 17% | <0.0001 |
| Laboratory findings | ||||
| BUN at admission (mg/dL) | 23 (17~32) | 27 (21~39) | 19 (16~26) | <0.0001 |
| BUN at discharge (mg/dL) | 25 (18~36) | 30 (22~44) | 22 (16~29) | <0.0001 |
| Serum sodium at admission (mEq/L) | 139 (137~141) | 139 (136~141) | 140 (137~142) | 0.0037 |
| Serum sodium at discharge (mEq/L) | 139 (136~141) | 138 (135~140) | 140 (137~141) | 0.0001 |
| Serum creatinine at admission (mg/dL) | 1.1 (0.9~1.5) | 1.3 (1.0~1.9) | 1.0 (0.8~1.3) | <0.0001 |
| Serum creatinine at discharge (mg/dL) | 1.1 (0.9~1.6) | 1.3 (1.0~1.9) | 1.0 (0.8~1.3) | <0.0001 |
| eGFR at admission (mL/min/1.73 m2) | 45 (31~60) | 36 (24~50) | 52 (40~65) | <0.0001 |
| eGFR at discharge (mL/min/1.73 m2) | 44 (30~59) | 35 (23~49) | 51 (37~63) | <0.0001 |
| Plasma BNP at admission (pg/mL) | 734 (460~1,280) | 835 (486~1,335) | 699 (445~1,188) | 0.1103 |
| Plasma BNP at discharge (pg/mL) | 204 (102~401) | 249 (125~501) | 182 (84~326) | 0.0001 |
| Discharge medication and device therapy | ||||
| Loop diuretics | 86% | 87% | 85% | 0.6357 |
| ACEI/ARB | 55% | 43% | 65% | <0.0001 |
| β-blocker | 89% | 85% | 92% | 0.0377 |
| Aldosterone antagonist | 34% | 41% | 28% | 0.0079 |
| Implantable cardioverter-defibrillator | 4% | 8% | 1% | 0.0019 |
| Cardiac resynchronization therapy | 4% | 6% | 1% | 0.0117 |
Values are presented as median (interquartile range) or %. Events represent all-cause death and unplanned hospitalization for worsening HF. *P values are based on comparisons between patients with and without events. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; BW, body weight (ΔBW, change in body weight between admission and discharge); CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CS, Clinical Scenario; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; HF, heart failure; HFmrEF, HF with mid-range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; ICM, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure; VHD, valvular heart disease.
Comparison of PV Indices
With regard to the PV indices, statistically significant differences between the 2 groups were seen in PVS at admission and discharge. There was no significant difference in the other PV indices between groups (Table 2).
Table 2.
Plasma Volume Indices of the Study Patients
| Characteristics | Patients with events (n=175) |
Patients without events (n=209) |
P value |
|---|---|---|---|
| %ΔPV (Strauss) (%) | 2.8 (−10.6~16.3) | 3.3 (−7.5~14.8) | 0.6778 |
| ePV at admission (Kaplan) (mL) | 2,254 (2,035~2,686) | 2,379 (2,008~2,811) | 0.1552 |
| ePV at discharge (Kaplan) (mL) | 2,061 (1,805~2,385) | 2,123 (1,861~2,532) | 0.1004 |
| ΔePV (Kaplan) (mL) | −235 (−371~−94) | −225 (−393~−81) | 0.8845 |
| %ΔPV (Kaplan) (%) | −10.5 (−15.8~−4.1) | −9.8 (−15.9~−3.7) | 0.8082 |
| ePV at admission (Hakim) (mL) | 1,971 (1,682~2,219) | 1,885 (1,649~2,155) | 0.1492 |
| ePV at discharge (Hakim) (mL) | 1,945 (1,614~2,198) | 1,848 (1,612~2,147) | 0.2795 |
| ΔePV (Hakim) (mL) | −36 (−132~52) | −35 (−125~56) | 0.6965 |
| %ΔPV (Hakim) (%) | −1.6 (−6.9~2.1) | −1.7 (−7.0~3.1) | 0.6681 |
| PVS at admission (%) | −9.0 (−19.8~2.1) | −19.4 (−30.9~−10.8) | <0.0001 |
| PVS at discharge (%) | 0.6 (−13.4~13.5) | −11.1 (−21.9~1.3) | <0.0001 |
| ΔPVS (%) | 9.0 (4.3~13.5) | 8.0 (4.3~13.5) | 0.5500 |
Values are presented as median (interquartile range) or %. Events represent all-cause death and unplanned hospitalization for worsening heart failure. ePV, estimated plasma volume; PVS, plasma volume status; ΔePV, change in estimated plasma volume between admission and discharge; ΔPVS, change in PVS between admission and discharge; %ΔPV, percent change in plasma volume between admission and discharge.
Prognostic Analysis
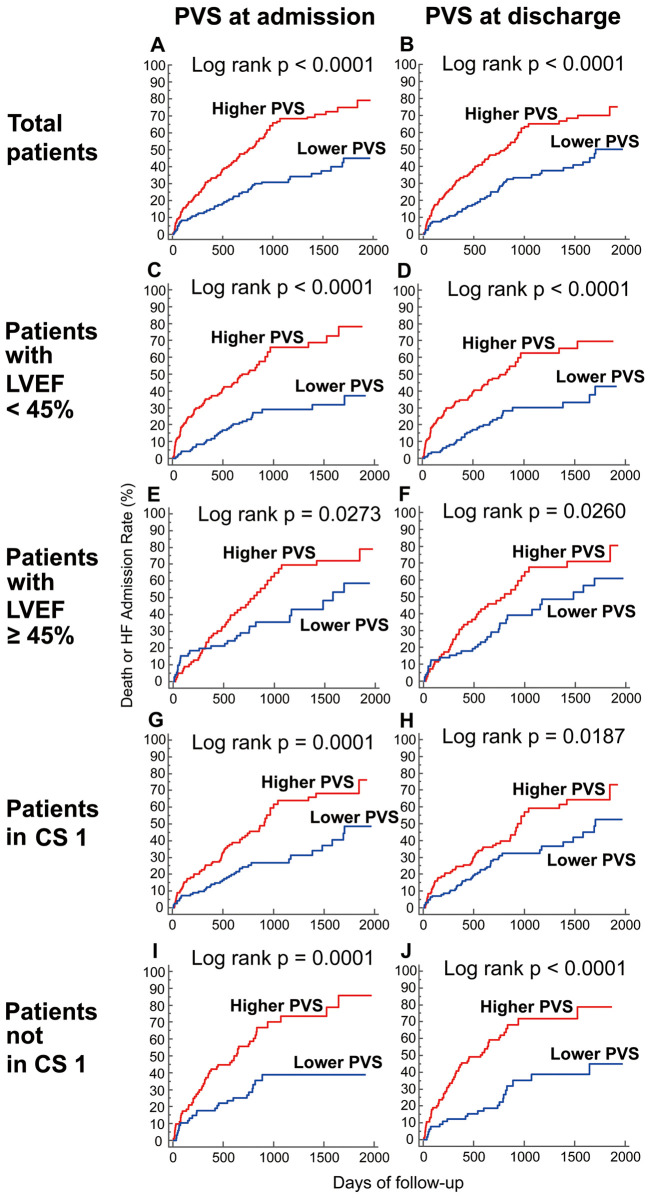
Univariate and multivariate Cox proportional hazard analyses for all-cause death and unplanned hospitalization for worsening HF are shown in Table 3. Among all of the PV indices tested, only the PVS values at admission and discharge were independent predictors of the primary endpoint in the multivariate Cox analysis, although ePV calculated with the Hakim formula also predicted the primary endpoint in the univariate analysis. Kaplan-Meier analysis showed that patients with a higher PVS had a significantly greater risk of all-cause death and unplanned hospitalization for worsening HF when they were stratified into 2 groups based on the median value of PVS at admission (−14.8%) or discharge (−6.8%) (Figure 1). ROC curve analysis showed that only the PVS at admission (AUC 0.682 [95% CI: 0.633 to 0.728], P<0.0001) and discharge (AUC 0.656 [95% CI: 0.606 to 0.704], P<0.0001) were significant predictors of the primary endpoint (Table 4).
Table 3.
Univariate and Multivariate Cox Analysis for All-Cause Death and Unplanned Hospitalization for Worsening HF
| HR (95% CI) | P value | |
|---|---|---|
| Cox analysis in total patients (n=384) | ||
| ePV at admission (Hakim) | ||
| Unadjusted | 1.0005 (1.0000~1.0009) | 0.0494 |
| Adjusted model* | 1.0002 (0.9994~1.0010) | 0.6549 |
| PVS at admission | ||
| Unadjusted | 1.0292 (1.0203~1.0382) | <0.0001 |
| Adjusted model* | 1.0166 (1.0039~1.0294) | 0.0103 |
| PVS at discharge | ||
| Unadjusted | 1.0241 (1.0162~1.0320) | <0.0001 |
| Adjusted model* | 1.0156 (1.0052~1.0261) | 0.0033 |
| Cox analysis in patients with LVEF <45% at admission (n=233) | ||
| PVS at admission | ||
| Unadjusted | 1.0323 (1.0209~1.0439) | <0.0001 |
| Adjusted model** | 1.0174 (1.0040~1.0310) | 0.0107 |
| PVS at discharge | ||
| Unadjusted | 1.0272 (1.0168~1.0377) | <0.0001 |
| Adjusted model** | 1.0129 (1.0012~1.0248) | 0.0303 |
| Cox analysis in patients with LVEF ≥45% at admission (n=150) | ||
| PVS at admission | ||
| Unadjusted | 1.0226 (1.0081~1.0374) | 0.0022 |
| Adjusted model** | 1.0229 (1.0063~1.0398) | 0.0068 |
| PVS at discharge | ||
| Unadjusted | 1.0188 (1.0067~1.0311) | 0.0023 |
| Adjusted model** | 1.0204 (1.0068~1.0343) | 0.0032 |
| Cox analysis in patients in CS1 (n=234) | ||
| PVS at admission | ||
| Unadjusted | 1.0281 (1.0158~1.0406) | <0.0001 |
| Adjusted model** | 1.0159 (1.0017~1.0304) | 0.0281 |
| PVS at discharge | ||
| Unadjusted | 1.0199 (1.0092~1.0308) | 0.0002 |
| Adjusted model** | 1.0094 (0.9978~1.0210) | 0.1126 |
| Cox analysis in patients not in CS1 (n=150) | ||
| PVS at admission | ||
| Unadjusted | 1.0298 (1.0170~1.0427) | <0.0001 |
| Adjusted model** | 1.0167 (1.0021~1.0315) | 0.0250 |
| PVS at discharge | ||
| Unadjusted | 1.0285 (1.0169~1.0402) | <0.0001 |
| Adjusted model** | 1.0191 (1.0059~1.0325) | 0.0044 |
*Adjusted for age, sex, hypertension, CAD, diabetes mellitus, atrial fibrillation, anemia, LVEF at admission, SBP at admission, NYHA functional class III or IV at discharge, BUN at discharge, serum sodium at admission, eGFR at admission, plasma brain natriuretic peptide at discharge, loop diuretic received at discharge, ACEI or angiotensin II type 1 receptor blocker received at discharge, and β-blocker received at discharge. **Adjusted for age, LVEF at admission, eGFR at admission, and plasma brain natriuretic peptide at discharge. CI, confidence interval; CS, Clinical Scenario; HR, hazard ratio; additional abbreviations as in Tables 1,2.
Figure 1.
Cumulative incidence of the primary endpoint according to plasma volume status (PVS) at admission and discharge. Kaplan-Meier curves for the primary endpoint (a composite of all-cause death and unplanned hospitalization for worsening heart failure) in total patients (A,B), patients with left ventricular ejection fraction (LVEF) <45% (C,D) and ≥45% (E,F), and patients in Clinical Scenario (CS) 1 (G,H) and not in CS 1 (I,J) when stratified according to PVS at admission (A,C,E,G,I) and discharge (B,D,F,H,J).
Table 4.
Receiver-Operating Characteristic Curve Analysis for the Primary Endpoint
| AUC (95% CI) | P value | |
|---|---|---|
| %ΔPV (Strauss) | 0.514 (0.463~0.565) | 0.6319 |
| ePV at admission (Kaplan) | 0.541 (0.490~0.592) | 0.1618 |
| ePV at discharge (Kaplan) | 0.548 (0.496~0.598) | 0.1042 |
| ΔePV (Kaplan) | 0.504 (0.453~0.556) | 0.8805 |
| %ΔPV (Kaplan) | 0.507 (0.456~0.558) | 0.8120 |
| ePV at admission (Hakim) | 0.543 (0.491~0.593) | 0.1508 |
| ePV at discharge (Hakim) | 0.532 (0.481~0.583) | 0.2838 |
| ΔePV (Hakim) | 0.512 (0.460~0.563) | 0.6966 |
| %ΔPV (Hakim) | 0.513 (0.461~0.564) | 0.6677 |
| PVS at admission | 0.682 (0.633~0.728) | <0.0001 |
| PVS at discharge | 0.656 (0.606~0.704) | <0.0001 |
| ΔPVS | 0.518 (0.466~0.569) | 0.5518 |
Values are presented as median (interquartile range) or %. Events represent all-cause death and unplanned hospitalization for worsening HF. AUC, area under the curve; additional abbreviations as in Tables 1–3.
We performed subgroup analyses in order to examine whether the prognostic value of PVS was affected by LVEF or CS classification (Table 3). One patient with missing LVEF data at admission was excluded from the subgroup analysis by LVEF. When the patients were divided into those with LVEF <45% and those with LVEF ≥45%, multivariate Cox analysis revealed that the PVS values at admission and discharge were independent predictors of the primary endpoint in both groups. Although the PVS at discharge lost statistical significance in the multivariate model in patients in CS1, the PVS at admission was independently associated with the primary endpoint in the patients in CS1 and those not in CS1. There was a significant difference in the risk of the primary endpoint when the patients were divided into 2 groups according to the median values of the admission PVS (−14.8%) or discharge PVS (−6.8%) in all subgroup analyses (Figure 1).
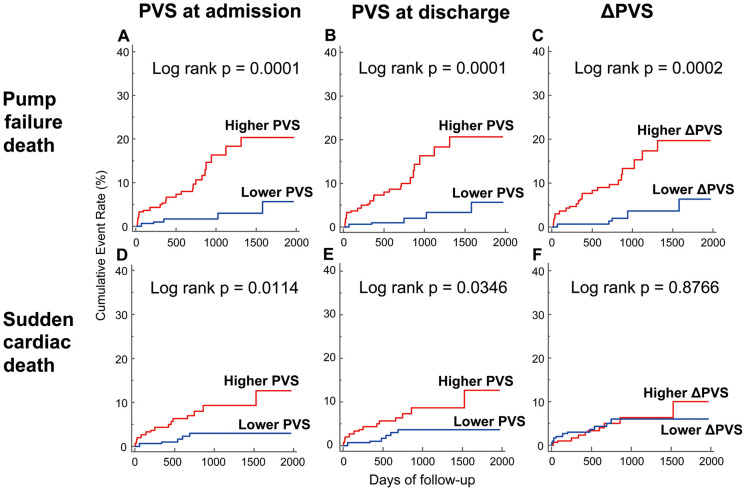
Based on the result that PVS was the most powerful predictor of the primary endpoint among all the PV indices, we subsequently investigated whether PVS could predict the mode of death (Table 5). The PVS at admission and discharge, and the change in PVS during hospital stay (∆PVS) were associated with PFD in multivariate analysis, even after adjustment for established prognosticators. The PVS values at admission and discharge were also independent predictors of SCD, although the relationship between the PVS at discharge and SCD was attenuated and lost statistical significance after adjustment for the eGFR at admission. In contrast, the ∆PVS had no association with SCD, even in the univariate analysis. Kaplan-Meier curves for PFD and SCD are shown in Figure 2. When patients were divided into 2 groups according to the median values of the admission PVS (−14.8%), discharge PVS (−6.8%), or the ∆PVS (8.4%), patients with a higher PVS at admission or discharge had a greater risk of both PFD and SCD, whereas a higher ∆PVS was only associated with a greater risk of PFD.
Table 5.
Univariate and Multivariate Cox Analysis for Pump Failure Death and Sudden Cardiac Death
| Model | Outcome | |||
|---|---|---|---|---|
| Pump failure death | Sudden cardiac death | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| PVS at admission | ||||
| Unadjusted | 1.0500 (1.0279~1.0726) | <0.0001 | 1.0379 (1.0123~1.0642) | 0.0035 |
| Model 1 | 1.0465 (1.0231~1.0705) | 0.0001 | 1.0407 (1.0144~1.0677) | 0.0022 |
| Model 2 | 1.0389 (1.0132~1.0652) | 0.0028 | 1.0382 (1.0112~1.0660) | 0.0053 |
| Model 3 | 1.0428 (1.0191~1.0670) | 0.0003 | 1.0348 (1.0080~1.0623) | 0.0107 |
| Model 4 | 1.0475 (1.0237~1.0718) | 0.0001 | 1.0402 (1.0133~1.0679) | 0.0032 |
| Model 5 | 1.0406 (1.0171~1.0647) | 0.0006 | 1.0342 (1.0071~1.0619) | 0.0131 |
| Model 6 | 1.0463 (1.0224~1.0708) | 0.0001 | 1.0406 (1.0143~1.0676) | 0.0023 |
| PVS at discharge | ||||
| Unadjusted | 1.0569 (1.0364~1.0779) | <0.0001 | 1.0280 (1.0051~1.0514) | 0.0164 |
| Model 1 | 1.0546 (1.0333~1.0764) | <0.0001 | 1.0289 (1.0053~1.0530) | 0.0163 |
| Model 2 | 1.0527 (1.0292~1.0768) | <0.0001 | 1.0274 (1.0030~1.0524) | 0.0274 |
| Model 3 | 1.0510 (1.0296~1.0729) | <0.0001 | 1.0225 (0.9983~1.0472) | 0.0683 |
| Model 4 | 1.0550 (1.0334~1.0771) | <0.0001 | 1.0266 (1.0024~1.0515) | 0.0312 |
| Model 5 | 1.0549 (1.0325~1.0777) | <0.0001 | 1.0246 (1.0003~1.0496) | 0.0474 |
| Model 6 | 1.0526 (1.0313~1.0744) | <0.0001 | 1.0290 (1.0054~1.0532) | 0.0158 |
| ΔPVS | ||||
| Unadjusted | 1.0730 (1.0365~1.1108) | 0.0001 | 0.9858 (0.9302~1.0447) | 0.6288 |
| Model 1 | 1.0934 (1.0492~1.1394) | <0.0001 | 0.9839 (0.9281~1.0431) | 0.5870 |
| Model 2 | 1.0993 (1.0517~1.1490) | <0.0001 | 0.9848 (0.9287~1.0444) | 0.6097 |
| Model 3 | 1.0788 (1.0362~1.1231) | 0.0002 | 0.9789 (0.9283~1.0323) | 0.4313 |
| Model 4 | 1.0919 (1.0463~1.1395) | 0.0001 | 0.9722 (0.9145~1.0336) | 0.3667 |
| Model 5 | 1.0954 (1.0530~1.1394) | <0.0001 | 0.9904 (0.9382~1.0455) | 0.7276 |
| Model 6 | 1.0910 (1.0488~1.1349) | <0.0001 | 0.9868 (0.9312~1.0457) | 0.6528 |
Model 1, adjusted for age and LVEF at admission; model 2, adjusted for age and plasma BNP at discharge; model 3, adjusted for age and eGFR at admission; model 4, adjusted for age and sodium at admission; model 5, adjusted for age and BUN at discharge; model 6, adjusted for age and NYHA class III/IV at discharge. Abbreviations as in Tables 1–3.
Figure 2.
Cumulative incidence of pump failure death and sudden cardiac death according to plasma volume status (PVS) at admission and discharge and the change in PVS (∆PVS). Kaplan-Meier curves for pump failure death (A–C) and sudden cardiac death (D–F) when stratified according to PVS at admission (A,D) and discharge (B,E) and ∆PVS during hospital stay (C,F).
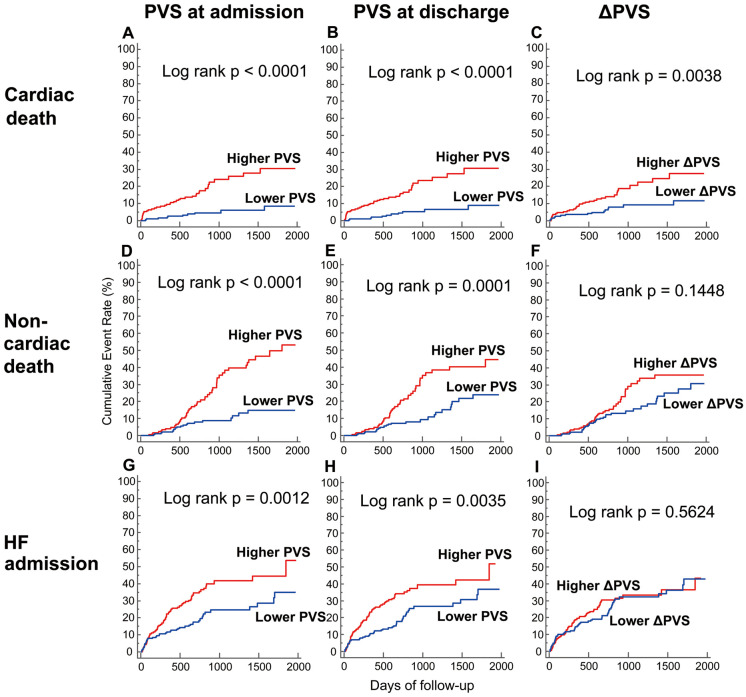
Lastly, we evaluated the predictive value of PVS on cardiac death, non-cardiac death, and unplanned hospitalization for worsening HF (Supplementary Table). The PVS values at admission and discharge were associated with cardiac death, non-cardiac death, and unplanned hospitalization for worsening HF in the multivariate analysis. Although the ∆PVS was associated with cardiac death in the multivariate analysis, the ∆PVS had no association with non-cardiac death or unplanned hospitalization for worsening HF. When patients were divided into 2 groups according to the median values of the admission PVS (−14.8%), discharge PVS (−6.8%) and the ∆PVS (8.4%), patients with a higher PVS at admission or discharge had greater risks of cardiac death, non-cardiac death, and unplanned hospitalization for worsening HF, whereas a higher ∆PVS was only associated with a greater risk of cardiac death (Figure 3).
Figure 3.
Cumulative incidence of cardiac death, non-cardiac death, and unplanned hospitalization for worsening heart failure (HF) according to plasma volume status (PVS) at admission and discharge and the change in PVS (∆PVS). Kaplan-Meier curves for cardiac death (A–C), non-cardiac death (D–F), and unplanned hospitalization for worsening HF (G–I) when stratified according to PVS at admission (A,D,G) and discharge (B,E,H) and ∆PVS during hospital stay (C,F,I).
Discussion
Increased intracardiac filling pressures without overt congestive signs and symptoms is termed ‘hemodynamic congestion’.5–7 HF patients with hemodynamic congestion have been reported to have outcomes as poor as those with overt signs and symptoms of congestion.27 Although clinical signs and symptoms of congestion have long been used as a marker of congestion,7,28 they are neither sensitive nor specific for congestion and might be non-cardiac in origin; therefore, relying on a single measure can be misleading.6,7 Moreover, the alleviation of signs and symptoms of congestion does not guarantee the resolution of hemodynamic congestion and improved prognosis in patients admitted for ADHF.5 Right heart catheterization is currently the most accurate way to assess hemodynamic congestion in HF patients, but its routine use is not recommended because of its invasive nature, and repeated assessment during admission is difficult.8 Therefore, a non-invasive tool to assess hemodynamic congestion is needed.
Several formulas for the estimation of PV and PVS have been developed using readily available data, and these methods can be used easily and repeatedly to assess subclinical hemodynamic congestion.9–13 However, little is known about the comparative prognostic value of these PV indices and their usefulness for the prediction of mode of death in patients with ADHF. To the best of our knowledge, this is the first report to demonstrate that the PVS is the best predictor of poor clinical outcome among the several PV indices. Moreover, the PVS is useful for the prediction of the mode of death in ADHF patients.
To date, the natriuretic peptides, such as BNP and N-terminal fragment pro-B-type natriuretic peptide,29 Hct,30 and BW31 have been used as non-invasive surrogate markers of congestion in ADHF patients. The natriuretic peptides remain the most studied biomarkers reflecting congestion in HF; however, their usefulness is limited because their production and release can be affected by non-cardiac factors such as age, renal function, and physical size.7 In addition, the predictive value of the PVS was unaffected by BNP in this study. Hct is a biological surrogate of PV, and its increase during hospital stay (i.e., hemoconcentration) is thought to reflect effective diuresis and sufficient fluid removal, leading to a better clinical outcome.6,30 Despite this, in the current study, hemoconcentration did not predict any endpoint, even in the univariate Cox analysis (data not shown). Although a change in BW has been used as a surrogate marker of decongestion,31 this might reflect factors other than decongestion such as malnutrition and cardiac cachexia. Echocardiography can also detect and monitor congestion,32 but has several drawbacks, including the length of the procedure and the need for an experienced cardiologist skilled in echocardiography.
Several studies have evaluated the prognostic value of formulas for the estimation of PV and PVS in ADHF patients.14–17 Using data from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), Duarte et al14 examined the prognostic value of the PV, as estimated by the Strauss, Kaplan, and Hakim formulas. They found that of these 3 formulas, the strongest association was found between the estimated PV variation calculated by the Strauss formula and poor clinical outcome. In addition, Bilchick et al found that the estimation of the PV change during admission by the Strauss formula was useful for risk stratification of ADHF patients in the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness trial (ESCAPE).16 However, we could not find any significant relationship between the PV change estimated by the Strauss formula and worse prognosis, which may been because of differences in the patients’ backgrounds. Furthermore, in contrast to the study by Duarte et al,14 we did not include patients with acute coronary syndrome. Moreover, there was no exclusion criterion for LVEF in our study, whereas patients were required to have LVEF ≤40% in EPHESUS and LVEF ≤30% in the ESCAPE trial at entry. Considering the fact that HF with preserved LVEF accounts for a significant proportion of patients hospitalized with ADHF,33 it could be considered that our study cohort was more reflective of a real-world population. Hudson et al also found that the PV change from admission to discharge calculated by the Strauss formula was useful for the prediction of prognosis in ADHF patients.15 However, as their study did not report the LVEF and BNP, it is unclear whether the PV change by the Strauss formula would have been significantly associated with poor outcome after adjustment for LVEF and BNP.
Our study results are consistent with those of Yoshihisa et al, who reported that the calculated PVS at admission was associated with worse clinical outcome in patients admitted with ADHF, even after adjustment for LVEF and BNP.17 In addition to those results, our study demonstrated that the PVS at discharge also provided prognostic value. The fact that only the PVS among the several PV indices predicted poor outcome suggests that it is not the absolute PV, but rather the deviation from the patient’s ideal PV, that is associated with postdischarge prognosis of ADHF patients. Although the PV change estimated by the Strauss formula relies on the assumption that changes in Hct and Hb are solely because of changes in PV, the production of red blood cells can be affected by many factors, including medications and bone marrow dysfunction induced by kidney dysfunction, malnutrition, and inflammation during admission, which may explain why, in our study, the PVS had better prognostic value than the PV change estimated by the Strauss formula.
In the original report from 2015, the PV validation cohort for PVS included chronic HF patients with reduced LVEF (≤45%).12 Although the LVEF was preserved in approximately half of the patients in the study by Yoshihisa et al,17 there was insufficient evidence to show that the PVS was useful for risk stratification of ADHF patients with preserved LVEF. In addition, as reported by Cotter et al, ADHF is thought to be initiated by a combination of 2 pathways, namely the cardiac and vascular pathways.34 Many ADHF patients with preserved LVEF, or those in CS 1, are primarily associated with the latter pathway, where central volume redistribution, rather than PV expansion, plays a causative role.22,34 Therefore, we performed subgroup analyses to confirm the prognostic value of PVS in patients with LVEF ≥45% and in those in CS 1. Although the PVS at discharge was not an independent predictor of the primary endpoint in the multivariate model in patients in CS 1, we found independent associations between the PVS at admission and discharge and the primary endpoint in multivariate analyses in the other subgroups. In addition, patients with a higher PVS had a significantly higher risk of the primary endpoint in all subgroups. Based on those results, it could be stated that PV expansion is associated with worse prognosis, even in ADHF patients with preserved LVEF, or in those with vascular failure.
Our data suggested that the PVS might be useful for the prediction of the mode of death in patients admitted with ADHF. Because there is very little data available on the mode of death in ADHF patients,35 we cannot fully explain why a lower ∆PVS was associated with a lower risk of PFD but not with a lower risk of SCD. Moreover, our data showed that the PVS was also useful for the prediction of cardiac death, non-cardiac death, and unplanned hospitalization for worsening HF, although the ∆PVS had no association with non-cardiac death or unplanned hospitalization for worsening HF. Thus, further study is needed to clarify the underlying mechanisms of the specific causes of death in ADHF patients.
Study Limitations
There are several to note. First, the small and empirically chosen sample size, and empirically chosen follow-up duration are major limitations. Second, this was a single-center study, so a possible ethnic difference must be considered when attempting to generalize the results to non-Japanese populations. Third, we could not measure PV using a validated method,36 and did not check the agreement among the PV methods in ADHF patients. Fourth, we could not evaluate the PV using other non-invasive tools such as lung ultrasound4 or bioimpedance vector analysis.37 Fifth, although calculation of the PVS does not rely solely on Hct, it is expected that the PVS value was affected by hematopoiesis, considering the impaired renal function of the patients with events in this study. Sixth, it is unknown whether the ideal PV can be calculated only from BW, even in patients with cardiac cachexia, and further study is needed to evaluate the prognostic value of PVS in this subgroup of patients. Seventh, as we did not have right heart catheterization data on the day of blood sampling, comparative analysis for the diagnostic ability to detect subclinical hemodynamic congestion between PVS and right heart catheterization data could not be performed in this study. Lastly, although this study was based on the assumption that volume overload is the primary cause of congestion in ADHF, recent studies have shown that decreased vascular capacitance and intercompartmental fluid shift also play a role in the occurrence of congestion.38 Therefore, we should pay attention not only to volume overload, but also to volume redistribution, when we assess the risk of HF exacerbation.
Conclusions
In this study, the calculated PVS was shown to be the best predictor of prognosis among several PV indices. Moreover, the calculated PVS was useful for the prediction of the mode of death in ADHF patients. The question of whether patient management using this index leads to better prognosis in ADHF patients should be addressed in future studies.
Acknowledgments / Disclosures
None.
Supplementary Files
Supplementary Table. Univariate and Multivariate Cox Analysis for Cardiac Death, Non-cardiac Death, and Unplanned Hospitalization for Worsening Heart Failure.
References
- 1. Rocha BM, Menezes Falcao L.. Acute decompensated heart failure (ADHF): A comprehensive contemporary review on preventing early readmissions and postdischarge death. Int J Cardiol 2016; 223: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 2. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al.. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 3. O’Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M.. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: Observations from the IMPACT-HF registry. J Card Fail 2005; 11: 200–205. [DOI] [PubMed] [Google Scholar]
- 4. Frassi F, Gargani L, Tesorio P, Raciti M, Mottola G, Picano E.. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail 2007; 13: 830–835. [DOI] [PubMed] [Google Scholar]
- 5. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al.. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur Heart J 2013; 34: 835–843. [DOI] [PubMed] [Google Scholar]
- 6. Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, et al.. Integrative assessment of congestion in heart failure throughout the patient journey. JACC Heart Fail 2018; 6: 273–285. [DOI] [PubMed] [Google Scholar]
- 7. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, et al.. Assessing and grading congestion in acute heart failure: A Scientific Statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 8. Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al.. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 2005; 294: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 9. Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD.. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol 2002; 39: 1901–1908. [DOI] [PubMed] [Google Scholar]
- 10. Kaplan AA.. A simple and accurate method for prescribing plasma exchange. ASAIO Trans 1990; 36: M597–M599. [PubMed] [Google Scholar]
- 11. Ismail N, Kiprov DD, Hakim RM.. Plasmapheresis. In: Daugirdis JT, Blake PG, Ing TS, editors. Handbook of dialysis, 4th edn. Philadelphia: Lippincott Williams & Wilkins, 2007; 276–299. [Google Scholar]
- 12. Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, et al.. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail 2015; 17: 35–43. [DOI] [PubMed] [Google Scholar]
- 13. Martens P, Nijst P, Dupont M, Mullens W.. The optimal plasma volume status in heart failure in relation to clinical outcome. J Card Fail 2019; 25: 240–248. [DOI] [PubMed] [Google Scholar]
- 14. Duarte K, Monnez JM, Albuisson E, Pitt B, Zannad F, Rossignol P.. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail 2015; 3: 886–893. [DOI] [PubMed] [Google Scholar]
- 15. Hudson SR, Chan D, Ng LL.. Change in plasma volume and prognosis in acute decompensated heart failure: An observational cohort study. J R Soc Med 2016; 109: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bilchick KC, Chishinga N, Parker AM, Zhuo DX, Rosner MH, Smith LA, et al.. Plasma volume and renal function predict six-month survival after hospitalization for acute decompensated heart failure. Cardiorenal Med 2017; 8: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshihisa A, Abe S, Sato Y, Watanabe S, Yokokawa T, Miura S, et al.. Plasma volume status predicts prognosis in patients with acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care 2018; 7: 330–338. [DOI] [PubMed] [Google Scholar]
- 18. McKee PA, Castelli WP, McNamara PM, Kannel WB.. The natural history of congestive heart failure: The Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 19. Kondo T, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, et al.. Serial change in serum chloride during hospitalization could predict heart failure death in acute decompensated heart failure patients. Circ J 2018; 82: 1041–1050. [DOI] [PubMed] [Google Scholar]
- 20. Hume R.. Prediction of lean body mass from height and weight. J Clin Pathol 1966; 19: 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Longo D.. Body fluids and other mass data [Table 218]. In: Longo DL, Fauci AS, Kasper DL et al, editors. Harrison’s principles of internal medicine, 18th edn. New York: McGraw-Hill, 2011; A-1. [Google Scholar]
- 22. Mebazaa A, Gheorghiade M, Pina IL, Harjola VP, Hollenberg SM, Follath F, et al.. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med 2008; 36: S129–S139. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al.. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 24. Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al.. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: Results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol 2009; 53: 426–435. [DOI] [PubMed] [Google Scholar]
- 25. Salah K, Pinto YM, Eurlings LW, Metra M, Stienen S, Lombardi C, et al.. Serum potassium decline during hospitalization for acute decompensated heart failure is a predictor of 6-month mortality, independent of N-terminal pro-B-type natriuretic peptide levels: An individual patient data analysis. Am Heart J 2015; 170: 531–542.e1. [DOI] [PubMed] [Google Scholar]
- 26. Salah K, Kok WE, Eurlings LW, Bettencourt P, Pimenta JM, Metra M, et al.. Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure. JACC Heart Fail 2015; 3: 751–761. [DOI] [PubMed] [Google Scholar]
- 27. Stevenson LW, Tillisch JH, Hamilton M, Luu M, Chelimsky-Fallick C, Moriguchi J, et al.. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol 1990; 66: 1348–1354. [DOI] [PubMed] [Google Scholar]
- 28. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al.. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 29. Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M; ADHERE Scientific Advisory Committee and Investigators.. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 2007; 49: 1943–1950. [DOI] [PubMed] [Google Scholar]
- 30. Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, et al.. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: Insights from the EVEREST trial. Eur J Heart Fail 2013; 15: 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, et al.. Body weight change during and after hospitalization for acute heart failure: Patient characteristics, markers of congestion, and outcomes: Findings from the ASCEND-HF Trial. JACC Heart Fail 2017; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirkpatrick JN, Vannan MA, Narula J, Lang RM.. Echocardiography in heart failure: Applications, utility, and new horizons. J Am Coll Cardiol 2007; 50: 381–396. [DOI] [PubMed] [Google Scholar]
- 33. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al.. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 34. Cotter G, Felker GM, Adams KF, Milo-Cotter O, O’Connor CM.. The pathophysiology of acute heart failure: Is it all about fluid accumulation? Am Heart J 2008; 155: 9–18. [DOI] [PubMed] [Google Scholar]
- 35. Parikh KS, Felker GM, Metra M.. Mode of death after acute heart failure hospitalization: A clue to possible mechanisms. Circ J 2016; 80: 17–23. [DOI] [PubMed] [Google Scholar]
- 36. International Committee for Standardization in Haematology.. Recommended methods for measurement of red-cell and plasma volume. J Nucl Med 1980; 21: 793–800. [PubMed] [Google Scholar]
- 37. Shochat MK, Shotan A, Blondheim DS, Kazatsker M, Dahan I, Asif A, et al.. Non-invasive lung impedance-guided preemptive treatment in chronic heart failure patients: A randomized controlled trial (IMPEDANCE-HF Trial). J Card Fail 2016; 22: 713–722. [DOI] [PubMed] [Google Scholar]
- 38. Fudim M, Hernandez AF, Felker GM.. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc 2017; 6: e006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table. Univariate and Multivariate Cox Analysis for Cardiac Death, Non-cardiac Death, and Unplanned Hospitalization for Worsening Heart Failure.