Abstract
Background: The ratio of N-terminal pro-brain natriuretic peptide (NT-proBNP) secretion from the heart to peripheral NT-proBNP remains unknown in patients with chronic kidney disease (CKD).
Methods and Results: We measured plasma NT-proBNP in the aortic root (AO; NT-proBNPAO) and in the coronary sinus (CS; NT-proBNPCS) in 544 patients. Patients were classified into 6 categories based on estimated glomerular filtration rate (eGFR): G1, n=44, eGFR ≥90 mL/min/1.73 m2; G2, n=221, 60≤eGFR<90 mL/min/1.73 m2; G3a, n=132, 45≤eGFR<60 mL/min/1.73 m2; G3b, n=77, 30≤eGFR<45 mL/min/1.73 m2; G4, n=34, 15≤eGFR<30 mL/min/1.73 m2; and G5, n=36, eGFR <15 mL/min/1.73 m2. In non-CKD patients, hemodynamics but not eGFR were independent predictors of log NT-proBNP. In CKD patients, eGFR and hemodynamics were independent predictors of log NT-proBNP. The ratio of NT-proBNP secretion from the heart to NT-proBNPAO significantly decreased with decreasing eGFR in 6 groups (P<0.0001): G1, 67±38%; G2, 50±24%; G3a, 40±21%; G3b, 30±16%; G4, 14.8±7.9%; and G5, 3.5±2.4%, respectively.
Conclusions: eGFR contributes to the value of NT-proBNP for prediction of hemodynamic overload in CKD patients but not in non-CKD patients, and the ratio of NT-proBNP secretion from the heart to peripheral NT-proBNP is markedly decreased in CKD patients, especially those with eGFR <30 mL/min/1.73 m2.
Key Words: Chronic heart failure, Chronic kidney disease, Estimated glomerular filtration rate, N-terminal pro-brain natriuretic peptide
Plasma brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP), as markers of ventricular wall-stress, are well-established biomarkers of hemodynamic abnormality, diagnosis and prognosis in patients with chronic heart failure (CHF).1–6 After secretion of BNP and NT-proBNP from the coronary sinus (CS), clearance of BNP occurs via enzymatic breakdown such as by neutral endopeptidase and dipeptidyl peptidase-4, and natriuretic receptor binding, or renal excretion,7 while NT-proBNP is mainly cleared by the kidneys. Interestingly, urinary NT-proBNP is significantly lower in CHF patients than in control subjects,8 suggesting that a marked decrease in urinary excretion of NT-proBNP contributes to a high plasma NT-proBNP due to tubular injury in CHF patients with chronic kidney disease (CKD). In addition, the extracardiac mechanism of elevation of plasma NT-proBNP depends on renal clearance and metabolism,8,9 indicating that the ratio of NT-proBNP secretion from the heart to peripheral NT-proBNP is potentially a marker of renal clearance of NT-proBNP.
NT-proBNP secretion from the heart is regulated by ventricular wall-stress, and peripheral NT-proBNP is mainly influenced by renal clearance and metabolism. Peripheral BNP is significantly decreased by 20% compared with that in the left ventricle.10 According to preliminary data, there was no difference between plasma NT-proBNP in the aortic root (AO; NT-proBNPAO) and that in the peripheral vein, suggesting that NT-proBNP is not cleared in systemic circulation and is mainly cleared by the kidneys. The ratio of NT-proBNP secretion from the heart to peripheral NT-proBNP, however, remains unknown in patients with CKD.
CKD is classified into 6 categories based on estimated glomerular filtration rate (eGFR), and the proportion of deaths from cardiovascular disease increases as eGFR decreases.11 Importantly, in patients with mild–moderate CKD (stages 3a, 3b), the incidence of cardiovascular mortality is much higher than the incidence of kidney failure.12,13 Therefore, cut-offs of biomarkers for the diagnosis and prognosis of CHF are important in these patients. Recently, Aimo et al reported that the cut-off of NT-proBNP for predicting hospitalization and death varied widely with CKD stage.14 We previously reported that NT-proBNP is more influenced by eGFR than is BNP by sampling blood from the AO and CS in CHF patients.15 The ratio of NT-proBNP secretion to peripheral NT-proBNP, however, remains unknown in patients with CKD, especially in severe and end-stage CKD patients. In addition, obesity is associated with the development of CKD and is a risk for CHF. Body mass index (BMI) is a marker of obesity, characterized by low NT-proBNP, but whether this NT-proBNP secretion is from the heart remains unclear.
Methods
Patients
The subjects consisted of 544 consecutive heart disease patients who underwent cardiac catheterization for clinical indications. Patients with acute coronary syndrome, aortic valve stenosis, mitral valve stenosis, hypertrophic cardiomyopathy, pericarditis, primary pulmonary hypertension, or lung disease were excluded. Patients on dialysis were not excluded. Patients with right-side heart disease were excluded and patients with mean pulmonary arterial pressure ≥25 mmHg and pulmonary capillary wedge pressure <15 mmHg were excluded because most patients had left-sided heart disease at the present institution. CHF was defined as symptomatic heart failure at sampling or hospitalization for heart failure in the previous 12 months. eGFR was used as an indicator of renal function based on the abbreviated Modification of Diet in Renal Disease study formula.16 Patients were classified into 6 categories based on eGFR:17 G1, n=44, eGFR ≥90 mL/min/1.73 m2; G2, n=221, 60≤eGFR<90 mL/min/1.73 m2; G3a, n=132, 45≤eGFR<60 mL/min/1.73 m2; G3b, n=77, 30≤eGFR<45 mL/min/1.73 m2; G4, n=34, 15≤eGFR<30 mL/min/1.73 m2; and G5, n=36, eGFR <15 mL/min/1.73 m2. Informed consent was obtained from all patients for participation in the study, according to a protocol approved by the institution Committee on Human Investigation.
Study Protocol
All patients were pre-medicated with an oral dose of diazepam (5 mg) and rested in bed in a supine position for at least 20 min. Right-sided and left-sided cardiac catheterization was performed and blood pressure was measured. Blood samples for measuring plasma NT-proBNP were collected simultaneously from the AO and CS (NT-proBNPAO and NT-proBNPCS). A 6-Fr catheter for blood sampling was positioned in the CS, and the position of the catheter was confirmed as previously reported.18
Measurement of NT-proBNP
Plasma NT-proBNP concentration was measured using the Elecsys proBNP sandwich immunoassay (Roche Diagnostics, Elecsys proBNP II), as previously reported.15
Statistical Analysis
All results are expressed as mean±SD or median (IQR). The chi-squared test or 1-way analysis of variance was used to determine differences between the 6 groups, and the differences were tested using Scheffe’s F-test. Univariate analysis was examined using Student’s t-test. Because NT-proBNP was not normally distributed, differences in mean NT-proBNP between the groups were detected on Wilcoxon rank-sum test with 2-tailed P<0.05, and log NT-proBNP was used in correlations and regression models. To evaluate the contribution of the transcardiac increase in NT-proBNP (i.e., log NT-proBNPCS–AO), and log NT-proBNPAO, univariate and stepwise multivariate analyses were used to compare 7 variables including hemodynamic parameters and eGFR. Linear regression analysis was used to determine the relationships between continuous variables. The difference in the intercept of the linear regression line between 2 groups was analyzed using ANCOVA. P<0.05 was regarded as significant.
Results
Patient Characteristics
Table 1 summarizes the patient characteristics according to CKD stage based on eGFR. There were no differences in NT-proBNPCS–AO, left ventricular ejection fraction (LVEF), or left ventricular end-diastolic pressure (LVEDP) in left-sided heart disease patients.
Table 1.
Characteristics of Patients With Left-Sided Heart Disease (n=544)
| Variables | CKD stage (eGFR: mL/min/1.73 m2) | P-value† | ||||||
|---|---|---|---|---|---|---|---|---|
| G1 (≥90) | G2 (60–89) | G3a (45–59) | G3b (30–44) | G4 (15–29) | G5 (<15) | |||
| Patients | 544 | 44 (8) | 221 (41) | 132 (24) | 77 (14) | 34 (6) | 36 (7) | |
| eGFR (mL/min/1.73 m2) | 58±24 | 102±16 | 72±8.3 | 53±4.4 | 38±3.8 | 25±4 | 6.5±3 | <0.0001 |
| Age (years) | 65±12 | 54±17 | 62±11 | 68±9 | 69±9.8 | 72±8.5 | 65±10 | <0.0001 |
| Sex (M/F) | 397/150 | 31/13 | 163/59 | 104/28 | 53/25 | 20/14 | 26/10 | NS |
| BMI (kg/m2) | 23±3.7 | 23±5.2 | 22.8±3.8 | 23.3±3.5 | 22.4±3.1 | 22.8±2.7 | 22.6±3.2 | NS |
| Heart failure | 393 (72) | 32 (71) | 155 (70) | 101 (77) | 68 (87) | 22 (65) | 36 (100) | 0.0002 |
| AF | 77 (14) | 5 (11) | 31 (14) | 18 (14) | 13 (17) | 8 (24) | 2 (6) | NS |
| Creatinine (mg/dL) | 1.5±2.0 | 0.6±0.1 | 0.8±0.1 | 1.0±0.1 | 1.3±0.2 | 2.0±0.5 | 8.2±3.2 | <0.0001 |
| NT-proBNPAO (pg/mL) | 669 (293–1,514) |
545 (215–868) |
437 (224–995) |
592 (259–1,261) |
800 (461–1,650) |
2,552 (1,245–7,081) |
10,206 (3,600–24,865) |
<0.0001 |
| NT-proBNPCS (pg/mL) | 931 (408–504) |
808 (376–1,668) |
634 (341–1,419) |
811 (369–1,736) |
979 (621–1,978) |
2,752 (1,463–8,454) |
10,554 (3,703–25,294) |
<0.0001 |
| NT-proBNPCS–AO (pg/mL) | 207 (102–459) |
268 (128–643) |
180 (96–437) |
200 (102–399) |
173 (110–434) |
349 (172–841) |
402 (105–496) |
0.07 |
| NT-proBNPCS–AO/ NT-proBNPAO (%) |
41±27 | 67±38 | 50±24 | 40±21 | 30±16 | 14.8±7.9 | 3.5±2.4 | <0.0001 |
| MBP (mmHg) | 89±16 | 85±16 | 89±16 | 88±15 | 87±14 | 90±19 | 97±17 | 0.01 |
| LVEF (%) | 47±14 | 47±17 | 48±14 | 47±14 | 47±13 | 44±15 | 52±11 | NS |
| LVEDP (mmHg) | 13±6.4 | 14±5.8 | 12.5±6.2 | 13.5±6.5 | 13.4±5.9 | 14±7.9 | 12±7.1 | NS |
| Etiology | NS | |||||||
| IHD | 317 (58) | 20 (42) | 135 (61) | 80 (61) | 42 (54) | 20 (59) | 20 (56) | NS |
| DCM | 106 (19) | 15 (31) | 43 (19) | 26 (20) | 15 (19) | 6 (18) | 1 (3) | NS |
| HHD | 61 (11) | 5 (10) | 25 (11) | 11 (8) | 12 (15) | 6 (18) | 2 (6) | NS |
| VHD | 68 (12) | 6 (13) | 23 (10) | 20 (15) | 10 (13) | 6 (18) | 3 (8) | NS |
| HT | 271 (50) | 16 (33) | 102 (46) | 70 (53) | 44 (56) | 10 (29) | 29 (81) | NS |
| HL | 258 (47) | 17 (35) | 116 (52) | 67 (51) | 35 (45) | 7 (21) | 16 (44) | NS |
| DM | 184 (34) | 11 (23) | 63 (28) | 49 (37) | 26 (33) | 10 (29) | 25 (69) | NS |
| Treatment | ||||||||
| ACEI or ARB | 399 (73) | 32 (67) | 159 (72) | 109 (83) | 62 (80) | 19 (56) | 18 (50) | NS |
| Ca blocker | 120 (22) | 10 (21) | 45 (29) | 19 (14) | 14 (18) | 7 (21) | 25 (69) | NS |
| Diuretics | 241 (44) | 17 (35) | 74 (33) | 66 (50) | 52 (67) | 16 (47) | 16 (44) | NS |
| Aldosterone blockers | 158 (29) | 9 (19) | 52 (23) | 46 (35) | 38 (49) | 12 (35) | 1 (3) | NS |
| β-blockers | 245 (46) | 21 (44) | 82 (37) | 71 (54) | 40 (51) | 16 (47) | 15 (42) | NS |
Data given as n (%), mean±SD or median (IQR). †ANOVA. ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AO, aortic root; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; CS, coronary sinus; DCM, dilated cardiomyopathy; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HHD, hypertensive heart disease; HL, hyperlipidemia; HR, heart rate; HT, hypertension; IHD, ischemic heart disease; LVEDP, left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction; MBP, mean blood pressure; NT-proBNP, N-terminal pro-brain natriuretic peptide; VHD, valvular heart disease.
Predictors of Plasma NT-proBNP in Left-Sided Heart Disease
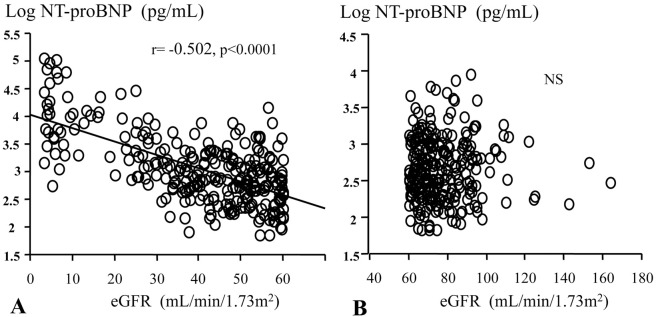
On stepwise multivariate analysis, LVEDP, LVEF, and BMI were independent predictors of logNT-proBNPCS–AO, and eGFR was not an independent predictor (Table 2). In non-CKD patients (n=264), LVEDP, LVEF, and BMI were independent predictors of log NT-proBNPAO, and eGFR was not an independent predictor (Table 3). In patients with CKD (n=280), LVEDP, LVEF and BMI, and eGFR were independent predictors of log NT-proBNPAO (Table 4). In non-CKD patients, there was no relationship between eGFR and log NT-proBNPAO. In patients with CKD there was a significant negative correlation between eGFR and log NT-proBNPAO (Figure 1).
Table 2.
Indicators of Transcardiac Increase in Log NT-proBNPCS–AO (n=544)
| Variables | Univariate correlation coefficient |
P-value | Multivariable β-coefficient (SE) |
P-value |
|---|---|---|---|---|
| Age (years) | −0.089 | 0.038 | ||
| Sex (male=1) | 0.057 | 0.187 | ||
| AF (AF=1) | 0.007 | 0.87 | ||
| BMI (kg/m2) | −0.131 | 0.0022 | −0.017 (0.005) | 0.0002 |
| LVEDP (mmHg) | 0.285 | <0.0001 | 0.017 (0.005) | <0.0001 |
| LVEF (%) | −0.330 | <0.0001 | −0.09 (0.001) | <0.0001 |
| eGFR (mL/min/1.73 m2) | −0.028 | 0.508 |
Abbreviations as in Table 1.
Table 3.
Indicators of Log NT-proBNPAO in 264 Non-CKD Patients
| Variables | Univariate correlation coefficient |
P-value | Multivariable β-coefficient (SE) |
P-value |
|---|---|---|---|---|
| Age (years) | −0.015 | 0.814 | ||
| Sex (male=1) | 0.143 | 0.025 | ||
| AF (AF=1) | 0.006 | 0.923 | ||
| BMI (kg/m2) | −0.171 | 0.0053 | −0.025 (0.006) | <0.0001 |
| LVEDP (mmHg) | 0.278 | <0.0001 | 0.021 (0.004) | <0.0001 |
| LVEF (%) | −0.233 | <0.0001 | −0.08 (0.002) | <0.0001 |
| eGFR (mL/min/1.73 m2) | 0.001 | 0.991 |
Abbreviations as in Table 1.
Table 4.
Indicators of Log NT-proBNPAO in 280 CKD Patients
| Variables | Univariate correlation coefficient |
P-value | Multivariable β-coefficient (SE) |
P-value |
|---|---|---|---|---|
| Age (years) | −0.027 | 0.648 | ||
| Sex (male=1) | 0.105 | 0.080 | ||
| AF (AF=1) | 0.020 | 0.737 | ||
| BMI (kg/m2) | −0.227 | 0.0001 | −0.027 (0.008) | 0.0005 |
| LVEDP (mmHg) | 0.238 | <0.0001 | 0.020 (0.004) | <0.0001 |
| LVEF (%) | −0.223 | 0.0002 | −0.10 (0.002) | <0.0001 |
| eGFR (mL/min/1.73 m2) | −0.645 | <0.0001 | −0.25 (0.002) | <0.0001 |
Abbreviations as in Table 1.
Figure 1.
Relationship between estimated glomerular filtration rate (eGFR) and plasma log N-terminal pro-brain natriuretic peptide (NT-proBNP) in (A) patients with chronic kidney disease (CKD) and (B) patients with non-CKD.
Log NT-proBNPCS–AO and Log NT-proBNPAO vs. CKD Stage
Between the 6 groups, there were no significant differences in NT-proBNPCS–AO (Table 1). NT-proBNPAO was significantly increased with increasing CKD stage (Table 1; Figure 2). The NT-proBNPCS–AO/NT-proBNPAO ratio significantly decreased with decreasing eGFR in the 6 groups (P<0.0001; G1, 67±38%; G2, 50±24%; G3a, 40±21%; G3b, 30±16%; G4, 14.8±7.9%; and G5, 3.5±2.4%, respectively; Figure 3). There were no differences in LVEF, LVEDP, or the transcardiac gradient of NT-proBNP, but plasma NT-proBNPAO in the CKD stage 3 patients was approximately double that of the non-CKD patients; and approximately 5-fold in the CKD stage 4, and approximately 10-fold in the CKD stage 5 patients compared with the non-CKD patients (Table 1).
Figure 2.
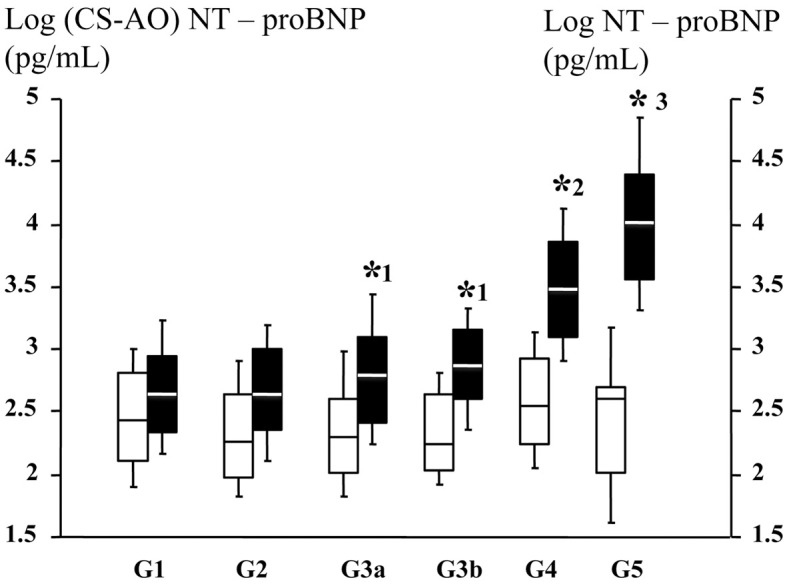
(□) Log N-terminal pro-brain natriuretic peptide (coronary sinus–aortic root; NT-proBNPCS–AO) and (■) log NT-proBNPAO vs. chronic kidney disease (CKD) stage. Crossbars, median; box, IQR. CKD stages: G1, estimated glomerular filtration rate (eGFR) ≥90 mL/min/1.73 m2; G2, 60≤eGFR<90 mL/min/1.73 m2; G3a, 45≤eGFR<60 mL/min/1.73 m2; G3b, 30≤eGFR<45 mL/min/1.73 m2; G4, 15≤eGFR<30 mL/min/1.73 m2; G5, eGFR <15 mL/min/1.73 m2. *1P<0.001 vs. G2, *2P<0.001 vs. G3a and G3b, *3P<0.001 vs. G4 with ANOVA regarding with log NT-proBNP in the AO.
Figure 3.
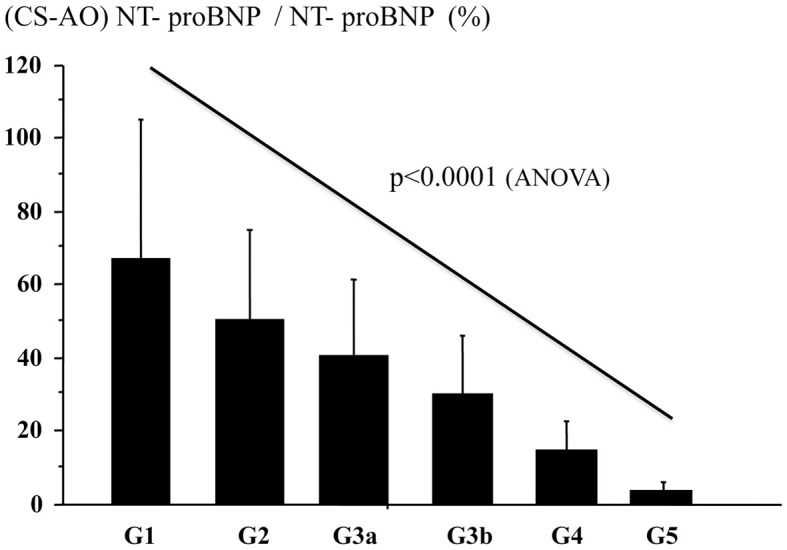
N-terminal pro-brain natriuretic peptide (coronary sinus–aortic root; NT-proBNPCS–AO)/NT-proBNPAO ratio vs. chronic kidney disease (CKD) stage. G1, estimated glomerular filtration rate (eGFR) ≥90 mL/min/1.73 m2; G2, 60≤eGFR<90 mL/min/1.73 m2; G3a, 45≤eGFR<60 mL/min/1.73 m2; G3b, 30≤eGFR<45 mL/min/1.73 m2; G4, 15≤eGFR<30 mL/min/1.73 m2; G5, eGFR <15 mL/min/1.73 m2.
Hemodynamics and NT-proBNP: Impact of Renal Function
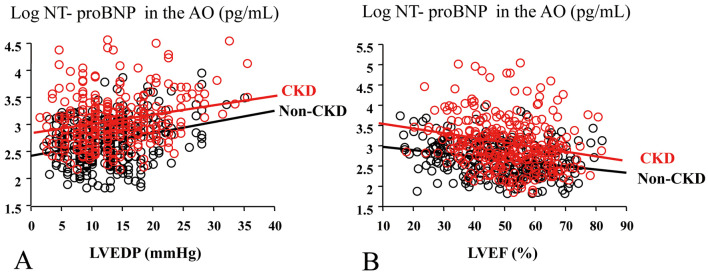
There were significant correlations between LVEDP, LVEF and the transcardiac increase in log NT-proBNP in non-CKD and CKD patients in both groups with the same regression line (data not shown). The regression line between LVEDP, LVEF and log NT-proBNPAO in CKD patients had a significant upward shift compared with that in non-CKD patients (P<0.001, Figure 4).
Figure 4.
Log N-terminal pro-brain natriuretic peptide in the aortic root (log NT-proBNPAO) vs. (A) left ventricular end-diastolic pressure (LVEDP) and (B) left ventricular ejection fraction (LVEF) in (black circles) non-chronic kidney disease (non-CKD) patients and (red circles) patients with CKD.
Discussion
Plasma NT-proBNP is a useful biomarker for mortality including cardiovascular death, not only in CHF patients but also in the general population.1,6,14,18 Given that NT-proBNP ranges widely in patients with CKD,14,19,20 the percentage of peripheral NT-proBNP of cardiac origin remains unknown in these patients. To address the problem, we measured the plasma NT-proBNP level in the AO and CS in 544 consecutive patients with left-sided heart disease and compared hemodynamic parameters in CKD patients. The present study suggests that (1) if we evaluate the severity of hemodynamic overload by plasma NT-proBNP, we should not account of eGFR in non-CKD patients; (2) in CKD patients (stages 3, 4, and 5), we should take eGFR into account in the evaluation of hemodynamic overload by plasma NT-proBNP (the NT-proBNPCS–AO/NT-proBNPAO ratio was significantly decreased [G1, 67±38%; G2, 50±24%; G3a, 40±21%; G3b, 30±16%; G4, 14.8±7.9%; and G5, 3.5±2.4%, respectively], especially in those with eGFR <30 mL/min/1.73 m2); and (3) BMI, a marker of obesity, is an independent factor of NT-proBNP secretion from the heart.
We are in the midst of a chronic epidemic of CHF and CKD worldwide. Obesity is associated with the development of CKD and progression to kidney failure. Additionally, obesity is predictive of cardiovascular disease and mortality in patients with CKD. BMI is a clinical marker of obesity. Many studies have reported that plasma NT-proBNP and BNP are low in obesity with or without CHF. The mechanism of low NT-proBNP and BNP, however, remains unknown. After BNP and NT-proBNP secretion from the CS, clearance of BNP via enzymatic breakdown and receptor binding may explain the low BNP in obesity,7 due to the upregulation of clearance receptor and neutral endopeptidase activity.21,22 NT-proBNP, however, is mainly cleared by the kidneys. The present study has shown that the low NT-proBNP secretion from the heart directly contributes to the low NT-proBNP in obesity.
After angiotensin receptor–neprilysin inhibitor (ARNI) treatment, cardiovascular death decreased by 20% compared with enalapril, with increased BNP and decreased NT-proBNP,23,24 suggesting that NT-proBNP may be recommended as a biomarker of CHF after ARNI treatment.24 In the present study, the cut-offs of BNP and NT-proBNP for cardiac events may be influenced by eGFR, especially in NT-proBNP.14 The cut-off of NT-proBNP for predicting hospitalization and death ranges widely across the CKD stages,14 and urinary NT-proBNP is significantly lower in CHF patients than in control subjects,8 suggesting that a marked decrease in urinary excretion of NT-proBNP contributes to a high plasma NT-proBNP by tubular injury in CHF patients with CKD.8,9
Because NT-proBNP has a long half-life and is cleared only by the kidneys, the NT-proBNPCS–AO/NT-proBNPAO ratio may be an indicator of renal clearance and metabolism of NT-proBNP in patients in stable conditions. The extracardiac mechanism of elevation of plasma NT-proBNP levels depends on the renal clearance and metabolism,8,9 indicating that both decreased renal blood flow and renal tubular injury may influence NT-proBNP, especially in stages 4 and 5.
In patients with eGFR-based CKD stage 4 and 5, renal clearance of NT-proBNP is approximately 15% and 3.5%, respectively (Table 1), indicating that approximately 85% and 95%, respectively, of NT-proBNP in the plasma is due to the decrease in renal clearance. If physicians evaluate hemodynamics according to the level of NT-proBNP, they should take into account eGFR in CKD patients, especially those with eGFR <30 mL/min/1.73 m2. There was no significant difference in the ratio of NT-proBNPCS–AO/NT-proBNPAO between CKD stage 5 patients with and without dialysis (data not shown). These results are consistent with previous reports noting a very high NT-proBNP in end-stage CKD patients with or without CHF.25,26 NT-proBNP is often O-glycosylated in cardiac myocytes,27 which may result in underestimation of total NT-proBNP level, which includes both glycosylated and non-glycosylated NT-proBNP, by the NT-proBNP assay system used in the present study.15 The NT-proBNPCS–AO/NT-proBNPAO ratio, however, may not be influenced by O-glycosylation.
Study Limitations
BMI in a general Japanese population is lower than in Western countries.28 The difference in BMI between the present patients and Western patients may influence the relationships between BMI and NT-proBNP. In the present study, we used creatinine to calculate eGFR. Cystatin C may be better than creatinine, and further studies are needed. The small numbers of patients with eGFR-based stages 4 and 5 was a further limitation. Finally, in the present study we did not measure renal blood flow, urinary NT-proBNP excretion, or the markers of renal tubular injury. Further studies are needed to clarify the relationship between the ratio of NT-proBNP secretion from the heart to peripheral NT-proBNP, the renal clearance of NT-proBNP in CKD and the mechanism of low NT-proBNP secretion in obesity.
Conclusions
eGFR influences the level of NT-proBNP for prediction of hemodynamic overload in CKD patients but not in non-CKD patients, and the ratio of NT-proBNP secretion from the heart to peripheral NT-proBNP is markedly decreased in CKD patients, especially those with eGFR <30 mL/min/1.73 m2. In addition, BMI, a marker of obesity, is an independent factor of NT-proBNP secretion from the heart.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
We wish to thank Atsuyuki Wada, MD, Masato Ohnishi, MD, Hiroshi Mabuchi, MD, Keiko Maeda, MD, Masayuki Yamaji, MD, and Gensyou Shichiri, MD for their advice on the study protocol. We also thank Ms. Ayane Murasaki for excellent technical assistance.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al.. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail 2017; 23: 628–651.28461259 [Google Scholar]
- 2. Tsutamoto T, Wada A, Maeda K, Hisanaga T, Maeda Y, Fukai D, et al.. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: Prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 1997; 96: 509–516. [DOI] [PubMed] [Google Scholar]
- 3. Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M.. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J 1998; 135: 825–832. [DOI] [PubMed] [Google Scholar]
- 4. Tsutamoto T, Wada A, Maeda K, Hisanaga T, Mabuchi N, Hayashi M, et al.. Plasma brain natriuretic peptide level as a biochemical marker of morbidity and mortality in patients with asymptomatic or minimally symptomatic left ventricular dysfunction. Eur Heart J 1999; 20: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 5. Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, et al.. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol 2000; 36: 1587–1593. [DOI] [PubMed] [Google Scholar]
- 6. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al.. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur Heart J 2006; 27: 330–337. [DOI] [PubMed] [Google Scholar]
- 7. Abuzaanona A, Lanfear D.. Pharmacogenomics of the natriuretic peptide system in heart failure. Curr Heart Fail Rep 2017; 14: 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linssen GC, Damman K, Hillege HL, Navis G, van Veldhuissen DJ, Voors AA.. Urinary N-terminal prohormone brain natriuretic peptide excretion in patients with chronic heart failure. Circulation 2009; 120: 35–41. [DOI] [PubMed] [Google Scholar]
- 9. Cortés R, Portolés M, Roselló-Lletí E, Martínez-Dolz L, Almenar L, Grigorian L, et al.. Impact of glomerular filtration rate on urinary BNP and NT-proBNP levels in heart failure. Peptides 2012; 33: 354–358. [DOI] [PubMed] [Google Scholar]
- 10. Haug C, Metzele A, Kochs M, Hombach V, Grünert A.. Plasma brain natriuretic peptide and atrial natriuretic peptide concentrations correlate with left ventricular end-diastolic pressure. Clin Cardiol 1993; 16: 553–557. [DOI] [PubMed] [Google Scholar]
- 11. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al.. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352. [DOI] [PubMed] [Google Scholar]
- 12. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al; for the CKD Prognosis Consortium.. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010; 375: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al; Chronic Kidney Disease Prognosis Consortium.. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes: A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aimo A, Januzzi JL Jr, Vergaro G, Ripoli A, Latini R, Masson S, et al.. High-sensitivity troponin T, NT-proBNP and glomerular filtration rate: A multimarker strategy for risk stratification in chronic heart failure. Int J Cardiol 2019; 277: 166–172. [DOI] [PubMed] [Google Scholar]
- 15. Tsutamoto T, Sakai H, Ishikawa C, Fujii M, Tanaka T, Yamamoto T, et al.. Direct comparison of transcardiac difference between increase brain natriuretic peptide (BNP) and N-terminal proBNP in patients with chronic heart failure. Eur J Heart Fail 2007; 9: 667–673. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al.. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 17. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al.. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014; 63: 713–735. [DOI] [PubMed] [Google Scholar]
- 18. Tsutamoto T, Wada A, Sakai H, Ishikawa C, Tanaka T, Hayashi M, et al.. Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol 2006; 47: 582–586. [DOI] [PubMed] [Google Scholar]
- 19. Scheven L, de Jong PE, Hillege HL, Lambers Heerspink HJ, van Pelt LJ, Kootstra JE, et al; PREVEND study group.. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J 2012; 33: 2272–2281. [DOI] [PubMed] [Google Scholar]
- 20. Kawagoe C, Sato Y, Toida T, Nakagawa H, Yamashita Y, Fukuda A, et al.. N-terminal-pro-B-type-natriuretic peptide associated with 2-year mortality from both cardiovascular and non-cardiovascular origins in prevalent chronic hemodialysis patients. Ren Fail 2018; 40: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al.. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004; 109: 594–600. [DOI] [PubMed] [Google Scholar]
- 22. McCord J, Mundy BJ, Hudson MP, Maisel AS, Hollander JE, Abraham WT, et al.. Relationship between obesity and B-type natriuretic peptide levels. Arch Intern Med 2004; 164: 2247–2252. [DOI] [PubMed] [Google Scholar]
- 23. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al; PARADIGM-HF Investigators and Committees.. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 24. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al; PARADIGM-HF Investigators and Coordinators.. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131: 54–61. [DOI] [PubMed] [Google Scholar]
- 25. Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, et al.. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: Relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis 2005; 46: 610–620. [DOI] [PubMed] [Google Scholar]
- 26. Kawagoe C, Sato Y, Toida T, Nakagawa H, Yamashita Y, Fukuda A, et al.. N-terminal-pro-B-type-natriuretic peptide associated with 2-year mortality from both cardiovascular and non-cardiovascular origins in prevalent chronic hemodialysis patients. Ren Fail 2018; 40: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuwahara K, Nakagawa Y, Nishikimi T.. Cutting edge of brain natriuretic peptide (BNP) research: The diversity of BNP immunoreactivity and its clinical relevance. Circ J 2018; 82: 2455–2461. [DOI] [PubMed] [Google Scholar]
- 28. Sugisawa T, Kishimoto I, Kokubo Y, Makino H, Miyamoto Y, Yoshimasa Y.. Association of plasma B-type natriuretic peptide levels with obesity in a general urban Japanese population: The Suita Study. Endocr J 2010; 57: 727–733. [DOI] [PubMed] [Google Scholar]