Abstract
Background: Many treatment options are available for pulmonary arterial hypertension (PAH), but specific recommendations for long-term treatment are unavailable. We compared prognosis in PAH patients receiving goal-oriented, sequential combination therapy evaluated using cardiopulmonary exercise testing (CPX) parameters or conventional empiric therapy.
Methods and Results: The Goal-Oriented Therapy Evaluated by Cardiopulmonary Exercise Testing for Pulmonary Arterial Hypertension (GOOD EYE) study was a multicenter, retrospective/prospective study in which a total of 129 patients with newly diagnosed PAH were enrolled (goal-oriented sequential combination therapy, n=42; conventional empiric therapy, n=87). Patients in the goal-oriented therapy group received sequential combination therapy, the efficacy of which was regularly evaluated using CPX parameters. Patients in the conventional empiric therapy group received conventional empiric therapy. The primary endpoint was cardiovascular death. In the goal-oriented therapy group, plasma brain natriuretic peptide, mean pulmonary arterial pressure, pulmonary vascular resistance, and 6-min walk test were significantly improved at 12 months compared with baseline. Survival in the goal-oriented therapy group at 1, 2, and 3 years (97.6%, 95.2%, and 86.0%, respectively) tended to be higher than that in the conventional empiric therapy group (P=0.082).
Conclusions: Goal-oriented sequential combination therapy evaluated using CPX parameters may be associated with a favorable prognosis compared with conventional empiric therapy in patients with newly diagnosed PAH.
Key Words: Combination therapy, Exercise capacity, Pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is associated with a high rate of morbidity and is fatal if left untreated. Current algorithms recommend an endothelin receptor antagonist (ERA)1–5 or phosphodiesterase-5 inhibitor (PDE-5I)6–8 as early first-line treatment in patients with PAH with a World Health Organization (WHO) functional class II or III.9
Hoeper et al proposed the use of goal-oriented therapy, evaluated on cardiopulmonary exercise (CPX) parameters or 6-min walk test (6MWT), for the treatment of PAH.10 6MWT, however, may not be an appropriate goal because walking distance is affected not only by cardiopulmonary fitness but also by factors such as age, sex, body weight, and height.11 Indeed, a meta-analysis has shown that improvement in 6MWT is not correlated with outcome in patients with PAH.12
There is increasing recognition of the potential of using CPX parameters to direct the treatment of PAH.10,13,14 For example, in 2002, Wensel et al showed that patients with peak oxygen uptake (peak V̇O2) <10.4 mL/min/kg and maximum systolic blood pressure (SBP) during exercise <120 mmHg had a much worse prognosis than patients who were able to meet those thresholds.15 In 2005, Hoeper et al examined the use of goal-oriented treatment evaluated on CPX parameters in PAH patients and reported that peak V̇O2 >10.4 mL/min/kg was associated with a better prognosis.10 Goal peak V̇O2 ≥15 mL/min/kg has been adopted in the 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines for the diagnosis and treatment of PAH.16
Currently, which combination therapy should be implemented – and how and in whom it should be implemented – remains a topic of debate.17,18 Compared with monotherapy, combination therapy improves exercise capacity and reduces the risk of clinical worsening in PAH patients.19
In the present study, we compared the effects on prognosis in patients with newly diagnosed PAH of either goal-oriented, sequential combination therapy evaluated on CPX parameters, or conventional empiric therapy. Thus, we investigated whether goal-oriented therapy based on CPX parameters is associated with better clinical outcome compared with conventional empiric therapy in patients with PAH.
Methods
Study Design and Subjects
The Goal-Oriented Therapy Evaluated by Cardiopulmonary Exercise Testing for Pulmonary Arterial Hypertension (GOOD EYE) study was a multicenter, retrospective-prospective study. The study protocol was registered in the University Hospitals Medical Information Network Clinical Trials Registry system (www.umin.ac.jp; no. UMIN000006174) before the start of the study. Patients who were 16–80 years old at study entry with newly diagnosed PAH of WHO functional class II–IV according to the Dana Point criteria20 were enrolled in the study. Patients with any of the following conditions at enrollment were excluded from the study: (1) pulmonary hypertension classified as group 2, 3, 4, or 5 according to the Dana Point classification; (2) pregnancy; (3) serum creatinine >2.0 mg/dL; (4) history of serious chronic obstructive pulmonary disease or restrictive lung disease; (5) unable to walk without assistance; (6) currently receiving PAH-targeted therapy such as an ERA, PDE-5I, or i.v. epoprostenol; or (7) other conditions that, according to the judgment of the physicians in charge, made enrollment inappropriate because of concerns regarding patient safety.
Informed Consent
Participating centers included affiliated hospitals led by cardiology specialists in and around Nagoya, Japan. All prospective participants provided written informed consent after receiving explanations from the physician in charge about study objectives, study protocol, possible adverse effects of the study drugs, measures for privacy protection, and study withdrawal. The study protocol was approved by the Ethics Review Board of Nagoya University School of Medicine (approval no. 1157). The Ethics Review Committee of Nagoya University School of Medicine and the relevant committee at each participating center also approved the study protocol.
Procedure
Historical Group (Group H) From June 2005 to December 2011, at the affiliated hospitals participating in the study, the therapeutic strategy for the treatment of PAH patients with WHO functional classification II–IV (without hemodynamic instability) consisted of either beraprost and/or an ERA (bosentan or ambrisentan) and/or a PDE-5I (sildenafil or tadalafil). Patients with WHO functional classification IV PAH (with hemodynamic instability) were treated immediately with i.v. epoprostenol, if indicated. Thus, this was a conventional empiric therapeutic strategy.
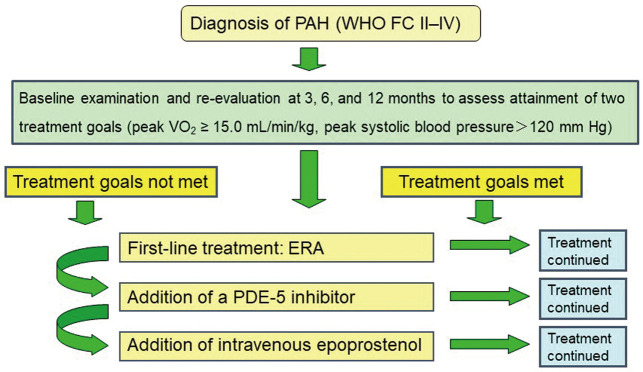
Goal-Oriented Group (Group G) From January 2012 to March 2015, the treatment strategy for PAH at the affiliated hospitals participating in the study was changed to follow a new treatment algorithm (Figure 1). Patients underwent an initial examination at diagnosis and then re-evaluation at 3, 6, and 12 months after diagnosis. Cardiac catheterization was performed at baseline and at 12 months; CPX was performed at baseline and at 3, 6, and 12 months. Two treatment goals were set: peak V̇O2 ≥15.0 mL/min/kg, which is defined as providing a “better prognosis” according to the 2009 ESC/ERS guidelines for the diagnosis and treatment of PAH;21 and peak SBP during exercise >120 mmHg. Patients were considered clinically stable when both treatment goals were reached. An ERA, either bosentan or ambrisentan, was used as first-line treatment. Add-on therapy was a PDE-5I, either sildenafil or tadalafil, followed by i.v. epoprostenol, if needed. Beraprost, diuretics, digitalis, and anticoagulants were excluded from this combination treatment protocol. Physicians expert in treating PAH determined the most appropriate pharmacological therapy for each patient. Patients were kept informed about all available treatment options. Whenever combination treatment was proposed, patients were informed of the potential risks and side-effects.
Figure 1.
Goal-based therapeutic algorithm used in the present study for newly diagnosed pulmonary arterial hypertension (PAH). ERA, endothelin receptor antagonist; PDE-5, phosphodiesterase-5; V̇O2, oxygen uptake; WHO FC, World Health Organization functional class.
Dosing Regimen
Bosentan was given at an initial dose of 62.5 mg twice daily for 4 weeks, which was titrated to 125 mg twice daily thereafter. Ambrisentan was started at 5 mg once daily for 4 weeks and increased gradually to 10 mg once daily thereafter in the absence of side-effects and with adequate tolerability. Sildenafil was titrated to a maximum of 20 mg 3 times daily. Tadalafil was started and maintained at 40 mg once daily. Given the individual variability in response, the choice of drug and dose was left to the discretion of the physicians. All drug regimens were adjusted as necessary to limit side-effects.
Measurements of Interest
Variables such as sex, age, smoking, comorbidities, associated connective tissue disease, HIV infection, portopulmonary hypertension, and congenital heart disease (CHD) were obtained at baseline. Physiological parameters, including WHO functional class, anthropometrics, blood pressure, and 6MWT, were evaluated at baseline. Blood examination, electrocardiogram, echocardiogram, respiratory function test, CPX, and right-heart catheterization were conducted at baseline. These measurements were repeated at 3, 6, and 12 months, except for right-heart catheterization, which was repeated at 12 months only. Blood examination included the following: plasma brain natriuretic peptide (BNP), uric acid, blood urea nitrogen, serum creatinine, sodium, potassium, and liver enzymes (including alanine aminotransferase, aspartate aminotransferase, γ-glutamyltranspeptidase, and bilirubin).
Exercise Capacity
The 6MWT is a measure of the distance a person walks in 6 min; in the present study, 6MWT was performed according to American Thoracic Society criteria.22 In addition, each patient underwent CPX at a progressively increasing work rate to maximum tolerance on a cycle ergometer. All patients started at 10 W for a 3-min warm-up, followed by a 10-W/min ramped-increment protocol. Twelve-lead electrocardiogram was monitored continuously, and arm blood pressure was automatically measured every minute during exercise and throughout the recovery period. After achieving peak workload, all patients pedaled at a load of 0 W for a cool-down period of at least 2 min to prevent excess venous pooling. Test termination criteria were patient request, volitional fatigue, ventricular tachycardia, ≥2 mm horizontal or down-sloping ST segment depression, or a drop in SBP ≥20 mmHg during exercise. A qualified exercise physiologist conducted each test under the supervision of a certified cardiologist. Respiratory gas exchange variables were acquired continuously throughout the test, and gas exchange data were obtained breath by breath. Before each test, the oxygen and carbon dioxide sensors were calibrated using gases with known oxygen, nitrogen, and carbon dioxide concentrations. The flow sensor was also calibrated before each test. Peak V̇O2 was recorded as the highest 30-s average obtained during the final stage of the exercise test, and peak respiratory exchange ratio as the highest 30-s average during the final stage of the test. Minute ventilation (V̇E) and CO2 output (V̇CO2) were obtained up to the respiratory compensation point during exercise, and V̇E/V̇CO2 slope was determined by means of a linear regression analysis up to the respiratory compensation point during exercise. Peak SBP was also recorded.
Study Organization
The Executive Committee created the study protocol and supervised the progression of the study. The Steering Committee approved the study protocol and made decisions about the management of the study. The Data and Safety Monitoring Board – consisting of a physician, an epidemiologist, a pulmonologist, and a cardiologist – provided the Steering Committee with advice when there were concerns about participant safety. The Endpoint Evaluation Committee consisted of a cardiologist and a pulmonologist who adjudicated the primary endpoints reported by physicians. Data collection via clinical records was managed centrally by the independent Data Management Group. The Statistical Analysis Board performed the statistical analyses independently from the aforementioned committees.
Statistical Analysis
All data are expressed as mean±SD. Differences between the 2 treatment groups were assessed using the chi-squared test for categorical variables and the Mann-Whitney rank-sum test for continuous variables. To evaluate the effect of sequential combination therapy in group G, hemodynamic parameters at baseline and at 12 months were compared using paired t-test for all patients. Repeated-measures analysis of variance was used to assess changes in the parameters over time in group G. Cumulative cardiac event-free survival estimates were calculated using the Kaplan-Meier method, and differences were evaluated with a stratified log-rank test. Expected survival (idiopathic PAH only) was calculated using an equation from the National Institutes of Health.23,24 All statistical analyses were carried out using SPSS 17.0 (SPSS, Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Table 1 lists the baseline clinical characteristics and important hemodynamic parameters for all patients; there were no significant differences between the 2 groups.
Table 1.
Patient Baseline Characteristics
| Group H (n=87) |
Group G (n=42) |
P-value | |
|---|---|---|---|
| Age (years) | 51.9±17.0 | 57.2±15.7 | 0.090 |
| Male | 29 (33) | 16 (38) | 0.800 |
| BSA (L/min/m2) | 1.51±0.22 | 1.57±0.19 | 0.144 |
| WHO II/III/IV | 27/41/19 | 11/19/12 | 0.504 |
| IPAH/CD/PPH/CHD | 38/31/4/14 | 17/12/7/6 | 0.142 |
| Laboratory data | |||
| BNP (pg/mL) | 238 (46–403) | 410 (69–573) | 0.084 |
| UA (mg/dL) | 6.6±2.2 | 7.2±2.9 | 0.196 |
| Cr (mg/dL) | 0.91±0.67 | 0.85±0.28 | 0.506 |
| Echocardiography | |||
| TRPG (mmHg) | 59.4±25.3 | 64.5±25.4 | 0.315 |
| TAPSE (mm) | 14.2±6.2 | 16.5±5.4 | 0.307 |
| Cardiac catheterization | |||
| PAWP (mmHg) | 11.3±4.3 | 10±4.7 | 0.141 |
| Systolic PAP (mmHg) | 73.6±30.1 | 70.3±19.6 | 0.455 |
| Diastolic PAP (mmHg) | 31.0±15.7 | 27.6±9.5 | 0.140 |
| Mean PAP (mmHg) | 48.2±19.6 | 44.2±11.9 | 0.153 |
| PVR (Wood unit) | 7.1±6.4 | 9.5±6.4 | 0.121 |
| RAP (mmHg) | 6.3±4.8 | 6.6±5.1 | 0.344 |
| SvO2 (%) | 65.5±12.3 | 64.2±9.8 | 0.633 |
| CO (L/min) | 4.79±1.75 | 4.38±1.5 | 0.205 |
| CI (L/min/m2) | 3.12±1.21 | 2.74±0.86 | 0.082 |
| Heart rate (beats/min) | 84±14 | 83±15 | 0.863 |
Data given as mean±SD, n (%) or median (range). BNP, brain natriuretic peptide; BSA, body surface area; CD, collagen disease; CHD, congenital heart disease; CI, cardiac index; CO, cardiac output; Cr, creatinine; Group G, goal-oriented sequential combination therapy; Group H, conventional empiric therapy; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension; PAP, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PPH, portopulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SvO2, mixed venous oxygen saturation; TAPSE, tricuspid annular plane systolic excursion: TRPG, trans regurgitation pressure gradient; UA, uric acid; WHO, World Health Organization.
Medication
The PAH patients in group G received sequential combination treatment (Table 2). At baseline, no patients had received either an ERA or PDE-5I; by the 3-month time point, 37 patients (88%) were receiving an ERA, and at the 6-month time point, a PDE-5I had been added in 28 cases (68%). At the 12-month time point, 35 patients (85%) were continuing to receive an ERA, 28 (68%) were continuing to receive a PDE-5I, and 1 (2%) was receiving i.v. prostaglandin I2. Table 3 lists the medication prescribed to the 2 groups of patients at the end of follow-up. There were no significant differences in the use of any medication between the 2 groups. At the end of the study, monotherapy and combination therapy were significantly lower and higher in group G than in group H, respectively.
Table 2.
Group G: Drugs Used to Treat PAH
| Medication | No. patients (%) | |||
|---|---|---|---|---|
| Baseline (n=42) |
3 months (n=42) |
6 months (n=41) |
12 months (n=41) |
|
| Oral PGI2 | 15 (37) | 15 (37) | 18 (44) | 19 (46) |
| ERA | 0 | 37 (88) | 35 (85) | 35 (85) |
| PDE-5I | 0 | 2 (5) | 28 (68) | 28 (68) |
| Epo-iv | 0 | 0 | 1 (2) | 1 (2) |
Epo-iv, i.v. epoprostenol; ERA, endothelin receptor antagonists; PDE-5I, phosphodestelase-5 inhibitor; PGI2, prostaglandin I2. Other abbreviations as in Table 1.
Table 3.
Medication at End of Study
| Group H (n=87) |
Group G (n=42) |
P-value | |
|---|---|---|---|
| Oral PGI2 | 53 (61) | 19 (45) | 0.110 |
| ERA | 65 (75) | 35 (83) | 0.231 |
| PDE-5I | 52 (60) | 28 (67) | 0.449 |
| Epo-iv | 16 (18) | 3 (7) | 0.109 |
| Anticoagulant | 27 (31) | 15 (36) | 0.595 |
| Diuretic | 48 (55) | 31 (74) | 0.065 |
| Digitalis | 18 (21) | 15 (36) | 0.960 |
| Monotherapy | 36 (41) | 8 (19) | 0.012 |
| Combination therapy | 45 (52) | 32 (76) | 0.007 |
Data given as n (%). Abbreviations as in Table 1.
Group G: Change in Clinical Parameters
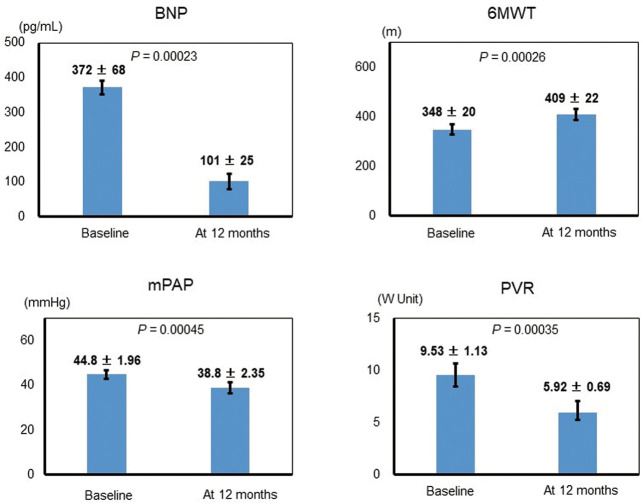
In group G, plasma BNP and 6MWT were significantly decreased and increased, respectively, at 12 months compared with at baseline (P=0.00023 and P=0.00026, respectively; Figure 2). In addition, mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance were significantly decreased at 12 months compared with at baseline; the 2 patients who died during the study were excluded from these 2 analyses (P=0.00045 and P=0.00035, respectively).
Figure 2.
Representative invasive and non-invasive assessments at baseline and at 12 months in the goal-oriented therapy group. 6MWT, 6-min walk test; BNP, brain natriuretic peptide; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance.
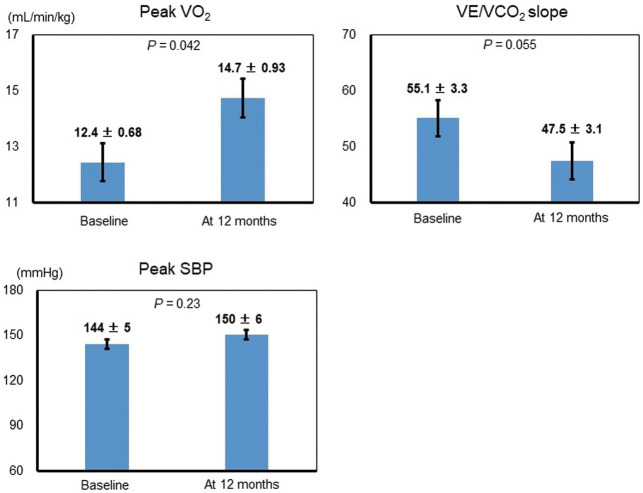
Figure 3 shows changes in exercise capacity in group G. There were no adverse incidents during CPX. Compared with baseline, peak V̇O2 was significantly increased (P=0.042). V̇E/V̇CO2 slope was decreased at 12 months compared with baseline, but this change was not statistically significant. Peak SBP was similar at baseline and at 12 months.
Figure 3.
Exercise capacity at baseline and at 12 months. SBP, systolic blood pressure in the goal-oriented therapy group; V̇E, minute ventilation; V̇CO2, CO2 output; V̇O2, oxygen uptake.
Prognosis
During the study period, cardiac death occurred in 5 patients (11.9%) in group G and in 21 (24.1%) in group H. The cumulative probability of survival was calculated for both groups using the Kaplan-Meier method (Figure 4). The survival rate at 1, 2, and 3 years in group G was 97.6%, 95.2%, and 86.0%, and that in group H was 91.9%, 79.6%, and 75.8%, respectively. The probability of survival was higher in group G than in group H, but this difference was not statistically significant (P=0.082). The probability of survival, however, was better in both groups than the predicted survival, as calculated using an equation from the National Institutes of Health,23,24 which predicted 1-, 2-, and 3-year survivals of 71.5%, 59.9%, and 50.0%, respectively.
Figure 4.
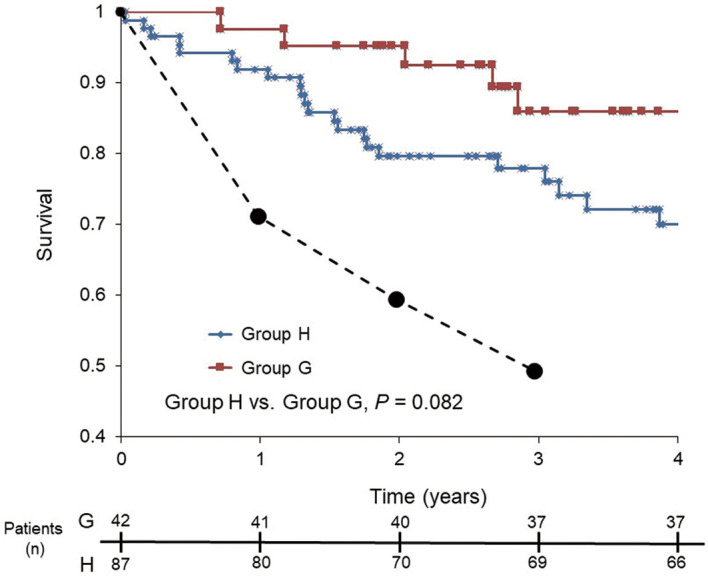
Kaplan-Meier estimates of survival in patients with newly diagnosed pulmonary arterial hypertension. Group G, goal-oriented sequential combination therapy; group H, conventional empiric therapy. Dotted line, expected survival as calculated with an equation from the National Institutes of Health.23,24
Discussion
This study investigated whether goal-oriented, sequential combination therapy evaluated using CPX parameters provided a better prognosis than conventional empiric therapy in newly diagnosed PAH patients. In the goal-oriented therapy group, mPAP was significantly improved at 12 months by 8.08±1.65 mmHg compared with baseline. In addition, peak V̇O2 was significantly improved at 12 months compared with baseline. Subsequently, goal-oriented, sequential combination therapy tended to provide an improved prognosis compared with conventional empiric therapy, suggesting that this approach may be better than conventional empiric therapy for treating patients with newly diagnosed PAH.
CPX Parameter-Guided Therapy
Most investigations into the treatment of PAH have focused on cardiac function at rest, but patients with PAH generally become breathless only during exercise. Indeed, peak V̇O2 is an independent prognostic indicator in patients with PAH.25 Hoeper et al proposed the use of a goal-oriented treatment strategy for the treatment of PAH that used the results of CPX and 6MWT to direct treatment (treatment goals: 6MWT, 380 m; peak V̇O2, >10.4 mL/min/kg; peak SBP during exercise, >120 mmHg).10 Using this approach, survival rates at 1, 2, and 3 years were 93%, 83%, and 80%, respectively.10 Similarly, the current ESC/ERS guidelines state that prognosis is improved when a treatment goal of peak V̇O2 ≥15 mL/min/kg is implemented.9 In the present study, we used a treatment goal of peak V̇O2 >15 mL/min/kg and obtained survival rates in the goal-oriented therapy group at 1, 2, and 3 years of 97.6%, 95.2%, and 86.0%, respectively, which are superior to the previously reported survival rates.10
In the present study, we removed 6MWT as a goal. The reason is that walking distance is affected not only by cardiopulmonary fitness but also by factors such as age, sex, body weight, and height.11 In addition, we could not find an established goal for peak SBP during exercise in patients with PAH, we thus used the same goal that was used in a previous study (peak SBP ≤120 mmHg).10,15 In the present study, no patients had peak SBP ≤120 mmHg and peak V̇O2 >15.0 mL/min/kg. Therefore, the therapeutic strategy depended on whether the peak V̇O2 goal was reached or not. In the present study peak SBP did not significantly change before or after treatment, suggesting that peak SBP during exercise should not be used to assess the efficacy of PAH treatment.
Sequential Combination Therapy
In Japan, oral ERA have been available since 2005 and PDE-5I have been available since 2008. Therefore, in the present study, we were able to use the same treatment in both groups, which allowed us to examine whether goal-oriented therapy was more useful than traditional empiric therapy for treating patients with newly diagnosed PAH.
In the present study, 2 female patients were judged by the expert physician to need early combination therapy. The first patient had portopulmonary hypertension, and, after achieving a favorable clinical outcome with the combination therapy, at 12 months she remained at WHO functional class I. The second patient had WHO functional class IV PAH with scleroderma and died due to right heart failure at 6 months.
I.v. epoprostenol was used for 1 patient in the 12-month period and in a total of 3 patients for >12 months and during follow-up in the goal-oriented therapy group.
PAH With CHD
In the present study, 6 group G patients had PAH with CHD. Ultimately, 4 of these patients could not be treated with sequential combination therapy because of worsening dyspnea or peripheral edema and were returned to monotherapy. The remaining 1 patient, who had Eisenmenger’s syndrome due to an atrial septal defect, was treated with combination therapy, and although he had poor exercise tolerance, he did not die during follow-up. In contrast, another patient with atrial septal defect needed only oral prostacyclin analog to retain good exercise capacity and improve from WHO functional class II to I. There are few published data on combination therapy for the treatment of PAH with CHD, but the recommended therapy is the same as in idiopathic PAH.26,27 Because there is a multitude of different clinical pictures of patients with PAH with CHD, stereotypical combination therapy might be difficult for PAH patients with CHD.
In the present study, exercise tolerance in teenage patients was relatively preserved regardless of the severity of PAH. Two of 3 teenage patients with mild PAH elected to receive only beraprost during the study period. The peak V̇O2 of 1 male patient (19 years old) who had idiopathic PAH and 1 female patient (16 years old) who had portopulmonary hypertension was 22.7 mL/min/kg and 19.0 mL/min/kg at baseline, respectively, and they reported no difficulties in daily life.
The results of this subanalysis suggest that goal-oriented therapy evaluated using CPX parameters might be most appropriate for middle-aged (40–60-year-old) patients with idiopathic PAH, collagen disease, or portopulmonary hypertension without CHD, or for patients newly diagnosed with PAH of WHO functional class II or III.
Prostacyclin Analogs
At the start of the present study, beraprost was already being used by 45% of the study cohort. Beraprost is the first chemically stable, orally active prostacyclin analog approved for the treatment of PAH,28,29 but its effects subside after 6 months of use.30,31 Beraprost is approved in Japan and South Korea for the treatment of PAH, but worldwide there is little evidence that beraprost is useful for the treatment of PAH. Therefore, in the present study, we excluded its use in the goal-oriented sequential combination therapy group. Recently, oral prostacyclin analogs, such as selexipag, which target the prostacyclin pathway, have been approved for the treatment of PAH.32 I.v. epoprostenol has been shown to improve hemodynamics, exercise capacity, and survival in patients with PAH.33 Therefore, i.v. epoprostenol should be used as soon as possible in cases of inadequate clinical results or in cases of deterioration by oral combination therapy. This means that PAH patients need to be stratified accurately.
New Therapeutic Combinations
COMPASS-2 did not demonstrate that adding bosentan to stable sildenafil therapy was superior to sildenafil monotherapy in delaying time to first morbidity/mortality event.34 Recently, the ERA macitentan and the oral prostanoids treprostinil and selexipag have been approved for the treatment of PAH.35 In addition, there have been significant advances in defining the role of upfront combination therapy in treatment-naïve PAH patients.9,36 Based on the results of the AMBITION trial,36 the 2015 ESC/ERS guidelines recommended that upfront combination therapy with tadalafil and ambrisentan should be offered as first-line therapy to treatment-naïve WHO group 1 PAH patients with functional class II or III symptoms.16 It should be noted, however, that the AMBITION trial did not conclusively demonstrate that upfront therapy was superior to sequential combination therapy.
Importance of Sequential Combination Therapy
An important finding of the present study is that to reach the predefined treatment goals, combination treatment eventually became necessary in almost half of the patients, indicating that monotherapy is likely insufficient for a large proportion of patients with PAH in whom the disease progresses despite active therapy. Hence, a primarily non-invasive treatment strategy and the use of combination treatment may yield acceptable results in the majority of patients with PAH. This goal-oriented strategy was based on several factors, including practicability and economical considerations. Sequential combination therapy evaluated using CPX parameters might be an appropriate therapy for the majority of PAH patients.
Study Limitations
The present study has several limitations. First, the number of patients in the goal-oriented group was small, but because the total number of newly diagnosed PAH patients worldwide is small, we believe that the data provided from the present goal-oriented group will be valuable for future studies of the use of goal-oriented strategies for the treatment of PAH. In addition, some data were missing from the conventional treatment group.
Second, because the mean patient age in the goal-oriented therapy group tended to be higher than that in the conventional empiric therapy group, cardiac death was more likely to occur in the goal-oriented therapy group. Thus, the cardiac death results obtained here are likely biased.
Third, to allow comparison with the conventional treatment group, we could use only ERA and PDE-5I such as bosentan, ambrisentan, sildenafil, and tadalafil in the goal-oriented treatment group. In addition, PDE-5I were not approved for use in Japan before 2008, therefore at that time physicians could not use triple-combination therapy. During the follow-up period, however, PDE-5I were approved for use; therefore, we list the medications in use at the end of the study in Table 3.
Fourth, recently, the oral prostanoids treprostinil37 and selexipag,32 several soluble guanylate cyclase stimulators,38 and the new ERA macitentan have been approved for use for the treatment of PAH in Japan; therefore, we now have more choices of medication for creating new goal-oriented treatment strategies for the treatment of PAH. Finally, we did not include exercise training for patients in the goal-oriented group. Exercise training, however, can improve functioning in patients with PAH.39
Conclusions
A goal-oriented therapeutic approach evaluated using CPX parameters provided reasonably favorable survival rates in patients with PAH compared with a conventional empiric treatment strategy.
Disclosures
The study was funded by Nagoya University Graduate School of Medicine. The Department of Cardiology, Nagoya University Graduate School of Medicine, reports receiving research promotion grants. A.H. used to belong to a department endowed by Actelion Pharmaceuticals Japan. T.K., S.A., and N.O. currently belong to a department endowed by Actelion Pharmaceuticals Japan. The research topics of these donation grants are not restricted. The Executive Committee had full access to all of the data at the end of the study and had final responsibility for the decision to submit for publication.
Funding / Support
Actelion Pharmaceuticals, Japan
Appendix
GOOD EYE Study Investigators
Principal Investigator: Toyoaki Murohara.
Executive Committee: Toyoaki Murohara (Chair), Takahisa Kondo, Akihiro Hirashiki.
Steering Committee: Lead investigators as above; Nagoya Graduate School of Medicine: Kimihiro Komori, Osamu Ito, Taichi Kato, Shoichi Maruyama, Tomio Suzuki, Yoshinao Muro, Kyosuke Takeshita; National Hospital Organization Nagoya Medical Center: Masao Katayama; Ogaki Municipal Hospital: Hideyuki Tsuboi.
Endpoint Evaluation Committee: Daiyukai General Hospital: Motomi Ando; Fujita Health University School of Medicine Banbuntane Houtokukai Hospital: Hideo Izawa; Fujita Health University School of Medicine: Kazuyoshi Imaizumi.
Writing and Subanalysis Study Committee: Toyoaki Murohara (Chair), Takahisa Kondo, Akihiro Hirashiki.
Clinical Research Coordinator and Data Management Group: Emiko Watanabe and investigators.
Participating Medical Institutions in the GOOD EYE Study
Aichi Medical University: Tetsuya Amano; Anjo Kosei Hospital: Masato Watarai, Masayoshi Koyasu; Chukyo Hospital: Masaya Kodera, Kenji Kada; Japanese Red Cross Nagoya Daiichi Hospital: Yoshihisa Shibata; Japanese Red Cross Nagoya Daini Hospital: Yukihiko Yoshida; Kainan Hospital: Manabu Miura; Komaki City Hospital: Seifuku Kyo, Taizo Kondo; Nagoya Ekisaikai Hospital: Daisuke Tanimura; National Hospital Organization, Nagoya Medical Center: Yasushi Tomita; Okazaki City Hospital: Toshikazu Tanaka; Tosei General Hospital: Masazumi Ajioka; Toyohashi Municipal Hospital: Kenshin Naruse; Toyota Memorial Hospital: Ryoji Ishiki; Yokkaichi Municipal Hospital: Masaaki Kanashiro.
Acknowledgments
The authors wish to express their sincere appreciation to all the patients, collaborating physicians, and other medical staff for their important contributions to the GOOD EYE study. The GOOD EYE Study Investigators and participating institutions are listed in Appendix.
References
- 1. Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, et al.. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 2. McLaughlin VV, Sitbon O, Badesch DB, Barst RJ, Black C, Galie N, et al.. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J 2005; 25: 244–249. [DOI] [PubMed] [Google Scholar]
- 3. Galie N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, et al.. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2005; 46: 529–535. [DOI] [PubMed] [Google Scholar]
- 4. Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al.. Ambrisentan for the treatment of pulmonary arterial hypertension: Results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008; 117: 3010–3019. [DOI] [PubMed] [Google Scholar]
- 5. Sasayama S, Kunieda T, Tomoike H, Matsuzaki M, Shirato K, Kuriyama T, et al.. Effects of the endothelin receptor antagonist bosentan on hemodynamics, symptoms and functional capacity in Japanese patients with severe pulmonary hypertension. Circ J 2005; 69: 131–137. [DOI] [PubMed] [Google Scholar]
- 6. Sastry BK, Narasimhan C, Reddy NK, Raju BS.. Clinical efficacy of sildenafil in primary pulmonary hypertension: A randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol 2004; 43: 1149–1153. [DOI] [PubMed] [Google Scholar]
- 7. Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S.. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: Comparison with inhaled nitric oxide. Circulation 2002; 105: 2398–2403. [DOI] [PubMed] [Google Scholar]
- 8. Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al.. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 9. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al.. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 10. Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J.. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J 2005; 26: 858–863. [DOI] [PubMed] [Google Scholar]
- 11. Enright PL, Sherrill DL.. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158: 1384–1387. [DOI] [PubMed] [Google Scholar]
- 12. Savarese G, Paolillo S, Costanzo P, D’Amore C, Cecere M, Losco T, et al.. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension?: A meta-analysis of 22 randomized trials. J Am Coll Cardiol 2012; 60: 1192–1201. [DOI] [PubMed] [Google Scholar]
- 13. Arena R, Lavie CJ, Milani RV, Myers J, Guazzi M.. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: An evidence-based review. J Heart Lung Transplant 2010; 29: 159–173. [DOI] [PubMed] [Google Scholar]
- 14. Triantafyllidi H, Kontsas K, Trivilou P, Orfanos SE, Lekakis J, Kremastinos D, et al.. The importance of cardiopulmonary exercise testing in the diagnosis, prognosis and monitoring of patients with pulmonary arterial hypertension. Hellenic J Cardiol 2010; 51: 245–249. [PubMed] [Google Scholar]
- 15. Wensel R, Opitz CF, Anker SD, Winkler J, Hoffken G, Kleber FX, et al.. Assessment of survival in patients with primary pulmonary hypertension: Importance of cardiopulmonary exercise testing. Circulation 2002; 106: 319–324. [DOI] [PubMed] [Google Scholar]
- 16. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al.. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 17. Channick RN.. Combination therapy in pulmonary arterial hypertension. Am J Cardiol 2013; 111: 16C–20C. [DOI] [PubMed] [Google Scholar]
- 18. Ogawa A, Matsubara H.. How long can we leave patients with pulmonary arterial hypertension on oral drug monotreatment? Circ J 2012; 76: 1089–1090. [DOI] [PubMed] [Google Scholar]
- 19. Zhu B, Wang L, Sun L, Cao R.. Combination therapy improves exercise capacity and reduces risk of clinical worsening in patients with pulmonary arterial hypertension: A meta-analysis. J Cardiovasc Pharmacol 2012; 60: 342–346. [DOI] [PubMed] [Google Scholar]
- 20. Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al.. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54: S43–S54. [DOI] [PubMed] [Google Scholar]
- 21. Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al.. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 22. Ross RM.. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167: 1451; author reply 1451. [DOI] [PubMed] [Google Scholar]
- 23. D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al.. Survival in patients with primary pulmonary hypertension: Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 24. Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, Beltran M, et al.. Survival in primary pulmonary hypertension: Validation of a prognostic equation. Circulation 1994; 89: 1733–1744. [DOI] [PubMed] [Google Scholar]
- 25. Wensel R, Francis DP, Meyer FJ, Opitz CF, Bruch L, Halank M, et al.. Incremental prognostic value of cardiopulmonary exercise testing and resting haemodynamics in pulmonary arterial hypertension. Int J Cardiol 2013; 167: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 26. Iversen K, Jensen AS, Jensen TV, Vejlstrup NG, Sondergaard L.. Combination therapy with bosentan and sildenafil in Eisenmenger syndrome: A randomized, placebo-controlled, double-blinded trial. Eur Heart J 2010; 31: 1124–1131. [DOI] [PubMed] [Google Scholar]
- 27. Manes A, Palazzini M, Leci E, Bacchi Reggiani ML, Branzi A, Galie N.. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: A comparison between clinical subgroups. Eur Heart J 2014; 35: 716–724. [DOI] [PubMed] [Google Scholar]
- 28. Okano Y, Yoshioka T, Shimouchi A, Satoh T, Kunieda T.. Orally active prostacyclin analogue in primary pulmonary hypertension. Lancet 1997; 349: 1365. [DOI] [PubMed] [Google Scholar]
- 29. Nagaya N, Uematsu M, Okano Y, Satoh T, Kyotani S, Sakamaki F, et al.. Effect of orally active prostacyclin analogue on survival of outpatients with primary pulmonary hypertension. J Am Coll Cardiol 1999; 34: 1188–1192. [DOI] [PubMed] [Google Scholar]
- 30. Galie N, Manes A, Branzi A.. Prostanoids for pulmonary arterial hypertension. Am J Respir Med 2003; 2: 123–137. [DOI] [PubMed] [Google Scholar]
- 31. Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, et al.. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2003; 41: 2119–2125. [DOI] [PubMed] [Google Scholar]
- 32. Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galie N, et al.. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 33. Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al.. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296–301. [DOI] [PubMed] [Google Scholar]
- 34. McLaughlin V, Channick RN, Ghofrani HA, Lemarie JC, Naeije R, Packer M, et al.. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015; 46: 405–413. [DOI] [PubMed] [Google Scholar]
- 35. Channick RN, Delcroix M, Ghofrani HA, Hunsche E, Jansa P, Le Brun FO, et al.. Effect of macitentan on hospitalizations: Results from the SERAPHIN trial. JACC Heart Fail 2015; 3: 1–8. [DOI] [PubMed] [Google Scholar]
- 36. Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al.. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 37. Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, et al.. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: A randomized, controlled trial. Circulation 2013; 127: 624–633. [DOI] [PubMed] [Google Scholar]
- 38. Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, et al.. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. [DOI] [PubMed] [Google Scholar]
- 39. Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, et al.. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006; 114: 1482–1489. [DOI] [PubMed] [Google Scholar]