Abstract
Purpose
Androgen deprivation therapy (ADT) is a treatment used in men with prostate cancer (PCa); however it is responsible for many adverse effects, with negative impact on quality of life. ADT causes loss of bone mineral density (BMD) and skeletal muscle mass, alteration of body composition, and cognitive function, which altogether lead to increased risk of accidental falls and fractures. This systematic review analyses the effectiveness of physical exercise (PE) in preventing accidental falls and fractures and reducing the loss of BMD in men with PCa receiving ADT.
Methods
We searched MEDLINE, EMBASE, CINAHL, and the Cochrane Library for articles between database inception and September 2, 2020. Eligible studies included randomized controlled trials (RCTs) investigating the effects of exercise on bone health in men with PCa receiving ADT.
Results
Nine RCTs were included. Experimental PE consisted in multicomponent programmes that involved aerobic, resistance, impact-loading exercise, and football training. None of the RCTs investigated the risk of accidental falls and fractures, while two trials reported beneficial effects of PE on lumbar spine, hip, and femoral shaft BMD. No further significant difference was detected in the outcomes investigated.
Conclusion
Evidence of the effectiveness of PE to prevent the risk of accidental falls and fractures and BMD loss is lacking. Nevertheless, clinical guidelines recommend PE as a part of the clinical management of men with PCa receiving ADT due to its known numerous health benefits. Research should focus on PE strategies to prevent accidental falls, a clinically relevant outcome in this vulnerable population.
Trial registration
The study protocol was registered with International Prospective Register of Systematic Reviews (PROSPERO, number CRD 42020158444) on 04/28/2020.
Electronic supplementary material
The online version of this article (10.1007/s00520-020-05830-1) contains supplementary material, which is available to authorized users.
Keywords: Prostatic neoplasms; Exercise; Accidental falls; Fractures, bone; Physical therapy modalities; Prevention
Introduction
Prostate cancer (PCa) is the most prevalent cancer among men worldwide, with 3.724.658 cases in 2018 [1].
Androgen deprivation therapy (ADT) is currently the standard systemic treatment in patients with metastatic or more aggressive PCa [2]. Often, ADT is used in combination with radiotherapy for localized advanced PCa with the aim to increase survival and control disease progression [2].
However, ADT is responsible for many adverse effects, with negative impact on quality of life [3]. Apart from the increased risk for cardiovascular events and metabolic syndrome [4, 5], ADT also alters the body composition, with loss in skeletal muscle mass that leads to a decrease in muscle strength [6, 7]. Also, patients on ADT manifest significant loss of bone mineral density (BMD), which occurs especially within the first year of treatment [8] and is associated with higher osteoporosis rates and risk of fractures [9]. Moreover, ADT seems responsible for cognitive dysfunction, although this finding has not been completely clarified and needs further investigation [10].
It is known that both reduction in muscle strength and cognitive dysfunction are predictors of higher fall rates and hospitalization in older adults [11, 12]. Thus, considering the loss of BMD, altogether these side effects of ADT explain the increased risk of accidental falls and fractures in this population [13].
In elderly adults, physical exercise (PE) has been proposed in different modalities as a strategy to produce several health benefits [14]. Recent guidelines addressing elderly adults recommend multicomponent exercise programmes, including resistance and neuromotor exercises, as a strategy to reduce the risk of accidental falls [14] as PE can prevent osteoporosis and improves body composition, muscle strength, and cognitive function [14]. Moderate-vigorous intensity programmes that include balance exercises seem to be particularly effective to reduce the risk of accidental falls [14].
Initial evidence indicates that in patients with cancer, PE may produce numerous benefits on physical performance, quality of life, and cancer-related fatigue [15]. In patients with PCa receiving ADT, exercise is beneficial to body composition, muscle strength, and physical performance [16–18], while its effects on bone health, cardiometabolic risk, quality of life, and cognitive functions remain uncertain [19, 20].
Patients receiving ADT are more exposed to the risk of accidental falls and fractures [9] due to the side effects of this drugs. As PE is recommended in healthy elderly adults to prevent these risks and also to prevent bone loss, we hypothesized that PE could be effective in preventing accidental falls and fractures even in men with PCa receiving ADT. Furthermore, recent evidence suggests PE as strategy to prevent osteoporosis in men receiving ADT when associated with pharmacological therapy [21]. Thus, we conducted this systematic review to search for evidence of the effectiveness of exercise on bone health in this population. Specifically, we searched for randomized controlled trials that implemented PE programmes to prevent accidental falls and fractures and/or to prevent the loss of BMD, in patients with PCa treated with ADT.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [22]. The study protocol was registered with International Prospective Register of Systematic Reviews (PROSPERO, number CRD 42020158444).
Search strategy and selection criteria
A systematic review of the literature was performed through sequential, individualized searches in MEDLINE, EMBASE, CINAHL, and the Cochrane Library. We searched for studies published up to September 2, 2020, without filters for study design or language. The search terms and strategies used are reported in online resource 1. Duplicates were removed in EndNote (version X7.5). Also, we performed a manual search in the reference lists of the studies included in this review to find any other relevant citation that may have been missed by the electronic search.
We included randomized controlled trials (RCTs) investigating the effects of supervised or unsupervised exercise on bone health in adult individuals with PCa receiving ADT.
Studies were eligible if the experimental intervention consisted of structured PE programmes compared with standard care or placebo active control. When exercise was associated with dietary supplements, studies were included if the exercise was clearly the predominant part of the experimental intervention. Furthermore, studies were eligible if they investigated the number of accidental falls or fractures that occurred in a specific timeframe or if they reported data on bone density by dual-energy X-ray absorptiometry (DEXA). Studies focusing on generalized advice and education on the benefit of exercise or studies that collected data on pathological (and not accidental) fractures were excluded.
Data analysis
Two investigators (B.B., M.C.) screened the title and abstract of all the citations retrieved to check their appropriateness related to the purpose of this review. The investigators also retrieved and checked for eligibility the full texts of studies deemed appropriate. Then, two investigators (B.B., S.C.) assessed the eligible studies for their methodological quality according to the Cochrane risk-of-bias tool [23]. In the whole process, any disagreement was resolved by discussion and consensus.
Two investigators (B.B., M.C.) extracted the following data from studies included: authors, year and country, sample size and average age, exclusion criteria, bone outcome measures collected and follow-up duration, general characteristics of the experimental intervention and standard care, drop-out rate. When essential data were missing, the investigators requested them from authors (at least three attempts).
Results
Bibliographic search results
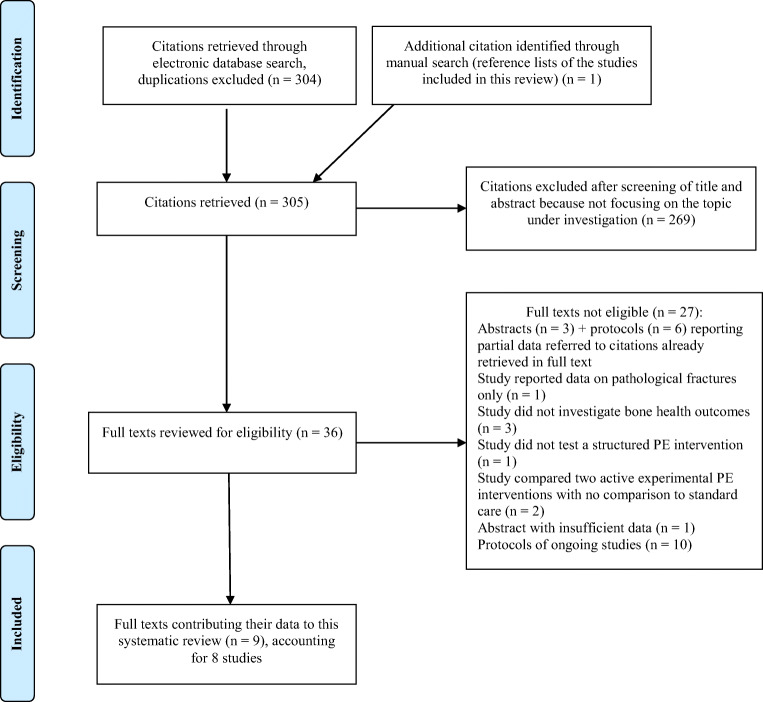
The electronic search yielded 304 citations, duplications excluded. One more citation was retrieved through the manual search, for a total of 305. According to the screening of title and abstract, 269 citations were excluded because they did not focus on the topic under investigation.
Thirty-six full texts were reviewed for eligibility, 27 of which were excluded for the following reasons: three conference abstracts and six study protocols referred to published full texts already retrieved [24–31]; four studies did not meet inclusion criteria with respect to the outcome, as one measured only pathological fractures [32], and the others did not report data on bone health [31, 33, 34]; one study did not test a structured physical exercise intervention, focusing instead on patient education [35]; two studies compared different structured physical exercise interventions, without comparison to standard care [36, 37]. Finally, eleven studies were also excluded since they reported insufficient data for analysis [38] or were protocols of ongoing studies [39–48]. We contacted the corresponding authors in order to obtain preliminary results (minimum three attempts), but the ones who replied said they had no data to share yet.
Thus, nine published full texts met the inclusion criteria and contributed their data to this review [24–30, 49, 50]. These full texts accounted for eight study designs, as the two by Uth et al. [28, 29] reported data collected at the 3- and 8-month follow-up, respectively, of the same study design and sample (Fig. 1). Of note, the study by Bjerre et al. [30] included patients with PCa regardless of their treatment with ADT. However, they reported specific data for the subgroup of patients on ADT and these data were considered in this review.
Fig. 1.
PRISMA flow chart of search and study selection process
Risk of bias of the included studies
The Cochrane risk-of-bias analysis of the included studies is reported in Fig. 2. Two studies did not report sufficient information to assess the adequacy of the random sequence generation [28, 29, 49, 51]. Due to the nature of the intervention, seven of the nine included studies did not provide blinding to group assignment for both participants and personnel [24–30, 49]. Moreover, four studies did not report enough information to judge blinding of outcome [24–26, 49]. Nevertheless, all the included studies were judged at low risk of detection bias since outcome measures were frequently objective. The analytical assessment of the risk of bias for each study included is reported in online resource 2.
Fig. 2.
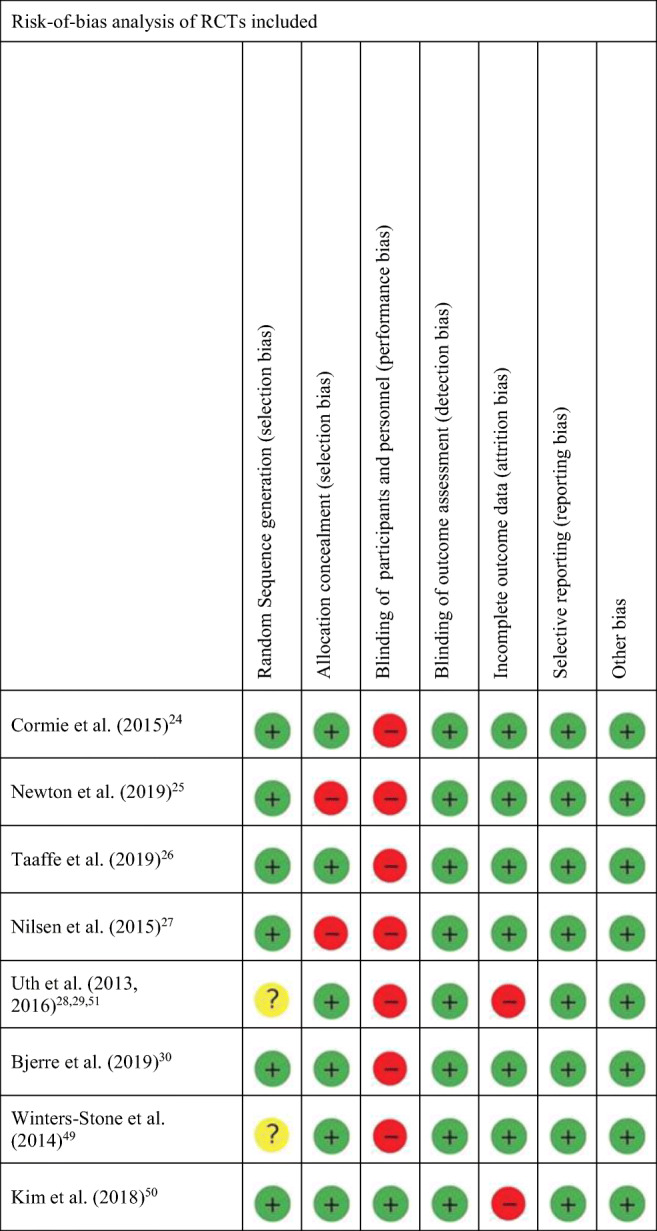
Risk-of-bias analysis of RCTs included
Study characteristics
Table 1 summarizes the characteristics of the studies included in this review. All were RCTs published in the last decade in different continents [24–30, 49, 50]. All studies allowed for the recruitment of patients treated at several specialized hospitals.
Table 1.
Characteristics of included studies
| First author (year of publication) | Country | Population | Exclusion criteria | Age (mean ± SD years) | Follow-up (months) | Drop-out rate | Bone outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EG | CG | Tot. | Tot. | EG | CG | ||||||
| Cormie et al. (2015) [24] | Australia | 32 | 31 | 63 |
Restriction to physical exercise based on clinicians’ judgement Musculoskeletal, cardiovascular, neurological disorders Prior exposure to ADT Bone metastasis |
68.3* | 69.6 (6.5) | 67.1 (6.5) | 3 | 13% | aBMD of whole body, lumbar spine (L2-L4) femoral neck |
| Newton et al. (2019) [25] | Australia | 57 (RE+IE) | 47 | 154 |
Restriction to physical exercise based on clinicians’ judgement Musculoskeletal, cardiovascular, neurological disorders Current regular resistance exercise Medication that affect bone metabolism Bone metastasis |
69.0 (9.0) | 68.7 (9.3) | 69.1 (8.4) | 6 | 19% | BMD of whole body, total hip, lumbar spine (L2-L4), femoral neck, trochanter |
| 50 (AE+RE) | 69.1 (9.4) | 6 | 14% | ||||||||
| Taaffe et al. (2019) [26] | Australia | 54 | 50 | 104 |
Restriction to physical exercise based on clinicians’ judgement Musculoskeletal, cardiovascular, neurological disorders Current regular resistance or aerobic exercise Prior exposure to ADT Osteoporosis Medication that affect bone metabolism Metastatic disease |
68.2* | 69.0 (6.3) | 67.5 (7.7) | 6 | 18% | BMD of whole body, total hip, lumbar spine |
| Nilsen et al. (2015) [27] | Norway | 28 | 30 | 58 |
Restriction to physical exercise based on clinicians’ judgement Musculoskeletal, cardiovascular, neurological disorders Current regular resistance exercise Medication that affect bone metabolism |
66.0 | 66.0 (6.6) | 66.0 (5.0) | 4 | 16% | aBMD of whole body, total hip, total lumbar spine, femoral neck, trochanter |
| Uth et al. (2016) [28] | Denmark | 29 | 28 | 57 |
Musculoskeletal, cardiovascular, neurological disorders Current regular resistance exercise Current chemotherapy Osteoporosis Other cancers |
67.0 | 67.1 (7.1) | 66.5 (4.9) | 3 | 14% | aBMD of whole body, legs, total hip, lumbar spine, femoral neck, femoral shaft |
| Uth et al. (2016) [29] | 8 | 28% | |||||||||
| Bjerre et al. (2019) [30] | Denmark | 46 | 41 | 87 |
Restriction to physical exercise based on clinicians’ judgement Osteoporosis |
NR | NR | NR | 6 | NR | BMD of whole body, total hip, lumbar spine, femoral neck |
| Winters-Stone et al. (2014) [49] | USA | 29 | 22 | 51 |
Restriction to physical exercise based on clinicians’ judgement Current regular resistance exercise Current chemotherapy Osteoporosis Medication that affect bone metabolism Bone metastasis |
70.2 | 69.9 (9.3) | 70.5 (7.8) | 12 | 29% | BMD of total hip, lumbar spine (L1-L4), femoral neck, greater trochanter |
| Kim et al. (2018) [50] | South Korea | 26 | 25 | 51 |
Restriction to physical exercise based on clinicians’ judgement Contraindications to unsupervised exercise Current regular resistance exercise Osteoporosis Medication that affect bone metabolism Bone metastasis Other cancers |
70.8 | 70.5 (5.0) | 71.0 (5.5) | 6 | 20% | BMD of total hip, lumbar spine (L1-L4), femoral neck |
aBMD areal bone mineral density, ADT androgen deprivation therapy, AE aerobic exercise, BMD bone mineral density, CG control group, EG experimental group, IE impact-loading exercise, NR not reported for the subgroup that received ADT, RE resistance exercise, SD standard deviation
*Estimated values
One study was a three-armed RCT comparing two active interventions with one control [25]. For the purposes of this review, we considered both the comparisons. Two studies were cross-over designs, and, for this review, we considered data of the first follow-up, before the cross-over, which was scheduled at 6 months for both studies [25, 26].
A high drop-out rate (≥ 28%) was registered in two studies [29, 49] and equal to 20% in a further one [50], whereas one study did not report this data point for the subgroup of patients receiving ADT [30]. The safety of interventions was assessed by recording the number and type of adverse events. Only two trials reported adverse events related to exercise [27, 28]: one partial Achilles tendon rupture [28], two fibula fractures [28], and five minor musculoskeletal injuries [27, 28]. Other trials registered generic health issues not related to the PE intervention that occurred both in the experimental and in the control group, such as hospitalization (n = 22) [25–27, 29, 49, 50], injury/accident (n = 10) [25–27], death (n = 4) [25, 49], and others (n = 4) [24, 26–29]. Bjerre et al. [30] analysed safety outcomes as number of falls, fractures, and serious adverse events occurring even in the subgroup of patients receiving ADT (respectively n = 5, n = 1, and n = 4), without any further detailed classification.
Participants
The sample size of the eight RCTs selected in this review ranged from 51 to 154 individuals, for a total of 625 participants, of whom 351 were randomized to receive experimental PE and 274 were randomized to receive standard care. The sample was made up of males aged from 66.0 to 70.8 years with local or metastatic PCa receiving ADT.
All the study designs excluded patients with restrictions to PE based on specific assessments (e.g., inability to walk 400 m [24–26], VO2 max < 35 ml/kg/min [28, 29], pain in the metastatic site associated to activity [28, 29]) or based on clinicians’ judgement [24–27, 30, 49, 50]. Five studies also excluded patients with musculoskeletal, cardiovascular, and neurological disorders that could inhibit them from exercising [24–29] or patients with contraindications to unsupervised exercise [50]. Further exclusion criteria have been summarized in detail in Table 1.
Characteristics of control group intervention and the experimental group intervention
Table 2 summarizes the characteristics of standard care and experimental PE programmes.
Table 2.
Characteristics of study intervention programmes
| First author (year of publication) | Sample | Duration of intervention (months) | EG Component | Experimental PE programme | Frequency (times/weeks) | Session duration (min) | Modality | CG | |
|---|---|---|---|---|---|---|---|---|---|
| Group | Single | ||||||||
| Cormie et al. (2015) [24] |
EG: 32 CG: 31 |
3 | AE+RE |
Supervised: AE: 20–30 min at 70–85% max HR RE: 6–12 RM, 1–4 sets, reps NR Progression: intensity and volume |
2 | 60 | x | Standard care | |
| Newton et al. (2019) [25] |
EG: 57 CG: 47 |
6 | RE+IE |
Supervised: RE: 6–12 RM, 2–4 sets, reps NR Progression: Intensity and volume IE: 3–5 times BW, 2–4 sets, 10 reps. Progression: Set and type of exercise |
2 | 60 | x | Printed booklet with information about exercise | |
|
Unsupervised: IE: two to four rotations of skipping (30 s), hopping, leaping, and drop jumping (all 10 times) |
2 | 60 | x | ||||||
|
EG: 50 CG: 47 |
6 | AE+RE |
Supervised: RE: 6–12 RM, 2–4 sets, reps NR Progression: Intensity and volume AE: 20–30 min at 60–85% max HR |
2 | 60 | x | |||
| Taaffe et al. (2019) [26] |
EG: 54 CG: 50 |
6 | AE+RE+IE |
Supervised: AE: 25–40 min of 60–85% max HR RE: 6–12 RM, 2–4 sets, reps NR Progression: intensity and volume IE: 3·4–5·2 times BW, 2–4 sets, 10 reps Progression: set and type of exercise |
3 | 60 | x | Standard care | |
| Nilsen et al. (2015) [27] |
EG: 28 CG: 30 |
4 | RE |
Supervised: RE: 6–10 RM, 1–3 sets, 10 reps. Progression: volume |
2 | 60 | x | Encouraged to maintain their habitual physical activity level and not to initiate strength training | |
|
Unsupervised: Home-based exercise session similar to supervised sessions performed at a moderate intensity |
1 | 60 | x | x | |||||
| Uth et al. (2016) [28] |
EG: 29 CG: 28 |
3 | FT |
Supervised: 15 min warm-up (drills, balance, strength exercise) 2–3 sets × 15 min Progression: set and frequency |
2–3 | 45–60 | x | Encouraged to maintain their habitual physical activity level | |
| Uth et al. (2016) [29] | 8 | ||||||||
| Bjerre et al. (2019) [30] |
EG: 46 CG: 41 |
6 | FT |
Supervised: 20 min warm-up 20 min dribbling, passing, and shooting 20 min of 5–7-a-side football Intensity and progression are NR |
2 | 60 | x | 15/30-min telephone session covering options for physical activity and free-of-charge rehabilitation delivery by the municipalities and subsequently an email with the same information | |
| Winters-Stone et al. (2014) [49] |
EG: 29 CG: 22 |
12 | RE+IE |
Supervised: RE upper body: 8–15 RM, 1–2 sets, 8–14 reps. RE lower body: 0–15% BW, sets NR, 8–12 reps IE: 0–10% BW, 1–10 sets, 10 reps Progression: Intensity and volume |
2 | 60 | x | Performed a series of whole body stretching and relaxation exercises | |
|
Unsupervised: Home-based exercise similar to class session but performed without weighted vests and replacing weights with resistance bands |
1 | 60 | x | ||||||
| Kim et al. (2018) [50] |
EG: 26 CG: 25 |
6 | AE+RE |
Unsupervised: Core program: Weight-bearing + RE RE: 0–10% BW, 2–3 sets, 8–15 reps. IE: weight-bearing exercise 11–15 RPE, 3–5 sets, 20–30 min Progression: intensity, volume, and type of exercise Optional programme: stabilization/balance exercise + Circuit Resistive Calisthenics (same dose as core program) Stabilization/balance exercise: intensity and volume NR |
2–5 | NR | NR |
Stretching 3–5 times/week (a total of 9 movements); Ten-minute telephone sessions (weekly for the first month and at monthly intervals thereafter) |
|
AE aerobic exercise, BW body weight, CG control group, EG experimental group, FT football training, HR heart rate, IE impact-loading exercise, NR not reported, PE physical exercise, RE resistance exercise, Reps repetitions, RM repetition maximum, RPE rate of perceived exertion, S seconds
The control group intervention consisted of stretching activities [49, 50] or educational material [25, 30, 50] or simply in encouraging patients to maintain their usual level of physical activity [27–29]. Taaffe et al. [26] provided all participants with standard daily supplementation of calcium (1000 mg/day) and vitamin D3 (800 IU/day).
Experimental PE interventions were characterized by multicomponent programmes [24–26, 28–30, 49, 50], with only one exception that implemented a single component of PE [27]. In most cases, PE consisted in aerobic exercise (AE) that could also be performed as weight-bearing activities and that was associated with resistance exercise (RE) [24–26, 50] and with impact-loading exercise (IE) [26]. Two study designs implemented football training (FT) as experimental PE [28–30]; although the full texts did not report this type of training in detail, it is likely that, by its nature, it included AE and IE, among others (e.g., RE, stretching).
Most experimental interventions were performed in 1-hour sessions repeated two or three times a week [24–30, 49]. Most of the studies described how the PE components were progressively modulated in terms of intensity, volume, and type of exercise [24–27, 49, 50]. Although the intensity of FT was not defined, this type of intervention was implemented through an initial warm-up followed by 2 matches lasting 15/20 min [28–30]. Uth et al. [28, 29] progressively increased the number of matches and the frequency of sessions per week.
Experimental PE were implemented as supervised exercise in clinics [24–26] or in sports facilities [28–30], as a combination of supervised and unsupervised (home-based) sessions [25, 27, 49], or as unsupervised home-based PE only [50].
Bone outcomes
Accidental falls and fractures
None of the studies selected for this review was designed to analyse the risk of accidental falls and/or fractures as an outcome measure of the effectiveness of experimental PE in reducing those risks. All study designs measured adverse events [24–30, 49, 50]. The two studies that tested FT also recorded fractures occurring during this kind of intervention to judge its safety due to the increased risk of collisions with other players and falls [28–30]. Although this was not the outcome we were interested in, we underline that all the experimented PE programmes were deemed safe [24–30, 49, 50].
Of note, several studies secondarily collected data on physical function through heterogeneous tests (e.g., Flamingo balance test, sit-to-stand test, etc.) [24, 27–29, 50]. The proof of effectiveness of experimental PE was demonstrated through the sit-to-stand test [24, 27, 50], which is valid to measure muscle power of the lower limbs; its validity in predicting accidental falls and fractures in patients with cancer, however, must still be demonstrated [52].
BMD
Table 3 reports the results of between-group comparisons of BMD at the various anatomical sites.
Table 3.
Between-group comparisons for BMD
| Follow-up (months) | BMD outcome | Mean change between groups (g/cm2) | 95% CI | p value | |
|---|---|---|---|---|---|
| Cormie et al. (2015) [24] | 3 | Whole body | − 0.002* | − 0.013 to 0.009 | 0.692 |
| Lumbar spine | − 0.009* | − 0.029 to 0.012 | 0.410 | ||
| Femoral neck | 0.000* | − 0.025 to 0.024 | 0.987 | ||
| Newton et al. (2019) [25] | 6 (RE+IE) | Whole body | 0.005* | − 0.002 to 0.011 | 0.174 |
| Total hip | 0.007* | − 0.002 to 0.016 | 0.128 | ||
| Lumbar spine | 0.014* | 0.001 to 0.027 | 0.039 | ||
| Femoral neck | 0.010* | 0.000 to 0.020 | 0.050 | ||
| Trochanter | − 0.003* | − 0.010 to 0.004 | 0.449 | ||
| 6 (AE+RE) | Whole body | 0.003* | − 0.007 to 0.0012 | 0.614 | |
| Total hip | 0.001* | − 0.009 to 0.011 | 0.807 | ||
| Lumbar spine | 0.004* | − 0.009 to 0.017 | 0.525 | ||
| Femoral neck | − 0.003* | − 0.014 to 0.008 | 0.571 | ||
| Trochanter | − 0.002* | − 0.01 to 0.007 | 0.699 | ||
| Taaffe et al. (2019) [26] | 6 | Whole body | NR | NR | 0.827 |
| Total hip | NR | NR | 0.848 | ||
| Lumbar spine | NR | NR | 0.111 | ||
| Nilsen et al. (2015) [27] | 4 | Whole body | 0.00* | − 0.02 to 0.01 | 0.520 |
| Total hip | 0.00* | − 0.01 to 0.01 | 0.690 | ||
| Total lumbar spine | 0.00* | − 0.02 to 0.01 | 0.847 | ||
| Femoral neck | 0.00* | − 0.02 to 0.01 | 0.467 | ||
| Trochanter | 0.00* | − 0.01 to 0.00 | 0.221 | ||
| Uth et al. (2016) [28] | 3 | Whole body | 0.01 | − 0.00 to 0.01 | 0.188 |
| Legs | 0.00 | − 0.00 to 0.01 | 0.336 | ||
| Uth et al. (2016) [29] | 8 | Total hip | R: 0.015 | 0.003 to 0.027 | 0.015 |
| L: 0.017 | 0.002 to 0.032 | 0.030 | |||
| Lumbar spine | 0.028 | − 0.010 to 0.065 | 0.144 | ||
| Femoral neck | R: 0.015 | − 0.002 to 0.031 | 0.078 | ||
| L: 0.015 | − 0.01 to 0.032 | 0.072 | |||
| Femoral shaft | R: 0.018 | 0.004 to 0.032 | 0.016 | ||
| L: 0.024 | 0·005 to 0·044 | 0.015 | |||
| Bjerre et al. (2019) [30] | 6 | Whole body | 0.005 | − 0.007 to 0.017 | 0.40 |
| Total hip | − 0.009* | − 0.033 to 0.014 | 0.43 | ||
| Lumbar spine | 0.0017* | − 0.019 to 0.053 | 0.34 | ||
| Femoral neck | 0.007* | − 0.009 to 0.023 | 0.39 | ||
| Winters-Stone et al. (2014) [49] | 12 | Total hip | NR | NR | 0.37 |
| Lumbar spine | NR | NR | 0.47 | ||
| Femoral neck | NR | NR | 0.77 | ||
| Greater trochanter | NR | NR | 0.58 | ||
| Kim et al. (2018) [50] | 6 | Total hip | NR | NR | 0.727 |
| Lumbar spine | NR | NR | 0.756 | ||
| Femoral neck | NR | NR | 0.888 |
AE aerobic exercise, BMD bone mineral density, CI confidence intervals, IE impact-loading exercise, L left, NR not reported, R right, RE resistance exercise
*Analyses adjusted for baseline values
All the RCTs selected for this review reported data on bone density measured by DEXA at different anatomical sites (Table 3). Lumbar spine BMD was collected in all the included studies, while femoral neck BMD was analysed in seven of them [24, 25, 27, 29, 30, 49, 50]. At the 6-month follow-up, Newton et al. [25] recorded a significant difference between groups for lumbar spine BMD (mean change 0.014 g/cm2, 95% CI 0.001–0.027, p = 0.039) and a positive trend for femoral neck BMD (mean change 0.010 g/cm2, 95% CI 0.000–0.020, p = 0.050), in favour of the experimental resistance and impact-loading PE compared with control group. No further significant difference was detected by any of the studies in these outcome measures. Of note, a per-protocol analysis performed by Winters-Stone et al. [49] at the level of single lumbar vertebra reported a significant difference in BMD only for L4 (p = 0.03), in favour of experimental PE.
Total hip BMD was measured by seven study designs [25–27, 29, 30, 49, 50], with significant differences recorded only by Uth et al. [29] on both hips at the 8-month follow-up (right 0.015 g/cm2, 95% CI 0.003–0.027, p = 0.015; left 0.017 g/cm2, 95% CI 0.002–0.032, p = 0.030). This study was the only one that collected data on BMD at the femoral shaft of both legs, recording a difference in favour of experimental PE on both sides (right 0.018 g/cm2, 95% CI 0.004–0.032, p = 0.016; left 0.024 g/cm2, 95% CI 0.005–0.044, p = 0.015) [29].
No further statistically significant difference was registered for BMD at any further anatomical site examined, such as whole body [24–28, 30], trochanter [25, 27, 49], or legs [28].
Considering the almost total absence of data in favour of PE with respect to this outcome, which was collected in various anatomical sites, we deemed it inappropriate to carry out a meta-analysis.
Further results: bone turnover markers
Six study designs also assessed several bone turnover markers (BTMs) such as markers of bone formation (alkaline phosphatase, procollagen type 1 amino-terminal propeptide and osteocalcin) or markers of bone resorption (C-terminal telopeptide of type I collagen, N-terminal telopeptide of type I collagen) [24–26, 28, 29, 49, 50].
At the 3-month follow-up, Uth et al. [28, 29] registered a statistically significant difference in favour of experimental PE for the markers of bone formation procollagen type 1 amino-terminal propeptide (36.6 μg/L, 95% CI 10.4–62.8, p = 0.008) and osteocalcin (8.6 μg/L, 95% CI 3.3–13.8, p = 0.002), but this result was not confirmed at the subsequent 8-month follow-up.
Discussion
The aim of this systematic review was to summarize the evidence regarding the effectiveness of PE programmes in reducing the risk of accidental falls and fractures, as a clinically relevant outcome in the PCa patient population.
Despite the strong existing evidence proving accelerated bone loss, additional muscle weakness, and cognitive dysfunction caused by ADT [7, 10] and the benefits of PE in reducing the risk of accidental falls in the healthy elderly population [14], no study has investigated the effects of exercise on these clinically relevant endpoints in PCa patients.
Furthermore, the effectiveness of PE in lessening or preventing the loss of BMD in this population is still uncertain due to the inconsistent results yielded by this systematic review. In particular, only two studies suggested that multicomponent PE, in particular resistance and impact-loading exercise or football training, may help achieve this outcome [25, 29]. These positive results could be explained by the longer period of training [25], as a minimum of 6–8 months is required to achieve bone remodelling [53], or by the high number of accelerations and decelerations and change of direction typically observed during the football training [29]. These characteristics of the PE intervention could provide sufficient bone-loading forces and osteogenic stimulus. However, both these study designs were affected by a certain degree of risk of bias. Therefore, although BMD was measured objectively, their results should be interpreted with caution.
Thus, to date, evidence of the beneficial effects of PE on bone health in men with PCa treated with ADT is still lacking, even though PE is beneficial in the healthy elderly for the same outcome [14].
The incidence of osteoporotic fractures increases with age, and it is estimated that more than 8.9 million osteoporotic fractures occur annually worldwide [54, 55]. In men over the age of 75, the most frequent site of fracture is the hip, the principal risk factor being low BMD [56]. Moreover, more than 30% of community-dwelling older adults over the age of 75 fall every year [57], leading to fractures, hospitalizations, and admission to nursing homes [58].
Therefore, the risk of falls in older adults increases morbidity, mortality, and financial burden for societies [59]. Indeed, the costs associated with fragility fractures account for €37 billion/year in Europe and are expected to increase [54], whereas in the USA, the overall number of healthy years of life lost (DALY) due to hip fractures is roughly 17,660 [60].
Considering the progressive aging of the population, recent guidelines suggest that future research should identify individuals at increased risk of fracture, to whom fracture prevention strategies should be targeted [61] in order to contain the increase in costs associated with this event [62]. We think that patients with PCa receiving ADT are among those individuals because the side effects they have affect bone and lead to double the healthcare cost per person [63]. Moreover, as fall prevention programmes are recommended to the elderly in general, as community-dwelling adults with cancer have greater accidental fall rates than do healthy elderly individuals and as patients undergoing active treatment are even more at risk [64], it seems logical to expect that the beneficial effects of PE would be greater in patients with PCa receiving ADT compared with healthy elderly individuals.
However, this systematic review demonstrated an almost complete lack of studies supporting this. Not only have clinically relevant outcomes never been investigated but also few of the RCTs included were powered to detect the effects of PE on BMD [25, 26, 49, 50].
We must say, however, that our review was limited to collecting evidence on PE. As we did not consider other types of interventions, such as nutritional and educational programmes that could help to prevent accidental falls and fractures, we cannot rule out that an evidence-based intervention different from stand-alone PE could be successfully applied. Furthermore, ten protocols of ongoing studies were retrieved by our search strategy; it is therefore very likely that in the next few years the conclusion drawn today, thanks to this extensive review conducted with rigorous methodology, will be outdated.
A final consideration regards the type of PE programmes tested in the studies included in this review: most combined different exercise modalities, such as resistance, weight-bearing endurance, and impact loading exercises, as recommended to provide benefits to bone health [53]. Moreover, PE programmes were of moderate-high intensity in all cases, suggesting that, according to the evidence and expertise, low-impact exercise may have no effect on BMD [53]. Despite this, the PE programmes did not produce the desired result on bone mass. This may be due to the insufficient power of some of the included studies, or it may be due to poor adherence to treatment, which is always an issue in studies involving lifestyle changes [65]. Poor adherence to treatment means that the expected dose of exercise is not achieved by participants. Thus, it can be difficult to determine the effect of the exercise on the outcomes of interest. It could also be that, however intense the programme, PE may not be sufficient to counteract the loss of bone mass induced by aging and ADT.
Thus, to conclude, experts recommend exercise as part of the treatment regimen of patients with cancer thanks to its large number of health benefits [19]. This review suggests that there is still no strong evidence to support this choice to prevent bone density loss in patients with PCa receiving ADT, according to recent literature [16, 21]. However, since exercise is an effective strategy to produce a large number of health benefits, future research should investigate the effects of PE to prevent the risk of accidental falls in this population, which is a clinically relevant outcome. For this purpose, PE should include coordination and balance exercises as well as muscle-strengthening activities. Evidence is needed regarding more precise training components, dose, and progression of exercise to prevent falls.
Electronic supplementary material
(DOCX 18 kb)
(DOCX 32 kb)
Acknowledgements
We thank Jacqueline M. Costa for the English language editing.
Author contributions
Barbara Bressi, Maribel Cagliari and Stefania Costi contributed to the conception and design of the work, literature search, selection of the trials, assessment of trial quality and data collection and contributed to the interpretation of data. They also drafted the work and approved the final version to be published. Massimiliano Contesini, Elisa Mazzini, Franco Antonio Mario Bergamaschi and Alfredo Moscato contributed to the conception and design of the work and to the interpretation of data. They revised the draft of the manuscript and approved the final version to be published. Maria Chiara Bassi contributed to the conception and design of work, the literature search and the selection of the trials and contributed to the interpretation of data. She also drafted the work and approved the final version to be published.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No ethical approval was required since we used only published data.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and material
No additional data available. Only published data were used. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. International Agency for Research on Cancer (2018) Cancer Today. https://gco.iarc.fr/today/home. Accessed 2 Sept 2020
- 2.Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D’Amico AV, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Kawachi MH, Kuettel M, Lee RJ, MacVicar GR, Malcolm AW, Miller D, Plimack ER, Pow-Sang JM, Richey S, Roach M, III, Rohren E, Rosenfeld S, Small EJ, Srinivas S, Stein C, Strope SA, Tward J, Walsh PC, Shead DA, Ho M. Prostate cancer, version 1.2014: Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:1471–1479. doi: 10.6004/jnccn.2013.0174. [DOI] [PubMed] [Google Scholar]
- 3.Alibhai SMH, Gogov S, Allibhai Z. Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: a systematic literature review. Crit Rev Oncol Hematol. 2006;60:201–215. doi: 10.1016/j.critrevonc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Carneiro A, Sasse AD, Wagner AA, Peixoto G, Kataguiri A, Neto AS, Bianco BAV, Chang P, Pompeo ACL, Tobias-Machado M. Cardiovascular events associated with androgen deprivation therapy in patients with prostate cancer: a systematic review and meta-analysis. World J Urol. 2014;33:1281–1289. doi: 10.1007/s00345-014-1439-6. [DOI] [PubMed] [Google Scholar]
- 5.Morote J, Gómez-Caamaño A, Alvarez-Ossorio JL, Pesqueira D, Tabernero A, Gómez Veiga F, Lorente JA, Porras M, Lobato JJ, Ribal MJ, Planas J, ANAMET Investigators Group José M Saladié, Gemma Sancho, Humberto Villavicencio, José Segarra, José Comet, José Francisco Suárez Dr., M José Ribal Dr., José Antonio Llorente Dr., Juan Uría Dr., Jesús Guajardo Dr., Antonio Gómez Caamaño Dr., Camilo García Freire Dr., Antonio Ojea Dr., Juan Mata Dr., Mª Luisa Vázquez Dr., Francisco Gómez Veiga Dr., Daniel Pesqueira Dr., Juan Pablo Ciria Dr., Roberto Llarena Dr., Jesús Miguel Unda Dr., Angel José Tabernero Dr., Angel Silmi Dr., Carlos Hernández Dr., Alfredo R The metabolic syndrome and its components in patients with prostate cancer on androgen deprivation therapy. J Urol. 2015;193:1963–1969. doi: 10.1016/j.juro.2014.12.086. [DOI] [PubMed] [Google Scholar]
- 6.Haseen F, Murray LJ, Cardwell CR, O’Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv. 2010;4:128–139. doi: 10.1007/s11764-009-0114-1. [DOI] [PubMed] [Google Scholar]
- 7.Galvão DA, Taaffe DR, Spry N, Joseph D, Turner D, Newton RU. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009;12:198–203. doi: 10.1038/pcan.2008.51. [DOI] [PubMed] [Google Scholar]
- 8.Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 9.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 10.McGinty HL, Phillips KM, Jim HSL, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014;22:2271–2280. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delbaere K, Kochan NA, Close JC, et al. Mild cognitive impairment as a predictor of falls in community-dwelling older people. Am J Geriatr Psychiatry. 2012;20:845–853. doi: 10.1097/JGP.0b013e31824afbc4. [DOI] [PubMed] [Google Scholar]
- 12.Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12:1–16. doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winters-Stone KM, Moe E, Graff JN, Dieckmann NF, Stoyles S, Borsch C, Alumkal JJ, Amling CL, Beer TM. Falls and frailty in prostate cancer survivors: current, past, and never users of androgen deprivation therapy. J Am Geriatr Soc. 2017;65:1414–1419. doi: 10.1111/jgs.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 15.Hasenoehrl T, Keilani M, Komandj S, et al. The effects of resistance exercise on physical performance and health-related quality of life in prostate cancer patients: a systematic review. Support Care Cancer. 2015;23:2479–2497. doi: 10.1007/s00520-015-2782-x. [DOI] [PubMed] [Google Scholar]
- 16.Edmunds K, Tuffaha H, Scuffham P, Galvão DA, Newton RU (2020) The role of exercise in the management of adverse effects of androgen deprivation therapy for prostate cancer: a rapid review. Support Care Cancer. 10.1007/s00520-020-05637-0 [DOI] [PubMed]
- 17.Keilani M, Hasenoehrl T, Baumann L, Ristl R, Schwarz M, Marhold M, Sedghi Komandj T, Crevenna R. Effects of resistance exercise in prostate cancer patients: a meta-analysis. Support Care Cancer. 2017;25:2953–2968. doi: 10.1007/s00520-017-3771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberga AS, Segal RJ, Reid RG, et al. Age and androgen-deprivation therapy on exercise outcomes in men with prostate cancer. Support Care Cancer. 2012;20:971–981. doi: 10.1007/s00520-011-1169-x. [DOI] [PubMed] [Google Scholar]
- 19.Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32:335–346. doi: 10.1200/JCO.2013.49.5523. [DOI] [PubMed] [Google Scholar]
- 20.Mundell NL, Daly RM, Macpherson H, Fraser SF. Cognitive decline in prostate cancer patients undergoing ADT: a potential role for exercise training. Endocr Relat Cancer. 2017;24:R145–R155. doi: 10.1530/ERC-16-0493. [DOI] [PubMed] [Google Scholar]
- 21.Joseph SJ, Lam V, Patel M. Preventing osteoporosis in men taking androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol. 2019;2:551–561. doi: 10.1016/j.euo.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. 10.1371/journal.pmed.1000097 [PMC free article] [PubMed]
- 23.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cormie P, Galvão DA, Spry N, Joseph D, Chee R, Taaffe DR, Chambers SK, Newton RU. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115:256–266. doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 25.Newton RU, Galvão DA, Spry N, et al. Exercise mode specificity for preserving spine and hip bone mineral density in prostate cancer patients. Med Sci Sports Exerc. 2019;51:607–614. doi: 10.1249/MSS.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 26.Taaffe DR, Galvão DA, Spry N, Joseph D, Chambers SK, Gardiner RA, Hayne D, Cormie P, Shum DHK, Newton RU. Immediate versus delayed exercise in men initiating androgen deprivation: effects on bone density and soft tissue composition. BJU Int. 2019;123:261–269. doi: 10.1111/bju.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsen TS, Raastad T, Skovlund E, Courneya KS, Langberg CW, Lilleby W, Fosså SD, Thorsen L. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54:1805–1813. doi: 10.3109/0284186X.2015.1037008. [DOI] [PubMed] [Google Scholar]
- 28.Uth J, Hornstrup T, Christensen JF, Christensen KB, Jørgensen NR, Helge EW, Schmidt JF, Brasso K, Helge JW, Jakobsen MD, Andersen LL, Rørth M, Midtgaard J, Krustrup P. Football training in men with prostate cancer undergoing androgen deprivation therapy: activity profile and short-term skeletal and postural balance adaptations. Eur J Appl Physiol. 2016;116:471–480. doi: 10.1007/s00421-015-3301-y. [DOI] [PubMed] [Google Scholar]
- 29.Uth J, Hornstrup T, Christensen JF, Christensen KB, Jørgensen NR, Schmidt JF, Brasso K, Jakobsen MD, Sundstrup E, Andersen LL, Rørth M, Midtgaard J, Krustrup P, Helge EW. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporos Int. 2016;27:1507–1518. doi: 10.1007/s00198-015-3399-0. [DOI] [PubMed] [Google Scholar]
- 30.Bjerre ED, Brasso K, Jørgensen AB, Petersen TH, Eriksen AR, Tolver A, Christensen JF, Poulsen MH, Madsen SS, Østergren PB, Borre M, Krustrup P, Johansen C, Rørth M, Midtgaard J. Football compared with usual care in men with prostate cancer (FC Prostate Community Trial): a pragmatic multicentre randomized controlled trial. Sports Med. 2019;49:145–158. doi: 10.1007/s40279-018-1031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alibhai SMH, Santa Mina D, Ritvo P, Tomlinson G, Sabiston C, Krahn M, Durbano S, Matthew A, Warde P, O’Neill M, Timilshina N, Segal R, Culos-Reed N. A phase II randomized controlled trial of three exercise delivery methods in men with prostate cancer on androgen deprivation therapy. BMC Cancer. 2019;19:1–11. doi: 10.1186/s12885-018-5189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton RU, Kenfield SA, Hart NH, Chan JM, Courneya KS, Catto J, Finn SP, Greenwood R, Hughes DC, Mucci L, Plymate SR, Praet SFE, Guinan EM, van Blarigan EL, Casey O, Buzza M, Gledhill S, Zhang L, Galvão DA, Ryan CJ, Saad F. Intense exercise for survival among men with metastatic castrate-resistant prostate cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open. 2018;8:e022899. doi: 10.1136/bmjopen-2018-022899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basaria S, Storer T. RESIST-ADT (androgen deprivation therapy). In: clinicaltrial.gov. Accessed 2 Sept 2020
- 34.Newton RU, Galvão DA, Spry N, Joseph D, Chambers SK, Gardiner RA, Hayne D, Taaffe DR. Timing of exercise for muscle strength and physical function in men initiating ADT for prostate cancer. Prostate Cancer Prostatic Dis. 2020;23:457–464. doi: 10.1038/s41391-019-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang DS, Jones JM, Samadi O, Shah S, Mitsakakis N, Catton CN, Jeon W, To J, Breunis H, Alibhai SMH. Healthy bones study: can a prescription coupled with education improve bone health for patients receiving androgen deprivation therapy?-a before/after study. Support Care Cancer. 2018;26:2861–2869. doi: 10.1007/s00520-018-4150-0. [DOI] [PubMed] [Google Scholar]
- 36.Santa Mina D, Ritvo P, Matthew AG, et al. Group exercise versus personal training for prostate cancer patients: a pilot randomized trial. J Cancer Ther. 2012;03:146–156. doi: 10.4236/jct.2012.32020. [DOI] [Google Scholar]
- 37.Papadopoulos E, Santa Mina D, Culos-Reed N, et al. Effects of six months of aerobic and resistance training on metabolic markers and bone mineral density in older men on androgen deprivation therapy for prostate cancer. J Geriatr Oncol. 2020;S1879-4068(19):30378–30379. doi: 10.1016/j.jgo.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Alibhai SM, Durbano S, O’Neill M, et al. Effects of a 6-month moderate-intensity exercise program on metabolic parameters and bone mineral density in men on androgen deprivation therapy for prostate cancer. J Clin Oncol. 2018;36:237. doi: 10.1200/JCO.2018.36.6_suppl.237. [DOI] [Google Scholar]
- 39.Alibhai SM (2014) An exercise trial and economic analysis in men with prostate cancer (ADT Ex RCT). In: clinicaltrial.gov. ClinicalTrials.gov. Accessed 7 Feb 2020
- 40.Wolin KY (2009) A pilot study of physical activity for improving quality of life in prostate cancer patients on androgen deprivation therapy (ADT) (MAP-P). In: clinicaltrial.gov. Accessed 7 Feb 2020
- 41.Lee CE, Leslie WD, Lau YKJ. A pilot study of exercise in men with prostate cancer receiving androgen deprivation therapy. BMC Cancer. 2012;12:103. doi: 10.1186/1471-2407-12-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinkman M, Trumpour S, Lyford K, Gray D. Choosing healthy eating choices and keeping active for men having the androgen therapy experience the Checkmate pilot study. BJU Int. 2018;122:16. doi: 10.1111/bju.14473. [DOI] [Google Scholar]
- 43.Owen PJ, Daly RM, Livingston PM, Mundell NL, Dalla Via J, Millar JL, Fraser SF. Efficacy of a multi-component exercise programme and nutritional supplementation on musculoskeletal health in men treated with androgen deprivation therapy for prostate cancer (IMPACT): study protocol of a randomised controlled trial. Trials. 2017;18:1–16. doi: 10.1186/s13063-017-2185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgetown University (2014) An exercise intervention for prostate cancer patients receiving androgen deprivation therapy (MIGHTY). In: clinicaltrial.gov. Accessed 7 Feb 2020
- 45.Baumann F, Großek A, Hafke R, Wrensch M (2016) Influences of targeted exercise therapy on treatment-related side effects of hormonetherapy in prostate cancer patients. In: Ger. Clin. trial Regist. https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00009975. Accessed 7 Feb 2020
- 46.Winters-Stone K. GET FIT Prostate: a randomized controlled exercise trial. In: clinicaltrial.gov. https://clinicaltrials.gov/ct2/show/NCT03741335?cond=get-fit&draw=2&rank=6. Accessed 7 Feb 2020
- 47.Murtola T (2019) Exercise and quality of life during androgen deprivation therapy. In: clinicaltrial.gov. https://clinicaltrials.gov/ct2/show/NCT04050397?cond=Exercise+and+quality+of+Life+during+androgen+deprivation+therapy&draw=2&rank=1. Accessed 7 Feb 2020
- 48.Capela A (2019) Walking football as a supportive medicine for patients with prostate cancer. In: clinicaltrial.gov.https://clinicaltrials.gov/ct2/show/NCT04062162?cond=Walking+football+as+a+supportive+medicine+for+patients+with+prostate+cancer&draw=2&rank=1 Accessed 7 Feb 2020
- 49.Winters-Stone KM, Dobek JC, Bennett JA, Maddalozzo GF, Ryan CW, Beer TM. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc. 2014;46:1482–1488. doi: 10.1249/MSS.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim SH, Seong DH, Yoon SM, Choi YD, Choi E, Song Y, Song H. The effect on bone outcomes of home-based exercise intervention for prostate cancer survivors receiving androgen deprivation therapy: a pilot randomized controlled trial. Cancer Nurs. 2018;41:379–388. doi: 10.1097/NCC.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 51.Uth J, Schmidt JF, Christensen JF, Hornstrup T, Andersen LJ, Hansen PR, Christensen KB, Andersen LL, Helge EW, Brasso K, Rørth M, Krustrup P, Midtgaard J. Effects of recreational soccer in men with prostate cancer undergoing androgen deprivation therapy: study protocol for the “FC Prostate” randomized controlled trial. BMC Cancer. 2013;13:1–10. doi: 10.1186/1471-2407-13-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams AD, Bird ML, Hardcastle SG, Kirschbaum M, Ogden KJ, Walters JA (2018) Exercise for reducing falls in people living with and beyond cancer. Cochrane Database Syst Rev 2018:. 10.1002/14651858.CD011687.pub2 [DOI] [PMC free article] [PubMed]
- 53.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. Physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–1996. doi: 10.1249/01.MSS.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 54.Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonafede M, Shi N, Barron R, Li X, Crittenden DB, Chandler D. Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch Osteoporos. 2016;11(1):26. doi: 10.1007/s11657-016-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cauley JA, Cawthon PM, Peters KE, Cummings SR, Ensrud KE, Bauer DC, Taylor BC, Shikany JM, Hoffman AR, Lane NE, Kado DM, Stefanick ML, Orwoll ES, for the Osteoporotic Fractures in Men (MrOS) Study Research Group Risk factors for hip fracture in older men: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2016;31:1810–1819. doi: 10.1002/jbmr.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 58.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 59.Heinrich S, Rapp K, Rissmann U, Becker C, König HH. Cost of falls in old age: a systematic review. Osteoporos Int. 2010;21:891–902. doi: 10.1007/s00198-009-1100-1. [DOI] [PubMed] [Google Scholar]
- 60.McGrath R, Al Snih S, Markides K, Hall O, Peterson M. The burden of health conditions for middle-aged and older adults in the United States: disability-adjusted life years. BMC Geriatr. 2019;19:1–13. doi: 10.1186/s12877-019-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanis JA, Cooper C, Rizzoli R, et al. Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int. 2017;28:2023–2034. doi: 10.1007/s00198-017-4009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Khoury F, Cassou B, Charles MA, Dargent-Molina P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f6234. doi: 10.1136/bmj.f6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krupski TL, Foley KA, Baser O, Long S, Macarios D, Litwin MS. Health care cost associated with prostate cancer, androgen deprivation therapy and bone complications. J Urol. 2007;178:1423–1428. doi: 10.1016/j.juro.2007.05.135. [DOI] [PubMed] [Google Scholar]
- 64.Bird ML, Cheney MJ, Williams AD. Accidental fall rates in community-dwelling adults compared to cancer survivors during and post-treatment: a systematic review with meta-analysis. Oncol Nurs Forum. 2016;43:E64–E72. doi: 10.1188/16.ONF.E64-E72. [DOI] [PubMed] [Google Scholar]
- 65.Bourke L, Smith D, Steed L, Hooper R, Carter A, Catto J, Albertsen PC, Tombal B, Payne HA, Rosario DJ. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;69:693–703. doi: 10.1016/j.eururo.2015.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)
(DOCX 32 kb)