Abstract
Isw1 and Chd1 are ATP-dependent nucleosome-spacing enzymes required to establish regular arrays of phased nucleosomes near transcription start sites of yeast genes. Cells lacking both Isw1 and Chd1 have extremely disrupted chromatin, with weak phasing, irregular spacing and a propensity to form close-packed dinucleosomes. The Isw1 ATPase subunit occurs in two different remodeling complexes: ISW1a (composed of Isw1 and Ioc3) and ISW1b (composed of Isw1, Ioc2 and Ioc4). The Ioc4 subunit of ISW1b binds preferentially to the H3-K36me3 mark. Here we show that ISW1b is primarily responsible for setting nucleosome spacing and resolving close-packed dinucleosomes, whereas ISW1a plays only a minor role. ISW1b and Chd1 make additive contributions to dinucleosome resolution, such that neither enzyme is capable of resolving all dinucleosomes on its own. Loss of the Set2 H3-K36 methyltransferase partly phenocopies loss of Ioc4, resulting in increased dinucleosome levels with only a weak effect on nucleosome spacing, suggesting that Set2-mediated H3-K36 trimethylation contributes to ISW1b-mediated dinucleosome separation. The H4 tail domain is required for normal nucleosome spacing but not for dinucleosome resolution. We conclude that the nucleosome spacing and dinucleosome resolving activities of ISW1b and Chd1 are critical for normal global chromatin organisation.
Subject terms: Molecular biology, Chromatin, Chromatin remodelling, Chromatin structure, Histone post-translational modifications, Nucleosomes
Introduction
Eukaryotic DNA is packaged into the nucleus in the form of chromatin. The structural subunit of chromatin is the nucleosome, which is composed of an octamer of core histones (two molecules each of H3, H4, H2A and H2B), around which is wrapped ~ 146 bp of DNA in ~ 1.7 superhelical turns1. Nucleosomes are regularly spaced along the DNA, like beads on a string, forming a fibre which spontaneously folds into higher-order chromatin structures2. Nucleosomes restrict access to DNA and are potent inhibitors of transcription and other DNA-dependent processes in vitro. Cells regulate access to their DNA in part by deploying ATP-dependent chromatin remodeling complexes that are capable of overcoming the nucleosome, either by removing it from the DNA or by sliding it along the DNA3–6.
The ISWI and CHD enzymes represent a major class of ATP-dependent chromatin remodelers conserved from yeast to mammals. They are primarily nucleosome sliding enzymes; many have nucleosome spacing activity in vitro7–13. In vivo, ISWI enzymes are important for chromatin organisation near promoters and other gene regulatory elements14–17. ISWI complexes have additional functions in chromatin assembly8,10,18, stress-induced gene repression19,20, transcript termination21,22 and quality control of mRNP biogenesis23.
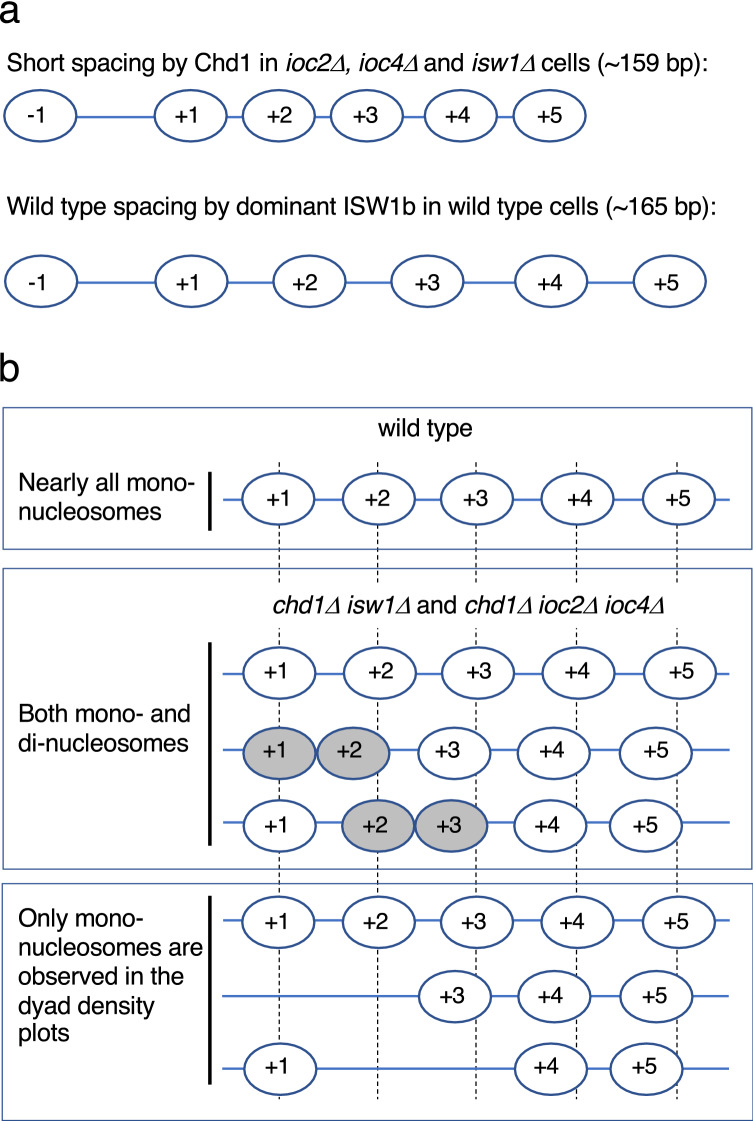
The budding yeast (Saccharomyces cerevisiae) possesses at least four ATP-dependent chromatin remodeling complexes capable of spacing nucleosomes in vitro: ISW1, ISW2, Chd1 and INO807,24–26. In vivo, global chromatin organisation in cells lacking Isw2 is very similar to wild type14,15, suggesting that ISW2 activity is more local than global. In contrast, cells lacking Ino8027–29 or Isw115,30 have shorter average nucleosome spacing than wild type cells. Cells lacking Chd1 have slightly shorter spacing and relatively poor nucleosome phasing14,15. Most impressively, cells lacking both Isw1 and Chd1 have extremely disrupted chromatin, indicating that both enzymes are required for proper chromatin organisation14,15. Recently, we showed that an important contributory factor to chromatin disruption in the chd1Δ isw1Δ double mutant is a tendency for nucleosomes at the 5′-ends of yeast genes to be packed close together, resulting in dinucleosomes with little or no linker DNA22. This observation suggests that Isw1 and/or Chd1 are critical for resolving dinucleosomes.
The yeast Isw1 ATPase subunit is found in two different complexes, termed ISW1a (containing Isw1 and Ioc3) and ISW1b (containing Isw1, Ioc2 and Ioc4)26,31. There is genetic evidence for antagonistic interactions between ISW1a and ISW1b, suggesting that ISW1a has a negative role in transcription that is suppressed by ISW1b32. The Ioc subunits appear to have regulatory functions, since the isolated Isw1 subunit is inactive in vitro26, unless the AutoN inhibitory domain is mutated33. The ISW1a complex is a potent nucleosome spacing enzyme in vitro26,34,35 and its structure has been determined, suggesting a mechanism involving separation of two nucleosomes using a protein ruler36. The ISW1a complex has higher nucleosome spacing activity than the ISW1b complex in vitro26 and contributes much more than ISW1b to nucleosomal array formation at promoters in a purified system25. Isw1 binding to chromatin is indirectly mediated by H3-K4 trimethylation37. The Ioc4 subunit of ISW1b has a PWWP domain which binds to H3-K36me3, a histone modification associated with active transcription38,39. The other auxiliary subunit of ISW1b, Ioc2, contains a putative PHD finger, which may bind to a methylated histone residue38. These observations suggest that H3-K4 and H3-K36 trimethylation may play a critical role in ISW1 function.
Although ISW1a and ISW1b have been studied in depth in vitro, relatively little is known about their contributions to ISW1 function in vivo. Here we have assessed the contributions of ISW1a and ISW1b to nucleosome spacing and separation of dinucleosomes. We find that ISW1b is the major spacing enzyme, whereas ISW1a plays a very minor role in global genic chromatin organisation. ISW1b, together with Chd1, is required to resolve dinucleosomes, whereas ISW1a makes little contribution.
Results
The ISW1b complex is the major nucleosome spacing enzyme in yeast
We have used MNase-seq to determine the chromatin organisation at yeast genes. Briefly, this technique involves micrococcal nuclease (MNase) digestion of the chromatin in purified nuclei to predominantly mononucleosomes. Suitably digested DNA samples are then used to construct libraries for paired-end sequencing. Alignment to the yeast genome indicates the location of each sequenced nucleosome. The midpoint of each sequence is assumed to represent the midpoint of the nucleosome (also called the dyad). An average plot for all 5770 yeast genes is obtained by aligning all genes on their transcription start site (TSS) or on their major + 1 nucleosome position, summing all of the nucleosome dyads on every gene, and normalizing to the genomic average (set at 1). A typical plot for wild type cells is shown in Fig. 1a. We define nucleosome spacing as the average distance between the dyad peaks for the + 1 to + 5 nucleosomes (i.e. the first five nucleosomes on the average gene), measured by the slope of the regression line. However, in some mutants, the phasing is so poor that the spacing cannot be measured accurately (see below). The degree of phasing is indicated by the height of the nucleosome peaks: higher peaks indicate more coincident dyads and therefore better nucleosome positioning.
Figure 1.
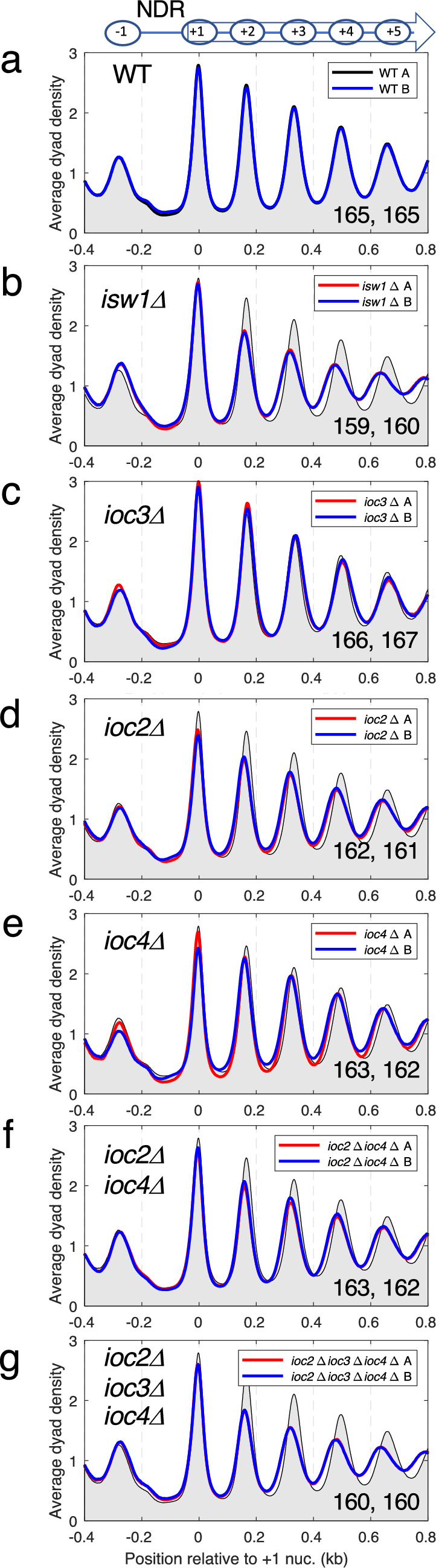
The ISW1b complex (Isw1-Ioc2-Ioc4) is required for setting wild type nucleosome spacing, whereas the ISW1a complex (Isw1-Ioc3) plays only a minor role. Average nucleosome dyad density plots for all genes: (a) wild type, (b) isw1∆, (c) ioc3∆, (d) ioc2∆, (e) ioc4∆, (f) ioc2∆ ioc4∆, (g) ioc2∆ ioc3∆ ioc4∆. All yeast genes were aligned on the midpoints of their + 1 nucleosomes. The dyad distribution was normalized to the global average (set at 1). For ease of comparison, wild type (WT) replicate A is shown as a black line with grey fill in all plots. Two biological replicate experiments (A and B) are shown for each strain in all of the plots (A: red line; B: blue line). The average spacing in bp is shown for replicates A and B in the bottom right corner (measured by regression analysis of the first 5 nucleosome peaks, beginning with the + 1 nucleosome).
We have shown previously15 that cells lacking Isw1 have weaker nucleosome phasing and reduced spacing relative to wild type cells (Fig. 1b). However, isw1Δ cells lack both the ISW1a and the ISW1b remodeling complexes. To assess the relative contributions of ISW1a and ISW1b to phasing and spacing, we examined the chromatin organisation in various ioc mutants. Cells lacking the ISW1a complex (ioc3Δ) show a slight increase in spacing (replicates: 166, 167 bp) with no change in phasing relative to wild type (165, 165 bp) (Fig. 1c). Cells lacking an ISW1b subunit (ioc2Δ or ioc4Δ) have intermediate nucleosome spacing (161–163 bp) (Fig. 1d,e): less than in wild type (165, 165 bp), but not as low as in isw1Δ cells (159, 160 bp), although the spacing is shorter on genes over 2 kb (see below). Small spacing differences are reproducible (compare biological replicate experiments; Fig. 1). The change in spacing becomes increasingly obvious downstream, because the nucleosome position shift is additive relative to the first (+ 1) nucleosome. For example, if the average spacing increases from 160 to 165 bp, then the downstream shift in average position for the + 2 nucleosome is 5 bp, it is 10 bp for the + 3 nucleosome, and 15 bp for the + 4 nucleosome, and so on. Phasing is weaker in the ioc2Δ mutant (Fig. 1d), but hardly affected in the ioc4Δ mutant (Fig. 1e). Cells lacking both ISW1b ancillary subunits (the ioc2Δ ioc4Δ double mutant) have similar chromatin organisation to the ioc2Δ single mutant, exhibiting the weaker phasing observed in ioc2Δ cells as well as the shorter spacing observed in both the ioc2Δ and ioc4Δ single mutants (Fig. 1f). The similar spacing in the ioc2Δ and ioc4Δ single mutants and the ioc2Δ ioc4Δ double mutant (~ 162 bp) suggests that both Ioc2 and Ioc4 are required for ISW1b spacing activity. Chromatin organisation in cells lacking all three ancillary subunits (the ioc2Δ ioc3Δ ioc4Δ triple mutant) is very similar to that in isw1Δ cells (Fig. 1g). This is expected, because both complexes should be inactive in both mutants, given that the Isw1 subunit by itself has no remodeling activity in vitro26.
Nucleosome phasing on a gene can be influenced by interference from a phasing signal emanating from downstream elements, such as a downstream promoter40. This effect can be eliminated by restricting the analysis to promoter-proximal nucleosomes on long genes40, defined here as genes with distances of > 2 kb between the TSS and the transcript termination site (1616 genes; Supplementary Fig. S1). We find that gene length has no effect on nucleosome spacing in wild type cells (165 bp on all genes and on long genes) or in ioc3Δ cells, which have slightly longer spacing than wild type cells (167 bp on all genes and on long genes). However, the ioc2Δ and ioc4Δ single mutants, the ioc2Δ ioc4Δ double mutant and the ioc2Δ ioc3Δ ioc4Δ triple mutant all exhibit shorter spacing on long genes than on all genes (see Fig. 1). In fact, the spacing on long genes is about the same in ioc2Δ (159 bp), ioc4Δ (160 bp), ioc2Δ ioc4Δ (159 bp), ioc2Δ ioc3Δ ioc4Δ (158 bp) and isw1Δ (159 bp) cells (Supplementary Fig. S1). Thus, long genes do not exhibit the intermediate spacing observed for all genes in the ioc mutants (Fig. 1d, e, f; Supplementary Fig. S1). These data suggest that the phasing potential of downstream elements is enhanced in the absence of ISW1b, resulting in phasing interference and altered spacing on short genes, but not on long genes. Taken together, our observations support the conclusion that the ISW1b complex is primarily responsible for wild type nucleosome spacing, whereas ISW1a plays a very minor role.
ISW1a and ISW1b spacing activities are not restricted to genes enriched in their respective Ioc subunits
Chromatin immunoprecipitation (ChIP-seq) experiments have shown that ISW1a (Ioc3), ISW1b (Ioc4) and Isw1 are enriched on different sets of genes, suggesting that these genes might be differentially affected by ISW1a or ISW1b27. We determined whether the chromatin organisation of these sets of genes is differentially affected by loss of ISW1a or ISW1b, as might be expected. In the case of ISW1a, we found that Ioc3-enriched genes show very slightly increased spacing (166 vs. 165 bp) in wild type cells and in ioc3Δ cells (167 vs. 166 bp), but these differences are probably negligible (Supplementary Fig. S2). Thus, ISW1a has little or no differential effect on the chromatin organisation of its putative target genes. Moreover, the spacing on Ioc3-bound genes is affected in ioc4Δ cells, indicating that ISW1a-enriched genes are affected by ISW1b (Ioc4) (Supplementary Fig. S2). In the case of ISW1b, Ioc4-enriched and non-enriched genes have the same spacing and phasing in wild type cells, suggesting that they are not differentially affected by ISW1b. In ioc4Δ cells, both sets of genes have shorter spacing than wild type (Supplementary Fig. S2). Ioc4-enriched genes may have slightly shorter spacing than non-enriched genes, but the effect is subtle (Supplementary Fig. S2). As expected, Ioc4-enriched genes are not affected in ioc3Δ cells (Supplementary Fig. S2). Thus, ISW1b affects the chromatin organisation of both sets of genes. Finally, Isw1-enriched genes have the same spacing as non-enriched genes in wild type cells and, although the phasing is slightly better on the Isw1-enriched genes, this effect is also observed in the absence of Isw1 (Supplementary Fig. S2). Isw1-enriched and non-enriched genes show similar changes in isw1Δ cells (short spacing and weaker phasing), although there is a slight difference in spacing of 1–2 bp between the two sets of genes (Supplementary Fig. S2). Overall, the Isw1 complexes affect the chromatin organisation of both enriched and non-enriched genes similarly, with only subtle differences at most. It is unclear why we do not observe obvious differences in chromatin organisation for putative target and non-target genes defined by Ioc3, Ioc4 or Isw1 enrichment; perhaps all genes have some bound ISW1b, such that the putative target genes are only modestly enriched relative to non-target genes. Alternatively, loss of one ISW1 complex might affect the distribution and/or activity of the other ISW1 complex.
ISW1b and Chd1 account for the extreme chromatin disruption in cells lacking both Isw1 and Chd1
Cells lacking Chd1 have a mild chromatin organisation defect14,15, characterized by somewhat shorter spacing than wild type (164, 163 bp vs. 165, 165 bp) and weaker phasing (Fig. 2a). This effect is slightly stronger on long genes (chd1Δ: 162 bp; wild type: 165 bp; Supplementary Fig. S1). In contrast, chromatin organisation in cells lacking both Chd1 and Isw1 (the chd1Δ isw1Δ double mutant) is extremely disrupted14,15 (Fig. 2b). The chd1Δ ioc2Δ ioc4Δ triple mutant (Fig. 2c) also has extremely disrupted chromatin, whereas the chd1Δ ioc3Δ double mutant is very similar to the chd1Δ single mutant (Fig. 2d). This observation is consistent with our conclusion that the ISW1b complex is much more important than the ISW1a complex for global nucleosome spacing (Fig. 1). However, chromatin organisation in the chd1Δ ioc2Δ ioc4Δ triple mutant is not quite as severely disrupted as in the chd1Δ isw1Δ double mutant: the phasing in the triple mutant is very poor, but somewhat stronger than in the double mutant (Fig. 2b,c). Measurement of the spacing in these two mutants is not appropriate because most of the peaks are too weak to measure accurately. The difference between the chd1Δ ioc2Δ ioc4Δ triple mutant and the chd1Δ isw1Δ double mutant is that the ISW1a complex (Isw1-Ioc3) is still present in the former mutant. This suggests that ISW1a contributes some residual order to genic chromatin in the absence of both Chd1 and ISW1b.
Figure 2.
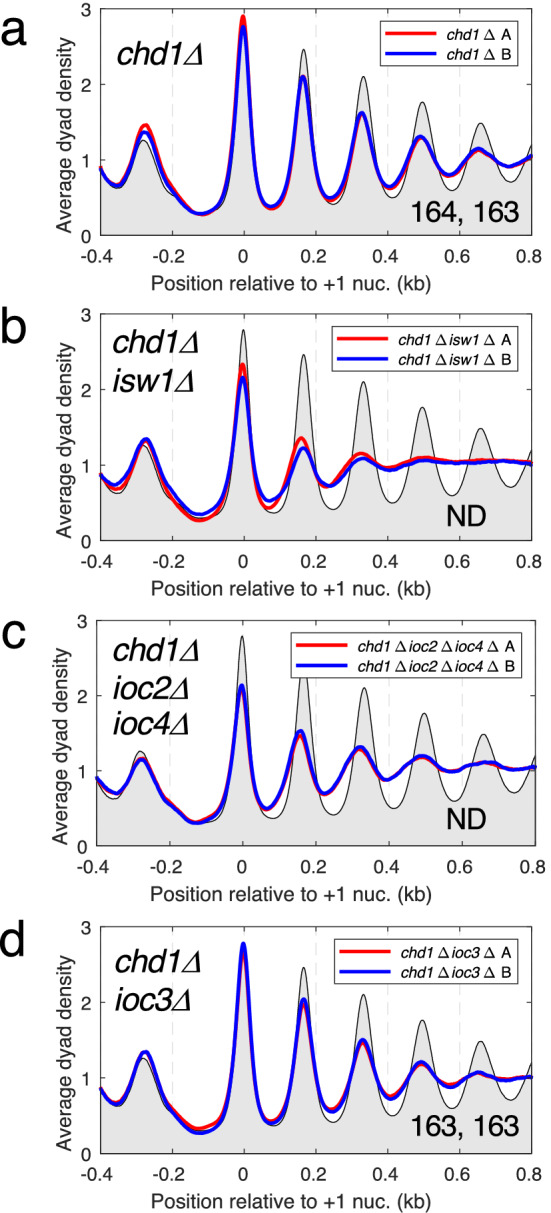
ISW1b and Chd1 are the major remodelers required for normal chromatin organisation. Average nucleosome dyad density plots for all genes: (a) chd1∆, (b) chd1∆ isw1∆, (c) chd1∆ ioc2∆ ioc4∆, (d) chd1∆ ioc3∆. All yeast genes were aligned on the midpoints of their + 1 nucleosomes. The dyad distribution was normalized to the global average (set at 1). For ease of comparison, wild type replicate A is shown as a black line with grey fill in all plots. Two biological replicate experiments (A and B) are shown for each strain in all of the plots (A: red line; B: blue line). The average spacing in bp is shown for replicates A and B in the bottom right corner (measured by regression analysis of the first 5 nucleosome peaks, beginning with the + 1 nucleosome); ND = not determined because the phasing is too weak for accurate measurement.
ISW1a and ISW1b have little effect on the chromatin of very active genes
Since ISW1b preferentially associates with transcriptionally active genes39, we examined whether ISW1a or ISW1b mediate specific effects at highly active genes. We defined highly active genes as those which have > 4 times the genomic average signal using our published ChIP-seq data for the Rpb3 subunit of Pol II15. Although this is a somewhat arbitrary threshold, it is clear from heat map analysis that relatively few genes have high levels of Pol II; most genes have relatively low Pol II levels (Supplementary Fig. S3). On average, the 300 most active genes have ~ eightfold higher Rpb3 density than the remaining genes. Accordingly, we compared the average chromatin structure of the most active genes with that of the remaining, much less active, 5470 genes (Supplementary Fig. S3). In wild type cells, the chromatin of the highly active genes is poorly organised, characterized by much reduced and irregular spacing, weak phasing and a much wider nucleosome-depleted region (NDR) at the promoter, which extends upstream41,42. Although the average spacing on the highly active genes cannot be measured accurately due to poor phasing, it is clearly shorter than the spacing on the other genes (Supplementary Fig. S3; compare nucleosome peak locations). The chromatin organisation of the highly active genes is not strongly differentially affected by any of the ioc∆ mutations or isw1∆ (Supplementary Fig. S3), whereas the less active genes have the spacing observed for all genes, as expected. We conclude that ISW1a and ISW1b have no differential effect on the chromatin organisation of highly active genes, with the caveat that their general state of disruption might obscure subtle effects. On the other hand, nucleosome phasing on the top 300 active genes is weaker in cells lacking Chd1 than in wild type or in any of the ioc mutants (Supplementary Fig. S3), suggesting that Chd1 is the most important spacing enzyme for highly active genes, and consistent with a direct association of Chd1 with transcript elongation factors43.
Both ISW1b and Chd1 are important for resolution of close-packed dinucleosomes
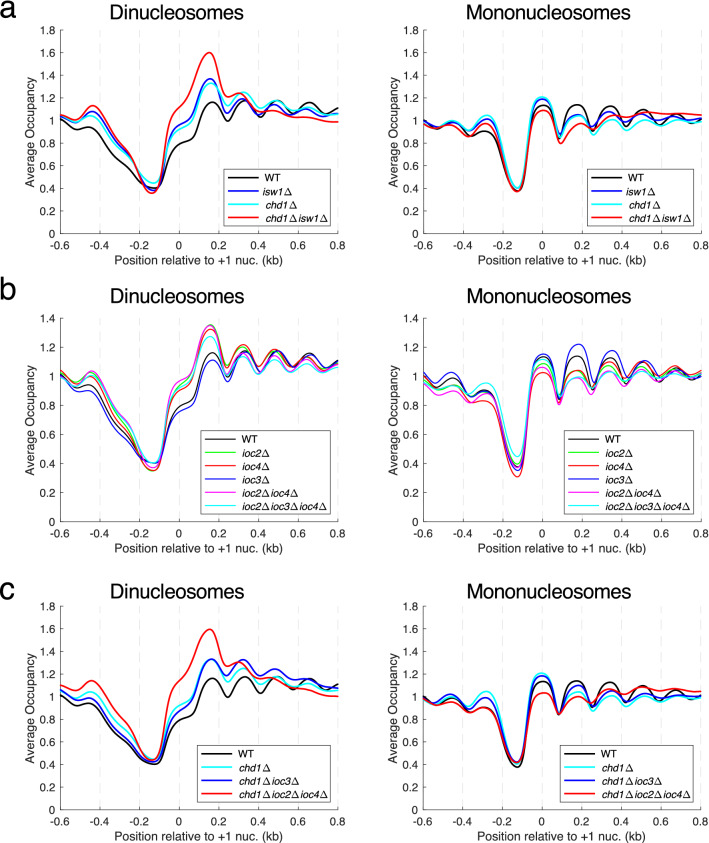
We reported previously that a major contributing factor to chromatin disruption in the chd1Δ isw1Δ double mutant is the presence of close-packed dinucleosomes, primarily involving the + 2 nucleosome (i.e. dinucleosomes containing either the + 1 and + 2 nucleosomes or the + 2 and + 3 nucleosomes)22 (Fig. 3a). These dinucleosomes are characterized by MNase-resistant DNA fragments of 250–350 bp, presumably representing two nucleosomes (or perhaps sub-nucleosomes) with little or no intervening linker DNA for MNase to cut. The dyad plots shown above (Figs. 1 and 2) include only mononucleosome data (the analysis was limited to DNA fragments of 120–180 bp). A dyad plot is not appropriate for dinucleosomes, since the midpoint of a dinucleosome would be located between the two nucleosomes. Instead, we used nucleosome occupancy (coverage) plots, in which the number of times each genomic base pair appears in either mononucleosomes or dinucleosomes is counted and normalized to the genomic average.
Figure 3.
Both ISW1b and Chd1 are important for separating close-packed dinucleosomes. Average nucleosome occupancy (coverage) plots for all genes. All yeast genes were aligned on the midpoint of their average + 1 nucleosome position. The occupancy was normalized to the global average (set at 1) for mononucleosomes (120–180 bp) or dinucleosomes (250–350 bp). Note different y-axis scales are used in different plots to separate the lines more clearly. Data for replicate A are shown (see Supplementary Fig. S4 for comparison of biological replicate experiments). (a) Dinucleosomes involving the + 2 nucleosome are enriched whereas + 2 mononucleosomes are depleted in chd1Δ isw1Δ cells; smaller effects are observed in chd1Δ and isw1Δ cells. (b) ISW1b (Isw1-Ioc2-Ioc4) accounts for the increased level of dinucleosomes in isw1Δ cells. (c) ISW1b and Chd1 are both required to resolve all dinucleosomes.
In the chd1Δ isw1Δ double mutant, there is a strong dinucleosome peak at the + 2 position and a depressed + 2 mononucleosome peak, consistent with the presence of a significant fraction of all + 2 nucleosomes in dinucleosomes22 (Fig. 3a; Supplementary Fig. S4). We determined the relative contributions of Chd1 and Isw1 to dinucleosome resolution. A dinucleosome peak is observed in both the chd1Δ and isw1Δ single mutants, but it is weaker than in the chd1Δ isw1Δ double mutant (Fig. 3a). The + 2 mononucleosome peak is also weaker in the single mutants relative to wild type. These data indicate that the Chd1 and Isw1 remodeling enzymes both contribute independently and make additive contributions to dinucleosome resolution, such that neither enzyme is capable of resolving all dinucleosomes on its own.
Next, we determined the separate contributions of the ISW1a and ISW1b complexes to Isw1-dependent dinucleosome resolution (Fig. 3b). The ioc2Δ and ioc4Δ single mutants have an enhanced dinucleosome peak and depressed mononucleosome peak, as observed for the isw1Δ single mutant, suggesting that both ancillary subunits of the ISW1b complex are required for Isw1-dependent dinucleosome resolution. Consistent with this conclusion, the ioc2Δ ioc4Δ double mutant has a dinucleosome peak similar to that of the single mutants. In contrast, there are fewer dinucleosomes in the ioc3Δ mutant, which has a slightly lower + 2 dinucleosome peak than wild type and somewhat higher levels of the + 2 and + 3 mononucleosomes (Fig. 3b; Supplementary Fig. S4), indicating that ISW1a is not important for dinucleosome resolution. These conclusions are supported by high levels of dinucleosomes in the chd1Δ ioc2Δ ioc4Δ triple mutant versus wild type, resulting from loss of both ISW1b and Chd1 (Fig. 3c). Similarly, the chd1Δ ioc3Δ double mutant has more dinucleosomes than wild type, but less than the chd1Δ ioc2Δ ioc4Δ triple mutant, which can be accounted for by the absence of Chd1 with little contribution from ISW1a (Fig. 3c). Thus, the ISW1b complex accounts quite well for the role of Isw1 in dinucleosome resolution.
Set2 contributes to dinucleosome separation
The Ioc4 PWWP domain binds preferentially to H3-K36me3 relative to unmethylated H3-K36 in vitro, suggesting that ISW1b may be regulated by H3-K36me338,39. The only H3-K36 methyltransferase in yeast is encoded by SET2. We examined whether ISW1b-mediated changes in nucleosome spacing and dinucleosome resolution depend on Set2. Nucleosome spacing in a set2Δ mutant (replicates: 165 and 163 bp) is slightly lower than wild type (165 and 165 bp), although this difference is probably not significant (Fig. 4a). Loss of Ioc4 has a stronger effect on nucleosome spacing (Fig. 1e; ioc4Δ replicates: 163 and 162 bp) than loss of Set2 (Fig. 4b), which is also true for long genes (Supplementary Fig. S1). Like ioc4Δ cells, set2Δ cells have higher dinucleosome levels than wild type cells (Fig. 4c). Interestingly, there are more + 3 and + 4 dinucleosomes in set2Δ cells than in ioc4Δ cells (Figs. 4c; 3b; Supplementary Fig. S4). However, there is no corresponding depression in the + 2, + 3 and + 4 mononucleosome peaks in set2Δ cells (Fig. 4c); instead, these mononucleosomes are higher than in wild type. There is an apparently compensatory reduction in NDR occupancy and the − 1 nucleosome (Fig. 4c), which is also observed in ioc4Δ cells but not in the other ioc mutants (Fig. 3b). Overall, these data suggest that Set2 and H3-K36me3 may be more important for the dinucleosome resolving activity of ISW1b than for its spacing activity.
Figure 4.
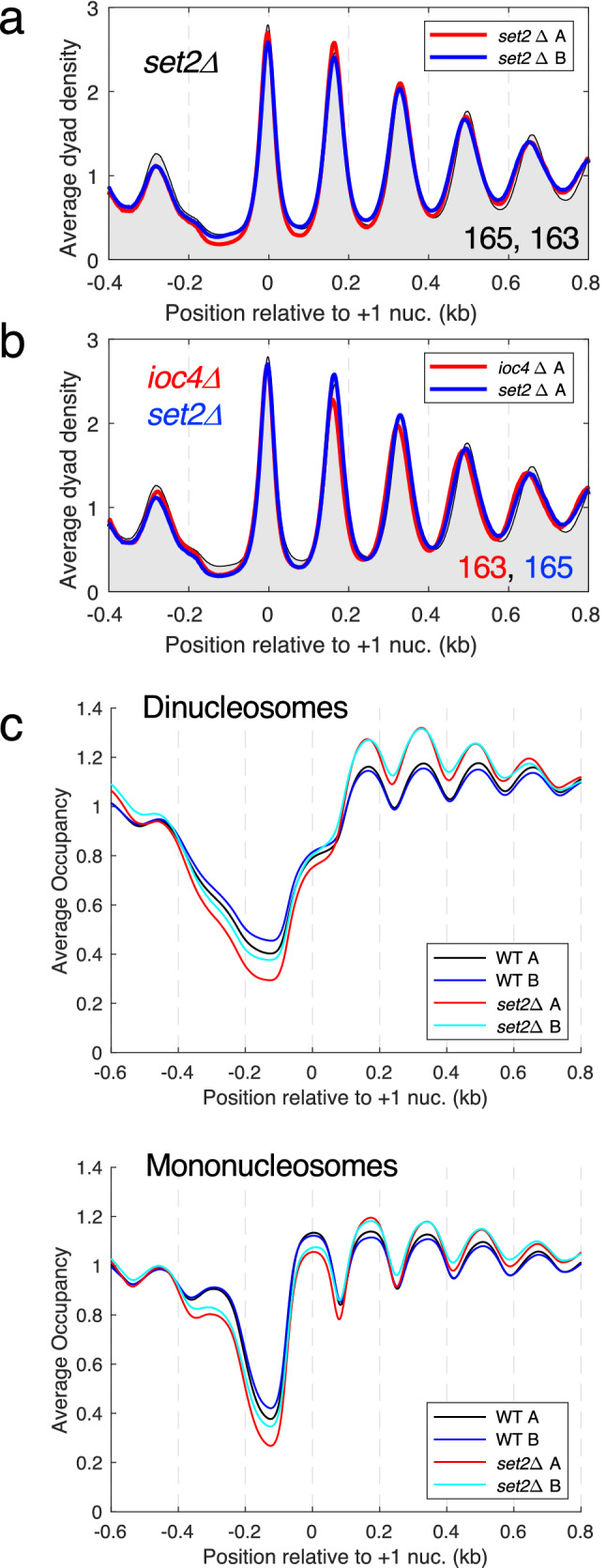
Set2 contributes to dinucleosome separation but has little effect on spacing. (a) Average nucleosome dyad density plot for all genes in set2Δ cells. Wild type replicate A is shown as a black line with grey fill. Two biological replicate experiments are shown: A (red line) B (blue line). The average spacing (bp) for replicates A and B is shown (bottom right). (b) Comparison of chromatin organisation in set2Δ cells (blue line) and ioc4Δ cells (red line) (replicates A). (c) Occupancy plots for dinucleosomes and mononucleosomes in set2Δ and wild type (WT) cells (see legend to Fig. 3).
Loss of Set1 has no effect on global chromatin organisation
The binding of ISW1 to chromatin is mediated indirectly through H3-K4 trimethylation37. On the other hand, a direct interaction might be possible, mediated by the putative PHD domain in the Ioc2 subunit of ISW1b38. Since Set1 is the only H3-K4 methyltransferase in yeast, we tested this possibility by examining set1Δ cells. However, chromatin organisation in set1Δ cells is essentially identical to wild type at the global level (Supplementary Fig. S5) and quite different from that in ioc2Δ cells (Fig. 1d). Dinucleosomes involving the + 3 and + 4 nucleosomes may be somewhat elevated in set1Δ cells, although this is unclear because the replicates are not consistent in this respect (Supplementary Fig. S4; Supplementary Fig. S5). In conclusion, Set1 and therefore H3-K4me3 are not required for ISW1-dependent nucleosome spacing.
Deletion of the H4 N-terminal tail domain results in shorter nucleosome spacing but does not increase dinucleosome levels
Nucleosome mobilisation in vitro by ISWI complexes isolated from different organisms requires the H4 N-terminal tail domain, specifically the “basic patch” (residues 16–19)33,44,45. In addition, genetic interactions between isw1 mutations and H4 mutations (point mutations and a tail deletion) suggest involvement in a common pathway19. We reasoned that deletion of the H4 N-tail might result in a chromatin organisation similar to that observed in the isw1Δ single mutant: reduced spacing, weaker phasing and increased dinucleosome formation (Figs. 1b, 3a). We constructed a yeast strain in which both H3/H4 gene loci (HHT1-HHF1 and HHT2-HHF2) were deleted and covered by a plasmid carrying wild type HHT1-HHF1 or HHT1-HHF1Δ21, in which the first 21 amino acid residues of the H4 N-tail had been deleted. This strain displayed a clear growth phenotype, with a doubling time of ~ 2.6 h, compared with ~ 1.8 h for the wild type strain, in synthetic complete (SC) medium. We find that nucleosome spacing is shorter in cells lacking the H4 N-tail (Fig. 5a), though not as short as in isw1Δ cells (Fig. 1b), and both mutants have poor phasing. These observations are consistent with the requirement of Isw1 for the H4 N-tail domain. However, removal of the H4 N-tail resulted in only a very slight increase in dinucleosome prevalence (Fig. 5b; Supplementary Fig. S4), unlike loss of Isw1 (Fig. 3a), indicating that loss of Isw1 and loss of the H4 N-tail are not equivalent.
Figure 5.
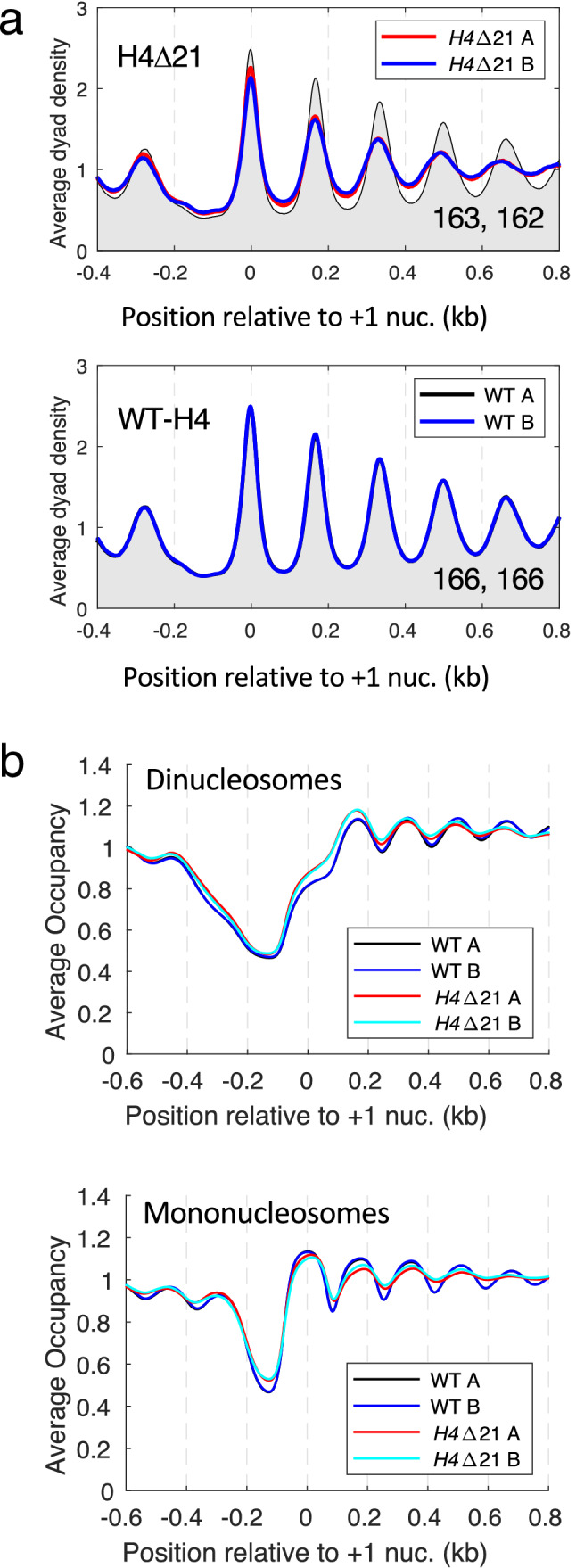
Deletion of the first 21 residues of the H4 N-terminal tail domain results in shorter global nucleosome spacing but little dinucleosome accumulation. (a) Comparison of the H4ΔN21 mutant with its isogenic wild type strain (WT-H4) (top panel). WT-H4 replicates (bottom panel). All yeast genes were aligned on the midpoints of their + 1 nucleosomes. The dyad distribution was normalised to the global average (set at 1). WT-H4 replicate A: black line with grey fill in both plots. The average spacing in bp is shown for each replicate in the bottom right corner. (b) Occupancy plots for dinucleosomes and mononucleosomes in H4Δ21 and wild type (WT) cells (see legend to Fig. 3).
Discussion
ISW1b is the primary nucleosome spacing enzyme in yeast
We and others have shown previously that Isw1 and Chd1 are both needed for normal global chromatin organisation in yeast14,15. We proposed that these two enzymes compete to set nucleosome spacing in wild type cells, with Isw1 being dominant, setting wild type spacing, and Chd1 directing shorter spacing15. In the absence of Isw1, the spacing is short, which we attributed to Chd1 activity. An important complication, which we have addressed here, is that there are two complexes containing the Isw1 ATPase subunit26. We have determined their respective roles in chromatin organisation in vivo: ISW1b and Chd1 are primarily responsible for global nucleosome spacing in wild type cells, whereas ISW1a has little effect on spacing (Fig. 6a). We note that Ino80C also affects global spacing27–29, but it is not yet clear how its activity meshes with those of ISW1b and Chd1.
Figure 6.
Dominant role of ISW1b in chromatin organisation. (a) Global average nucleosome spacing is primarily determined by ISW1b (wild type cells), since loss of ISW1a has little effect. Short spacing is attributed to Chd1, because the chd1∆ isw1∆ double mutant has highly disrupted chromatin. (b) Dinucleosomes disrupt phasing and reduce spacing. Model to show the effects of dinucleosomes on chromatin organisation. Top panel: Average nucleosome positions on a gene in wild type cells with regular spacing. Middle: Close-packed dinucleosomes (grey ovals) are high in the absence of both ISW1b and Chd1. These dinucleosomes preferentially involve the + 2 nucleosome i.e., they are mostly + 1/+ 2 or + 2/+ 3 dinucleosomes. Nucleosomes farther down the gene (+ 3 etc.) may be regularly spaced relative to the dinucleosome, but because a linker is missing in the dinucleosome, downstream nucleosomes are out of phase with other nucleosomal arrays, resulting in poor phasing due to interference patterns from the different arrays. Bottom: Dinucleosomes are absent from the mononucleosome dyad density plots, resulting in depressed, flattened peaks. These effects are strongest in cells lacking both ISW1b and Chd1. Dinucleosomes that cannot be resolved by ISW1b can account for shorter spacing in chd1∆ cells.
A difficulty for our competition model is that, in the absence of Chd1, ISW1b is expected to win the competition, resulting in wild type spacing. Instead, we observe that spacing is somewhat shorter in cells lacking Chd115 (Fig. 2a; Supplementary Fig. S1). One possible explanation is that the dominance of ISW1b depends on Chd1 and so, in its absence, we see an increased contribution from ISW1a, such that the spacing represents the average of ISW1a and ISW1b activities. If so, cells having only ISW1b (the chd1Δ ioc3Δ double mutant) are expected to have wild type spacing. However, the result is intermediate spacing, very similar to the chd1Δ single mutant (Fig. 2). Alternatively, ISW1b might only be able to create nucleosome arrays with wild type spacing if Chd1 has already made arrays with short spacing, although this model is inconsistent with in vitro data showing that purified ISW1b can space nucleosomes by itself26.
A more satisfying explanation involves dinucleosomes (Fig. 6b). Consider a single gene. If there are regularly spaced nucleosomes downstream of a close-packed + 1/+ 2 dinucleosome (i.e. no linker), these nucleosomes will be out of phase by one linker length with nucleosomes in cells with no dinucleosome on this gene. Other cells may have a + 2/+ 3 dinucleosome instead, which will alter the positions of regularly spaced downstream nucleosomes to give a different phasing. The result is generally weaker phasing (peak flattening) and a shift to shorter average spacing. Moreover, since the dinucleosomes are not counted in the mononucleosome phasing pattern, there will be missing occupancy around the + 2 nucleosome, resulting in more pattern disruption (primarily a depressed + 2 nucleosome peak). These effects will increase as the fraction of genes having a dinucleosome increases: the chd1Δ isw1Δ double mutant and the chd1Δ ioc2Δ ioc4Δ triple mutant both have high dinucleosome levels and extremely poor phasing. The chd1Δ single mutant has fewer dinucleosomes, resulting in a smaller shift in the peaks to shorter spacing. These considerations can account for the shift to shorter spacing in the absence of Chd1 and for the major disruption of chromatin in the absence of both Chd1 and ISW1b. Thus, we propose that the spacing is shorter in cells lacking Chd1 because ISW1b cannot resolve all of the dinucleosomes.
Previously, we showed that Isw1 and/or Chd1 is required to resolve dinucleosomes, or to prevent their formation22. Here we show that both enzymes are important for separating dinucleosomes and that, as observed for spacing activity, ISW1b is much more important than ISW1a. In vitro, both ISW1a and ISW1b can move nucleosomes reconstituted on a pair of 601 nucleosome positioning sequences farther apart, even if separated by a linker of only 4 bp46, indicating that both ISW1 complexes probably have close-packed dinucleosome resolving activity.
The Set2 methyltransferase contributes to dinucleosome separation
We observe that the ancillary subunits of ISW1b, Ioc2 and Ioc4, are both necessary for ISW1b-dependent chromatin organisation, although Ioc2 makes a greater contribution, since it affects phasing as well as spacing. We suggest that Ioc2 and Ioc4 cooperate to influence the remodeling activity of the Isw1 subunit. Both may be linked to specific histone modifications: Ioc4 binds to reconstituted nucleosomes carrying H3-K36me3 with higher affinity than to unmethylated nucleosomes, suggesting that Set2-mediated H3-K36me3 might be an important regulator of ISW1b38,39,47. Our data provide some evidence to support this proposal, because although loss of Set2 has only a subtle effect on nucleosome spacing, it does result in increased dinucleosome levels, similar to that observed for loss of Ioc4. We propose that Set2-mediated trimethylation of H3-K36 is important for ISW1b-mediated dinucleosome resolution, through interaction of H3-K36me3 with its Ioc4 subunit.
We note that neither ISW1 complex requires H3-K36me3 to space nucleosomes in vitro, since they are active on nucleosomes containing unmodified recombinant histones. On the other hand, in vivo, H3-K36me3 may have local effects on ISW1b activity, rather than global effects, which we have not detected. These are probably not occurring at the most active genes because their chromatin organisation in set2Δ cells is similar to wild type (Supplementary Fig. S3). H3-K36me3 recognition may also affect other functions of ISW1b, such as gene repression20 or mRNP quality control23.
The fact that Ioc2 has a putative PHD domain suggests that ISW1b may interact with an additional methylated histone residue, although there is no evidence for this at present38. It is unlikely to be H3-K4me3, because our data indicate that the nucleosome spacing activity of the ISW1b complex is independent of Set1 and therefore of H3-K4me3. H3-K4me3 may also bind to one of the chromodomains of Chd148, although this is controversial49. If Chd1 does indeed bind to H3-K4me3, this binding is not critical for its nucleosome spacing function, since we find that loss of Set1 does not result in global chromatin defects, unlike loss of Chd1.
The H4 N-terminal tail domain is required for normal nucleosome spacing
The H4 tail domain is generally required by ISWI-like enzymes for activity4,5,44,50. The ISWI ATPase subunit has an inhibitory AutoN domain that resembles the H4 tail, which is displaced by the H4 tail when ISWI binds to a nucleosome4,5. The isolated yeast Isw1 subunit is inactive without Ioc subunits unless the AutoN domain is inactivated by mutation33, implying that the Ioc subunits may regulate Isw1 activity through the AutoN domain. We find that the H4 tail deletion mutant exhibits shorter spacing, consistent with inactivation of ISW1b, but there is little increase in dinucleosomes. The most likely explanation for the non-equivalence of the H4Δ21 and isw1Δ mutations is that the H4 tail domain is involved in multiple processes, including interactions with other remodelers and proteins. This possibility is supported by the fact that the H4Δ21 mutant has a strong growth defect, unlike the various ioc mutants and the isw1Δ mutant, which have no obvious growth defect. Since Chd1 also makes contacts with the basic patch in the H4 tail51,52, its remodeling activity may also be compromised, although H4Δ21 chromatin is clearly not as disrupted as chd1Δ isw1Δ chromatin. The H4 N-tail is also required by ISW2 in vitro53, although global chromatin organisation is unaffected in isw2Δ cells14,15. The low prevalence of dinucleosomes in the H4Δ21 mutant suggests that the H4 tail might be required for dinucleosome formation, possibly because the increased negative charge on nucleosomes lacking the H4 tail might limit how closely nucleosomes can approach one another54,55. Deletion of the H4 tail also results in the loss of multiple post-translational modification sites, preventing interaction with many chromatin factors.
Methods
Plasmid construction
The primers used in this study are listed in Supplementary Table S1. pRS-HHT1-HHF1 (p368) was constructed by insertion of the 1930-bp SacI-PstI fragment containing the HHT1-HHF1 locus from p36756 at the same sites in pRS317 (CEN ARS LYS2) (ATCC 77157). pRS-HHT1-HHF1Δ21 (p730) was constructed by replacing the 752-bp AfeI-SacI fragment in p368 with a 689-bp AfeI-SacI fragment with the H4 N-terminal tail deletion (H4 begins Met-Leu22…) made by ligating two PCR fragments together (obtained using p368 as template with primers 1770/1771 and 1773/1812). The sequence was confirmed.
Yeast strain construction
The yeast strains used in this study are listed in Supplementary Table S2. YDC507 (H4 N-terminal tail deletion) was constructed by transforming ROY128157, which carries plasmid pCC67 (2 micron origin URA3 HHT1-HHT1 HTA1-HTB1)58, with p730 (CEN ARS LYS2 HHT1-HHF1Δ21), selection on plates made with synthetic complete medium without lysine (SC-lys) and then counter-selection against URA3 with 5-fluoro-orotic acid (5-FOA) to evict pCC67. A wild type control strain (YDC101) was constructed in the same way using p368 (CEN ARS LYS2 HHT1-HHF1) instead of p730. The diploid strain YPE600 was made by crossing YTT1967 with YDC11159. YPE606 (ioc3Δ) was constructed by transforming YPE600 with an ioc3Δ::KanMX fragment made using primers 1918/1919 and genomic DNA from YTT645, followed by G418 selection on YPD plates and sporulation. YPE608 (ioc4Δ) was constructed by transforming YPE600 with an ioc4Δ::HPH1 fragment made using primers 1922/1923 and genomic DNA from YTT827, followed by hygromycin selection on YPD plates and sporulation. YPE636 (ioc2Δ) was constructed by transforming YPE600 with an ioc2Δ::URA3 fragment made using primers 1961/1962 and extended using primers 1965/1966 and wild type genomic DNA as template, followed by selection on SC-ura plates and sporulation. YPE654 (ioc2Δ ioc3Δ) was constructed by transforming YPE636 with the ioc3Δ::KanMX DNA fragment (see above). YPE655 (ioc2Δ ioc4Δ) was constructed by transforming YPE636 with the ioc4Δ::HPH1 fragment (see above). YPE657 (ioc2Δ ioc3Δ ioc4Δ) was constructed by transforming YPE654 with the ioc4Δ::HPH1 fragment. YPE712 (chd1Δ ioc3Δ) was obtained by crossing YJO48615 with YPE607. YJO487 was obtained by sporulation of YJO50515. YPE715 (chd1Δ ioc2Δ ioc4Δ) was constructed by crossing YJO487 with YPE655. YPE602 (set1Δ) was constructed by transforming YPE600 with a set1Δ::NAT1 fragment made using primers 1926/1927 and genomic DNA from YTT1986, followed by nourseothricin selection on YPD plates and sporulation. YPE604 (set2Δ) was constructed by transforming YDC111 with a set2Δ::TRP1 fragment made using primers 1914/1915 extended with 1916/1917 and plasmid pFA6a-TRP160, followed by selection on SC-trp plates.
MNase-seq
Nuclei were prepared as described61. MNase digestion of nuclei and construction of paired-end libraries was as described62, except that mononucleosomal DNA was not gel-purified, in order to retain mononucleosomes and dinucleosomes in the correct proportions22. Two biological replicate experiments were performed for all strains (i.e., the replicate experiments for each strain were performed entirely independently). MNase-seq data were analysed using scripts originally described by22; modified code is provided (Supplementary Code).
Supplementary Information
Acknowledgements
We thank Alan Hinnebusch for helpful comments on the manuscript and Răzvan Chereji for help with the bioinformatics. We thank Alex Naim for constructing p368, Toshi Tsukiyama for yeast strains, and Rohinton Kamakaka and Namrita Dhillon for pCC67 (pRO149) and ROY1281. This research was supported by the Intramural Research Program of the NIH (NICHD). We thank the NHLBI Core Facility (Yan Luo, Poching Liu and Jun Zhu) for paired-end sequencing. This study utilized the high performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health.
Author contributions
P.R.E.: Acquisition, analysis and interpretation of data. D.J.C.: Analysis, interpretation of data and manuscript preparation.
Funding
Open Access funding provided by the National Institutes of Health (NIH).
Data availability
The datasets generated during the current study are available in the NCBI Gene Expression Omnibus (GEO) repository [GSE156224]. Published data sets used in the current study are available in the GEO repository (MNase-seq data for YJO484: GSE11751422; Rpb3 ChIP-seq data: GSE6940015).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82842-9.
References
- 1.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Thoma F, Koller T, Klug A. Involvement of histone H1 in the organisation of the nucleosome and of the salt-dependent superstructures of chromatin. J. Cell Biol. 1979;83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chereji RV, Clark DJ. Major determinants of nucleosome positioning. Biophys. J. 2018;114:2279–2289. doi: 10.1016/j.bpj.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapier CR, Iwasa J, Cairns BR, Peterson CL. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 2017;18:407–422. doi: 10.1038/nrm.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul S, Bartholomew B. Regulation of ATP-dependent chromatin remodelers: accelerators/brakes, anchors and sensors. Biochem. Soc. Trans. 2018;46:1423–1430. doi: 10.1042/BST20180043. [DOI] [PubMed] [Google Scholar]
- 6.Prajapati HK, Ocampo J, Clark DJ. Interplay among ATP-dependent chromatin remodelers determines chromatin organisation in yeast. Biology (Basel) 2020;9:190. doi: 10.3390/biology9080190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukiyama T, Palmer J, Landel CC, Shiloach J, Wu C. Characterization of the imitation switch subfamily of ATP-dependent chromatin-remodeling factors in Saccharomyces cerevisiae. Genes Dev. 1999;13:686–697. doi: 10.1101/gad.13.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lusser A, Urwin DL, Kadonaga JT. Distinct activities of CHD1 and ACF in ATP-dependent chromatin assembly. Nat. Struct. Mol. Biol. 2005;12:160–166. doi: 10.1038/nsmb884. [DOI] [PubMed] [Google Scholar]
- 9.Stockdale C, Flaus A, Ferreira H, Owen-Hughes T. Analysis of nucleosome repositioning by yeast ISWI and Chd1 chromatin remodeling complexes. J. Biol. Chem. 2006;281:16279–16288. doi: 10.1074/jbc.M600682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T, Bulger M, Pazin MJ, Kobayashi R, Kadonaga JT. ACF, an ISWI-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell. 1997;90:145–155. doi: 10.1016/S0092-8674(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 11.Pointner J, et al. CHD1 remodelers regulate nucleosome spacing in vitro and align nucleosomal arrays over gene coding regions in S. pombe. EMBO J. 2012;31:4388–4403. doi: 10.1038/emboj.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieleg C, et al. Nucleosome spacing generated by ISWI and CHD1 remodelers is constant regardless of nucleosome density. Mol. Cell. Biol. 2015;35:1588–1605. doi: 10.1128/MCB.01070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torigoe SE, Patel A, Khuong MT, Bowman GD, Kadonaga JT. ATP-dependent chromatin assembly is functionally distinct from chromatin remodeling. elife. 2013;2:e00863. doi: 10.7554/eLife.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gkikopoulos T, et al. A role for Snf2-related nucleosome-spacing enzymes in genome-wide nucleosome organisation. Science. 2011;333:1758–1760. doi: 10.1126/science.1206097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ocampo J, Chereji RV, Eriksson PR, Clark DJ. The ISW1 and CHD1 ATP-dependent chromatin remodelers compete to set nucleosome spacing in vivo. Nucleic Acids Res. 2016;44:4625–4635. doi: 10.1093/nar/gkw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiechens N, et al. The Chromatin Remodelling Enzymes SNF2H and SNF2L Position Nucleosomes adjacent to CTCF and Other Transcription Factors. PLoS Genet. 2016;12:e1005940. doi: 10.1371/journal.pgen.1005940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barisic D, Stadler MB, Iurlaro M, Schübeler D. Mammalian ISWI and SWI/SNF selectively mediate binding of distinct transcription factors. Nature. 2019;569:136–140. doi: 10.1038/s41586-019-1115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vary JC, Fazzio TG, Tsukiyama T. Assembly of yeast chromatin using ISWI complexes. Methods Enzymol. 2004;375:88–102. doi: 10.1016/S0076-6879(03)75006-X. [DOI] [PubMed] [Google Scholar]
- 19.Lindstrom KC, Vary JC, Parthun MR, Delrow J, Tsukiyama T. Isw1 functions in parallel with the NuA4 and Swr1 complexes in stress-induced gene repression. Mol. Cell. Biol. 2006;26:6117–6129. doi: 10.1128/MCB.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinskaya M, Nair A, Clynes D, Morillon A, Mellor J. Nucleosome remodeling and transcriptional repression are distinct functions of Isw1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 2009;29:2419–2430. doi: 10.1128/MCB.01050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morillon A, et al. Isw1 chromatin remodeling ATPase coordinates transcription elongation and termination by RNA polymerase II. Cell. 2003;115:425–435. doi: 10.1016/S0092-8674(03)00880-8. [DOI] [PubMed] [Google Scholar]
- 22.Ocampo J, Chereji RV, Eriksson PR, Clark DJ. Contrasting roles of the RSC and ISW1/CHD1 chromatin remodelers in RNA polymerase II elongation and termination. Genome Res. 2019;29:407–417. doi: 10.1101/gr.242032.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babour A, et al. The chromatin remodeler ISW1 Is a quality control factor that surveys nuclear mRNP biogenesis. Cell. 2016;167:1201–1214.e15. doi: 10.1016/j.cell.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Udugama M, Sabri A, Bartholomew B. The INO80 ATP-dependent chromatin remodeling complex is a nucleosome spacing factor. Mol. Cell. Biol. 2011;31:662–673. doi: 10.1128/MCB.01035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krietenstein N, et al. Genomic nucleosome organisation reconstituted with pure proteins. Cell. 2016;167:709–721.e12. doi: 10.1016/j.cell.2016.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vary JC, et al. Yeast Isw1p forms two separable complexes in vivo. Mol. Cell. Biol. 2003;23:80–91. doi: 10.1128/MCB.23.1.80-91.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yen K, Vinayachandran V, Batta K, Koerber RT, Pugh BF. Genome-wide nucleosome specificity and directionality of chromatin remodelers. Cell. 2012;149:1461–1473. doi: 10.1016/j.cell.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Bakel H, et al. A compendium of nucleosome and transcript profiles reveals determinants of chromatin architecture and transcription. PLoS Genet. 2013;9:e1003479. doi: 10.1371/journal.pgen.1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu H, et al. Chromatin remodeler Ino80C acts independently of H2A.Z to evict promoter nucleosomes and stimulate transcription of highly expressed genes in yeast. Nucleic Acids Res. 2020 doi: 10.1093/nar/gkaa571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tirosh I, Sigal N, Barkai N. Widespread remodeling of mid-coding sequence nucleosomes by Isw1. Genome Biol. 2010;11:R49. doi: 10.1186/gb-2010-11-5-r49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellor J, Morillon A. ISWI complexes in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2004;1677:100–112. doi: 10.1016/j.bbaexp.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Lafon A, Petty E, Pillus L. Functional antagonism between Sas3 and Gcn5 acetyltransferases and ISWI chromatin remodelers. PLoS Genet. 2012;8:e1002994. doi: 10.1371/journal.pgen.1002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan L, Wu H, Li X, Gao N, Chen Z. Structures of the ISWI-nucleosome complex reveal a conserved mechanism of chromatin remodeling. Nat. Struct. Mol. Biol. 2019;26:258–266. doi: 10.1038/s41594-019-0199-9. [DOI] [PubMed] [Google Scholar]
- 34.Richmond TJ. Nucleosome recognition and spacing by chromatin remodelling factor ISW1a. Biochem. Soc. Trans. 2012;40:347–350. doi: 10.1042/BST20110748. [DOI] [PubMed] [Google Scholar]
- 35.Gangaraju VK, Bartholomew B. Dependency of ISW1a chromatin remodeling on extranucleosomal DNA. Mol. Cell. Biol. 2007;27:3217–3225. doi: 10.1128/MCB.01731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada K, et al. Structure and mechanism of the chromatin remodelling factor ISW1a. Nature. 2011;472:448–453. doi: 10.1038/nature09947. [DOI] [PubMed] [Google Scholar]
- 37.Santos-Rosa H, et al. Methylation of histone H3 K4 mediates association of the Isw1p ATPase with chromatin. Mol. Cell. 2003;12:1325–1332. doi: 10.1016/S1097-2765(03)00438-6. [DOI] [PubMed] [Google Scholar]
- 38.Smolle M, et al. Chromatin remodelers Isw1 and Chd1 maintain chromatin structure during transcription by preventing histone exchange. Nat. Struct. Mol. Biol. 2012;19:884–892. doi: 10.1038/nsmb.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltby VE, et al. Histone H3 lysine 36 methylation targets the Isw1b remodeling complex to chromatin. Mol. Cell. Biol. 2012;32:3479–3485. doi: 10.1128/MCB.00389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganguli D, Chereji RV, Iben JR, Cole HA, Clark DJ. RSC-dependent constructive and destructive interference between opposing arrays of phased nucleosomes in yeast. Genome Res. 2014;24:1637–1649. doi: 10.1101/gr.177014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiner A, Hughes A, Yassour M, Rando OJ, Friedman N. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 2010;20:90–100. doi: 10.1101/gr.098509.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole HA, Ocampo J, Iben JR, Chereji RV, Clark DJ. Heavy transcription of yeast genes correlates with differential loss of histone H2B relative to H4 and queued RNA polymerases. Nucleic Acids Res. 2014;42:12512–12522. doi: 10.1093/nar/gku1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simic R, et al. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 2003;22:1846–1856. doi: 10.1093/emboj/cdg179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clapier CR, Längst G, Corona DF, Becker PB, Nightingale KP. Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol. Cell. Biol. 2001;21:875–883. doi: 10.1128/MCB.21.3.875-883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clapier CR, Nightingale KP, Becker PB. A critical epitope for substrate recognition by the nucleosome remodeling ATPase ISWI. Nucleic Acids Res. 2002;30:649–655. doi: 10.1093/nar/30.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krajewski WA. Yeast Isw1a and Isw1b exhibit similar nucleosome mobilisation capacities for mononucleosomes, but differently mobilise dinucleosome templates. Arch. Biochem. Biophys. 2014;546:72–80. doi: 10.1016/j.abb.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Smolle M, Workman JL. Transcription-associated histone modifications and cryptic transcription. Biochim. Biophys. Acta. 2013;1829:84–97. doi: 10.1016/j.bbagrm.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pray-Grant MG, Daniel JA, Schieltz D, Yates JR, Grant PA. Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation. Nature. 2005;433:434–438. doi: 10.1038/nature03242. [DOI] [PubMed] [Google Scholar]
- 49.Sims RJ, et al. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J. Biol. Chem. 2005;280:41789–41792. doi: 10.1074/jbc.C500395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamiche A, Kang JG, Dennis C, Xiao H, Wu C. Histone tails modulate nucleosome mobility and regulate ATP-dependent nucleosome sliding by NURF. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14316–14321. doi: 10.1073/pnas.251421398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundaramoorthy R, et al. Structure of the chromatin remodelling enzyme Chd1 bound to a ubiquitinylated nucleosome. elife. 2018;7:e35720. doi: 10.7554/eLife.35720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferreira H, Flaus A, Owen-Hughes T. Histone modifications influence the action of Snf2 family remodelling enzymes by different mechanisms. J. Mol. Biol. 2007;374:563–579. doi: 10.1016/j.jmb.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dang W, Kagalwala MN, Bartholomew B. Regulation of ISW2 by concerted action of histone H4 tail and extranucleosomal DNA. Mol. Cell. Biol. 2006;26:7388–7396. doi: 10.1128/MCB.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark DJ, Kimura T. Electrostatic mechanism of chromatin folding. J. Mol. Biol. 1990;211:883–896. doi: 10.1016/0022-2836(90)90081-V. [DOI] [PubMed] [Google Scholar]
- 55.Blank TA, Becker PB. Electrostatic mechanism of nucleosome spacing. J. Mol. Biol. 1995;252:305–313. doi: 10.1006/jmbi.1995.0498. [DOI] [PubMed] [Google Scholar]
- 56.Eriksson PR, et al. Global regulation by the yeast Spt10 protein is mediated through chromatin structure and the histone upstream activating sequence elements. Mol. Cell. Biol. 2005;25:9127–9137. doi: 10.1128/MCB.25.20.9127-9137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhillon N, Kamakaka RT. A histone variant, Htz1p, and a Sir1p-like protein, Esc2p, mediate silencing at HMR. Mol. Cell. 2000;6:769–780. doi: 10.1016/S1097-2765(00)00076-9. [DOI] [PubMed] [Google Scholar]
- 58.Clark-Adams CD, Norris D, Osley MA, Fassler JS, Winston F. Changes in histone gene dosage alter transcription in yeast. Genes Dev. 1988;2:150–159. doi: 10.1101/gad.2.2.150. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y, McLaughlin N, Lindstrom K, Tsukiyama T, Clark DJ. Activation of Saccharomyces cerevisiae HIS3 results in Gcn4p-dependent, SWI/SNF-dependent mobilisation of nucleosomes over the entire gene. Mol. Cell. Biol. 2006;26:8607–8622. doi: 10.1128/MCB.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 61.Cole HA, Howard BH, Clark DJ. Activation-induced disruption of nucleosome position clusters on the coding regions of Gcn4-dependent genes extends into neighbouring genes. Nucleic Acids Res. 2011;39:9521–9535. doi: 10.1093/nar/gkr643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chereji RV, Ocampo J, Clark DJ. MNase-sensitive complexes in yeast: nucleosomes and non-histone barriers. Mol. Cell. 2017;65:565–577.e3. doi: 10.1016/j.molcel.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available in the NCBI Gene Expression Omnibus (GEO) repository [GSE156224]. Published data sets used in the current study are available in the GEO repository (MNase-seq data for YJO484: GSE11751422; Rpb3 ChIP-seq data: GSE6940015).