Abstract
Background:
Childhood asthma developmental programming is complex. Maternal asthma is a strong risk factor for childhood asthma, while vitamin D (VD) has emerged as a modifiable prenatal exposure.
Objective:
To examine the combined effect of early and late prenatal VD status in pregnancies with and without asthma on childhood asthma/recurrent wheeze development.
Methods:
We conducted a cohort study using prospectively collected data from the Vitamin D Antenatal Asthma Reduction Trial (VDAART), a randomized, double-blinded, placebo-controlled vitamin D supplementation trial in pregnant women at high risk of offspring asthma (N=806 mother-offspring pairs). 25-hydroxyvitamin-D [25(OH)D] was measured in early and late pregnancy. Our main exposure was an ordered variable representing early and late prenatal VD sufficiency [25(OH)D≥30ng/mL)] status in pregnancies with and without asthma. The primary outcome was offspring asthma or recurrent wheeze by age 3 years. We also examined the effect of prenatal VD on early life asthma/recurrent wheeze progression to active asthma at age 6 years.
Results:
Among maternal asthmatics, compared to those with early and late prenatal VD insufficiency, those with early or late VD sufficiency (aOR=0.56; 95% CI 0.31-1.00) or early and late VD sufficiency (aOR=0.36; 95% CI 0.15-0.81) had lower risk of offspring asthma/recurrent wheeze by age 3 years (Pfor trend=0.008). This protective trend was reiterated in asthma/recurrent wheeze progression to active asthma from age 3 to 6 years (Pfor trend=0.04).
Conclusion:
This study implicates a protective role for VD sufficiency throughout pregnancy, particularly in attenuating the risk conferred by maternal asthma on childhood asthma/recurrent wheeze development.
Clinical Implication:
Early identification and correction of vitamin D insufficiency in asthmatic pregnant women have implications for the prevention of early offspring asthma/recurrent wheeze and the persistence of offspring asthma to later childhood.
Capsule summary:
Our data support the role of early and late-pregnancy vitamin D sufficiency in attenuation of the risk of offspring asthma or/and recurrent wheeze in early childhood and the progression to active asthma at later childhood.
Keywords: pregnancy, maternal asthma, prenatal, vitamin D, childhood, recurrent wheeze, asthma
Graphical Abstract

Introduction
Childhood asthma is a complex, chronic disease of the airways with multiple associated risk factors that potentially interact with each other. It is the most common chronic disease of childhood, affecting approximately 6.2 million children (age <18 years) in the United States with a prevalence of 8.3% of children in 2017; similar rates are reported globally1-4. The high economic cost of asthma was estimated at over $81 billion in 20135. The medical burden of asthma and wheezing illness in childhood may extend into adolescence and adulthood, with sequelae including lung function abnormalities6, 7. Therefore, preventive measures targeting modifiable risk factors in at-risk populations could reduce the global burden of asthma.
Maternal asthma is a well-established risk factor for childhood wheezing and asthma8. Nevertheless, the developmental programming of childhood asthma is far more complex, encompassing pre- and post-natal environmental factors. Among these factors, the role of vitamin D in childhood asthma is increasingly highlighted as more is known about its pleiotropic effects on immunomodulation, lung development, and the microbiome9-12. In pregnancy, vitamin D is now known to exert effects throughout pregnancy and perhaps as early as the pseudoglandular stage (5-17 weeks)10. Cord blood vitamin D (VD) status is dependent on prenatal VD status, and its deficiency is associated with early and late-childhood asthma and wheeze13-15, suggesting a role for VD sufficiency from the early to late prenatal period, potentially extending throughout childhood. The Vitamin D Antenatal Asthma Reduction Trial (VDAART) was designed to investigate the effect of vitamin D supplementation during pregnancy on the prevention of childhood asthma and wheeze in high-risk pregnancies for childhood asthma and atopy16. The data from the VDAART cohort by age of 3 years is consistent with prior investigations that maternal asthma is a strong predictor of early childhood recurrent wheeze and asthma and that cord blood VD sufficiency could also have a role in the prevention of the early life respiratory adverse outcomes15, 17. However, the combined effects of maternal asthma status and prenatal VD sufficiency status, beginning in early pregnancy, on childhood asthma and wheeze have not been assessed to date.
Here, we examined the effect of VD status across multiple prenatal time points combined with the effect of maternal asthma status on the development of childhood asthma or recurrent wheeze (asthma/recurrent wheeze) by age 3 years. We hypothesized that children born to mothers with VD sufficiency during pregnancy have a reduced risk of childhood asthma/recurrent wheeze compared to mothers with VD insufficiency during early and late pregnancy.
Methods
Study design and participants
The present study is a secondary analysis of the VDAART. The details of VDAART are previously described16, 18. In brief, VDAART is a randomized, double-blind, placebo-controlled trial of pregnant women randomized to intervention (4400 IU vitamin D daily) or placebo (multivitamin containing 400 IU vitamin D daily). Maternal-offspring pairs with offspring follow-up data available for the primary trial outcome of “asthma/recurrent wheeze” by age 3 years were used as the source population for this analysis (N=806, intention-to-treat [ITT] population; Figure I). Children continued to be followed for the assessment of their asthma status at age 6. Eligible participants in the VDAART were non-smoking, pregnant women aged 18-39 years at 10-18 weeks of gestation; with a personal history of asthma or atopy or partner’s (biologic father of the child) history of asthma or atopy; and intent to participate for at least four years. All participants provided written consent to participate in the trial. Supplemental File I provides further details on the eligibility and exclusion criteria of the VDAART participants. VDAART was approved by the Institutional Review Boards of the three clinical sites and of the data coordinating center, the Channing Division of Network Medicine at Brigham and Women’s Hospital.
Figure I.
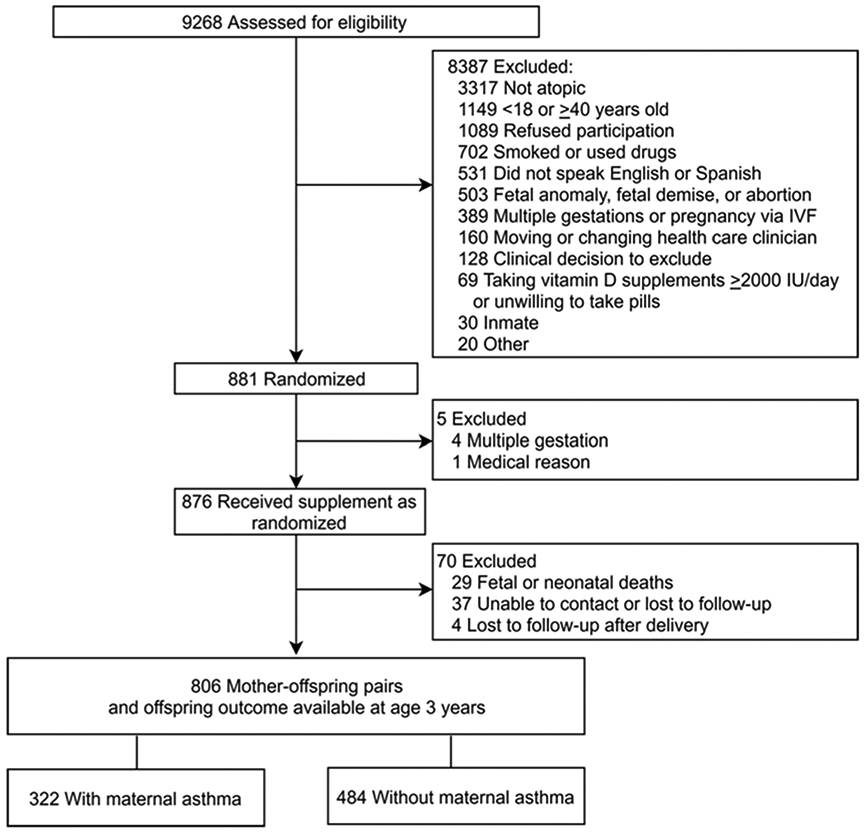
CONSORT diagram
Vitamin D measurement
When discussing VD, we refer to 25-hydroxyvitamin D [25(OH)D], the major circulating form of VD. VD levels in the prenatal period were collected at early pregnancy [10-18 weeks of gestational age (wga)] and late pregnancy (32-38 wga). Prenatal VD levels were measured per VDAART protocol16. Maternal prenatal 25(OH)D levels were obtained using the DiaSorin LIAISON® method, a validated chemiluminescence assay, performed at the Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA19. The assay is co-specific for 25(OH)D3 and 25(OH)D2. Inter-assay CV’s for 25(OH)D2 and 25(OH)D3 were all ≤ 6.8% at various concentrations of samples processed and total 25(OH)D values were used for the analysis reported here.
Primary outcome: Offspring asthma/recurrent wheeze
The main outcome of this secondary analysis is the same as the primary outcome of the initial VDAART report, i.e., combined outcome of asthma/recurrent wheeze by age 3 years16. Offspring asthma was defined as a parental report of physician-diagnosed asthma. Recurrent wheeze was defined by one of the following definitions: 1) parental report of wheeze before and after the child’s second birthday; 2) parental report of wheeze before child’s second birthday and use of asthma medication (inhaled corticosteroids, leukotriene modifiers, oral steroids) after the second birthday; 3) two or more distinct parental reports of wheeze after the child’s second birthday; 4) two or more distinct reports of asthma medications as noted above after the child’s second birthday; 5) one parental report of wheeze and episode of asthma medication (as noted above) at separate time points after the child’s second birthday16.
Children assessed for the primary outcome were followed up to determine their asthma status at age 6. Therefore, we additionally examined the outcome of asthma/recurrent wheeze by age 3 years persisting as active asthma at age 6 years. Active asthma was defined per the VDAART 6-year follow-up report: physician-diagnosed asthma at any time in the first 6 years of life plus a report of child’s use of any asthma medication or report of wheeze after the 5th birthday20.
Main study variables
Maternal asthma status and prenatal VD sufficiency status were the main exposures of interest. Completed questionnaires at enrollment and monthly maternal health questionnaires thereafter provided the information on the presence or absence of maternal asthma. A woman was considered to have asthma if she reported physician-diagnosed asthma at any time in her life. VD sufficiency was defined at a 25(OH)D threshold of ≥30 ng/mL based on the Endocrine Society’s recommendations for maximum health benefits21 and prior observation in the relationship of pregnancy VD status and risk of childhood asthma and other adverse pregnancy outcomes17, 22, 23; otherwise, subjects were considered VD insufficient.
Prenatal VD status (in the following order: early pregnancy at 10-18 wga, late pregnancy at 32-38 wga) was represented using a composite ordered variable with three exposure levels: early and late prenatal insufficiency, partial prenatal sufficiency (sufficiency at either early or late pregnancy time point), early and late prenatal sufficiency. To evaluate the effect of the early versus late prenatal time point in a sensitivity analysis, we additionally examined a composite, 4-level ordered exposure level: early and late prenatal insufficiency, early sufficiency and late prenatal insufficiency, early insufficiency and late prenatal sufficiency, early and late prenatal sufficiency.
Additional study variables
Additional study variables were selected as a priori determinants or potential confounders in the relationship of the outcome and main variables of interest: maternal age, maternal income, maternal atopy, paternal asthma, trial arm, study site, preterm birth (<37 wga: yes or no), child gender and child race.
Statistical analysis
The main study groups were mothers with and without a personal history of asthma. For the comparison of baseline characteristics between maternal subjects with and without asthma, we used the Student’s t-test and chi-square or Fisher’s exact test, as appropriate. The primary analysis was performed in three steps. Firstly, we assessed the risk of offspring asthma/recurrent wheeze by age of 3 years in high-risk pregnancies with asthma relative to low-risk pregnancies without asthma, regardless of the VD status during pregnancy. Secondly, we examined how the risk of offspring asthma/recurrent wheeze might differ across the ordered variable of VD status representing the prenatal sufficiency status (at early and late gestation) among pregnancies with and without asthma. Thirdly, to examine whether the postulated protective effect of prenatal vitamin D extended into later childhood, we examined whether prenatal VD status influenced the risk of offspring asthma/recurrent wheeze by age 3 years progressing into active asthma at age 6 years.
The trend of risk estimates was assessed using an extended Mantel-Haenszel chi-square test for linear trend with correction for continuity. We used multivariable logistic regression to obtain the adjusted odds of the outcome with inclusion of covariates and potential confounders and the trend across ordered levels of exposure variable was re-examined. We tested for an interaction between prenatal VD status and maternal asthma. Crude and adjusted odds ratios (OR and aOR) were calculated with 95% confidence intervals (CI) and corresponding P-values were obtained. The Hosmer-Lemeshow (H-L) test was used to assess logistic regression model goodness-of-fit for all models. All statistical analysis was performed using the R statistical software version 3.6.2 (packages: effects, performance). All tests were two-sided and a P-value less than a pre-specified alpha of 0.05 was considered statistically significant.
Sensitivity analysis
To assess the effect of early vs late VD sufficiency on the risk of the primary outcome of asthma/recurrent wheeze, we firstly examined a 4-level composite, prenatal VD exposure as defined above as well as the individual effects of baseline and 3rd trimester VD on a continuous scale. Next, to assess the robustness of our definition of offspring asthma, we examined the outcome of asthma and recurrent wheeze (offspring who had a physician diagnosis of asthma and also recurrent wheeze) by age 3 years.
Results
Baseline Characteristics and Vitamin D Status by Study Time Points
The VDAART ITT population included 806 mother-offspring pairs (Figure I), of whom, 322 mothers (322/806 = 40.0%) had a history of physician-diagnosed asthma. Maternal asthma status and offspring asthma/recurrent wheeze outcomes by age 3 years were available for all subjects. Baseline characteristics were balanced between subjects with and without maternal asthma except for younger maternal age at enrollment among mothers with asthma compared to those without asthma (mean difference – 0.9 years, SD 5.5; Table I). The 6-year follow-up data for active asthma status was available in 87.7% of the ITT population (707/806), of whom, 83.5% (673/806) had early and late VD measures.
Table I.
Baseline characteristics in the VDAART ITT population by maternal asthma status (N=806) a
| Maternal asthma (n=322) |
No maternal asthma (n=484) |
P value | |
|---|---|---|---|
| Maternal age (mean, SD) | 26.7 (5.4) | 27.8 (5.5) | <0.01 |
| Maternal income | 0.06 | ||
| Less than <$50,000 | 151 (46.9) | 190 (39.3) | |
| $50,000 or greater | 94 (29.2) | 175 (36.2) | |
| Prefer not to answer or do not know | 77 (23.9) | 119 (24.6) | |
| Maternal education | 0.29 | ||
| Less than college | 144 (44.7) | 197 (40.7) | |
| Some college, college graduate or graduate school | 178 (55.3) | 287 (59.3) | |
| Treatment arm: intervention (vitamin D 4400 IU/day) | 171 (53.1) | 234 (48.3) | 0.21 |
| Site name | 0.89 | ||
| Boston | 93 (28.9) | 147 (30.4) | |
| San Diego | 112 (34.8) | 162 (33.5) | |
| St. Louis | 117 (36.3) | 175 (36.2) | |
| Maternal atopy: yes | 252 (78.3) | 358 (74.0) | 0.19 |
| Vitamin D sufficiency statusb | |||
| Baseline sufficiency (10-18 wga) | 70 (21.9) | 106 (22.0) | 1.00 |
| Missingness (n) | 2 | 3 | |
| 3rd trimester sufficiency (32-38 wga) | 175 (57.2) | 247 (53.2) | 0.32 |
| Missingness (n) | 16 | 20 | |
| Preterm delivery (<37 weeks) | 27 (8.4) | 44 (9.1) | 0.81 |
| Child gender: male | 178 (55.3) | 243 (50.2) | 0.18 |
| Child race | 0.39 | ||
| Black | 165 (51.6) | 225 (47.0) | |
| White | 98 (30.6) | 167 (34.9) | |
| Other | 57 (17.8) | 87 (18.2) |
Abbreviations: ITT: intention to treat; SD: standard deviation
Data are given as number (percentage) of individuals, unless otherwise specified.
Vitamin D sufficiency defined as 25(OH)D≥30ng/mL
SI conversion factor: To convert 25(OH)D from ng/mL to nmol/L, multiply by 2.496.
Abbreviations: wga, weeks of gestational age
Overall, 766 subjects (766/806=95.04%) had VD measurement for both prenatal time points and were included for this analysis. The proportion of subjects with VD sufficiency was higher in the 3rd trimester than at baseline (420/766 = 54.8% vs 166/766 = 21.7% respectively, P<0.001). There was no significant difference in VD sufficiency status between those with and without asthma at either of the prenatal time points.
Maternal Asthma and Risk of “Offspring Asthma/Recurrent Wheeze”
Two hundred and two out of 766 offspring (26.4%) had physician-diagnosed asthma/recurrent wheeze prior to age 3 years. The proportion of offspring asthma/recurrent wheeze by age of 3 years was higher among children whose mothers were asthmatic versus non-asthmatic (34.2% versus 21.2%, P<0.001; respectively). Accordingly, children whose mothers were asthmatic had almost two times higher odds of developing asthma/recurrent wheeze by age of 3 years than those whose mothers were not asthmatic, even after adjusting for maternal age, maternal income, maternal atopy, paternal asthma, treatment arm, study site, preterm birth, child gender and child race (Table II). The Hosmer-Lemeshow test for this and all subsequent models demonstrated adequate goodness of fit (P ≥0.05).
Table II.
Risk of “offspring asthma/recurrent wheeze” by age 3 years according to maternal asthma status and prenatal vitamin D status among pregnant women with and without asthma
| Offspring asthma/recurrent wheeze |
Univariable | Multivariable a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Total (case %) |
OR (95% CI) | P-value | P for trend | Adjusted OR (95% CI) | P-value | P for trend | |
| Maternal asthma status | |||||||||
| No maternal asthma | 98 | 364 | 462 (21.2) | Ref | -- | -- | Ref | -- | -- |
| Maternal asthma | 104 | 200 | 304 (34.2) | 1.93 (1.40, 2.68) | <0.001 | -- | 2.03 (1.43, 2.87) | <0.001 | -- |
| Total | 202 | 564 | 766 (26.4) | ||||||
| Prenatal vitamin D status in mothers without asthma | |||||||||
| Early & late prenatal insufficiency b (insufficient & insufficient) c | 44 | 151 | 195 (22.6) | Ref | -- | 0.40 | Ref | -- | 0.75 d |
| Partial prenatal sufficiency (sufficient or sufficient) | 40 | 148 | 188 (21.3) | 0.93 (0.57, 1.51) | 0.76 | 1.23 (0.69, 2.17) | 0.48 | ||
| Early & late prenatal sufficiency (sufficient & sufficient) | 14 | 65 | 79 (17.7) | 0.74 (0.37, 1.41) | 0.38 | 1.05 (0.46, 0.23) | 0.91 | ||
| Total | 98 | 364 | 462 (21.2) | ||||||
| Prenatal vitamin D status in mothers with asthma | |||||||||
| Early & late prenatal insufficiency (insufficient & insufficient) | 54 | 66 | 120 (45) | Ref | -- | <0.001 | Ref | -- | 0.008 e |
| Partial prenatal sufficiency (sufficient or sufficient) | 39 | 89 | 128 (30.5) | 0.54 (0.32, 0.90) | 0.02 | 0.56 (0.31, 1.00) | 0.05 | ||
| Early & late prenatal sufficiency (sufficient & sufficient) | 11 | 45 | 56 (19.6) | 0.30 (0.14, 0.62) | 0.002 | 0.36 (0.15, 0.81) | 0.02 | ||
| Total | 104 | 200 | 304 (34.2) | ||||||
Abbreviations: OR, odds ratio.
Adjusted for maternal age, maternal income, maternal atopy, paternal asthma, treatment arm, study site, preterm birth, child gender and child race.
Vitamin D sufficiency defined as 25(OH)D ≥ 30 ng/mL
In the following order: baseline (10-18 weeks gestational age), 3rd trimester (32-38 weeks gestational age)
H-L goodness-of-fit test: P ≥0.05. McFadden’s R2: 0.15.
H-L goodness-of-fit test: P ≥0.05. McFadden’s R2: 0.18.
Effect of Early and Late Pregnancy VD Sufficiency Status on “Offspring Asthma/Recurrent Wheeze” by Maternal Asthma Status
In examining the effect of early and late prenatal VD status on offspring asthma/recurrent wheeze, a significant interaction was detected between prenatal VD and maternal asthma status (Pinteraction=0.03). Among pregnant women with asthma, a decreasing trend in risk of having a child with asthma/recurrent wheeze across the composite, ordered variable representing prenatal VD status indicated a benefit from partial (early or late) prenatal VD sufficiency during pregnancy or maintenance of VD sufficiency in early and late pregnancy (Pfor trend <0.001; Table II). In the adjusted model, the trend across the ordered levels of VD exposure remained significant (Pfor trend=0.008) with the highest decrease (≈ 65%) in risk of offspring asthma/recurrent wheeze for asthmatic mothers who had early and late prenatal VD sufficiency compared with those with VD insufficiency at both early and late pregnancy (Table II).
In a sensitivity analysis of the effect of the early versus late prenatal time point, we utilized an expanded 4-level prenatal VD exposure. Among with women with asthma, compared to those with early and late VD insufficiency, women with early VD insufficiency, but late sufficiency had a 50% reduced risk of offspring asthma/recurrent wheeze (aOR=0.52; 95% CI 0.28, 0.94; P=0.03); the greatest risk reduction (≈ 65%) was observed among women with VD sufficiency in early and late pregnancy (aOR=0.35; 95% CI 0.15, 0.81; P=0.02). There was no significant effect among women with VD sufficiency only in early pregnancy or among women without asthma.
Among pregnant women without asthma, a trend in risk reduction of offspring asthma/recurrent wheeze was not observed across the ordered variable representing 3 levels of prenatal VD exposure status (P for trend = 0.75, Table II). Children born to mothers without asthma who had VD sufficiency at both early and late pregnancy did not show a lower risk of asthma/recurrent wheeze as compared to those born to pregnancies without asthma who were VD insufficient at both early and late pregnancy (Table II). There was no difference in the proportion of offspring asthma/recurrent wheeze in pregnant women with and without asthma who had VD sufficiency in both early and late pregnancy (11/56 = 19.6% versus 14/79 = 17.7%, P=0.95).
Effect of Early and Late Pregnancy VD Sufficiency Status on “Offspring Asthma and Recurrent Wheeze”
One hundred and fourteen out of 766 offspring (14.9%) had asthma by age 3 years, of which, 86 offspring also had recurrent wheeze (86/766 = 11.2%). Considering “offspring asthma and recurrent wheeze” as the outcome, we observed a similar trend in risk reduction of the outcome with increasing prenatal VD exposure (Pfor trend =0.01) among offspring of mothers with asthma, with the greatest risk reduction in those with early and late prenatal VD sufficiency (aOR=0.19; 95% CI: 0.41, 0.64; Supplemental File II-Table SI).
Furthermore, both baseline and 3rd trimester VD levels separately demonstrated an inverse relationship with the risk of offspring asthma and recurrent wheeze among women with asthma (Supplemental File III-Figure SI). In the adjusted model, the effect of 3rd trimester, but not baseline, VD remained significant among women with asthma. Every 10-unit increase in 3rd trimester VD level was associated with 22% reduction in risk of offspring asthma and recurrent wheeze (aOR10-unit=0.78; 95% CI 0.62, 0.96).
Progression of Early Childhood Asthma/Recurrent Wheeze to Active Asthma at Age 6 Years
A total of 673 out of the 766 offspring (87.9%) with year 3 outcomes and prenatal VD values available also had year 6 outcomes ascertained. Among mother-offspring pairs with early & late prenatal insufficiency, 42 out of 211 offspring (24.9%) had the outcome of “early asthma/recurrent wheeze by age of 3 years and active asthma at age 6 years”. In contrast, 6 out of 93 offspring (6.5%) with early & late prenatal sufficiency had progression of asthma/recurrent wheeze to active asthma. In a model adjusted for the same covariates and additionally maternal asthma status, a linear trend towards reduced risk of the outcome was observed across the ordered variable representing prenatal VD status with the greatest reduction among those with sufficiency at both prenatal time points (Pfor trend=0.04; Table III).
Table III.
Effect of prenatal vitamin D sufficiency status on “asthma/recurrent wheeze by age 3 years and active asthma at 6 years”
| Offspring asthma/recurrent wheeze by age 3 years and active asthma by age 6 years |
Univariable | Multivariable a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No d | Total (case %) |
OR (95% CI) | P-value |
P for trend |
Adjusted OR (95% CI) | P-value | P for trend | |
| Prenatal vitamin D status in mothers | |||||||||
| Early & late prenatal insufficiency b (insufficient & insufficient) c | 42 | 169 | 211 (24.9) | Ref | -- | 0.003 | Ref | -- | 0.04 e |
| Partial prenatal sufficiency (sufficient or sufficient) | 32 | 193 | 225 (14.2) | 0.67 (0.40, 1.10) | 0.12 | 0.68 (0.37, 1.25) | 0.26 | ||
| Early & late prenatal sufficiency (sufficient & sufficient) | 6 | 87 | 93 (6.5) | 0.28 (0.10, 0.63) | 0.005 | 0.37 (0.12, 0.97) | 0.05 | ||
| Total | 80 | 449 | 529 (15.1) f | ||||||
Abbreviations: OR, odds ratio.
Adjusted for maternal asthma, maternal age, maternal income, maternal atopy, paternal asthma, maternal asthma, treatment arm, study site, preterm birth, child gender and child race.
Vitamin D sufficiency defined as 25(OH)D ≥ 30 ng/mL
In the following order: baseline (10-18 weeks gestational age), 3rd trimester (32-38 weeks gestational age)
Defined as offspring without asthma/recurrent wheeze by age 3 years and without active asthma at age 6 years.
H-L goodness-of-fit test: P≥0.05; McFadden’s R2: 0.26
A total of 529 out of 673 subjects with prenatal VD and year 3 and year 6 outcome met the definition for the selective phenotype above (footnote c), thus excluding 144 offspring with only asthma/recurrent by age 3 years or active asthma at age 6 years.
Discussion
This study contributes to the existing literature as it examines the effects of prenatal VD sufficiency status and maternal asthma status in pregnancies with and without asthma on childhood asthma/recurrent wheeze. Asthmatic mothers had a two times higher risk of having a child with asthma/recurrent wheeze prior to the age of 3 years compared to non-asthmatic mothers. However, this risk among asthmatic mothers was substantially attenuated if they were VD sufficient (≥30ng/mL) at early and late pregnancy. Therefore, women with asthma who start their pregnancies with high levels of VD and remain VD sufficient throughout pregnancy are likely to experience a reduced risk of asthma/recurrent wheeze in their children prior to age 3 years. Yet, based on our sensitivity analysis with a 4-level prenatal VD exposure to evaluate the weight of the early versus late time point, pregnant women with asthma and VD insufficiency at their early pregnancy might also benefit from the improvement of their VD status during pregnancy.
The optimal dosage of vitamin D supplementation and the definition of VD sufficiency have generated much debate. At present, there is no consensus as to what constitutes VD sufficiency for non-bone related outcomes. Indeed, sufficient levels may vary depending on the health outcome of interest, with vitamin D exerting differential effects on skeletal and extra-skeletal health, and between the pregnant versus non-pregnant state23. Our investigation of prenatal VD sufficiency at a threshold of 30 ng/mL demonstrated the greatest risk reduction of offspring asthma/recurrent wheeze in mothers with asthma, though there was a role for partial prenatal VD sufficiency. Considering vitamin D’s pleiotropic effects, it is also possible that there may be distinct or interacting mechanisms by which it influences fetal lung programming depending on baseline VD levels and in a dose-response fashion as we have observed. Our study suggests that a threshold of 30 ng/mL sufficiency in early pregnancy appears to be a minimum threshold with regards to the risk of early childhood asthma/recurrent wheeze development. Given the observed dose-response effects of VD at the early and particularly late pregnancy, higher levels may be more protective in pregnancies with high-risk for early childhood asthma. Nevertheless, this observation is a matter of further investigation for effectiveness and safety.
A regulatory role for vitamin D has been implicated as early as the 2nd week of gestation in murine experiments of fetal lung development and as early as the pseudoglandular stage of lung development (5-17 weeks) in human fetal lung programming, potentially more so affecting developing lung transcriptomes among asthmatics10, 24. However, what has been unclear is the temporal contribution of vitamin D to lung development in early versus late pregnancy. Collectively with our results, these findings highlight a role for ensuring early prenatal VD sufficiency status, and additionally, the maintenance of sufficient VD levels through pregnancy in providing the greatest reduction of childhood adverse respiratory outcome development.
The literature supports a role for vitamin D supplementation, most probably among those with prenatal VD deficiency, which is common in pregnancy25,26. It should be also noted that observational studies following 25(OH)D levels across pregnancy have shown that 25(OH)D levels remain either unchanged or decrease over time27, 28. A randomized controlled trial of vitamin D supplementation in pregnant women found considerable VD deficiency at the time of delivery as well, with median serum 25(OH)D levels of 24 nmol/L (10 ng/mL) consistent with the deficiency in the control arm, versus 65 nmol/L (26 ng/mL) in the intervention arm, which was supplemented with vitamin D beginning at 20 weeks of gestation with dosing based on the serum 25(OH)D levels at the time of study entry25.
Notably, in our study, the protective effect of VD sufficiency in pregnancy on offspring outcomes by age 3 years was seen only among mothers with asthma. This observation implicates a distinct role for VD sufficiency throughout pregnancy and maternal asthma beyond the heritability due to maternal asthma status alone, which has not previously been reported.
Specifically, variation in genetic pathways of immune response and lung development may modulate the effect of VD sufficiency in pregnancy. However, in a sensitivity analysis and considering the outcome of “asthma/recurrent wheeze by age 3 years and active asthma at age 6 years”, we observed a linear trend in prenatal VD status effect independent of maternal asthma status.
Vitamin D has been associated with reductions in asthma exacerbations and respiratory infections29-31. This effect may be of particular importance in asthmatic pregnant women as impairment in asthma control and an increase in asthma severity during pregnancy are associated with perinatal morbidities, including pre-term labor, preeclampsia, and low birth weight32,33. These observations, along with our findings, warrant further studies to investigate the underlying mechanisms of our observed effect of vitamin D in risk reduction of offspring asthma/recurrent wheeze, particularly among children born to mothers who had asthma.
Suggested roles for prenatal vitamin D supplementation include an effect on transient early childhood wheeze through the reduction of viral lower respiratory tract infections rather than a reduction in childhood asthma34. Indeed, the diagnosis of asthma in pre-school children is difficult and often made in retrospect, making it difficult to predict which children will develop later childhood asthma on the basis of early life wheezing. The recently published results of the VDAART 6-year ITT follow-up indicate that, while there was no significant effect of VD supplementation on the development of school-age asthma/recurrent wheeze by age 6 years, an effect was seen in airway resistance measures on impulse oscillometry in offspring of mothers receiving high dose of vitamin D supplementation using the ITT analysis. Here, we report a trend towards a reduced risk of early life asthma/recurrent wheeze progressing into school-age active asthma with improvement and maintenance of VD sufficiency during pregnancy. This observation suggests that prenatal VD supplementation may yet have an effect on childhood asthma outcomes, at least among those with low VD status. Measures such as level-based assessment of VD effect, early vitamin D supplementation, and assessment in populations at highest risk of offspring asthma may help further define the role of vitamin D in supplementation trials for prevention of childhood asthma.
There were several strengths and limitations to this study. This analysis was performed using data collected from a clinical trial of women and infants closely followed longitudinally. All asthma diagnoses are based on the reported physician diagnosis of asthma, which is used most commonly. We further increased the precision of this diagnosis by examining the outcome of asthma and recurrent wheeze. Notably, by following the effect of prenatal vitamin D out to age 6 years, we observed a trend in risk reduction of early life asthma/recurrent wheeze to later childhood asthma influenced by early and late VD sufficiency on the progression of this phenotype. This implicates a role for prenatal vitamin D in prevention of childhood asthma and wheezing phenotypes beyond transient wheeze. VD exposure was directly measured instead of indirectly estimated using surrogate measures such as dietary intake and were collected at multiple time points. We did not observe the same pattern of risk reduction among women without asthma, potentially due to the fact that a larger sample size might be required to capture a prenatal effect of VD exposure, if any, throughout pregnancy. This is a limitation particularly given the lower baseline risk of offspring asthma among pregnancies without asthma as compared to those with maternal asthma, the strongest risk factor for childhood asthma. It is noted that the VDAART was designed for evaluating prenatal vitamin D supplementation and its effect on the maternal VD levels and trial outcome of asthma/recurrent wheeze by age of 3 years. Therefore, we considered the prenatal VD status in early and late pregnancy as the main exposure for this study. The question of how prenatal and postnatal vitamin D supplementation, as well as induced status in mother and child, affect childhood asthma is a matter of further investigation. We also acknowledge that maternal asthma status did not differentiate between current or prior asthma, though our estimates of the effect of maternal asthma status on offspring asthma/recurrent wheeze are in line with what has been reported in the literature8,35. Finally, some studies suggest a weaker effect of paternal asthma on the risk of childhood asthma compared with the effect of maternal asthma35. While we did examine the effect of paternal asthma on our results, the results for maternal asthma and vitamin D did not change as a function of paternal asthma, as was also previously shown in the VDAART10.
In conclusion, this study implicates a protective effect of prenatal VD sufficiency throughout pregnancy on the development of childhood asthma/recurrent wheeze by age 3 years, particularly in attenuating the increased baseline risk conferred by maternal asthma. Further studies may shed light on the underlying mechanisms as this effect appears to interact with the genetic contribution from maternal asthma risk. Furthermore, the protective trend of early and late prenatal VD sufficiency appears to extend from the progression of early life asthma/recurrent wheeze to asthma at age 6 years. These investigations have implications for identifying populations of pregnant women at greater risk for offspring asthma and recurrent wheeze outcomes due to combined VD insufficiency and a history of maternal asthma.
Supplementary Material
Acknowledgements:
We thank the participants of the VDAART for their contributions and support.
Funding: The Vitamin D Antenatal Asthma Reduction Trial (VDAART) was funded by the National Heart, Lung, and Blood Institute (U01 HL091528 and R01 HL091528 to S.T.W and A.A.L.). ML has received research support from the National Heart, Lung, and Blood Institute (T32 HL007427). HM has received research support from the National Heart, Lung, and Blood Institute (2L30 HL129467-02A1, U01 HL091528, and 1 K01HL146977 01A1).
Abbreviations
- VDAART
Vitamin D Antenatal Asthma Reduction Trial
- 25(OH)D
25-hydroxyvitamin D
- ITT
intention-to-treat
- wga
weeks gestational age
- H-L
Hosmer-Lemeshow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: This study is an ancillary analysis from the VDAART, which is registered with ClinicalTrials.gov (NCT00902621).
Disclosure of conflict of interest: The authors declare no potential conflict of interest pertaining to their contribution and this submission.
References
- 1.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital Signs: Asthma in Children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep 2018; 67:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics 2016; 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sears MR. Trends in the prevalence of asthma. Chest, 2014; 145:219–225. [DOI] [PubMed] [Google Scholar]
- 4.Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Respiratory Medicine 2017; 5:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc 2018; 15:348–56. [DOI] [PubMed] [Google Scholar]
- 6.Duijts L, Granell R, Sterne JA, Henderson AJ. Childhood wheezing phenotypes influence asthma, lung function and exhaled nitric oxide fraction in adolescence. Eur Respir J 2016; 47:510–9. [DOI] [PubMed] [Google Scholar]
- 7.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med 2016; 374:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirzakhani H, Carey VJ, Zeiger R, Bacharier LB, O'Connor GT, Schatz MX, et al. Impact of parental asthma, prenatal maternal asthma control, and vitamin D status on risk of asthma and recurrent wheeze in 3-year-old children. Clinical and Experimental Allergy 2019; 49:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornsby E, Pfeffer PE, Laranjo N, Cruikshank W, Tuzova M, Litonjua AA, et al. Vitamin D supplementation during pregnancy: Effect on the neonatal immune system in a randomized controlled trial. J Allergy Clin Immunol 2018; 141:269–78 e1. [DOI] [PubMed] [Google Scholar]
- 10.Kho AT, Sharma S, Qiu W, Gaedigk R, Klanderman B, Niu S, et al. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med Genomics 2013; 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, et al. Factors influencing the infant gut microbiome at age 3-6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol 2017; 139:482–91 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss ST. Bacterial components plus vitamin D: The ultimate solution to the asthma (autoimmune disease) epidemic? Journal of Allergy and Clinical Immunology 2011; 127:1128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawes BL, Bonnelykke K, Jensen PF, Schoos AM, Heickendorff L, Bisgaard H. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: the COPSAC2000 birth cohort study. PLoS One 2014; 9:e99856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollams EM, Teo SM, Kusel M, Holt BJ, Holt KE, Inouye M, et al. Vitamin D over the first decade and susceptibility to childhood allergy and asthma. J Allergy Clin Immunol 2017; 139:472–81 e9. [DOI] [PubMed] [Google Scholar]
- 15.Mirzakhani H, Al-Garawi AA, Carey VJ, Qiu W, Litonjua AA, Weiss ST. Expression network analysis reveals cord blood vitamin D-associated genes affecting risk of early life wheeze. Thorax 2019; 74:200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016; 315:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirzakhani H, O'Connor G, Bacharier LB, Zeiger RS, Schatz MX, Weiss ST, et al. Asthma control status in pregnancy, body mass index, and maternal vitamin D levels. J Allergy Clin Immunol 2017; 140:1453–6 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials 2014; 38:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ersfeld DL, Rao DS, Body JJ, Sackrison JL Jr., Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem 2004; 37:867–74. [DOI] [PubMed] [Google Scholar]
- 20.Litonjua AA, Carey VJ, Laranjo N, Stubbs BJ, Mirzakhani H, et al. Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction. N Engl J Med 2020; 382:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:1911–30. [DOI] [PubMed] [Google Scholar]
- 22.Mirzakhani H, Litonjua AA, McElrath TF, O'Connor G, Lee-Parritz A, Iverson R, et al. Early pregnancy vitamin D status and risk of preeclampsia. J Clin Invest 2016; 126:4702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollis BW, Wagner CL. New insights into the vitamin D requirements during pregnancy. Bone Res 2017; 5:17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med 2011; 183:1336–43. [DOI] [PubMed] [Google Scholar]
- 25.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? Journal of Steroid Biochemistry and Molecular Biology 2014; 144:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sablok A, Batra A, Thariani K, Batra A, Bharti R, Aggarwal AR, et al. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol (Oxf) 2015; 83:536–41. [DOI] [PubMed] [Google Scholar]
- 27.Ozias MK, Kerling EH, Christifano DN, Scholtz SA, Colombo J, Carlson SE. Typical prenatal vitamin D supplement intake does not prevent decrease of plasma 25-hydroxyvitamin D at birth. J Am Coll Nutr 2014; 33:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang JY, Lucey AJ, Horgan R, Kenny LC, Kiely M. Impact of pregnancy on vitamin D status: a longitudinal study. Br J Nutr 2014; 112:1081–7. [DOI] [PubMed] [Google Scholar]
- 29.Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA Jr., Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med 2017; 5:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. S5 Vitamin d for the management of asthma: cochrane systematic review and meta-analysis. Thorax 2016; 71:A6.1–A6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017; 356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giles W, Murphy V. Asthma in pregnancy: a review. Obstet Med 2013; 6:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grzeskowiak LE, Smith B, Roy A, Dekker GA, Clifton VL. Patterns, predictors and outcomes of asthma control and exacerbations during pregnancy: a prospective cohort study. ERJ Open Res 2016; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Mutius E, Martinez FD. Vitamin D Supplementation during Pregnancy and the Prevention of Childhood Asthma. N Engl J Med 2020; 382:574–575. [DOI] [PubMed] [Google Scholar]
- 35.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One 2010; 5:e10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


