Abstract
Background
Extracts of milk thistle, Silybum marianum (L.) Gaertn., are used as dietary supplements for their hepatoprotective, anti-inflammatory, and anti-tumor activities.
Objective
An assay based on UHPLC-MS/MS was developed and validated for the quantitative analysis of six major milk thistle flavonolignans extracted from human serum.
Methods
Ethyl acetate containing 0.1% formic acid was used to extract flavonolignans from human serum. A 10-min UHPLC-MS/MS method using selected reaction ion monitoring was developed for measuring extracts for silybin A, silybin B, isosilybin A, isosilybin B, silychristin, and silydianin.
Results
The quantitative method was validated with respect to selectivity, specificity, accuracy, linearity, precision, LOD, and LLOQ. Extraction efficiency for the quality control standards at LLOQ, low, medium, and high concentrations ranged between 81% and 109%, and the calibration curves were linear (R2 > 0.997) for all flavonolignans. The method precision was determined using coefficients of variation, which were <15%. The method accuracy was assessed using percent relative error which was <15%.
Conclusions
The UHPLC-MS/MS assay is fast, precise, sensitive, selective, accurate, and useful for the analysis of milk thistle flavonolignans in human serum.
Highlights
The UHPLC-MS/MS assay is suitable for rapid quantitative analysis of milk thistle flavonolignans in human serum.
Milk thistle (Silybum marianum (L.) Gaertn.) is an annual or biennial plant indigenous to Europe and the Mediterranean belonging to the daisy family (Asteraceae). Silymarin, which is a mixture of flavonolignans and their precursor flavonoids, is the main component of extracts of milk thistle seeds (1). Milk thistle and silymarin are widely used as dietary supplements due to their anti-inflammatory and anti-tumor activities (2–4) as well as hepatoprotective effects (5).
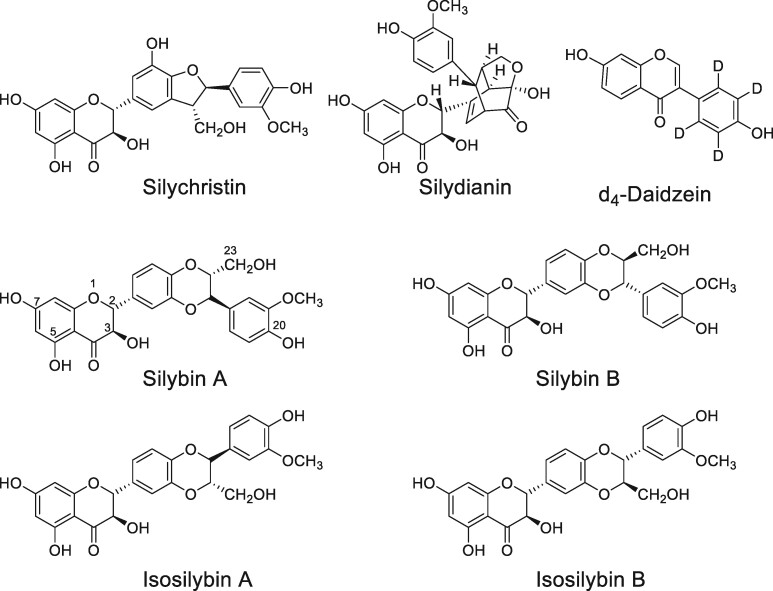
Defatted lipophilic extracts from the seeds/fruits of milk thistle contain flavonoids and flavonolignans such as silybin A, silybin B, isosilybin A, isosilybin B, silydianin, and silychristin (Figure 1). The silybins and isosilybins are isomers (6), and the silybins have been reported to be more abundant than isosilybins in the seed extract (7). Although many clinical trials of milk thistle have been carried out (for example, >50 studies are listed on ClinicalTrials.gov using milk thistle or silymarin), many have suffered from inadequately standardized test materials (8). This limitation leads to inconsistent results due to variable or irreproducible levels of silymarin constituents, each of which might have different pharmacological activities. Such lack of standardization leads to inconsistent or unknown dosages which may affect the validity of conclusions drawn from the clinical studies (9, 10). A validated method for the measurement of milk thistle flavonolignans in test materials and human serum is necessary to support pharmacokinetic analyses and to enable accurate correlation between pharmacological concentrations of individual compounds and the observed in vivo effects. A validated analytical standardization method for milk thistle flavonolignans (silymarin) may be used to ensure product consistency and clinical study reproducibility.
Figure 1.
Structures of milk thistle flavonolignans and d4-daidzein (internal standard) used in the study.
Previous analytical methods to measure flavonolignans in milk thistle dietary supplements have utilized HPLC-UV, UHPLC-UV, capillary electrophoresis-UV, HPLC-MS, and UHPLC-MS/MS. Each of these published methods has limitations that can be overcome by using an appropriately validated UHPLC-MS/MS assay. For example, Quaglia, et al. (11) used HPLC and capillary electrophoresis to measure flavonoids in dried fruits of milk thistle but could not separate the diastereomers of silybin and isosilybin. The HPLC-MS method of Lee, et al. (6) used low-resolution single-stage mass spectrometry on a triple quadrupole mass spectrometry instead of tandem mass spectrometry with selected reaction monitoring. Wang, et al. (12) used UHPLC-MS/MS and Liu, et al. (13) used UHPLC-UV for the rapid separation (<10 min) and detection of 6 major flavonolignans from milk thistle (Figure 1), but neither method was quantitative.
In response to the AOAC International call for a single-laboratory method validation for quantification of flavonolignans in milk thistle dietary supplements, Mudge, et al. (14) reported a HPLC-UV method for all six flavonolignans. Although appropriately validated, this method utilized a 37-min HPLC method instead of faster UHPLC separation and used UV for quantification instead of tandem mass spectrometric detection. We developed and validated a faster, more sensitive, and more selective UHPLC-MS/MS approach that is suitable for the measurement of milk thistle flavonolignans in human serum.
Although there have been several methods reported for the measurement of milk thistle flavonolignans in dietary supplements, there are few quantitative methods for these compounds in human serum or plasma. In a rare example, Brinda, et al. (15) used HPLC-MS/MS to measure six silymarin active compounds extracted from human plasma. Although validated, this method required 30 min per chromatographic separation, and each flavonolignan peak was over 1 min wide. Our new UHPLC-MS/MS method uses a 10 min separation with sharper chromatographic peaks and greater sensitivity for the measurement of all 6 major milk thistle flavonolignans in human serum.
Methods
Materials and Reagents
Silychristin and silydianin (≥ 98.0% purity) were purchased from ApexBio (Boston, MA, USA), silybin B, isosilybin A and isosilybin B (≥ 98.0% purity) were purchased from Sigma Aldrich (St. Louis, MO, USA), silybin A (50:50 mixture of silybin A and B), and d4-daidzein (≥ 99.0% purity) were purchased from Cayman Chemicals (Ann Arbor, MI, USA). HPLC-MS grade acetonitrile, methanol, and formic acid were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Ultrapure water was prepared using a Milli-Q water purification system (Millipore, MA, USA). All other reagents and solvents were reagent grade or better and were purchased from VWR (Visalia, CA, USA).
Separation of Silybin A and Silybin B
Silybin A was prepared from a 50:50 (w/w) mixture of silybins A and B using a method adapted from Kim, et al. (16). Briefly, silybin A was separated from silybin B using a Waters (Milford, MA, USA) Delta 600 preparative HPLC system equipped with a photodiode detector and a Phenomenex (Torrance, CA, USA) Ultremex C18 column (250 × 10 mm, 5 µm). The isocratic mobile phase contained methanol/water (50:50; v/v) at a flow rate of 3 mL/min. The injection volume was 50 µL, and UV absorbance detection was monitored at 286 nm. Silybin A eluted at 14 min, and the retention time of silybin B was 16 min. Peaks corresponding to silybin A collected from multiple injections were combined, evaporated to dryness using a rotary evaporator, and then lyophilized to give a white powder. Identification of silybin A was confirmed using high-resolution mass spectrometry as described below.
Preparation of Calibration Standards, Quality Controls, and Internal Standards
Stock solutions of milk thistle flavonolignan standards (1 mg/mL) in methanol were prepared, aliquoted, and stored at -20°C until use. The working solutions for the calibration standards were prepared by diluting the stock solutions with methanol/water (50:50, v/v). Quality control (QC) standards at LLOQ, low, medium and high concentrations in methanol/water (50:50; v/v) were prepared from 1 mg/mL stock solutions. Standard working solutions (20 µL) of varying concentrations of the milk thistle flavonolignans were spiked into human serum for use as standards. The final concentration of each calibration curve standard is shown in Table 1.
Table 1.
UHPLC retention times (RT), negative ion electrospray MS/MS selected reaction monitoring (SRM) transitions, collision energies (CE), and concentrations (ng/mL) of standards spiked into blank human serum for calibration curvesa
| Spiked concentrations (ng/mL) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | RT (min) | SRM transition m/z | CE (V) | (1) | ( 2 ) | ( 3 ) | ( 4 ) | ( 5 ) | ( 6 ) | ( 7) | ( 8 ) | ( 9 ) | ( 10 ) | ( 11 ) |
| Silychristin | 1.9 |
481 → 125 481 → 325 |
29 22 |
1.2 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 |
| Silydianin | 2.4 |
481 → 179 481 → 125 |
26 31 |
1.2 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 |
| Silybin A | 5.1 |
481 → 125 481 → 301 |
28 21 |
0.4 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 |
| Silybin B | 5.5 |
481 → 125 481 → 301 |
29 21 |
0.4 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 |
| Isosilybin A | 6.9 |
481 → 125 481 → 453 |
29 20 |
0.4 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 |
| Isosilybin B | 7.2 |
481 → 125 481 → 453 |
27 19 |
0.4 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 |
| d4-Daidzein | 3.6 |
257 → 212 257 → 136 |
31 39 |
|||||||||||
The first SRM transition of each milk thistle flavonolignan was used as the quantifier while the second SRM transition was used as a qualifier in the UHPLC-MS/MS assay.
A stock solution of the internal standard d4-daidzein (1 mg/mL) was prepared by dissolving an accurately weighed sample in dimethylsulfoxide. The stock was diluted with methanol/water (50:50, v/v) to obtain a working solution of 125 ng/mL. Because stable isotopically labeled flavonolignans were unavailable, d4-daidzein was used as the internal standard due to its similar solubility and extraction efficiency.
Extraction of Milk Thistle Flavonolignans from Human Serum
Human serum (100 µL) was mixed with 80 µL internal standard working solution in a 1.5 mL microcentrifuge tube. The flavonolignan standards at varying concentrations (20 µL) were then added. Four volumes (800 µL) of ethyl acetate containing 0.1% formic acid was added, and the mixture was vortexed for 2 min followed by centrifugation for 30 min at 18 000×g and 4°C. The organic layer was transferred to a clean centrifuge tube, dried under vacuum and reconstituted in 200 µL of mobile phase for UHPLC-MS/MS analysis.
High-Resolution UHPLC-MS and MS/MS of Milk Thistle Flavonolignan Standards
High-resolution mass spectra and tandem mass spectra of all flavonolignan standards and d4-daidzein internal standard were obtained using a Shimadzu (Kyoto, Japan) 9030 Q-ToF tandem mass spectrometer equipped with a Shimadzu Nexera UHPLC system. The electrospray ionization interface temperature was 300°C, and the voltage was -3.5 kV for negative ion mode. The heat block and desolvation line temperatures were 400°C and 250°C, respectively. Nitrogen was used as a drying gas at a flow rate of 10 L/min, for nebulization at 3 L/min and as a heating gas at 10 L/min. Mass spectra and product ion tandem mass spectra were acquired every 100 ms over the scan range of m/z 70–700. Product ion tandem mass spectra were obtained using a collision energy of 35 V with an energy spread of 17 V. A 5-µL aliquot was injected into the UHPLC-MS for quantitative analysis.
UHPLC-MS/MS Quantitative Analysis
Aliquots (5 µL) of each milk thistle sample were analyzed by using UHPLC-MS/MS. Chromatographic separation of the milk thistle flavonolignans was carried out using a Shimadzu Nexera UHPLC system fitted with a Waters Acquity UPLC BEH C18 column (1.7 μm, 130 Å, 2.1 mm × 50 mm). The column oven temperature was 40°C and the autosampler was 10°C. The mobile phase consisted of a gradient from water (A) to methanol (B), each containing 0.01% formic acid, as follows: 40% B for 2 min, 40–45% from 2–4.5 min; 45% B from 4.5–5.5 min; 45–50% B from 5.5–6.0 min; 50–55% B from 6.0–6.5 min; 55–60% B from 6.5–6.6 min; and 60–65% B from 6.6–8.0 min. The column was equilibrated at the initial condition of 40% B for 2 min prior to the next injection. The total UHPLC-MS/MS cycle for the separation of milk thistle flavonolignans was 10 min. The flow rate was 0.35 mL/min.
The UHPLC system was coupled to a Shimadzu LCMS-8060 triple quadruple mass spectrometry equipped with electrospray operating in negative ion mode. Nitrogen was used as drying gas at a flow rate of 5 L/min and for nebulization at 3 L/min. The interface and desolvation line temperatures were 400°C and 300°C, respectively. The milk thistle flavonolignans were measured using collision-induced dissociation with selected reaction monitoring (SRM). The SRM dwell time for each transition was 15 ms, and the collision gas pressure was 230 kPa. Data acquisition, integration, and linear standard curve fitting were carried out using Shimadzu Lab Solutions software (version 5.7). The coefficients of variation and relative errors were calculated using Microsoft Excel software (Seattle, WA, USA).
Validation of the UHPLC-MS/MS Analytical Method
Analytical method validation was performed following the U.S. FDA guidelines for bioanalytical method validation (17) and AOAC single-laboratory validation of chemical methods for dietary supplements and botanicals (18). The method was validated for recovery, matrix effect, selectivity, specificity, sensitivity, linearity, limit of detection (LOD), lower limit of quantitation (LLOQ), upper limit of quantitation (ULOQ), precision, accuracy, and stability. The method recovery was determined by comparing the peak areas obtained from blank human serum samples spiked prior to extraction with the peak areas obtained from spiked post-extraction serum samples. Matrix effects were assessed using six different batches of blank human serum. Briefly, a sample of each blank human serum batch was extracted with ethyl acetate (contained 0.1% formic acid) as described above and spiked with QC standards at low and high concentrations. Internal standard solution was added, and the solvent was removed under vacuum. Separately, QC standards at low and high concentrations in neat solution (methanol/water, 50:50; v/v) were mixed with 4 volumes of acidified ethyl acetate and processed similarly to the spiked samples.
Selectivity and specificity were evaluated by comparing retention times and ratios of SRM MS/MS responses (quantifier and qualifier signals) of standards in neat solution with standards spiked into blank human serum. Sensitivity and linearity were assessed by plotting the area ratio of the standard peak to that of internal standard versus the theoretical concentration of the standard. The LOD was determined using signal-to-noise ratio between 3 and 5. The LLOQ was defined as the lowest amount of analyte that could be determined in a sample with sufficient signal-to-noise ratio between 5 and 10. The ULOQ was defined as the highest concentration of analyte where the relationship between the MS signal and the concentration was linear, which was determined by the coefficient of determination (R2).
The interday and intraday precision and accuracy were determined by calculating the coefficient of variation (CV) and relative error (RE), respectively, of spiked QC standards in serum matrix at LLOQ, low, medium, and high concentrations. The stability of the milk thistle flavonolignans was determined by analyzing QC samples at low, medium, and high concentrations. The autosampler stability was assessed by re-analyzing the processed samples 24 h after the first injection. The freeze-thaw stability was determined for each QC level in serum that had been stored at −20°C for 36 h and thawed at room temperature. When completely thawed, aliquots were measured by UHPLC-MS/MS, and the remaining samples were re-frozen for at least 12 h. The freeze-thaw cycle was then repeated. The compounds were considered stable when the difference between the freshly prepared samples and those tested for stability was <15%.
Results and Discussion
Selection of Milk Thistle Flavonolignans
The six flavonolignans selected for study are the main constituents of silymarin extract from milk thistle seeds and are antioxidants with pharmacological activity (19). These flavonolignans are constitutional isomers, with the molecular formula C25H22O10 (Figure 1). Thermodynamic and pKa studies have identified the hydroxyl groups at positions 7 and 20 as the sites of radical based oxidation in polar and non-polar solvents, respectively (20, 21), and these groups are involved in the antioxidant mechanisms of silymarin (21, 22). Silybin A was purchased as a mixture of silybin A and B and was separated through preparative HPLC to afford ≥ 98% pure silybin A (Supplemental Figure S1). All other flavonolignan standards were purchased as single compounds and were determined to have purities of ≥ 98%.
Extraction of Milk Thistle Flavonolignans from Human Serum
Flavonolignan extraction efficiency and sample clean-up was carried out using a combination of protein precipitation and liquid extraction using acidified ethyl acetate and was compared with protein precipitation using acetonitrile. The addition of formic acid-enabled protein precipitation while the flavonolignans were extracted into the ethyl acetate layer. In addition to efficient sample clean-up and short sample preparation time (< 2 h), higher flavonolignan recoveries were obtained using acidified ethyl acetate than when using acetonitrile (see Matrix effects and recovery below and Supplemental Table S1).
UHPLC-MS/MS Method Optimization
Different chromatographic columns were tested for their effect on peak shape and separation. These included Phenomenex Luna Omega C18 UHPLC (1.6 μm, 100 Å, 2.1 mm × 50 mm) (Torrance, CA), Advanced Chromatography Technologies (Aberdeen, Scotland) ACE Super C18 UHPLC (1.7 μm, 90 Å, 2.1 mm × 100 mm), and Waters Acquity UPLC BEH C18 (1.7 μm, 130 Å, 2.1 × 50 mm) (Milford, MA) column. Each column was tested using different mobile phase compositions including 0.1% formic acid in water (aqueous phase) and acetonitrile or methanol (organic phase). The Waters Acquity UPLC column was selected for all subsequent experiments because it provided the best separation for all milk thistle flavonolignan standards and the d4-daidzein internal standard.
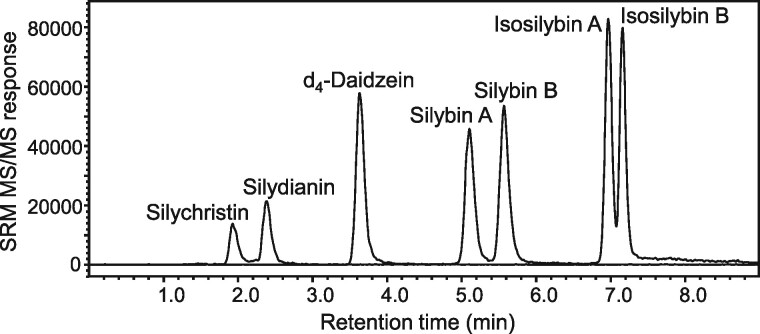
Chromatographic separation of the analytes was optimized by evaluating different mobile phase compositions and various additives. Gradient separations were evaluated using 10 mM ammonium formate, 10 mM ammonium acetate, or formic acid (0.01% or 0.1%) in water as mobile phase A, and methanol or acetonitrile were compared as mobile phase B. Rapid separation (< 8 min) of all analytes (Figure 2) was achieved without carryover between analyses using a linear gradient consisting of 0.01% formic acid in water and 0.01% formic acid in methanol at a flow rate of 0.35 mL/min.
Figure 2.
UHPLC-MS/MS chromatogram of milk thistle flavonolignan standards (8 ng/mL) spiked into blank human serum. d4-Daidzein was used as an internal standard.
Negative ion electrospray produced better signal-to-noise for each flavonolignan than did positive ion mode. For tandem mass spectrometric optimization, the two most abundant fragments of each deprotonated milk thistle flavonolignan were chosen for selected reaction monitoring (SRM) transitions, and the collision-induced dissociation energies were adjusted to produce maximum SRM signals. The most abundant product ion for each flavonolignan was used as the quantifier SRM transition while the second most abundant product ion was used as the qualifier SRM transition. The SRM transitions and their corresponding collision energies are detailed in Table 1.
Validation of UHPLC-MS/MS Method
(a) Matrix effects and recovery
Blank serum samples were extracted, spiked with standards, and analyzed for potential peak suppression or enhancement by endogenous matrix components. The percent matrix effects for all flavonolignan analytes and d4-daidzein internal standard assessed at 5 ng/mL (low QC) and 1000 ng/mL (high QC) indicate that ion suppression or ion enhancement was < 15% in 6 different human serum batches which is within the guidelines recommended by the FDA. The extraction recovery studies were performed at LLOQ, low, medium, and high QC concentrations, and the peak areas of the serum samples spiked with the standards pre- and post-extraction were compared. The extraction efficiencies for all the milk thistle flavonolignans at the different QC concentrations were between 81% and 109% (Table 2).
Table 2.
Percent recovery of spiked milk thistle flavonolignan standards in blank human serum matrix
| Analyte | Concentration (ng/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| LLOQ | Recovery % | Low | Recovery % | Medium | Recovery % | High | Recovery % | |
| Silychristin | 1.2 | 81.1 | 5 | 94.0 | 50 | 93.4 | 1000 | 97.7 |
| Silydianin | 1.2 | 97.5 | 5 | 91.2 | 50 | 93.3 | 1000 | 96.2 |
| Silybin A | 0.4 | 83.6 | 1.2 | 97.5 | 50 | 97.0 | 1000 | 102 |
| Silybin B | 0.4 | 83.1 | 1.2 | 103 | 50 | 96.3 | 1000 | 102 |
| Isosilybin A | 0.4 | 85.2 | 1.2 | 98.4 | 50 | 91.0 | 1000 | 98.2 |
| Isosilybin B | 0.4 | 109 | 1.2 | 97.7 | 50 | 91.3 | 1000 | 97.9 |
(b) Selectivity and specificity
The SRM chromatograms of extracted blank serum did not show any peaks corresponding to the flavonolignans or internal standard. Additionally, there were no peaks observed at the same retention times as the standards, which indicated that there was no endogenous substance in the blank human serum interfering in the analysis of flavonolignans. These results show that the UHPLC-MS/MS assay was selective and specific for the intended milk thistle flavonolignan analysis.
(c) Sensitivity and linearity
The UHPLC-MS/MS assay showed a linear response from 0.4–1024 ng/mL for silybin A, silybin B, isosilybin A and isosilybin B, and from 1.2–1024 ng/mL for silychrisin and silydianin. The correlation coefficients were > 0.997 for all the analytes (Supplemental Figures S2–S7). The limits of detection were 0.07 ng/mL for silybin A, silybin B, isosilybin A and isosilybin B, and were 0.4 ng/mL for silychristin and silydianin, while the lower limits of quantitation ranged from 0.4– 1.2 ng/mL (Table 3). Therefore, the UHPLC-MS/MS assay showed excellent sensitivity as well as a wide dynamic range, which would be suitable for analyses of human serum from clinical pharmacokinetics studies requiring measurements over a wide range of concentrations.
Table 3.
Calibration curve linear range, the coefficient of determination (R2), accuracy, and precision for the UHPLC-MS/MS assay of six milk thistle flavonolignans
| Analyte | Linear range (ng/mL) | R2 | Theoretical | Intraday (n = 5) |
Interday (n = 12) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Measured | Accuracy | Precision | Measured | Accuracy | Precision | ||||
| QC (ng/mL) | QC (ng/mL) | RE (%) | CV (%) | QC (ng/mL) | RE (%) | CV (%) | |||
| Silychristin | 1.2–1024 | 0.9999 | 1.2 | 1.23 | 2.6 | 9.9 | 1.19 | 0.743 | 10.4 |
| 5 | 4.91 | 1.75 | 6.78 | 5.04 | 0.875 | 8.23 | |||
| 50 | 50.6 | 1.28 | 5.6 | 50.2 | 0.37 | 7.8 | |||
| 1000 | 1022 | 2.2 | 2.48 | 1029 | 2.91 | 4.79 | |||
| Silydianin | 1.2–1024 | 0.9968 | 1.2 | 1.16 | 3.28 | 7.56 | 1.09 | 8.96 | 10.5 |
| 5 | 5.03 | 0.54 | 2.05 | 4.97 | 0.577 | 5.46 | |||
| 50 | 52.5 | 4.98 | 4.99 | 53.5 | 7.04 | 8.49 | |||
| 1000 | 1010 | 0.962 | 2.74 | 1005 | 0.481 | 5.82 | |||
| Silybin A | 0.4–1024 | 0.9995 | 0.4 | 0.439 | 9.75 | 10.1 | 0.424 | 6.08 | 12.4 |
| 5 | 5.06 | 1.14 | 3.51 | 5.29 | 5.83 | 5.05 | |||
| 50 | 55.7 | 11.5 | 3.8 | 55.9 | 11.9 | 6.21 | |||
| 1000 | 993 | 0.724 | 3.23 | 955 | 4.51 | 4.16 | |||
| Silybin B | 0.4–1024 | 0.9995 | 0.4 | 0.39 | 2.55 | 8.35 | 0.402 | 0.396 | 7.23 |
| 5 | 5.04 | 0.824 | 5.22 | 5.03 | 0.662 | 6.25 | |||
| 50 | 53.3 | 6.52 | 3.69 | 53.5 | 7 | 5.28 | |||
| 1000 | 1023 | 2.34 | 3.14 | 980 | 1.97 | 7.03 | |||
| Isosilybin A | 0.4–1024 | 0.9999 | 0.4 | 0.39 | 2.55 | 8.72 | 0.398 | 0.396 | 14.6 |
| 5 | 5.74 | 14.9 | 8.17 | 5.63 | 12.6 | 8 | |||
| 50 | 56 | 12.1 | 6.05 | 57.3 | 14.5 | 6.77 | |||
| 1000 | 1004 | 0.36 | 2.36 | 1025 | 2.51 | 3.45 | |||
| Isosilybin B | 0.4–1024 | 0.9998 | 0.4 | 0.395 | 1.2 | 8.58 | 0.402 | 0.521 | 8.89 |
| 5 | 5.55 | 11 | 4.54 | 5.58 | 11.6 | 6.18 | |||
| 50 | 53.9 | 7.78 | 5.79 | 56.3 | 12.7 | 5.83 | |||
| 1000 | 1004 | 0.364 | 1.78 | 1005 | 0.457 | 3.31 | |||
(d) Accuracy and precision
The accuracy (%RE) and precision (%CV) of the analytical method were determined using QC samples at LLOQ, low, medium, and high concentrations in at least 5 replicates for intraday and 12 replicates for interday assays. At the LLOQ of the flavonolignans, the intraday accuracy ranged between 1.20 and 9.75% with precision in the range of 7.56 to 10.0%. The interday accuracy ranged from 0.396 to 8.96%, and the precision range was 7.23 to14.6% (Table 3). Overall, the interday and intraday precision for all QC concentrations were found to be within acceptable limits (< 15% CV) as indicated in the U.S FDA guidelines for analytical method development (17). The interday and intraday accuracies for all flavonolignans at all QC concentrations were < 15%, which was also within the acceptable range defined by the FDA guidelines (17). These results demonstrate that the UHPLC-MS/MS assay for the milk thistle flavonolignan analyzed in this study was reproducible and reliable.
(e) Stability
The stabilities of milk thistle flavonolignans at low and high QC concentrations were investigated under different conditions (including autosampler temperature and repeated freeze thaw cycles). The stability in the autosampler at 10°C for 24 h ranged from 93 to 110% for low QC concentration compounds and 80 to 95% at high QC concentrations (Table 4). Repeated freeze thaw cycles did not significantly affect the stability of the analytes. The stability data suggest that accurate results would be obtained by analyzing the samples within 24 h after extraction.
Table 4.
Stability of milk thistle flavonolignans during storage and handling, measured using UHPLC-MS/MS
| Stability (%) – 5 ng/mL |
Stability (%) – 1000 ng/mL |
|||||
|---|---|---|---|---|---|---|
|
Analyte
(n = 3) |
24 h | Freeze-Thaw cycle |
24 h | Freeze-Thaw cycle |
||
| 1 | 2 | 1 | 2 | |||
| Silychristin | 104 ± 4 | 110 ± 5 | 83.4 ± 3.2 | 80.3 ± 5.9 | 87.9 ± 5.7 | 88.4 ± 9.1 |
| Silydianin | 110 ± 8 | 108 ± 6 | 75.1 ± 9.6 | 94.0 ± 6.7 | 87.2 ± 5.1 | 86.7 ± 9.1 |
| Silybin A | 99.3 ± 4.3 | 106 ± 7 | 94.9 ± 5.1 | 88.2 ± 4.2 | 91.1 ± 5.4 | 98.2 ± 8.3 |
| Silybin B | 93.4 ± 5.8 | 97.1 ± 8.8 | 95.9 ± 1.3 | 91.4 ± 4.4 | 92.9 ± 4.7 | 98.3 ± 7.7 |
| Isosilybin A | 98.6 ± 5.0 | 104 ± 2 | 105 ± 2 | 91.1 ± 4.7 | 94.0 ± 4.8 | 100 ± 7 |
| Isosilybin B | 101 ± 5 | 102 ± 5 | 103 ± 5 | 94.8 ± 6.3 | 97.1 ± 3.2 | 105 ± 6 |
Conclusions
A method based on UHPLC-MS/MS for the quantitative analysis of milk thistle flavonolignans was developed and validated using human serum. The assay is accurate, reproducible and free from matrix interference. The assay is also sensitive, precise and accurate and has a wide linear range of milk thistle flavonolignans concentrations.
Supplemental Information
Supplemental information is available on the J. AOAC Int. website.
Supplementary Material
Acknowledgments
The authors would like to thank Shimadzu for providing the UHPLC-MS/MS systems used in this study. This work was supported by a P50AT000155 administrative supplement grant from the NIH National Center for Complementary and Integrative Health.
Contributor Information
Ruth N Muchiri, Linus Pauling Institute, Oregon State University, 2900 SW Campus Way, Corvallis, OR 97331, USA; Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Corvallis, OR 97331, USA.
Richard B van Breemen, Linus Pauling Institute, Oregon State University, 2900 SW Campus Way, Corvallis, OR 97331, USA; Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Corvallis, OR 97331, USA.
References
- 1. Csupor D., Csorba A., Hohmann J. (2016) J. Pharm. Biomed. Anal. 130, 301–317 [DOI] [PubMed] [Google Scholar]
- 2. Smith T., Kawa K., Eckl V., Morton C., Stredney R. (2017) HerbalGram 115, 56–65 [Google Scholar]
- 3. Wing Ying Cheung C., Gibbons N., Wayne Johnson D., Lawrence Nicol D. (2010) Anti-Cancer Agents Med. Chem. 10, 186–195 [DOI] [PubMed] [Google Scholar]
- 4. Deep G., Agarwal R. (2007) Integr. Cancer Ther. . 6, 130–145 [DOI] [PubMed] [Google Scholar]
- 5. Mayer K., Myers R., Lee S. (2005) J. Viral Hepat. 12, 559–567 [DOI] [PubMed] [Google Scholar]
- 6. Lee J.I., Narayan M., Barrett J.S. (2007) J. Chromatogr. B 845, 95–103 [DOI] [PubMed] [Google Scholar]
- 7. Hoh C.S.L., Boocock D.J., Marczylo T.H., Brown V.A., Cai H., Steward W.P., Berry D.P., Gescher A.J. (2007) J. Agric. Food Chem. 55, 2532–2535 [DOI] [PubMed] [Google Scholar]
- 8. Ball K.R., Kowdley K.V. (2005) J. Clin. Gastroenterol. 39, 520–528 [DOI] [PubMed] [Google Scholar]
- 9. Tamayo C., Diamond S. (2007) Integr. Cancer Ther. 6, 146–157 [DOI] [PubMed] [Google Scholar]
- 10. Fenclova M., Novakova A., Viktorova J., Jonatova P., Dzuman Z., Ruml T., Kren V., Hajslova J., Vitek L., Stranska-Zachariasova M. (2019) Sci. Rep. 9, 11118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quaglia M., Bossu E., Donati E., Mazzanti G., Brandt A. (1999) J. Pharm. Biomed. Anal. 19, 435–442 [DOI] [PubMed] [Google Scholar]
- 12. Wang K., Zhang H., Shen L., Du Q., Li J. (2010) J. Pharm. Biomed. Anal. 53, 1053–1057 [DOI] [PubMed] [Google Scholar]
- 13. Liu H., Du Z., Yuan Q. (2009) J. Chromatogr. B 877, 4159–4163 [DOI] [PubMed] [Google Scholar]
- 14. Mudge E., Paley L., Schieber A., Brown P.N. (2015) Anal. Bioanal. Chem. 407, 7657–7666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brinda B.J., Zhu H.-J., Markowitz J.S. (2012) J. Chromatogr. B 902, 1–9 [DOI] [PubMed] [Google Scholar]
- 16. Kim N.-C., Graf T.N., Sparacino C.M., Wani M.C., Wall M.E. (2003) Org. Biomol. Chem. 1, 1684–1689 [DOI] [PubMed] [Google Scholar]
- 17.(2018) US FDA Guidance for Industry: Bioanalytical Method Validation, Silver Spring, MD. www.fda.gov [accessed on January 21, 2020] [Google Scholar]
- 18.(2013) Appendix K: Guidelines for Dietary Supplements and Botanicals, AOAC Int. Gaithersburg, MD. www.eoma.aoac.org [accessed on January 21 2020] [Google Scholar]
- 19. Abenavoli L., Izzo A.A., Milić N., Cicala C., Santini A., Capasso R. (2018) Phytother. Res. 32, 2202–2213 [DOI] [PubMed] [Google Scholar]
- 20. van Wenum E., Jurczakowski R., Litwinienko G. (2013) J. Org. Chem. 78, 9102–9112 [DOI] [PubMed] [Google Scholar]
- 21. Biedermann D., Vavříková E., Cvak L., Křen V. (2014) Nat. Prod. Rep. 31, 1138–1157 [DOI] [PubMed] [Google Scholar]
- 22. Wellington K., Jarvis B. (2001) BioDrugs 15, 465–489 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.