Abstract
SARS-CoV-2 infection can associate diverse neurological manifestations. Several studies have provided proof to support the theory of neurotropic involvement of SARS-CoV-2. Alpha-synuclein has been described as a native antiviral factor within neurons, and upregulation of this protein can be seen in animals that suffered other neuroinvasive infections. To assess if increased expression of this protein takes place in COVID-19 patients with neurological symptoms, we analyzed serum total alpha-synuclein levels in three groups: seven COVID-19 patients with myoclonus, Parkinsonism and/or encephalopathy; thirteen age- and sex-matched COVID-19 patients without neurological involvement and eight age- and sex-matched healthy controls. We did not find differences among them. In a subset of four patients, the change in serum alpha-synuclein before and after the onset of neurological symptoms was not significant either. Cerebrospinal fluid alpha-synuclein levels were also similar between neurological COVID-19 and healthy controls. Overall, these results cannot support the hypothesis of alpha-synuclein upregulation in humans with neurological symptoms in COVID-19. Further research taking into account a larger group of COVID-19 patients including the whole spectrum of neurological manifestations and disease severity is needed.
Keywords: COVID-19, Alpha-synuclein, Myoclonus, Parkinsonism, Virus, SARS-CoV-2
Introduction
At the end of 2019, a cluster of cases of pneumonia was reported in Wuhan, China [1, 2]. A novel β-coronavirus (CoV), subsequently named SARS-CoV-2, was identified as the causative agent of the disease, termed COVID-19 [1]. Initially thought to be restricted to the respiratory tract, several reports demonstrated that neurological features in COVID-19 can be relatively common, including large vessel stroke, Guillain–Barre syndrome, encephalopathy, and meningoencephalitis [3–5].
We previously reported three COVID-19 patients with generalized myoclonus [6] and another one who developed generalized myoclonus, opsoclonus, and an asymmetric hypokinetic-rigid syndrome after a severe SARS-CoV-2 infection [7]. Although rare, other reports have described COVID-19-associated myoclonic syndrome [8–13] and Parkinsonism [14, 15]. Direct central nervous system (CNS) damage by SARS-CoV-2 or post infectious/immune-mediated pathogenesis has been considered in those cases. Several studies have provided proof to support the theory of neurotropic involvement of SARS-CoV-2 [16–18].
Alpha-synuclein (αSyn) could be part of the first line of defense against pathogens acting as a natural antimicrobial peptide [19], maybe preventing neuron-to-neuron spread of virus [20, 21], as shown by an increased neuronal expression of αSyn following acute West Nile virus infection, another enveloped RNA virus [22, 23]. It has been theorized that similar αSyn upregulation might occur with SARS-CoV-2 infection [24, 25].
To assess this hypothesis, we analyzed total αSyn in serum and cerebrospinal fluid (CSF), when available, from patients with COVID-19 who also presented neurological signs and symptoms, including four patients already reported [6, 7] and another three with a similar clinical course (myoclonus and encephalopathy). We compared those results with total αSyn levels in COVID-19 patients with no neurological manifestations, and in healthy age- and sex-matched controls.
Methods
Ethics
Study protocol received ethical approval by Hospital Universitario 12 de Octubre Ethics Committee (Protocol number 20/342), in accordance to Declaration of Helsinki. All COVID-19 alive patients gave their written informed consent to the study during their clinical follow-up. Healthy controls donated CSF and serum samples to the i + 12 Laboratory of Neuroscience after obtaining informed consent at the time of sample collection.
Study population
In this retrospective study, we defined three groups of comparison: COVID-19 with neurological symptoms (COVID-19-NRL), COVID-19 without neurological involvement, and healthy controls.
We reviewed all COVID-19 patients older than 16 years who were admitted to Hospital Universitario 12 de Octubre, Madrid, Spain, from March 1st to April 30th 2020, who also had a consultation with Neurology Department. To delineate COVID-19-NRL group, we selected those patients with generalized myoclonus (with encephalopathy or Parkinsonism) related to SARS-CoV-2 infection, who received a thorough assessment to rule out another explanation for their neurological symptoms, such as metabolic disturbances, hypoxia, or drugs. We found a total of seven patients with moderate-to-severe SARS-CoV-2 infection who matched that criteria.
To define the COVID-19 group free of neurological symptoms, we retrospectively selected thirteen moderate-to-severe COVID-19 age- and sex-matched patients free of any kind of neurological complaints (including milder ones such as anosmia or headache), who were admitted to the Hospital Universitario 12 de Octubre in the same period. SARS-CoV-2 infection was confirmed in all of them with RT-PCR.
Another control group was configured with eight age- and sex-matched healthy controls, recruited before the COVID-19 outbreak from the Neurology Department of Hospital Universitario 12 de Octubre, who voluntarily donated serum and CSF samples for research purposes.
Biological samples and analysis
For the COVID-19-NRL group, we selected those serum samples obtained closer to the onset of neurological manifestations as part of their routine clinical care, which were stored in the Hospital serum bank and available for a new analysis. Four individuals also had stored serum extracted after the onset of COVID-19 symptoms but before the occurrence of neurological involvement. A subset of three patients also had CSF available for laboratory analysis. Other eight serum samples from this group, obtained in different moments of the disease, were also investigated. For the COVID-19 group free of neurological manifestations, we analyzed available serum samples collected closer to the date of symptom onset. All participants from the healthy control group had available serum and CSF samples, obtained at the same time, and stored in a biobank.
Venous blood from participants was collected in serum separating tubes and centrifuged at 1500 g for 10 min. Serum supernatant was aliquoted in small volumes and stored at − 80 ºC. CSF samples were collected by lumbar puncture after obtaining informed consent from participants. All samples were spun at 3000 rpm at 4 °C for 10 min to remove any cells and debris, aliquoted in small volumes, and stored in low bind polypropylene tubes at − 80 °C.
Serum and CSF total αSyn measurements were performed in the Laboratory of Neuroscience from Hospital 12 de Octubre Research Institute (i + 12). Analysis was performed using commercially available total αSyn ELISA kits (Invitrogen, KHB0061) as described by the manufacturer. The concentration of total αSyn was determined by spectrometric measurement at 450 nm in an appropriate microplate reader (EnSpire Multimode Plate Reader, Perkin Elmer, USA). Each sample was analyzed in duplicate. Intra-assay coefficients of variation were < 10% for all samples.
Serum enolase was quantified at Clinical Analysis Laboratory of Hospital 12 de Octubre, using Elecsys® NSE electrochemiluminescence automated immunoassay on the Cobas e801 (Roche Diagnostics). The limit of quantification of this assay is 0.15 ng/ml (measuring range 0.075–300 ng/mL).
Statistical analysis
Statistical analysis and graphs were performed using Stata/IC software (Stata 16.1, StataCorp LLC, USA) and Prism (GraphPad Software version 8.00, La Jolla, CA).
All data are reported as mean and SD unless otherwise indicated. The normality of the variable distribution was assessed using the Shapiro–Wilk test. A parametric test for comparison of two means was applied when possible. Comparison of αSyn among the three groups was done using Kruskal–Wallis test. Changes in αSyn and enolase serum levels before and after the onset of neurological symptoms were analyzed with paired t tests. Associations were measured with Spearman correlation.
Results
Demographics
All three groups (COVID-19 with and without neurological symptoms, and individuals without an acute infection) were similar in age and sex distribution (Table 1). All patients with SARS-CoV-2 infection included in this study had a moderate-to-severe bilateral pneumonia requiring oxygen supplementation (Table 2). Among those patients requiring intensive care, median intensive-care unit (ICU) stay was similar (median: 16 days in patients with neurological symptoms and 17 days in those without; Table 2). Nevertheless, neurological COVID-19 patients required longer hospitalization stay (median: 43 days) than the other group (median: 8 days) (Table 2).
Table 1.
Total alpha-synuclein (αSyn) and enolase levels in serum and cerebrospinal fluid
| COVID-19 with neurological symptoms | COVID-19 without neurological symptoms | Healthy controls | p value | |
|---|---|---|---|---|
| Age, years | 65.0 (61.4–76.3)a | 66.5 (65.9–68.4)a | 66.5 (64–70.5)a | – |
| Sex, M/F | 5/2 | 8/5 | 6/2 | – |
| Serum αSyn, ng/ml | 15.64 (13.3) (n = 7) | 20.92 (18.1—26.9)a (n = 13) | 26.82 (18.90) (n = 8) | 0.27 |
| CSF αSyn, pg/ml | 324.2 (223.4) (n = 3) | – | 256.5 (265.6) (n = 8) | 0.71 |
| Serum enolase, ng/ml | 9.31 (4.7) (n = 7) | 14.35 (8.9) (n = 13) | – | 0.18 |
All data are displayed as Mean (SD), unless otherwise indicated
CSF cerebrospinal fluid, F: female, M male
aMedian (interquartile interval)
Table 2.
COVID-19 patient’s characteristics
| ID | Age (years) | Sex | Neurological manifestations | Systemic manifestations | ICU stay (days) | Death | Time from symptom onset until admission (days) | Time until neurologic symptoms (days) | Time until serum extraction (days) | Length of hospitalization (days) | Brain imaging scans | CSF | EEGa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | M |
Myoclonus Mild encephalopathy |
Bilateral pneumonia + respiratory failure | 16 | No | 2 | 8 | 12 | 43 |
MRI Normal |
WC: 0 /µl RBC: 2 /µl Proteins: 0.43 g/l |
Mild diffuse slowing (reactive) |
| 2 | 76 | M |
Myoclonus Mild encephalopathy |
Bilateral pneumonia + respiratory failure | - | No | 2 | 2 | 6 | 20 |
MRI Normal |
No | Mild diffuse slowing (reactive) |
| 3 | 88 | F |
Myoclonus Mild encephalopathy |
Bilateral pneumonia + respiratory failure | - | Yes | 4 | 25 | 31 | 34 |
CT Normal |
No | Mild diffuse slowing |
| 4 | 58 | M |
Parkinsonism Myoclonus Mild encephalopathy |
Bilateral pneumonia + respiratory failure | 28 | No | 7 | 31 | 49 | 47 |
MRI Normal DaT-SPECT Altered |
WC: 8 /µl RBC: 150 /µl Proteins: 0.82 g/l |
Normal |
| 5 | 61 | M |
Encephalopathy Myoclonus |
Bilateral pneumonia + respiratory failure | 7 | No | 12 | 15 | 17 | 23 |
MRI Normal |
No | Diffuse slowing |
| 6 | 68 | M |
Myoclonus Encephalopathy |
Bilateral pneumonia + respiratory failure | 24 | No | 7 | 33 | 34 | 48 |
CT Normal |
No | Mild diffuse slowing |
| 7 | 65 | F |
Myoclonus Encephalopathy |
Bilateral pneumonia + respiratory failure | 14 | No | 4 | 18 | 68 | 75 |
MRI Normal |
WC: 0 /µl RBC: 22 /µl Proteins: 0.18 g/l | Mild diffuse slowing |
| 8 | 64 | M | – | Bilateral pneumonia + respiratory failure | - | No | 8 | – | 10 | 13 | – | – | – |
| 9 | 64 | M | – | Bilateral pneumonia + respiratory failure | 27 | Yes | 7 | – | 9 | 29 | – | – | – |
| 10 | 65 | M | – | Bilateral pneumonia + respiratory failure | – | No | 14 | – | 15 | 5 | – | – | – |
| 11 | 65 | M | – | Bilateral pneumonia + respiratory failure | – | No | 5 | – | 10 | 8 | – | – | – |
| 12 | 65 | F | – | Bilateral pneumonia + respiratory failure | – | No | 5 | – | 12 | 8 | – | – | – |
| 13 | 66 | M | – | Bilateral pneumonia + respiratory failure | – | No | 20 | – | 22 | 8 | – | – | – |
| 14 | 66 | M | – | Bilateral pneumonia + respiratory failure | – | Yes | 4 | – | 6 | 7 | – | – | – |
| 15 | 66 | M | – | Bilateral pneumonia + respiratory failure | 16 | Yes | 7 | – | 10 | 27 | – | – | – |
| 16 | 67 | F | – | Bilateral pneumonia + respiratory failure | 18 | No | 14 | – | 15 | 49 | – | – | – |
| 17 | 68 | F | – | Bilateral pneumonia + respiratory failure | 12 | Yes | 15 | – | 17 | 22 | – | – | – |
| 18 | 72 | M | – | Bilateral pneumonia + respiratory failure | – | No | 7 | – | 13 | 8 | – | – | – |
| 19 | 76 | F | – | Bilateral pneumonia + respiratory failure | – | No | 15 | – | 16 | 5 | – | – | – |
| 20 | 80 | F | – | Bilateral pneumonia + respiratory failure | – | Yes | 9 | – | 10 | 6 | – | – | – |
CSF cerebrospinal fluid, CT computed tomography, DaT-SPECT dopamine transporter single photon emission computed tomography, EEG electroencephalogram, MRI magnetic resonance imaging. RBC red blood cells, WC white cells
aAll patients showed no epileptiform activity on EEG
All selected patients exhibited a similar clinical picture, consistent in generalized myoclonus as shown in patient one [6] with variable degrees of severity (Table 2). We also observed the development of subacute Parkinsonism in one patient (patient four) [7]. A detailed clinical and work-up description has been reported elsewhere for four of the seven patients of this group [6, 7]. The remaining three had a similar clinical course than those already published.
Notwithstanding the clinical severity of the neurological group, the prognosis was good for most of them, and some exhibited marked improvement without a specific treatment, including the patient who developed the subacute Parkinsonism. The proportion of deaths was only of 14% in this group, while it was of 38% in the group without neurological involvement (Table 2).
Alpha-synuclein testing
Serum total αSyn levels did not differ significantly among the three groups (Kruskal–Wallis Ho = 2.624, df = 2, p = 0.27; Table 1 and Fig. 1). We also did not find differences when comparing αSyn levels in the CSF of COVID-19-NRL and healthy controls (d = 67.6 pg/ml, IC 95%: − 325.7–461.0 pg/ml, p = 0.71; Table 1; Fig. 2).
Fig. 1.
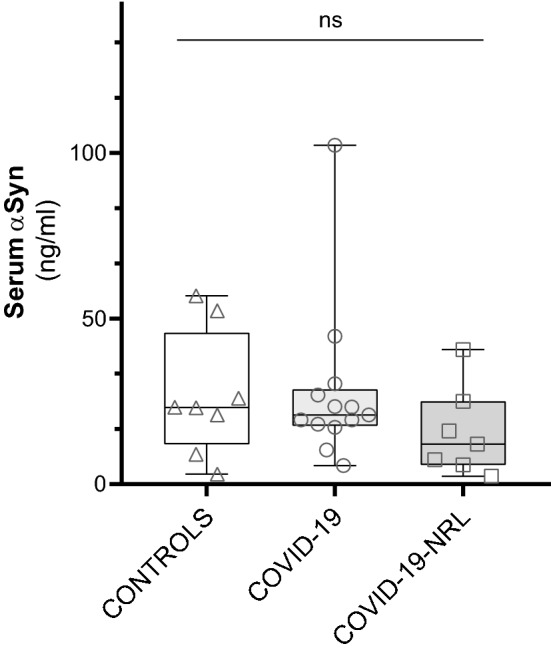
Serum total α-synuclein (αSyn) in controls, COVID-19 patients with neurological manifestations (COVID-19-NRL), and COVID-19 patients
Fig. 2.
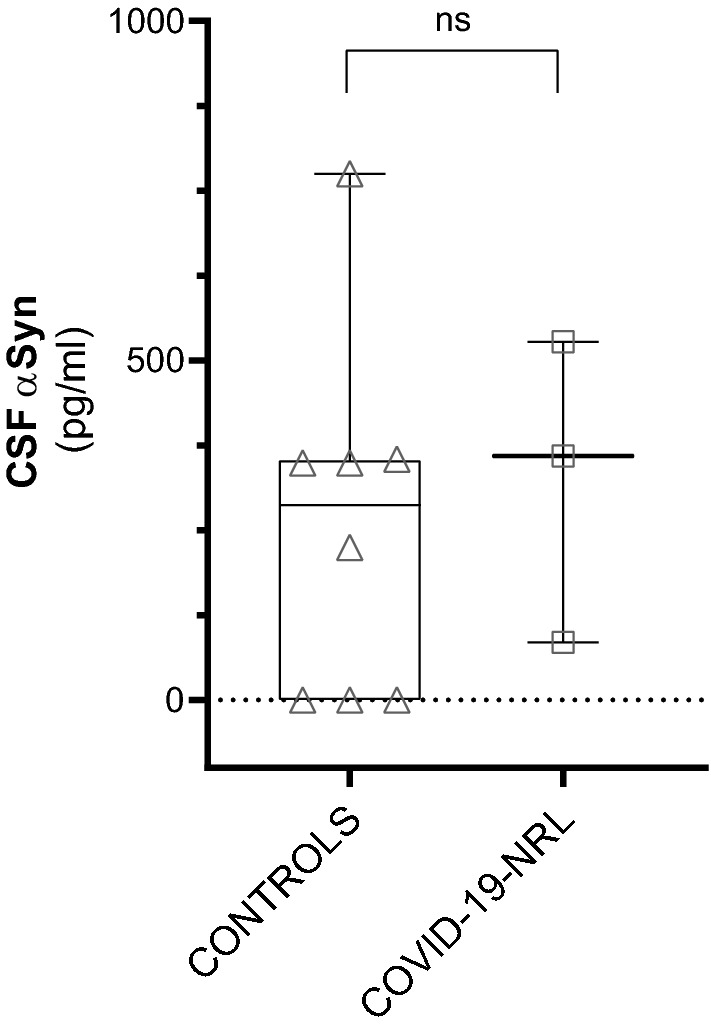
Cerebrospinal fluid (CSF) total alpha-synuclein (αSyn) in controls and COVID-19 with neurological manifestations (COVID-19-NRL)
αSyn measurements were also very similar in the subgroup that required intensive care, compared to those who did not (z = 0.266, p = 0.82). Serum sample analyzed in COVID-19-NRL was extracted later in the course of the disease (median days since symptom onset: 31; Table 2) than in non-neurologic COVID-19 (median: 12 days; Table 2). Nevertheless, no statistically significant correlation was found with serum αSyn concentration and the days elapsed since the beginning of the disease (rs = − 0.21, p = 0.26, n = 32; Fig. 3) or since the onset of neurological symptoms (rs = 0.07, p = 0.78, n = 19).
Fig. 3.
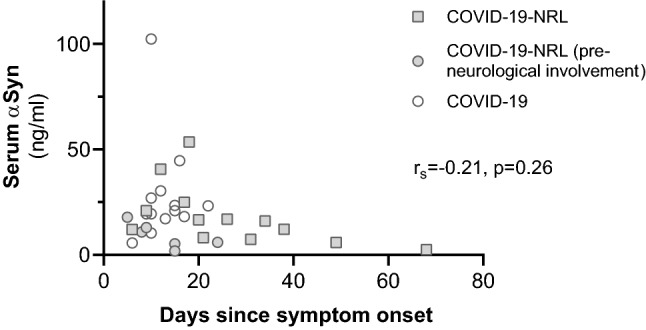
Serum total alpha-synuclein (αSyn) levels and days since COVID-19 symptom onset. COVID-19-NRL: COVID-19 patients with neurological symptoms
We also tested for αSyn levels before and after the onset of neurological symptoms in a subgroup of four patients who also had available serum close to the moment of admission. No significant difference was found between the two groups (change = − 3.78 ng/ml, IC 95%: − 21.2–13.7 ng/ml, p = 0.54). An individual graphical representation of values can be found in Fig. 4.
Fig. 4.
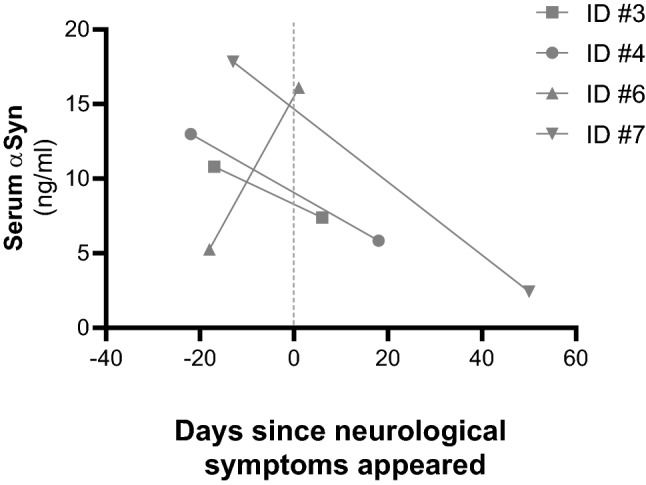
Change in serum total alpha-synuclein (αSyn) levels before and after the onset of neurological manifestations
To assess whether COVID-19 patients with neurological involvement suffered from an underappreciated CNS injury, we also analyzed serum neuron specific enolase (NSE), a validated biomarker for CNS injury available in our institution, in all COVID-19 patients (Table 1). There were no differences between groups with or without neurological involvement (z = 1.39, p = 0.18). This validated marker of neuronal injury was within the normal limits in all but one measurement corresponding to a non-neurologic COVID-19 patient who subsequently died (normal range: < 20 ng/ml, altered reading: 38.3 ng/ml, patient 14). In addition, we did not find a significant change of NSE when neurological involvement appeared (change = − 4.45 ng/ml, IC 95%: − 12.1–3.13, p = 0.16) in the subgroup of four patients with samples pre- and post-neurological symptoms.
Discussion
Our results show no significant differences in serum total αSyn levels measured on patients with COVID-19 and neurological involvement in the form of generalized myoclonus and encephalopathy, compared with age- and sex-matched COVID-19 patients free of neurological symptoms and healthy controls. We also did not find a significant change in serum αSyn concentration before and after the emergence of neurological manifestations in a subset of four patients where pre- and post-samples were available. Furthermore, CSF total αSyn levels were similar in three patients with neurological involvement compared with those from healthy controls without acute infection.
Abnormal αSyn accumulation in peripheral fluids (serum, plasma, and CSF) may mirror brain abnormalities of Parkinson’s disease (PD) patients, making this protein a candidate biomarker in PD [26] as well as in other synucleinopathies [27]. Moreover, the role of infectious etiologies in the development of Parkinsonism, PD, and other neurodegenerative diseases has been proposed elsewhere [19, 28, 29]. It has been theorized that a neurotropic pathogen can gain access to the CNS and trigger diverse mechanisms of neurodegeneration [19, 28]. However, as far as we know, this is the first study that quantify serum αSyn in patients with a neurotropic virus infecting the CNS.
αSyn is nearly exclusively expressed in neurons, and its physiological function is not completely understood [30]. Recent studies support the hypothesis that αSyn can function as a restriction factor for RNA virus infections within neurons, inhibiting viral growth and injury to the CNS [22, 23]. However, we did not find serum or CSF αSyn change in patients with SARS-CoV-2 infection affecting the CNS.
First, our results could suggest that generalized myoclonus in COVID-19 (with associated encephalopathy and parkinsonism in some of the patients described) may not be the consequence of direct CNS damage by SARS-CoV-2, but an immune-mediated pathomechanism, and further studies should consider this possibility. Nevertheless, neither evidence of inflammation nor of infection was described in CSF or neuroimaging from none of the reported cases [8–15]. As we have previously argued [6, 7], these findings do not exclude the possibility that SARS-CoV-2 entered the CNS, since some animal models showed no histologic lesions or encephalitis after viral neuroinvasion [31, 32], including SARS-CoV [33], and virus could not be detected in the CSF [32]. Even more, RT-PCR testing in the CSF could have suboptimal sensitivity for the detection of SARS-CoV-2, as it occurs with other neuroinvasive viruses, such asenterovirus-D68, rabies virus, and West Nile virus [4].
Second, no upregulation in serum could not rule out an intraneuronal increase in αSyn aggregates [34]. In some animal models, cytoplasmic αSyn aggregates were found strictly in conjunction with the presence of viral antigens [34]. Therefore, SARS-CoV-2 RNA detection and immunohistochemistry [16] and αSyn immunohistochemical staining on brains from patients who died from COVID-19 could shed light on it. Even more, other putative antimicrobial peptides such as APP/amyloid-β [35] or tau [36] could be involved in neurons protection before the activation of the innate and adaptive immune system take place [19, 31, 33]. Indeed, autopsy cases of postencephalitic Parkinsonism, a chronic complication of encephalitis lethargica, showed tau immunoreactivity and the absence of αSyn pathology [37].
This study has several limitations. First, it is a retrospective study with an extremely limited number of patients. As an explorative study, we thus focused on COVID-19 with major neurological symptoms such as generalized myoclonus. We made a special effort to include only those cases where other alternative explanations (metabolic disturbances, drugs, etc.) were improbable, and that conduced to a very low number of participants. Therefore, further research is necessary, taking into account a larger group of COVID-19 patients including the whole spectrum of neurological manifestations and disease severity.
Second, although sample manipulation was similar for all of them and there were no relevant macroscopic changes, other confounders such as hemolysis and platelet contamination that may affect αSyn levels were not systematically assessed in the healthy control group.
Third, the age of participants cannot rule out the possibility that other mechanisms affecting αSyn clearance and regulation have already turned on.
In conclusion, we did not find any significant difference in serum and CSF total αSyn levels among COVID-19 patients with and without neurological symptoms and healthy controls. Overall, these results cannot support the hypothesis of αSyn upregulation in humans when an infectious pathogen gains access to the CNS. We hope that this small study encourages further research to investigate the neural function of αSyn and other possible biological markers of COVID-19 neurological manifestations.
Author contributions
VAB-P: conception, organization and execution of the research project, statistical analysis and interpretation of data, laboratory alpha-synuclein analysis, and drafting and revising the manuscript. FJA-D: acquisition of data and revising the manuscript for intellectual content. MR-O: acquisition of data and revising the manuscript for intellectual content. ML-G: acquisition of data and revising the manuscript for intellectual content. PR-S: acquisition of data and revising the manuscript for intellectual content. AM-G: acquisition of data and revising the manuscript for intellectual content. MA-R: laboratory enolase analysis, acquisition of samples, and revising the manuscript for intellectual content. JLE: laboratory enolase analysis, acquisition of samples, and revising the manuscript for intellectual content. AP-R: acquisition of samples, revising the manuscript for intellectual content. ÁM-L: acquisition of data and revising the manuscript for intellectual content. MR-F: revising the manuscript for intellectual content. EC: revising the manuscript for intellectual content. JGA: conception, organization and execution of the research project, interpretation of data, and drafting and revising the manuscript.
Funding
Dr. Víctor Antonio Blanco-Palmero is supported by the Instituto de Salud Carlos III (ISCIII, Spanish Biomedical Research Institute) through a “Río Hortega” contract (CM 18/0095).
Data availability
Individual and detailed clinical and laboratory data from each patient can be provided upon request.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no competing financial interests and report no disclosures.
Ethics approval
Study protocol received ethical approval by Hospital Universitario 12 de Octubre Ethics Committee (Protocol number 20/342), in accordance to Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all alive patients.
References
- 1.(2020) WHO | Novel Coronavirus – China. WHO
- 2.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghannam M, Alshaer Q, Al-Chalabi M, et al. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267:3135–3153. doi: 10.1007/s00415-020-09990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, et al. Generalized myoclonus in COVID-19. Neurology. 2020;95:e767–e772. doi: 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Méndez-Guerrero A, Laespada-García MI, Gómez-Grande A, et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95:e2109–e2118. doi: 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- 8.Anand P, Zakaria A, Benameur K, et al. Myoclonus in patients with coronavirus disease 2019: a multicenter case series. Crit Care Med. 2020;48:1664–1669. doi: 10.1097/CCM.0000000000004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuhna P, Herlin B, Vassilev K, et al. Movement disorders as a new neurological clinical picture in severe SARS-CoV-2 infection. Eur J Neurol. 2020;27:e88–e90. doi: 10.1111/ene.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkstra F, Van den Bossche T, Willekens B, et al. Myoclonus and cerebellar ataxia following COVID-19. Mov Disord Clin Pract. 2020;7:974–976. doi: 10.1002/mdc3.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoo A, McLoughlin B, Cheema S, et al. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J Neurol Neurosurg Psychiatry. 2020;91:1013–1014. doi: 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- 12.Schellekens MMI, Bleeker-Rovers CP, Keurlings PAJ, et al. Reversible myoclonus-ataxia as a postinfectious manifestation of COVID-19. Mov Disord Clin Pract. 2020;7:977–979. doi: 10.1002/mdc3.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muccioli L, Rondelli F, Ferri L, et al. Subcortical myoclonus in Coronavirus Disease 2019: comprehensive evaluation of a patient. Mov Disord Clin Pract. 2020;7:971–973. doi: 10.1002/mdc3.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faber I, Brandão PRP, Menegatti F, et al. Coronavirus disease 2019 and Parkinsonism: a non-post-encephalitic case. Mov Disord. 2020;35:1721–1722. doi: 10.1002/mds.28277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen ME, Eichel R, Steiner-Birmanns B, et al. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19:804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267:2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2020 doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 19.Tulisiak CT, Mercado G, Peelaerts W, et al. Can infections trigger alpha-synucleinopathies? In: progress in molecular biology and translational science. Amsterdam: Elsevier; 2019. pp. 299–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massey AR, Beckham JD. Alpha-synuclein, a novel viral restriction factor hiding in plain sight. DNA Cell Biol. 2016;35:643–645. doi: 10.1089/dna.2016.3488. [DOI] [PubMed] [Google Scholar]
- 21.Stolzenberg E, Berry D, Yang D, et al. A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun. 2017;9:456–463. doi: 10.1159/000477990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beatman EL, Massey A, Shives KD, et al. Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol. 2016;90:2767–2782. doi: 10.1128/JVI.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesteberg KE, Beckham JD. Immunology of west nile virus infection and the role of alpha-synuclein as a viral restriction factor. Viral Immunol. 2019;32:38–47. doi: 10.1089/vim.2018.0075. [DOI] [PubMed] [Google Scholar]
- 24.Brundin P, Nath A, Beckham JD. Is COVID-19 a Perfect Storm for Parkinson’s Disease? Trends Neurosci. 2020;43:3. doi: 10.1016/j.tins.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavel A, Murray DK, Stoessl AJ. COVID-19 and selective vulnerability to Parkinson’s disease. Lancet Neurol. 2020;19:719. doi: 10.1016/S1474-4422(20)30269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bougea A, Stefanis L, Paraskevas GP, et al. Plasma alpha-synuclein levels in patients with Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci. 2019;40:929–938. doi: 10.1007/s10072-019-03738-1. [DOI] [PubMed] [Google Scholar]
- 27.Cong S, Xiang C, Wang H, Cong S. Diagnostic utility of fluid biomarkers in multiple system atrophy: a systematic review and meta-analysis. J Neurol. 2020;2:4. doi: 10.1007/s00415-020-09781-9. [DOI] [PubMed] [Google Scholar]
- 28.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limphaibool N, Iwanowski P, Holstad MJV, et al. Infectious etiologies of Parkinsonism: pathomechanisms and clinical implications. Front Neurol. 2019;10:2. doi: 10.3389/fneur.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villar-Piqué A, Lopes da Fonseca T, Outeiro TF. Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J Neurochem. 2016;139:240–255. doi: 10.1111/jnc.13249. [DOI] [PubMed] [Google Scholar]
- 31.Jang H, Boltz D, McClaren J, et al. Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice. J Neurosci. 2012;32:1545–1559. doi: 10.1523/JNEUROSCI.5123-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Wit E, Siegers JY, Cronin JM, et al. 1918 H1N1 influenza virus replicates and induces proinflammatory cytokine responses in extrarespiratory tissues of ferrets. J Infect Dis. 2018;217:1237–1246. doi: 10.1093/infdis/jiy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netland J, Meyerholz DK, Moore S, et al. Severe acute respiratory syndrome Coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marreiros R, Müller-Schiffmann A, Trossbach SV, et al. Disruption of cellular proteostasis by H1N1 influenza A virus causes α-synuclein aggregation. Proc Natl Acad Sci USA. 2020;117:6741–6751. doi: 10.1073/pnas.1906466117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosztyla ML, Brothers HM, Robinson SR. Alzheimer’s Amyloid-β is an antimicrobial peptide: a review of the evidence. J Alzheimer’s Dis. 2018;62:1495–1506. doi: 10.3233/JAD-171133. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi N, Masuda J, Kudoh J, et al. Binding sites on tau proteins as components for antimicrobial peptides. Biocontrol Sci. 2008;13:49–56. doi: 10.4265/bio.13.49. [DOI] [PubMed] [Google Scholar]
- 37.Jellinger KA. Absence of α-synuclein pathology in postencephalitic parkinsonism. Acta Neuropathol. 2009;118:371–379. doi: 10.1007/s00401-009-0537-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual and detailed clinical and laboratory data from each patient can be provided upon request.


