Abstract
The limitations of cisplatin, a standard chemotherapy for lung cancer, have been documented with serious adverse effects and drug resistance. To address the need for novel therapy, this study firstly reveals the potential of peptide from Lentinus squarrosulus (Mont.) as a chemotherapeutic adjuvant for cisplatin treatment. The purified peptide from L. squarrosulus aqueous extracts was obtained after eluting with 0.4 M NaCl through FPLC equipped with anion exchange column. Preincubation for 24 h with 5 µg/mL of the peptide at prior to treatment with 5 µM cisplatin significantly diminished %cell viability in various human lung cancer cells but not in human dermal papilla and proximal renal cells. Flow cytometry indicated the augmentation of cisplatin-induced apoptosis in lung cancer cells pretreated with peptide from L. squarrosulus. Preculture with the peptide dramatically inhibited colony formation in lung cancer cells derived after cisplatin treatment. Strong suppression on integrin-mediated survival was evidenced with the diminution of integrins (β1, β3, β5, α5, αV) and down-stream signals (p-FAK/FAK, p-Src/Src, p-Akt/Akt) consequence with alteration of p53, Bax, Blc-2 and Mcl-1 in cisplatin-treated lung cancer cells preincubated with peptide from L. squarrosulus. These results support the development of L. squarrosulus peptide as a novel combined chemotherapy with cisplatin for lung cancer treatment.
Subject terms: Molecular medicine, Drug development
Introduction
Despite several decades of intensive research, lung cancer remains to be a global health burden and the leading cause of cancer-related death1. Among various available remedies, standard chemotherapy is still one of the major treatment options due to its known efficacy and relatively good patient compliance2. Cisplatin, a platinum-based compound, is a first-line chemotherapeutic drug for the treatment of lung cancer3. In combination with other drugs, it is commonly used as adjuvant therapy for the advanced stages of lung cancer and after tumor resection4. However, the aggressive features and distinct mechanisms observed in lung cancer cells lead to chemotherapeutic resistance and treatment failure5,6. Moreover, adverse effects such as renal toxicity and alopecia further compromise patients’ quality of life and limit the use of cisplatin7. To improve compliance and clinical outcomes, a significant amount of research has been conducted to find novel therapies to restore cisplatin sensitivity in cancer cells without increasing toxicity to normal cells8,9.
While the aim is to maximize cisplatin efficacy, evidence shows that cisplatin resistance is associated with reduced apoptosis induction in lung cancer cells10. Current research trends reveal that targeting Bcl-2 family proteins is a promising strategy to overcome cisplatin resistance11,12. The upregulation or overexpression of the anti-apoptosis protein, Bcl-2 (B-cell lymphoma 2), is related to the reduced susceptibility to cisplatin-induced apoptosis10,13. Moreover, evidence suggests that the upregulation of another anti-apoptosis protein, Mcl-1 (Myeloid cell leukemia 1) is correlated to the persistence of tumor pathology in lung cancer patients14. On the other hand, the downregulation of Mcl-1 protein has been found to effectively restore drug sensitivity in cisplatin-resistant lung cancer cells15. Meanwhile, the decreased protein level of pro-apoptosis Bax (Bcl-2 associated X) protein causes hyperplasia of lung tumor and resistance to various apoptosis stimuli16. Evidently, the modulation of Bcl-2 family proteins to reverse cisplatin resistance is worth investigating further. Another strategy is the regulation of tumor suppressor p53 which has been a reported mechanism of several natural chemosensitizers9.
Dysregulation of survival signaling pathways has also been previously linked to cisplatin resistance. Elevated level of FAK (Focal adhesion kinase) also protects lung cancer cells from cisplatin-induced apoptosis17. The complexation between transmembrane molecules, integrins, and extracellular matrix (ECM) initiates the survival cascade via stimulation of FAK/Src (Proto-oncogene tyrosine-protein kinase) consequently activating the downstream PI3K (phosphoinositide 3-kinase)/Akt (Protein kinase B) signaling pathway18. The overexpression of integrins especially β1, β3, β5, α5 and αV, have particularly been implicated in lung cancer progression and cisplatin treatment failure19–21. In addition, the upregulation of PI3K/Akt-related survival molecules by integrin-ECM interaction results in the activation of anti-apoptosis proteins, inhibition of p53 and disruption of apoptosis cell death22,23. Therefore, the regulation of integrins and corresponding survival molecules has emerged as another strategy to augment cisplatin potency24–26.
Due to the obvious need of innovation in cancer treatment, multi-targeted natural chemosensitizers used in combination with standard anticancer drugs have recently been highlighted for their capacity to enhance efficacy and reduce toxicities of chemotherapy9,27. Among various natural resources, peptide extracts from edible mushroom have gained an attention due to anti-tumor potential and promising safety profile28. Recently, peptides extracted from Lentinus squarrosulus (Mont.) have been revealed to induce apoptosis in human lung cancer cells29. However, the reported extracts composed of various peptides ranging from 11 to 75 kDa which may contain some toxicity. In order to improve efficacy of these peptide extracts, the purification of L. squarrosulus peptide extracts have been performed through fast protein liquid chromatography (FPLC) in this study. Furthermore, this study is the first to investigate the novel chemosensitizing activity of the peptide extracted from L. squarrosulus to enhance cisplatin efficacy without causing toxicity to non-cancer cells. The investigation seeks to provide information which could encourage the further development of chemotherapeutic adjuvants with good safety profile for lung cancer treatment.
Results
Purified peptide isolated from L. squarrosulus extracts
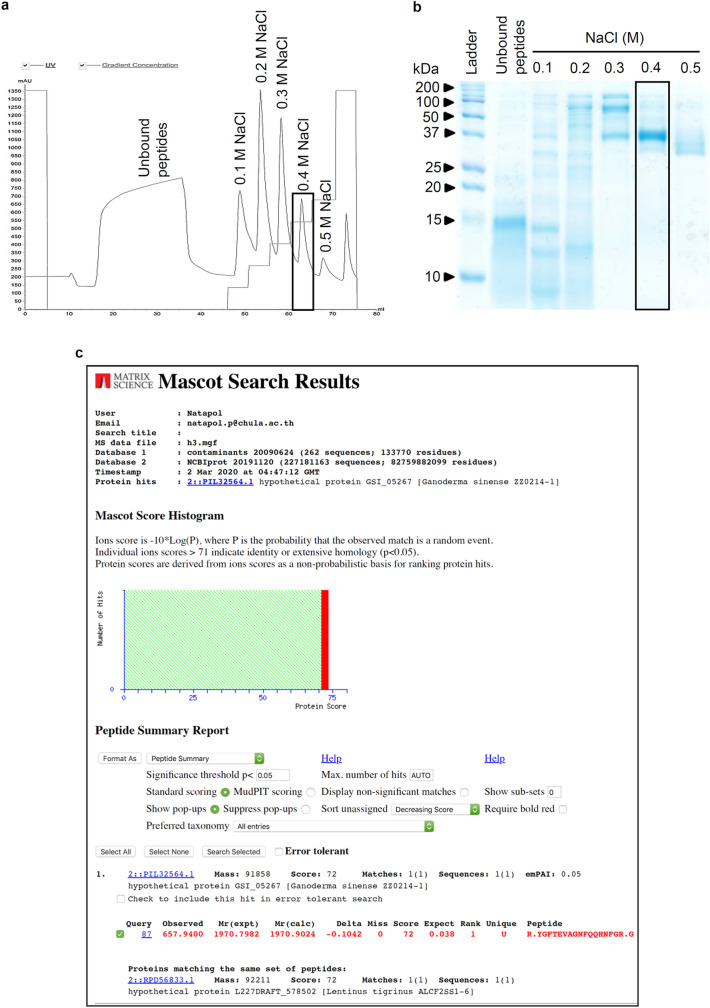
The peptide pellets were precipitated out from the homogenized aqueous solution of L. squarrosulus fruiting bodies by adding 40–80% (NH4)2SO4. These crude peptide extracts were then resolubilized in PBS (pH 7.4) and further purified through FPLC coupled with anion exchange column. Figure 1a shows a representative FPLC chromatogram of different peptide fractions which were eluted through a stepwise increase in concentration of NaCl solution (0.1, 0.2, 0.3, 0.4 and 0.5 M). The eluted peptide profile was composed of six peaks including unbound peptides. The composition of each peptide fraction was evaluated through SDS-PAGE analysis. Among the six peptide fractions, the most purified peptide appears in the fraction eluted with 0.4 M NaCl, which presented an intensively stained single band of protein at ~ 37 kDa (Fig. 1b). It is worth noting that protein bands appearing with less intensity and various molecular weight were presented in the other peptide fractions. Therefore, the peptide of 0.4 M NaCl fraction was selected for further investigations on chemosensitizing effect to cisplatin.
Figure 1.
Purified peptide from aqueous extract of Lentinus squarrosulus (Mont.). (a) Chromatogram of eluted peptides obtained from FPLC at different concentrations of NaCl. (b) Peptide composition of different fractions from L. squarrosulus extracts analyzed by SDS-PAGE. The rectangular box indicates the purest fraction, 0.4 M NaCl peptide. (c) The Mascot search web service indicated 72 similarity score of proteomic analysis of 0.4 M NaCl peptide with extracellular metalloproteinase from Ganoderma sinense ZZ0214-1 and Lentinus tigrinus ALCF2SS1-6.
Proteomics analysis of L. squarrosulus purified fraction
Despite hundreds of peptide fragments separated through LC–MS/MS, the search results reported only one matched query. Fragments with observed mass at 657.9400 were uniquely matched (p < 0.05) with the peptide sequence (R.YGFTEVAGNFQQHNFGR.G) from hypothetical protein GSI_05267 of Ganoderma sinense ZZ0214-1 and the same set of peptides was also matched to hypothetical protein L227DRAFT_578502 of Lentinus tigrinus ALCF2SS1-6 as shown in Fig. 1c. The peptide sequence of matched fragments was then 100% identity matched to “A0A2G8SFL4_9APHY—Extracellular metalloproteinase Ganoderma sinense ZZ0214-1” and “A0A5C2S0B7_9APHY—Extracellular metalloproteinase Lentinus tigrinus ALCF2SS1-6” from UniProt database.
Cytotoxicity of L. squarrosulus peptide
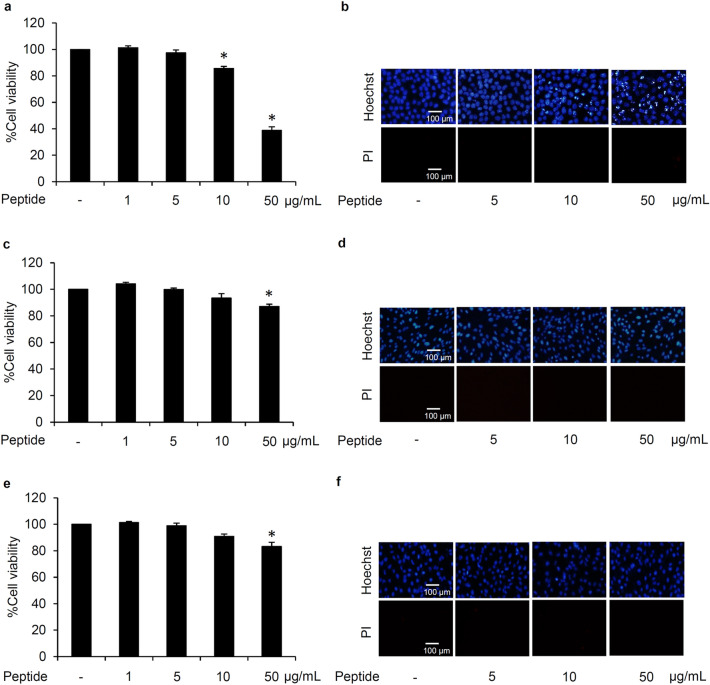
To clarify the optimum concentrations for further use in the study, the cytotoxic effect of 0.4 M NaCl L. squarrosulus peptide fraction was investigated in both human lung cancer and normal cells. After incubation with various concentrations (0–50 µg/mL) of 0.4 M NaCl peptide for 24 h, the reduction of %cell viability was significantly noted in human lung cancer H460 cells treated with 10 and 50 µg/mL of the peptide (Fig. 2a). Corresponding with the viability assay results, apoptosis as characterized by the bright blue fluorescence of Hoechst33342 nuclear stain was evidently increased in the cells cultured with 10–50 µg/mL of 0.4 M NaCl peptide (Fig. 2b). Notably, there were no red fluorescence cells signifying necrosis in all peptide-treated lung cancer cells.
Figure 2.
Cytotoxicity profile of L. squarrosulus peptide from 0.4 M NaCl fraction in human lung cancer and normal cells. The non-toxic concentration range of the peptide was determined in (a) human lung cancer H460, (c) dermal papilla DPCs, and (e) proximal renal HK-2 cells cultured with 0.4 M NaCl peptide (0–50 µg/mL) for 24 h via MTT viability assay. Costaining with Hoechst33342 and propidium iodide (PI) indicated no detectable apoptosis and necrosis (b) H460, (d) DPCs, and (f) HK-2 cells after treatment with 5 µg/mL of 0.4 M NaCl peptide for 24 h. Notably, no cell death was observed in DPCs and HK-2 cells at higher concentrations of the peptide while apoptosis cells were visually in Hoechst33342-stained H460 cells cultured with 10–50 µg/mL of 0.4 M NaCl peptide. Values are means of three independent experiments ± SD. *p < 0.05 versus non-treated control cells.
Because hair loss and renal failure are major side effects of cisplatin30–32, the cytotoxicity of 0.4 M NaCl peptide was also investigated in human dermal papilla DPCs cells and human proximal renal HK-2 cells. Figure 2c,e depict the viability in DPCs and HK-2 cells as determined through MTT assay. The %cell viability remained approximately 100% in both cell types after treatment with 0–10 µg/mL of L. squarrosulus peptide for 24 h. Although significant reduction of cell viability was detected, apoptosis and necrosis were barely observed in DPCs and HK-2 cells cultured with peptide of 0.4 M NaCl fraction at 50 µg/ml for 24 h (Fig. 2d,f). Remarkably, the peptide extract possesses selective cytotoxicity towards lung cancer cells which is evident in the lower %cell viability and increased apoptosis observed in human lung cancer H460 cells after treatment with 0.4 M NaCl peptide at 10–50 µg/ml compared with both DPCs and HK-2 cells.
To clearly clarify chemosensitizing effect, pretreatment of cancer cells with chemotherapeutic adjuvant at non-toxic concentration is suggested33. Therefore, 5 µg/mL of 0.4 M NaCl peptide which did not exhibit cytotoxicity in cancer and non-cancer cells was selected as the optimum non-toxic concentration for further experiments related to sensitization to cisplatin-induced cell death in human lung cancer cells.
L. squarrosulus peptide sensitizes cisplatin-induced apoptosis in human lung cancer cells
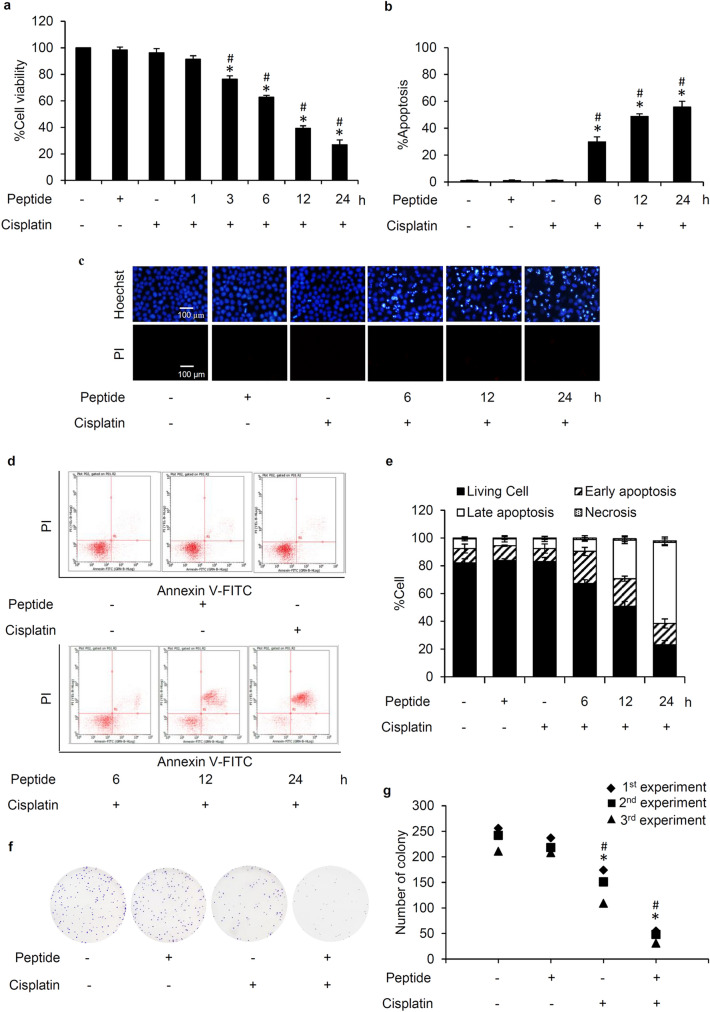
The chemosensitizing effect of L. squarrosulus peptide was determined in time-dependent investigation in human lung cancer cells. Lung cancer H460 cells were pretreated with 0.4 M NaCl peptide prior to exposure of 5 µM cisplatin. The cell viability of H460 cells further decreased in a time-dependent manner in response to pretreatment (3–24 h) of 0.4 M NaCl peptide (5 µg/mL) as indicated in Fig. 3a. Figure 3b presents the time-dependent increment of %apoptosis in H460 cells preincubated with 0.4 M NaCl peptide. Correspondingly, nuclear costaining with Hoechst33342 and propidium iodide (PI) also showed that apoptosis cell death was remarkably noted in L. squarrosulus peptide-pretreated H460 cells in a time (6–24 h) -dependent manner (Fig. 3c).
Figure 3.
Sensitizing effect of L. squarrosulus peptide on cisplatin-induced cytotoxicity in human lung cancer H460 cells. (a) The reduction of viability in cisplatin-treated H460 cells was observed in time-dependent manner in response to 0.4 M NaCl peptide at 5 µg/mL. (b) Higher %apoptosis detected by (c) bright blue fluorescence of Hoechst33342 staining was demonstrated in H460 cells preincubated with the peptide at 5 µg/mL for 6–24 h compared with the cells treated with cisplatin alone. Notably, there was no necrosis characterized by propidium iodide (PI) red fluorescence observed in all treated cells. (d) Flow cytometry histograms of annexin V-FITC/PI showed the augmentation of apoptosis in H460 cells preincubated with 0.4 M NaCl peptide (5 µg/mL) then treated with 5 µM of cisplatin for 24 h. (e) The higher %cells in both early and late apoptosis was noted in the cells pretreated with L. squarrosulus peptide for 6–24 h compared with the cells treated only with cisplatin. Pretreatment with 0.4 M NaCl peptide suppresses colony formation of human lung cancer cells derived after cisplatin treatment. (f) Capability to form new colony of lung cancer H460 cells was evaluated via clonogenic assay. (g) Lower number of forming colony was demonstrated in cisplatin treated-H460 cells that were precultured with 5 µg/mL of 0.4 M NaCl fraction from L. squarrosulus peptides compared with both non-treated control and only cisplatin treated groups. It was worth noting that treatment alone with 0.4 M NaCl peptide did not alter colony formation in lung cancer H460 cells which confirms the non-toxic effect of L. squarrosulus peptide at 5 µg/mL. Values shown are means of three independent experiments ± SD. *p < 0.05 versus non-treated control cells, #p < 0.05 versus the cells only treated with cisplatin.
Flow cytometry with annexin V-FITC/PI was performed to further clarify mode of cell death and evaluate the sensitizing effect of L. squarrosulus peptide on cisplatin-induced apoptosis in human lung cancer H460 cells. Labelled annexin V-FITC allows the accurate tracking of the early apoptosis cells due to specific binding to the exposed phosphatidylserine on the surface of cell membrane34. Annexin V-FITC is used in conjugation with PI to further track the cells undergoing late apoptosis and necrosis, cell integrity is compromised thereby allowing PI internalization35. The obtained histograms indicated that majority of the cisplatin-treated and peptide-treated H460 were viable cells, comparable to the untreated cell population, as indicated by the large accumulation of non-stained cells (Annexin V-/PI-) in the three groups (Fig. 3d). On the other hand, there was remarkable increase in the percentage of cells in both early (Annexin V + /PI-) and late (Annexin V + /PI +) apoptosis in human lung cancer cells pretreated with 5 µg/mL of 0.4 M NaCl peptide for 6–24 h following with further 24 h treatment of 5 µM cisplatin (Fig. 3e). These results indicate that the L. squarrosulus peptide augments cisplatin-induced apoptosis when non-toxic concentrations were used for both cisplatin and the peptide, thus providing further evidence of a strong chemosensitization effect of L. squarrosulus peptide.
Diminution of colony formation in cisplatin-treated H460 cells precultured with L. squarrosulus peptide
The ability of lung cancer cells to form new colonies after cisplatin treatment alone or with 0.4 M NaCl fraction from L. squarrosulus extracts was investigated through clonogenic assay, which is an in vitro assay often used to gauge the effect of a treatment on cell survival and proliferation36. Viable H460 cells were collected after preincubation with 0–5 µg/mL of 0.4 M NaCl peptide for 24 h following with the further incubation of 5 µM cisplatin for another 24 h. A single cell suspension was prepared and seeded onto 6-well plate at a density of 250 cells/well, and the culture was maintained for 7 days. Figure 3f depicts that in comparison with L. squarrosulus peptide-treated H460 cells and the untreated control group which presented evidently higher colony number, colony formation was diminished in cisplatin-treated cells. Remarkably, the reduction of colony formation was greater in cisplatin-treated H460 cells which were preincubated with L. squarrosulus peptide (Fig. 3g).
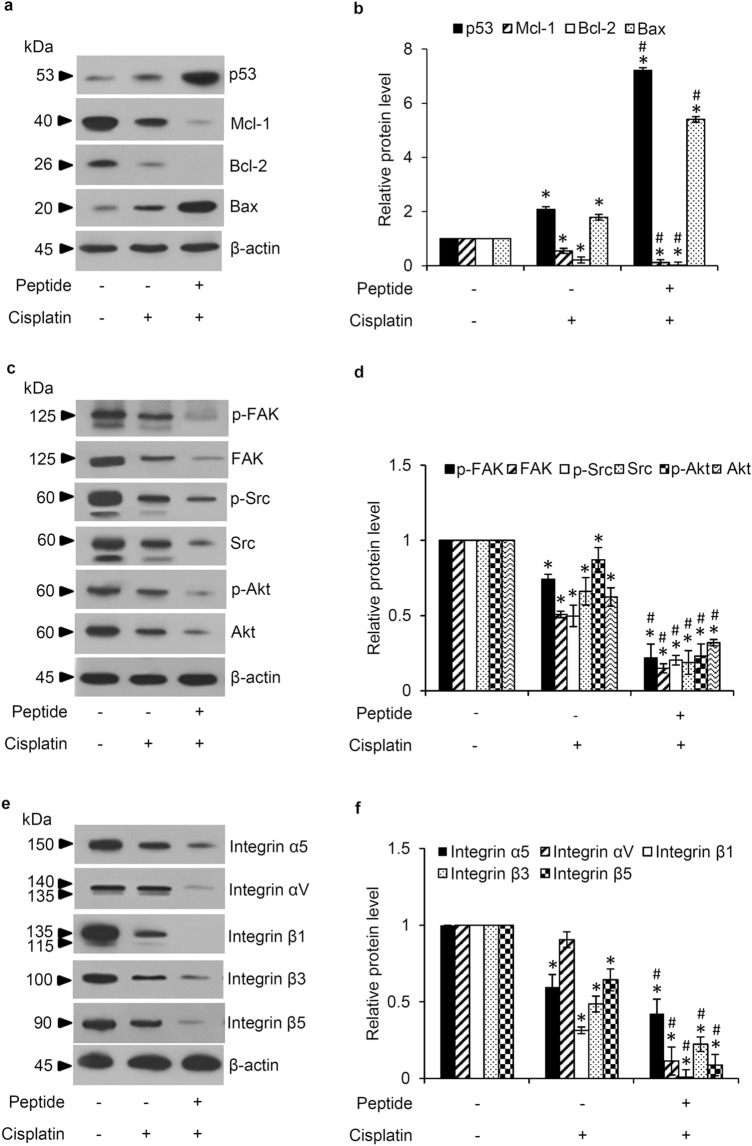
L. squarrosulus peptide modulates apoptosis and integrin-mediating survival pathway
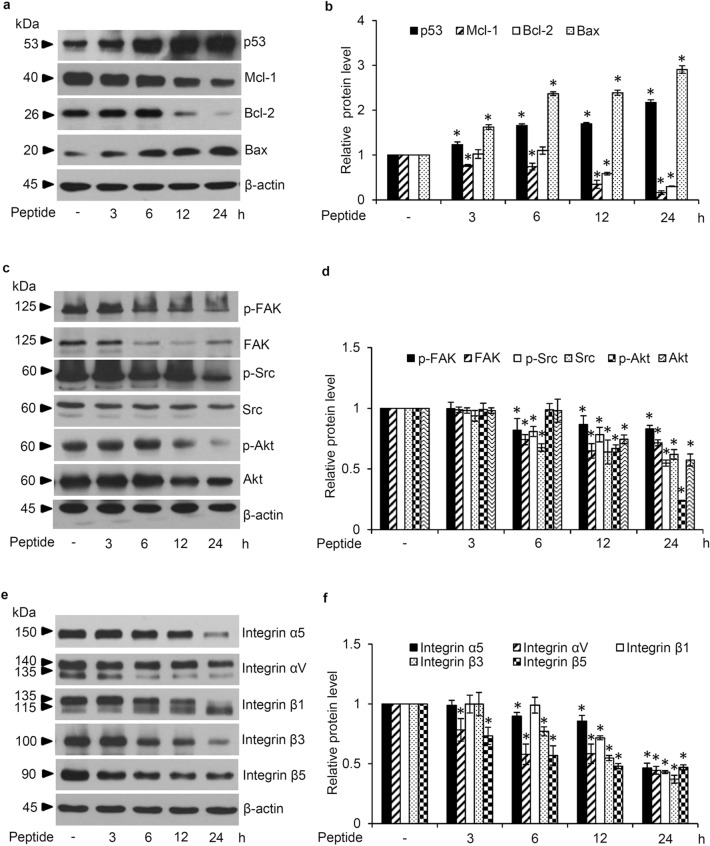
The alteration on apoptosis-regulating proteins was examined in human lung cancer cells cultured with L. squarrosulus peptide for varying time periods (0–24 h). Augmentation of tumor suppressor p53 and pro-apoptosis Bax proteins as well as downregulation of anti-apoptosis Mcl-1 protein were time-dependently observed in H460 cells incubated with 0.4 M NaCl peptide at 5 µg/mL for 3–24 h (Fig. 4a). The alteration of these apoptosis-regulating proteins correlated well with the chemosensitizing activity of L. squarrosulus peptide. Moreover, the expression of Bcl-2 anti-apoptosis protein was significantly decreased in lung cancer cells after treatment with the peptide for 12–24 h compared with non-treated control cells (Fig. 4b).
Figure 4.
L. squarrosulus peptide modulates apoptosis and survival pathway in human lung cancer cells. (a) Western blot analysis revealed the reduction of anti-apoptosis proteins (Mcl-1, Bcl-2) in H460 cells cultured with 5 µg/mL of L. squarrosulus peptide from 0.4 M NaCl fraction for 12–24 h. (b) However, the significant upregulation of p53 and pro-apoptosis Bax protein was observed after 3 h of peptide treatment. The downregulation of (c) survival proteins and (e) upstream regulatory molecules, integrins, was also detected in L. squarrosulus peptide treated-cells. (d) The expression level of survival p-FAK, FAK, p-Src, Src, p-Akt and Akt protein was time-dependently decreased which corresponded with (f) the diminution of integrin β1, β3, β5, αV and α5 in H460 cells incubated with 5 µg/mL of 0.4 M NaCl peptide. Values are means of three independent experiments ± SD. *p < 0.05 versus non-treated control cells.
The evaluation on modulatory role of L. squarrosulus peptide on survival signaling pathways in human lung cancer cells was further performed. Western blot analysis revealed incrementally decreased expression of survival-related proteins including FAK, p-FAK, Src, p-Src, Akt, p-Akt in H460 cells cultured with 0.4 M NaCl peptide at 5 µg/mL for 6–24 h (Fig. 4c,d). Moreover, the diminished levels of β1, β3, β5, α5 and αV integrins, which are up-stream regulatory molecules, were time-dependently observed following the treatment of L. squarrosulus peptide (Fig. 4e,f). These results indicated that L. squarrosulus peptide at non-toxic concentration effectively alters apoptosis and integrin-mediating survival signals in human lung cancer cells.
Anticancer mechanisms of cisplatin promoted by L. squarrosulus peptide
The results in Fig. 4 show that incubation with L. squarrosulus peptide alone modulated the expression levels of signaling proteins involved in apoptosis and survival pathways in human lung cancer cells. These proteins were likewise investigated in L. squarrosulus peptide-preincubated H460 cells which received cisplatin treatment. Culture with 5 µM of cisplatin alone for 24 h significantly altered the level of Bcl-2 family proteins including Bax, Mcl-1 and Bcl-2 showing that intrinsic apoptosis pathway is activated in response to cisplatin treatment (Fig. 5a). The upregulation of p53 and Bax as well as the reduction of Mcl-1 and Bcl-2 were significantly greater in the 0.4 M NaCl peptide-pretreated H460 cells subjected to cisplatin treatment in comparison to the cisplatin-treated cells (Fig. 5b). Similarly, the suppression on integrin-mediating survival pathway was also indicated in cisplatin-treated H460 cells. The decreased levels of integrin β1, β3, β5 and α5 and the corresponding diminution of survival signaling proteins including FAK, p-FAK, Src, p-Src, Akt and p-Akt were more pronounced in the cisplatin treated-H460 cells that were precultured with 0.4 M NaCl peptide compared with the cells treated with only cisplatin (Fig. 5c,d,e,f). Intriguingly, the downregulation of integrin αV in human lung cancer cells was only observed in response to pretreatment with L. squarrosulus peptide followed by the exposure to cisplatin.
Figure 5.
Pretreatment with 0.4 M NaCl peptide obtained from L. squarrosulus significantly sensitizes cisplatin-induced cell death through regulation of apoptosis and integrin-mediated survival pathway in human lung cancer H460 cells. (a, b) The protein levels of Mcl-1 and Bcl-2 were significantly decreased while tumor suppressor p53 and pro-apoptosis Bax protein levels were obviously increased in comparison with H460 cells treated with cisplatin alone. (c, d) Related survival proteins (FAK, p-FAK, Src, p-Src, Akt, p-Akt) and (e, f) integrins (α5, αV, β1, β3, β5) were considerably decreased as a result of peptide preincubation in cisplatin-treated cells compared to the cells treated with cisplatin alone. Values are means of three independent experiments ± SD. *p < 0.05 versus non-treated control cells, #p < 0.05 versus the cells only treated with cisplatin.
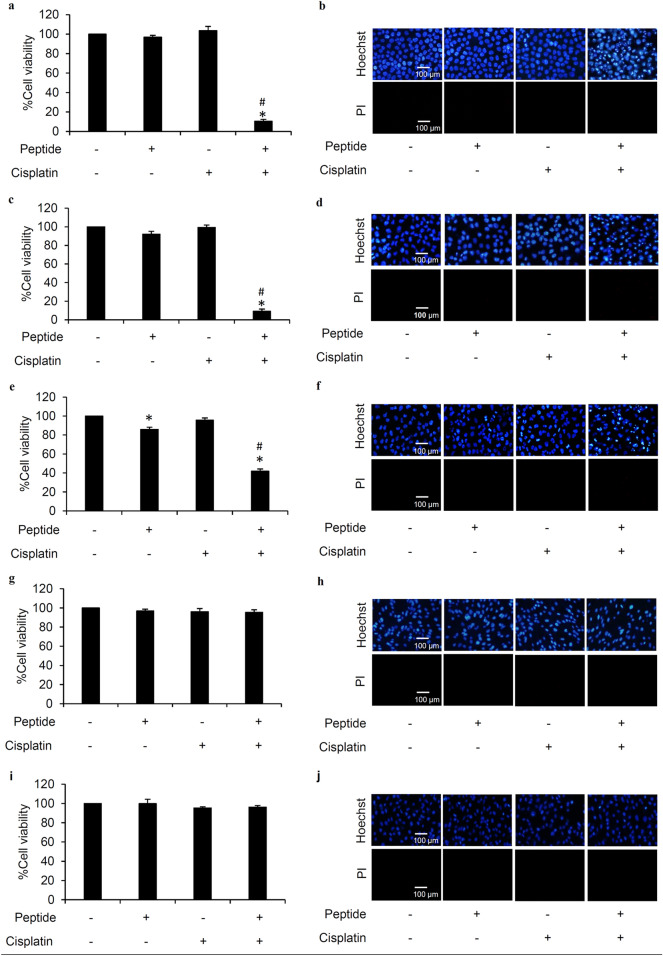
Selective chemosensitizing effect of L. squarrosulus peptide in human lung cancer cells
To further assess the chemosensitizing effect previously observed in H460 (p53 and KRas wild-type) (Fig. 3), other human lung cancer cells including H292 (p53 wild-type), H23 (p53 and KRas mutant) and A549 (KRas mutant) were either pretreated with 5 µg/mL of 0.4 M NaCl peptide for 24 h or left untreated before further incubation with non-toxic concentration of cisplatin at 5 µM for 24 h. Figure 6a,c,e, respectively revealed the greater reduction of %cell viability in cisplatin-treated H23, H292 and A549 lung cancer cells which were preincubated with L. squarrosulus peptide compared with the cells treated with cisplatin alone. While there were no observable apoptosis and necrosis cells detected after nuclear staining with Hoechst33342 and PI in lung cancer cells cultured with cisplatin or 0.4 M NaCl peptide alone for 24 h, apoptosis was strongly augmented in cisplatin-treated cells that were preincubated with L. squarrosulus peptide (Fig. 6b,d,f).
Figure 6.
Peptide from Lentinus squarrosulus Mont. shows high safety prolife. Chemosensitizing effect of 0.4 M NaCl peptide from L. squarrosulus was presented in various human lung cancer cells including (a) H23, (c) H292 and (e) A549 cells. In comparison to cells with or without cisplatin treatment, peptide preincubation combined with cisplatin treatment augmented observed apoptosis cells which are characterized by condensed DNA/fragmented nuclei stained by bright blue fluorescence of Hoechst33342 in (b) H23, (d) H292 and (f) A549 cells. MTT assay revealed no alteration of cell viability in (g) human dermal papilla DPCs cells and (i) human proximal renal HK-2 cells after preculture with 5 µg/mL of 0.4 M NaCl peptide for 24 h following with treatment with 5 µM cisplatin for another 24 h. No detectable apoptosis and necrosis were noted via costaining with Hoechst33342/propidium iodide (PI) in all treatments of (h) DPCs and (j) HK-2 cells. Values are means of three independent experiments ± SD. *p < 0.05 versus non-treated control cells, #p < 0.05 versus the cells treated only with cisplatin.
Due to notable cisplatin-modulated serious adverse effects such as hair loss and nephrotoxicity30–32, the effect of L. squarrosulus peptide on cisplatin-induced toxicity was also evaluated in human dermal papilla DPCs and proximal renal HK-2 cells. Figure 6g,h revealed that 24 h pretreatment with 0.4 M NaCl peptide at 5 µg/mL did not cause significant alteration in viability and death in DPCs incubated with cisplatin (5 µM) for 24 h when compared with untreated control and cells with cisplatin treatment alone. Furthermore, there was no cisplatin-induced toxicity as reflected by the MTT viability assay and Hoechst33342/PI costaining performed in HK-2 cells precultured with L. squarrosulus peptide prior exposure to cisplatin (Fig. 6i,j). These results suggested that L. squarrosulus peptide selectively sensitized cisplatin toxicity in human lung cancer cells.
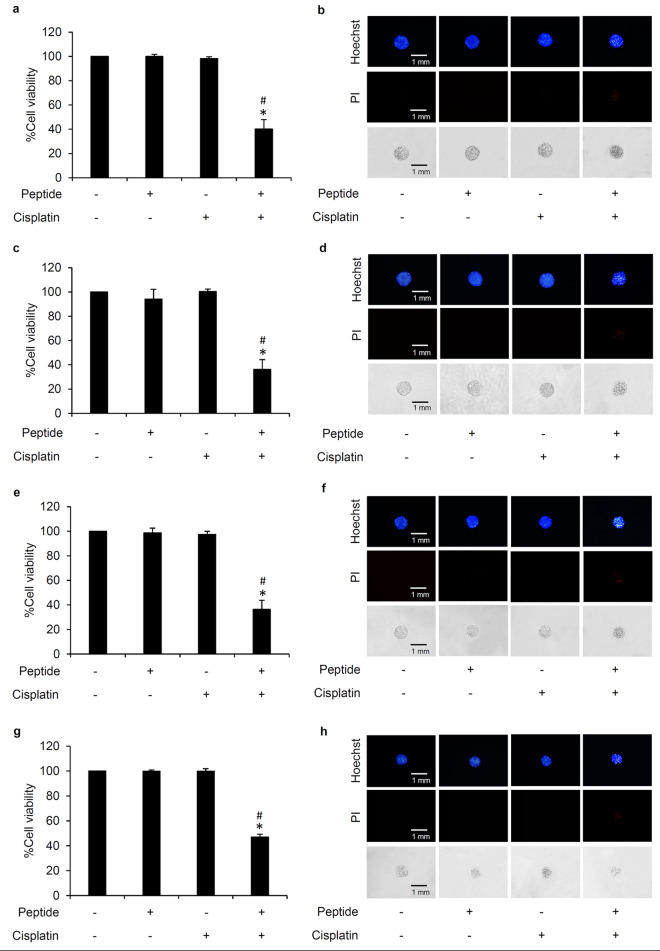
Additionally, chemosensitizing effect of the peptide from L. squarrosulus was validated in three-dimensional cancer spheroids. The in vitro multicellular cancer spheroids better reflect in vivo tumor behavior and thus the spheroid culture has been recognized as a good model for evaluation of anticancer activity37,38. Similar to the effects seen in two-dimension cell culture, preincubation with 0.4 M NaCl peptide at 5 µg/mL for 24 h prior to treatment with cisplatin remarkably diminished %cell viability of cancer spheroids derived from lung cancer H460 (Fig. 7a), H23 (Fig. 7c), H292 (Fig. 7e) and A549 (Fig. 7g) cells. Meanwhile, culture alone either with 0.4 M NaCl peptide (5 µg/mL) or cisplatin (5 µM) for 24 h did not alter viability in all lung cancer spheroids. Nuclear staining obviously depicted apoptosis cell death evidenced by the bright blue fluorescence of Hoechst33342 in the spheroids treated with L. squarrosulus peptide following with cisplatin treatment (Fig. 7b,d,f,h). These results strongly support the chemosensitizing effect of L. squarrosulus peptide on cisplatin-induced apoptosis in human lung cancer cells.
Figure 7.
Chemosensitizing effect of L. squarrosulus peptide in multicellular lung cancer spheroids. PrestoBlue assay presented the significant reduction of %cell viability in cancer spheroids obtained from lung cancer (a) H460, (c) H23, (e) H292 and (g) A549 cells that were cultured with 0.4 M NaCl peptide (5 µg/mL) for 24 h prior to 24 h treatment of 5 µM cisplatin. Nuclear staining clearly depicted the elevation of apoptosis observed as bright blue fluorescence of Hoechst33342 in multicellular spheroids of (b) H460, (d) H23, (f) H292 and (h) A549 cells after coculture with L. squarrosulus peptide and cisplatin. Notably, the presentation of both bright blue Hoechst33342 fluorescence and red fluorescence of propidium iodide (PI) indicated late stage of apoptosis detected in the spheroids after preincubation with 0.4 M NaCl peptide followed by cisplatin treatment. Values are means of three independent experiments ± SD. *p < 0.05 versus non-treated control cells, #p < 0.05 versus the cells treated only with cisplatin.
Discussion
Cisplatin is one of the most clinically effective anticancer agents and it remains to be a component of first-line treatment for advanced non-small cell lung cancer39. However, drug resistance and numerous undesirable side effects limit the use of cisplatin. To combat these problems and obtain greater therapeutic outcome, the combination of cisplatin with other drugs has been an available option40. Chemosensitization by natural compounds to increase cisplatin potency and decrease substantial toxicity is gaining interest as a novel anticancer strategy27. Thus, this study evaluated the chemosensitizing effect of the peptide extracted from L. squarrosulus, an edible mushroom, on cisplatin-induced cell death in human lung cancer cells. Time-dependent chemosensitizing activity was evidenced by the augmentation of cisplatin-induced apoptosis detected with Hoechst33342/PI costaining (Fig. 3b,c) and annexin V-FITC/PI assay through flow cytometry (Fig. 3d,e) of human lung cancer cells pretreated with 5 µg/ml of purified 0.4 M NaCl peptide from L. squarrosulus extracts for 6–24 h.
Cisplatin cytotoxicity is mediated by the propagation signals produced in response to DNA damage which in turn activate downstream proteins such as p53 and pro-apoptosis Bax, ultimately resulting in apoptosis40,41. The dysregulation of apoptosis, especially through the intrinsic pathway controlled by the Bcl-2 family proteins, could contribute to the resistance to cisplatin-induced toxicity observed in cancer cells41. Indeed, the elevated level of anti-apoptosis proteins in the Bcl-2 family has been well recognized as an underlying mechanism of chemotherapeutic resistance42. The downregulation of p53 and Bax in lung cancer cells is also associated with increased cisplatin resistance and reduced apoptosis10. Moreover, tumor progression is tightly correlated with decreased level of tumor suppressor p53 protein43. The treatment with L. squarrosulus peptide alone in human lung cancer cells caused not only the reduction of anti-apoptosis Mcl-1 and Bcl-2 proteins but also the augmentation of p53 and Bax in a time-dependent manner (Fig. 4a). Besides that, pretreatment with the peptide dramatically enhanced the effects of cisplatin treatment at non-toxic concentration, such as reduction of Mcl-1 and Bcl-2 levels as well as the elevation of p53 and Bax in cisplatin-treated lung cancer cells (Fig. 5a).
Integrins are heterodimeric transmembrane adhesion proteins which serve as survival initiating molecules. Cellular signaling generated from the interaction between ECM and integrins activates down-stream survival proteins including FAK and Src which subsequently propagates the PI3K/Akt pathway and its strong survival-related effects18. Downregulation of integrins (β1, β3, β5, α5 and αV) corresponds to the suppression of activated forms of survival signaling molecules, p-FAK, p-Src and p-Akt in lung cancer cells cultured with L. squarrosulus peptide (Fig. 4c-f). Interestingly, it has been continuously reported that various natural peptides could effectively repress the integrin-regulated survival pathways44,45. Integrins, especially the β1, β3, β5, α5 and αV subunit, and the related survival proteins have been shown to contribute to uncontrollable lung cancer progression and chemotherapeutic failure, as their dysregulation could promote rapid cloning of cancer cells and increase cell survival against various stresses such as cisplatin toxicity19,20,46. Coinciding with decreased survival signaling, the capability of individual cells to generate new cancer colonies was clearly more suppressed in cisplatin-treated lung cancer cells which were then preincubated with L. squarrosulus peptide as compared to both untreated control and cisplatin-treated groups (Fig. 3e,g). This inhibitory activity of L. squarrosulus peptide on colony formation correlated well with observed lower level of integrin-regulated survival proteins in lung cancer cells preincubated with L. squarrosulus following with cisplatin treatment in comparison with cells cultured only with cisplatin (Fig. 5c-f).
Integrin signaling allows the survival of lung cancer cells against drug-induced apoptosis. Thus, targeting of integrins presents good therapeutic potential for lung cancer cells treatment24,46. Downregulation of integrins and associated survival signals induces apoptosis and suppresses survival in diverse lung cancer cell, as observed in both p53 wild-type and mutants lung cancer cells46,47. Moreover, activation of integrins (β1, β3, αV) is also associated with aggressive features of oncogenic KRas dependent lung cancer cells and antagonizing these integrins has been shown to sensitize the resistant lung cancer cells to chemotherapy48–50. The presented results indicate that L. squarrosulus peptide may provide chemosensitizing activity against wild-type and mutant lung cancer cells through inhibition of integrins as demonstrated by the peptide’s effects on H460 (p53 and KRas wild-type), H292 (p53 wild-type), H23 (p53 and KRas mutant) and A549 (KRas mutant) (Figs. 3, 6, 7).
In line with the results presented in this study, there is much evidence which suggest that integrin is a likely target molecule affected by anticancer peptides44,45,51. The disruption on integrin-ECM interaction can trigger the internalization and degradation of transmembrane integrins52,53. Although downregulated level of integrins and consequent repression of downstream survival signals support that integrin is a potential therapeutic target of L. squarrosulus peptide, other related mechanisms of anticancer peptides such as perturbation of cell membrane and penetration to intracellular organelles are worthy of further elucidation54.
Not only selective anticancer activity against human lung cancer cells (Fig. 2) but also specific chemosensitizing effect of the obtained L. squarrosulus peptide was clearly evident as the results indicate that the enhancement of cisplatin-induced apoptosis occurred only in human lung cancer cells but not in non-cancer DPCs and HK-2 cells (Fig. 6). These results suggest that the L. squarrosulus peptide may provide protective effect against cisplatin side effects such as hair loss and nephrotoxicity which correlate with increased cell death in dermal papilla and proximal renal cells, respectively31,32. The selective chemo-enhancing activity against human lung cancer cells of L. squarrosulus peptide would thus be beneficial in reducing the dose-limiting cisplatin toxicity observed in normal cells.
Although initial assessment suggests the eluted 0.4 M NaCl fraction is composed of a ~ 37 kDa peptide (Fig. 1), the proteomics analysis revealed many amino acid sequences that did not match with available peptides in the library. Further improvement in purification process might facilitate the identification of the novel chemosensitizing peptide. However, the highest recorded similarity score in the proteomics analysis corresponded to extracellular metalloproteinase Ganoderma sinense and Lentinus tigrinus (Fig. 1c), confirming the mushroom origins of this chemotherapeutic adjuvant peptide and encouraging greater attention to these natural sources.
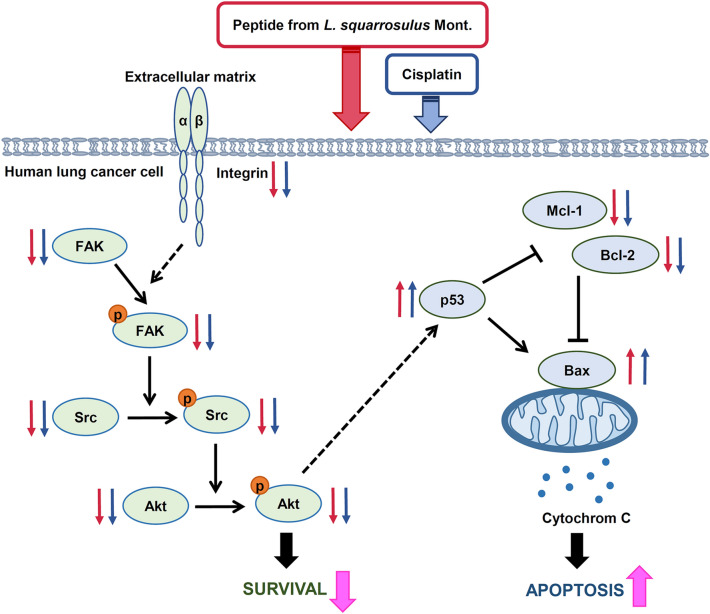
In summary, the current study revealed selective chemosensitizing effect of L. squarrosulus peptide extract to cisplatin-induced apoptosis in human lung cancer cells via suppression of integrin/FAK/Src/Akt survival signaling, downregulation of anti-apoptosis proteins (Mcl-1, Bcl-2), and upregulation of p53 as well as pro-apoptosis Bax proteins (Fig. 8). Subsequently, the data presented herein would support the development of L. squarrosulus peptides as a novel chemosensitizer.
Figure 8.
Proposed regulatory machinery of chemosensitizing effect of peptide extracted from Lentinus squarrosulus on cisplatin-induced apoptosis in human lung cancer cells. The peptide enhances the suppressive activity of cisplatin on integrin-mediating survival which resultes in the downregulation of integrin and down-stream signaling proteins including p-FAK/FAK, p-Src/Src and p-Akt/Akt as well as activation of apoptosis mediated by overexpression of p53 which correlates with increased Bax and diminution of anti-apoptosis Mcl-1 and Bcl-2 proteins. The effects of the peptide and cisplatin at non-toxic concentrations is indicated by red and blue small arrows, respectively. Big pink arrows represent the augmented cytotoxicity of cisplatin after pretreatment of human lung cancer cells with 0.4 M NaCl fraction of L. squarrosulus peptides.
Methods
Chemical reagents
Hoechst33342, propidium iodide (PI), dimethylsulfoxide (DMSO), cisplatin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Tween-20, acetonitrile, formic acid, coomassie brilliant blue R-250, isopropanol, acetic acid and skim milk powder were bought from Sigma-Aldrich Chemical (St. Louis, MO, USA). Annexin V-Fluorescein Isothiocyanate (FITC) apoptosis detection kit and PrestoBlue reagent were procured from Thermo Fisher Scientific (Rockford, IL, USA). Roswell Park Memorial Institute (RPMI) medium, Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), l-glutamine, penicillin/streptomycin solution and trypsin–EDTA (0.25%) were purchased from Gibco (Gaithersburg, MA, USA). Ammonium sulfate ((NH4)2SO4), potassium dihydrogen phosphate (KH2PO4), ammonium bicarbonate (NH4HCO3), dithiothreitol, iodoacetamide, methanol (CH3OH) and ethanol (CH3CH2OH) were obtained from Merck (Darmstadt, Germany). Di-sodium hydrogen orthophosphate anhydrous (Na2HPO4), potassium chloride (KCl) and sodium chloride (NaCl) were obtained from Univar (Ajax, Australia). Primary antibodies specific for integrins α5, αV, β1, β3, and β5, FAK, p-FAK (Tyr 397), Src, p-Src (Tyr 416), Akt, p-Akt (Thr 308), p53, Bax, Mcl-1, Bcl-2, β-actin as well as the horseradish peroxidase (HRP)-labeled secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Immobilon Western chemiluminescent HRP substrate was obtained from Milipore (Billerica, MA, USA). A bicinchoninic acid (BCA) protein assay kit was purchased from Thermo scientific (Rockford, IL, USA).
Preparation of extracted peptide
-
Isolation of crude peptides
The edible mushrooms, L. squarrosulus, were homogenized with deionized water (3 mL/g). Proteins were salted out slowly on ice by adding (NH4)2SO4 to the aqueous filtrate until 40–80% final saturation. Next, centrifugation at 6,500 rpm for 1 h (4 °C) was performed to acquire crude protein pellets which were re-solubilized with phosphate buffer solution (PBS; pH 7.4) containing Na2HPO4 and KH2PO4 on ice. Then, the solution was dialyzed overnight with the phosphate buffer at 4 °C to eliminate (NH4)2SO4 as previously described29.
-
Peptide purification
Fast protein liquid chromatography (FPLC) with HiTrap DEAE FF 1 mL anion exchange column pre-equilibrated with PBS (pH 7.4) was used for further peptide purification. After elution of the unbound peptides, the different adsorbed peptides were released from the positively charged adsorbent via stepwise increase of NaCl concentration (0.1, 0.2, 0.3, 0.4 and 0.5 M) in PBS at a flow rate of 1 mL/min. The eluted peptides were further concentrated via lyophilization. The eluates were then analyzed for quantity and homogeneity of peptides by BCA assay kit and SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), respectively.
-
Determination of protein content
BCA assay kit was used to measure the total protein content of each concentrated peptide extract, according to manufacturer’s instructions. The developed product was evaluated through microplate reader (Anthros, Durham, NC, USA) at 570 nm. The protein concentration was calculated based on the calibration curve of the BSA standard.
-
Evaluation on homogeneity of purified peptide fraction
The homogeneity of purified peptide fraction was determined by SDS-PAGE using 15% (w/v) gel as previously described29. Briefly, the mixtures containing 15 µg protein content of each peptide fraction and loading dye were added onto SDS-PAGE after heating at 95 °C for 5 min. After complete separation, the gel was stained with coomassie brilliant blue R-250 solution overnight and then destained with isopropanol: acetic acid: water (10: 10: 80% v/v) solution.
Identification of peptide in purified fraction from L. squarrosulus extracts
The most purified fraction from L. squarrosulus extracts presenting only one major protein band via SDS-PAGE analysis was selected for further peptide identification. The SDS-PAGE gel area of interested protein was cut and washed with sterile deionized water. After destaining with 25 mM NH4HCO3 in 50% methanol, the gel was washed three times with sterile deionized water. Before incubation with 10 mM dithiothreitol (in 10 mM NH4HCO3) at room temperature for 1 h, the gel was dehydrated by soaking in acetonitrile. After adding 5 mM dithiothreitol (in 50 mM iodoacetamide), the gel was subsequently incubated for 1 h in the dark. Then, the gel was washed with acetonitrile (2 time × 5 min) and digested with 10 ng/μL of proteomics-grade trypsin (Sigma-Aldrich Chemical, St. Louis, MO, USA) in 50% acetonitrile/10 mM NH4HCO3 at 37 °C for 12 h. The supernatant was collected and concentrated through vacuum-drying. The peptide sample was kept at - 80 °C until performing proteomics analysis. Before the sample was subjected to mass spectrum analysis, it was solubilized in 0.1% formic acid. LC–MS/MS with nanocolumn was set at a flow rate of 300 nL/min using the HCT Ultra PTM Discovery System (Bruker Daltonics Ltd., Hamburg, Germany) coupled to an UltiMate 3000 LC System (Dionex Ltd., Sunnyvale, CA, USA). A multistep gradient of 10–70% acetonitrile (in 0.1% formic acid) was used as mobile phase to elute peptides55. The mass spectrum fingerprint of the eluted peptides was searched against Contaminants database version 20090624 (262 sequences, 133770 residues) and NCBIprot database version 20191120 (227181163 sequences; 82759882099 residues) using Mascot search web service (Matrix Science, London, UK) with the following search parameters; Type of search: MS/MS Ion Search, Enzyme: Trypsin, Fixed modifications: Carbamidomethyl (C), Variable modifications: Oxidation (M), Mass values: Monoisotopic, Protein Mass: Unrestricted, Peptide Mass Tolerance: ± 1.2 Da, Fragment Mass Tolerance: ± 0.6 Da, Max Missed Cleavages: 1, Instrument type: ESI-TRAPT. Protein hits was considered positively identified when a component peptide had a statistically significant Mascot score (p < 0.05). The matched peptide sequences were searched against UniProtKB reference proteomes plus Swiss-Prot database in UniProt release 2020_03 for protein annotation.
Cell culture
Human lung cancer H460, H23 and H292 cells (ATCC, Manassas, VA, USA) were cultured in RPMI medium. Meanwhile, human lung cancer A549 cells (ATCC, Manassas, VA, USA) and human renal proximal tubular HK-2 cells (ATCC, Manassas, VA, USA) were cultured in DMEM. Prigrow III medium (Applied Biological Materials Inc., Richmond, BC, Canada) was used for the culture of human dermal papilla cells DPCs obtained from Applied Biological Materials Inc. (Richmond, BC, Canada). All culture mediums were supplemented with l-glutamine (2 mM), FBS (10%) and penicillin/streptomycin (100 units/mL). The cells were maintained in humidified atmosphere containing 5% CO2 with temperature set at 37 °C until 70–80% confluence for further experiments.
Cytotoxicity assay
Cells were seeded onto 96-well plate at a density of 1 × 104 cells/well for overnight. After indicated treatment, the culture medium was replaced with 0.4 mg/mL of MTT and the cells were further incubated for 3 h at 37 °C in dark place. Then, the supernatant was removed and DMSO was added to dissolve the formazan product. A microplate reader (Anthros, Durham, NC, USA) was used to measure the intensity of formazan color at 570 nm. The absorbance ratio of treated to non-treated control cells was calculated and presented as percent cell viability.
Nuclear staining assay
Mode of cell death was evaluated via nuclear staining assay. The treated cells were costained with Hoechst33342 (0.02 µg/mL) and PI (0.01 µg/mL) at 37 °C for 30 min. A fluorescence microscope (Olympus IX51 with DP70, Olympus, Tokyo, Japan) was used to visualize the cells and characterize the mode of death. The Hoechst33342 dye was allowed for the detection of nuclear condensation which characterizes apoptosis while the necrosis cells were distinguished by their uptake of PI.
Flow cytometric analysis
The percentages of cells undergoing apoptosis and necrosis were also quantified through flow cytometry after double staining using annexin V-FITC/PI assay kit, following manufacturer’s instructions. Briefly, lung cancer H460 cells were cultured at the density of 1.5 × 105 cells/well in 6-well plate. The cells were preincubated with the peptide extract for varying times (0, 6, 12, 24 h) before further culture with 5 µM of cisplatin for 24 h. Then, the cells were detached and centrifuged at 5,000 rpm (4 °C) for 5 min. The annexin V-FITC and PI working solution were added into respective single cell suspensions which were prepared in binding buffer solution. The proportions of living, apoptosis and necrosis cells in each prepared suspension were determined with Guava easyCyte 5 benchtop flow cytometer with the GuavaSoft version 2.7 software (Merck, Darmstadt, Germany).
Clonogenic assay
Human lung cancer cells (1.5 × 105 cells/well) in 6-well plate were pretreated with peptide (5 µg/mL) for 24 h before exposure with 5 µM of cisplatin. After 24 h, single cell suspensions of 250 viable cells derived from each treatment were seeded again onto 6-well plate. The colony formation in each plate was examined after 7 days of incubation under 5% CO2 humidified atmosphere at 37 °C. The resulting cancer colonies were counted after staining with 0.05% w/v crystal violet in 4% formaldehyde36.
Establishment of three-dimensional multicellular cancer spheroids
Human lung cancer cells were seeded at a density of 1 × 104 cells/well and maintained with appropriate media containing 10% FBS, 2 mM l-glutamine and 100 units/mL penicillin/streptomycin in 96-well round bottom ultra-low attachment plate (Corning, Tewksbury, MA, USA). After 4-day incubation, the lung cancer spheroids were subjected to the evaluation of chemosensitizing activity. To examine viability of lung cancer cells, the three-dimensional spheroids were incubated with PrestoBlue reagent (10 µL) for 3 h. Then, the fluorescence intensity of resorufin, the reduced form of resazurin was detected with fluorescence microplate reader (CLARIOstar Plus Microplate Reader, BMG Labtech, Baden-Wurttemberg, Germany) at excitation wavelength of 570 nm and emission wavelength of 610 nm. The percent cell viability was derived from the relative fluorescence intensity between treated to non-treated spheroids. Moreover, mode of cell death was observed under fluorescence microscope (Olympus IX51 with DP70, Olympus, Tokyo, Japan) after costaining tumor spheroids with Hoechst33342 (0.02 µg/mL) and PI (0.01 µg/mL) at 37 °C for 30 min56.
Western blot analysis
Human lung cancer H460 cells were seeded onto 6-well plate at the density of 1.5 × 105 cells/well. After indicated treatments, the cells were incubated with lysis buffer containing 1X radio-immunoprecipitation assay (RIPA) buffer (Thermo scientific, Rockford, IL, USA) and cocktail protease inhibitor (Roche Applied Science, Indianapolis, IN, USA) for 45 min on ice. To collect the clear supernatant, the cell lysates were centrifuged at 10,000 rpm at 4 °C for 15 min. BCA protein assay kit was used to determine total protein content. Equal amount of protein from each sample was adjusted to appropriate volume using lysis buffer and then mixed with loading buffer. The samples were heated at 95 °C for 5 min to speed up protein denaturation. Then, 35 µg protein from each sample was loaded onto a 10% SDS-PAGE. After separation, the cellular proteins were transferred onto 0.45 µm nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked with 5% non-fat dry milk in Tris buffered saline with Tween 20 (TBST) at room temperature for 45 min. The primary antibodies were added onto the membranes and incubated at 4 °C overnight. Then, the membranes were washed with TBST for three times (7 min). The membranes were further incubated with specific horseradish peroxidase (HRP)-linked secondary antibodies for 2 h at 25 °C. Finally, the signals from specific proteins were detected using chemiluminescent substrates. The analyst/PC densitometric software (Bio-Rad Laboratory, Hercules, CA, USA, version 6.0.1, 2017) was used to quantify the density of protein signal.
Statistical analysis
All the data were averaged from three independent experiments. Statistical data analysis was carried out by one-way ANOVA and Tukey HSD post hoc test using SPSS Statistic 22 version (Armonk, NY, USA). Statistical significance was considered at p < 0.05.
Supplementary Information
Acknowledgements
This research was supported by the 90th Anniversary of Chulalongkorn University under Rachadapisek Somphot Fund and the research fund from Faculty of Pharmaceutical Sciences, Chulalongkorn University (Grant No. Phar2563-RG006). HK would like to thank Chulalongkorn University for the provision of the Scholarship for International Graduate Students in ASEAN Countries.
Author contributions
Conceptualization, C.C.; methodology, C.C.; formal analysis, H.E.E.K, G.A.U.E., N.Po., E.P., P.C. and C.C; experimental measurement, H.E.E.K, G.A.U.E., S.R., N.Ph., E.P., P.C. and C.C; writing-original draft preparation, H.E.E.K, G.A.U.E., S.R., N.Po. and C.C.; editing, C.C.; project administration, C.C.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Data availability
All data generated or analyzed during this study are included in this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83606-1.
References
- 1.Ferlay J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Patel N, Adatia R, Mellemgaard A, Jack R, Møller H. Variation in the use of chemotherapy in lung cancer. Br. J. Cancer. 2007;96:886–890. doi: 10.1038/sj.bjc.6603659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger DS, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Ne. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 4.Besse B, Le Chevalier T. Adjuvant or induction cisplatin-based chemotherapy for operable lung cancer. Oncology (Williston Park) 2009;23:520–527. [PubMed] [Google Scholar]
- 5.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 6.d'Amato TA, Landreneau RJ, McKenna RJ, Santos RS, Parker RJ. Prevalence of in vitro extreme chemotherapy resistance in resected nonsmall-cell lung cancer. Ann. Thorac. Surg. 2006;81:440–447. doi: 10.1016/j.athoracsur.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun CY, Zhang QY, Zheng GJ, Feng B. Phytochemicals: Current strategy to sensitize cancer cells to cisplatin. Biomed. Pharmacother. 2019;110:518–527. doi: 10.1016/j.biopha.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira Júnior RG, et al. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia. 2018;129:383–400. doi: 10.1016/j.fitote.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Han JY, et al. The relationship between cisplatin-induced apoptosis and p53, Bcl-2 and Bax expression in human lung cancer cells. Korean J. Intern. Med. 1999;14:42–52. doi: 10.3904/kjim.1999.14.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta SV, Shukla SN, Vora HH. Overexpression of Bcl2 protein predicts chemoresistance in acute myeloid leukemia: Its correlation with FLT3. Neoplasma. 2013;60:666–675. doi: 10.4149/neo_2013_085. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, et al. Prognostic value of Bcl-2 expression in patients with non-small-cell lung cancer: A meta-analysis and systemic review. Onco. Targets Ther. 2015;8:3361–3369. doi: 10.2147/OTT.S89275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar Biswas S, Huang J, Persaud S, Basu A. Down-regulation of Bcl-2 is associated with cisplatin resistance in human small cell lung cancer H69 cells. Mol. Cancer Ther. 2004;3:327–334. [PubMed] [Google Scholar]
- 14.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS. Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, et al. Simultaneous targeting of ATM and Mcl-1 increases cisplatin sensitivity of cisplatin-resistant non-small cell lung cancer. Cancer Biol. Ther. 2017;18:606–615. doi: 10.1080/15384047.2017.1345391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milas I, et al. Epidermal growth factor receptor, cyclooxygenase-2, and BAX expression in the primary non-small cell lung cancer and brain metastases. Clin. Cancer Res. 2003;9:1070–1076. [PubMed] [Google Scholar]
- 17.Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015;146:132–149. doi: 10.1016/j.pharmthera.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Bergmann S, et al. Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. J. Cell Sci. 2009;122:256–267. doi: 10.1242/jcs.035600. [DOI] [PubMed] [Google Scholar]
- 19.Dingemans AM, et al. Integrin expression profiling identifies integrin alpha5 and beta1 as prognostic factors in early stage non-small cell lung cancer. Mol. Cancer. 2010;9:152. doi: 10.1186/1476-4598-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wayner EA, Orlando RA, Cheresh DA. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J. Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiuthed A, Chanvorachote P. Cisplatin at sub-toxic levels mediates integrin switch in lung cancer cells. Anticancer Res. 2014;34:7111–7117. [PubMed] [Google Scholar]
- 22.Santos AR, et al. β1 integrin-focal adhesion kinase (FAK) signaling modulates retinal ganglion cell (RGC) survival. PLoS ONE. 2012;7:e48332. doi: 10.1371/journal.pone.0048332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen DM, et al. Enhancement of depsipeptide-mediated apoptosis of lung or esophageal cancer cells by flavopiridol: Activation of the mitochondria-dependent death-signaling pathway. J. Thorac. Cardiovasc. Surg. 2003;125:1132–1142. doi: 10.1067/mtc.2003.180. [DOI] [PubMed] [Google Scholar]
- 24.Aksorn N, Chanvorachote P. Integrin as a molecular target for anti-cancer approaches in lung cancer. Anticancer Res. 2019;39:541–548. doi: 10.21873/anticanres.13146. [DOI] [PubMed] [Google Scholar]
- 25.Raab-Westphal S, Marshall JF, Goodman SL. Integrins as therapeutic targets: Successes and cancers. Cancers (Basel) 2017;9:110. doi: 10.3390/cancers9090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MY, et al. Novel monoclonal antibody against beta 1 integrin enhances cisplatin efficacy in human lung adenocarcinoma cells. J. Biomed. Res. 2016;30:217–224. doi: 10.7555/JBR.30.2016K0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016;40–41:160–169. doi: 10.1016/j.semcancer.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman M. Mushroom polysaccharides: Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods. 2016;5:80. doi: 10.3390/foods5040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prateep A, Sumkhemthong S, Suksomtip M, Chanvorachote P, Chaotham C. Peptides extracted from edible mushroom: Lentinus squarrosulus induces apoptosis in human lung cancer cells. Pharm. Biol. 2017;55:1792–1799. doi: 10.1080/13880209.2017.1325913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, et al. Identification of microRNA-mRNA networks involved in cisplatin-induced renal tubular epithelial cells injury. Eur. J. Pharmacol. 2019;851:1–12. doi: 10.1016/j.ejphar.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Yan L, Zhu Q, Shao F. Puerarin attenuates cisplatin-induced rat nephrotoxicity: The involvement of TLR4/NF-κB signaling pathway. PLoS ONE. 2017;12:e0171612. doi: 10.1371/journal.pone.0171612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botchkarev VA. Molecular mechanisms of chemotherapy-induced hair loss. J. Investig. Dermatol. Symp. Proc. 2003;8:72–75. doi: 10.1046/j.1523-1747.2003.12175.x. [DOI] [PubMed] [Google Scholar]
- 33.Puchsaka P, Chaotham C, Chanvorachote P. α-Lipoic acid sensitizes lung cancer cells to chemotherapeutic agents and anoikis via integrin β1/β3 downregulation. Int. J. Oncol. 2016;49:1445–1456. doi: 10.3892/ijo.2016.3624. [DOI] [PubMed] [Google Scholar]
- 34.Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011;50:2597. doi: 10.3791/2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wlodkowic D, Skommer J, Darzynkiewicz Z. Flow cytometry-based apoptosis detection. Methods Mol. Biol. 2009;559:19–32. doi: 10.1007/978-1-60327-017-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 37.Nunes AS, Barros AS, Costa EC, Moreira AF, Correia IJ. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2018;116:206–226. doi: 10.1002/bit.26845. [DOI] [PubMed] [Google Scholar]
- 38.Zanoni M, et al. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016;6:19103. doi: 10.1038/srep19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fennell DA, et al. Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat. Rev. 2016;44:42–50. doi: 10.1016/j.ctrv.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto M, Nakajima W, Seike M, Gemma A, Tanaka N. Cisplatin-induced apoptosis in non-small-cell lung cancer cells is dependent on Bax- and Bak-induction pathway and synergistically activated by BH3-mimetic ABT-263 in p53 wild-type and mutant cells. Biochem. Biophys. Res. Commun. 2016;473:490–496. doi: 10.1016/j.bbrc.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 42.Hata AN, Engelman JA, Faber AC. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol. Cancer Res. 2014;12:3–13. doi: 10.1158/1541-7786.MCR-13-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–188. doi: 10.7150/thno/v01p0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakrabarti S, Jahandideh F, Wu J. Food-derived bioactive peptides on inflammation and oxidative stress. Biomed. Res. Int. 2014;2014:608979. doi: 10.1155/2014/608979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aoudjit F, Vuori K. Integrin signaling in cancer cell survival and chemoresistance. Chemother. Res. Pract. 2012;2012:283181. doi: 10.1155/2012/283181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantovani F, Collavin L, Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Symonds JM, Ohm AM, Tan AC, Reyland ME. PKCδ regulates integrin αVβ3 expression and transformed growth of K-ras dependent lung cancer cells. Oncotarget. 2016;7:17905–17919. doi: 10.18632/oncotarget.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamidi H, Ivaska J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seguin L, et al. An integrin β3-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arosio D, Manzoni L, Corno C, Perego P. Integrin-targeted peptide- and peptidomimetic-drug conjugates for the treatment of tumors. Recent Pat. Anticancer Drug Discov. 2017;12:148–168. doi: 10.2174/1574892812666170203151930. [DOI] [PubMed] [Google Scholar]
- 52.Sancey L, et al. Clustering and internalization of integrin alphavbeta3 with a tetrameric RGD-synthetic peptide. Mol. Ther. 2009;17:837–843. doi: 10.1038/mt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas M, et al. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J. Biol. Chem. 2010;285:23842–23849. doi: 10.1074/jbc.M109.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deslouches B, Di YP. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget. 2017;8:46635–46651. doi: 10.18632/oncotarget.16743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akekawatchai C, Roytrakul S, Phaonakrop N, Jaresitthikunchai J, Jitrapakdee S. Proteomic analysis of the anoikis-resistant human breast cancer cell lines. Methods Mol. Biol. 2020;2138:185–193. doi: 10.1007/978-1-0716-0471-7_11. [DOI] [PubMed] [Google Scholar]
- 56.Ekert JE, et al. Three-dimensional lung tumor microenvironment modulates therapeutic compound responsiveness in vitro–implication for drug development. PLoS ONE. 2014;9:e92248. doi: 10.1371/journal.pone.0092248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article.