Abstract
Plants are one of a prefect source of natural effective compounds that have antimicrobial, and other activities. This study investigated the activity of the aqueous extract for three wild edible plants (Sonchus oleraceus, Cichorium pumilum, and Portulaca oleracea) at three concentrations (1.5, 2.5 and 5 mg/ml) as antifungal and antitoxigenic. Many functional groups such as alcohols, phenols, alkanes and alkenes, etc were appeared in aqueous extracts by Fourier Transform Infrared Spectroscopy (FTIR) analysis. Where an extract of Portulaca oleracea gave a greater total phenolic and flavonoids were 210.4 ± 1.15 and 36.7 ± 0.79 mg/mL, respectively, followed by Sonchus oleraceus (192.3 ± 2.11 mg/mL) and Cichorium pumilum (186.4 ± 2.18 mg/mL). The results indicated that increasing the concentration of the extract, the area of inhibition zone increased with all treatments, where the highest inhibition zone was observed using 5 mg/ml for Portulaca oleracea extract was 17.1 ± 1.7, 26.5 ± 1.5 and 22.8.±2.3 mm against Aspergillus flavus, Aspergillus ochraceus and Aspergillus parasiticus, respectively, while the lowest antifungal activity was marked with Cichorium pumilum extract with all tested fungi. The results have also indicated that the aqueous extract has inhibited formed of aflatoxin B1 (AFB1) and ochratoxin A (OTA), where the percentages of inhibition AFB1 were 78.03, 68.8 and 81.7% after treated yeast extract sucrose (YES) media by 5 mg crude extract for extract Sonchus oleraceus, Cichorium pumilum and Portulaca oleracea, respectively. In contrast, the inhibitory effect against OTA at the same concentration was 77.5, 72.3, and 85.2% in the same order for plants. Finally, these plants provide an aqueous extract that contains many effective compounds that enable to play the role of antifungal and antitoxigenic.
Keywords: Antifungal activity, Antitoxigenic, Wild plants, Aqueous and extract
Antifungal activity; Antitoxigenic; Wild plants; Aqueous and extract
1. Introduction
Aspergillus spp is ubiquitous fungi that usually inhabit in indoor air environments especially dust, soils and flotsam of the plants. Aspergillus contamination of food and feed cause's major economic problems worldwide, many Aspergillus species such as A. flavus and A. parasiticus, A. nomius, A. bombycis and A. ochraceus produce various mycotoxins, which aflatoxin B1 (AFB1) and ochratoxin A (OTA) [1, 2, 3]. These are mostly produced by A. flavus and A. ochraceus, respectively, as well as they, are classified as carcinogens by the International Agency for Research on Cancer (IARC) [4]. These fungi grow with wide range temperature between 20 to 40 °C with a 10–20% of moisture content and 70–90% of relative humidity in the air, therefore associated with a tropical and subtropical climate, which includes Egypt [5]. Many extracts and compounds obtained from natural sources, such as plants, algae, microalgae and bacteria were screened for the ability to inhibit of growth of fungal and produced toxins [6, 7, 8]. Accordingly, A lot of studies reported that the natural sources for inhibitors of biosynthesis for AFB1 and OTA were the common bioactive compounds in plant extract for example phenols, flavonoids, alkaloids, tannins, terpenes and resins which that all possess antifungal properties [9, 10, 11, 12]. Many wild plants grow in Egypt, which many Egyptians used to eat especially in rural areas, or use in treating certain diseases as digestive, purgative and emollient [13]. Three wild edible plants (Sonchus oleraceus) known as Sow Thistle and (Cichorium pumilum called common chicory) belonging to the Asteraceae family. Portulaca oleracea is an annual succulent in the family Portulacaceae called in Egypt “Rejlah" [14, 15]. Therefore, this study aimed to evaluate an aqueous extract for leaves of three wild edible plants as anti-fungal and anti-mycotoxins produced by Aspergillus spp including (A. flavus, A ochraceus and A. parasiticus). Through, examined of the impact of these extracts on the growth and the ability to produce AFB1 and OTA.
2. Materials and methods
2.1. Plant material
The plants (Sonchus oleraceus, Cichorium pumilum and Portulaca oleracea) were collected from Kafr El-Sheikh government –Egypt. The fresh leaves plant were dried in an oven at 45–50 °C for 12 h, then ground to a fine powder and stored in plastic vials.
2.2. Fungal strains
Three fungal strains from Aspergillus spp were used in this study A. flavus (ATCC 28542), A ochraceus (ATCC 22947) and A. parasiticus (ATCC 26692).
2.3. Chemicals and solvents
Potato Dextrose Agar (PDA) and yeast extract agar were obtained from Sigma- Aldrich, Lyon, France. The standards of toxins (AFB1 and OTA) and solvents were purchased from Sigma chemical Co. (St. Louis, MO).
3. Methods
3.1. Preparation of aqueous extract
Five grams of powder plants leaves were extracted by 100 ml of distilled water at room temperature for 24 h, then centrifuged at 3000 rpm for 15 min, and evaporated to near dryness, and the resulting viscous powder was dissolved to obtain stock solution.
3.2. Determination of total phenolic content
The total phenolic content in aqueous extract for leaves was determined using Folin – Ciocalteau method as follows: Briefly, 1 ml of extract in a volumetric flask was diluted with distilled water to 46 ml. 1 ml of Folin-Ciocalteu reagent was added and the contents of the flask were blended completely. After 3 min, 3 ml of Na2CO3 (2%) was added, then mixture left for 2 h. The absorbance of mixture was measured at 760 nm. Total phenolic content was measured by plotting the calibration curve of a Gallic acid equivalent (GAE) standard [16].
3.3. Determination of total flavonoid content
The total flavonoid content was measured according to the method Yan-Hwa et al, 2000 [17]. as follows: 1 ml of extract was diluted with 4.3 ml of 80 % aqueous ethanol containing 0.1 ml of 10 % Al(NO3)3 and 0.1 ml of 1 M aqueous CH3COOK. After 40 min at room temperature, the absorbance was determined spectrophotometrically at 415 nm. The total flavonoid content was measured by us using calibration curve of a quercetin equivalent (QE) standard.
3.4. Determination of antioxidant activities
Determination of DPPH radical scavenging activity: The antioxidant activity of extracts, based on the scavenging activity of the stable DPPH free radical was determined by the method described by Lee et al, 2015 [18].
3.5. Assay of antifungal activity
The antifungal activity was tested on the three strains of fungi using agar well diffusion technique Perez et al, 1990 [19]. The strains were cultivated on PDA slants at 28 °C for 7 days. Spores were harvested by adding 10 ml of sterile distilled water containing 0.05% Tween 20 and scraping the surface of the culture to free the spores. One mL of spore suspension was inoculated into each plate. Wells of 5 mm diameter was made on the PDA surface and filled with the three gradual with three concentrations of (1.5, 2.5 and 5 mg/ml), obtained from diluting the stock solutions were used. Wells containing with (100 μl pure solvent (water) were used as a negative control, while wells containing with Nystatin (1000 Unit/ml were considered as positive control. The inoculated plates were incubated at 27 °C for 48 h, and then the antifungal activity was assessed by measuring the zone of inhibition (mm). The results average was calculated from at least three replicates for each treatment.
3.6. Control of producing AFB1 and OTA by aqueous extract in YES medium
The yeast extract sucrose (YES) culture medium (2% yeast extract and 15% sucrose/liter distilled water) was used in this experiment. YES was poured into 250 ml Erlenmeyer flask and autoclaved at 121 °C for 15 min, then cooled and inoculated with spores suspension of A. flavus and A. ochraceus both separately. Gradual concentrations of (1.5, 2.5 and 5 %) from aqueous extract were added to YES medium and incubated at 28 °C for 14 days. After the end of the incubation period, the AFB1 was extracted then determined using HPLC as following: AFB1 was extracted from YES medium by chloroform (20 ml twice with 10 ml YES media) then homogenization for 3 min in a separation funnel. Finally filtration of the chloroform phase, then evaporation to dryness to use with HPLC.
3.7. Determination of AFB1 by HPLC
The HPLC system consists of Waters Binary Pump Model 1525, a Model Waters 1500 Rheodyne manual injector, a Watres 2475 Multi- Wavelength Fluorescence Detector, and a data workstation with software Breeze 2. A phenomenex C18 (250 × 4.6 mm i.d.), 5 μm from Waters corporation (USA). The mobile phase consists of (water/methanol/acetonitrile (240:120:40v/v/v) with flow rate of 1.0 ml/min. The fluorescence detector was operated at wavelength of 360 nm and 440 for excitation and emission, respectively. Concentrations of AFB1 in samples were calculated individual for each sample through using compare peak area for sample with standard with 20 μl as injection volume for both standard solutions and sample extracts [20]. The percentage of inhibition was calculated as following equation. The percentage of inhibition AFB1 = .where: C is concentration of toxin in the positive sample that inoculated by spores of fungus only. T is concentration of toxin in the sample containing spores of fungus and aqueous extract (treatment sample).
3.8. Extraction and determination of OTA
Ten milliliters of YES medium was filtered, and extracted with 20 ml of chloroform. The chloroform phase was filtered and concentrated then dryness under a nitrogen to dry film, it was dissolved in 1 ml water/acetonitrile (3: 1 v/v) and mixed well by vortex for 30 s. The mixture was used for HPLC analysis. The HPLC system consisted of Waters Binary Pump Model 1525, Model Waters 1500 Rheodyne manual injector and Waters 2475 Multi- Wavelength Fluorescence Detector (Waters Pacific Pte Ltd, Science Park Road, Singapore). The data workstation with software Breeze TM 2 phenomenex C18 column (250 9 4_6 mm i.d.), 5 lm from Waters Corporation (Milford, MA). An isocratic system with acetonitrile: water: acetic acid (55: 43: 2) was used with flow rate of 1.0 ml/min. The injection volume was 20μl for both standard solutions and sample. The fluorescence detector was operated at wavelength of 335 nm and 465 nm for excitation and emission, respectively. OTA concentrations in samples were calculated individual for each sample through using compare peak area for sample with standard [21]. The percentage of inhibition was calculated as previously mentioned.
3.9. Statistical analysis
General Linear Model procedure of the SPSS ver. 18 (IBM Corp, NY) was used to statistically analysed. The significance of the differences among treatment groups was determined by Waller–Duncan k-ratio. All statements of significance were depended on the probability of P-value ≤ 0.05 was considered to be statistically significant.
4. Results and discussion
4.1. Total phenols and flavonoids in the aqueous extract
Data recorded in Table 1 shown an aqueous extract of P. oleracea has a greater total phenolic and flavonoids were 210.4 ± 1.15 and 36.7 ± 0.79 mg/mL, respectively, followed by S. oleraceus (192.3 ± 2.11 mg/mL) and finally C. pumilum (186.4 ± 2.18 mg/mL). On the other hand DPPH free radical scavenging activity was ranged 53.8–65.6% with three plants extract. These compounds affected by many factors such as water, air, soil, elevation, geographical variation, plant growth stage and differences between species, as well as extraction methods [22, 23, 24].
Table 1.
The content of aqueous extract from phenolic, flavonoid and antioxidant.
| Wild plants | Total phenolic (mg/mL) | Total flavonoids (mg/mL) | Antioxidant activity (%) |
|---|---|---|---|
| S.oleraceus | 192.3 ± 2.11 | 25.1 ± 1.33 | 57.3 |
| C. pumilum | 186.4 ± 2.18 | 24.3 ± 1.53 | 53.8 |
| P. oleracea | 210.4 ± 1.15 | 36.7 ± 0.79 | 65.6 |
Fourier Transform Infrared Spectroscopy (FTIR).
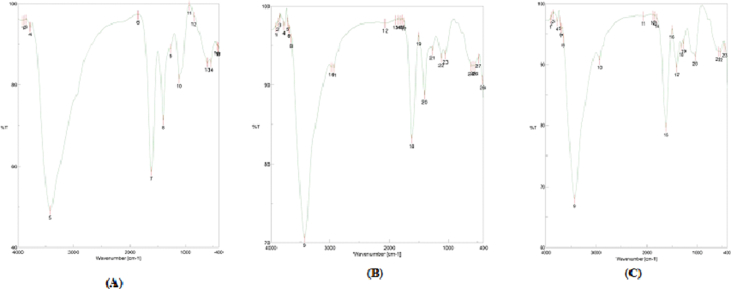
4.2. Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR analysis of bonds and functional groups in aqueous extract for three plants were presented in Tables 2, 3, and 4. The results indicated that many functional groups appeared in the aqueous leaves extract with the FTIR analysis such as alcohols, phenols, alkanes, carboxylic acids, aldehydes and alkenes. All the three plant extracts contains high absorbance at 3421 to 3430.74 cm−1 (OH stretching and N–H) and 1614.13 to 1624 cm−1 (C=O stretching) as shown in Figure 1. The general the extract of these plants contains phenolic and flavonoid compounds, which include more than 400 compounds, as well as other chemical constituents such as steroids, vitamins, minerals, fatty acids, alkaloids, saponins, etc. these compounds, especially flavonoids have a fifteen carbon, which consists of two phenyl rings bound together by three carbon atoms to form an oxygenated heterocycle [25, 26].
Table 2.
FTIR spectrum of bond in the aqueous extract for S. oleraceus.
| NO. of peak | Band position (cm−1) | bond and functional group |
|---|---|---|
| 1 | 3913.82 | O–H Hydroxy group |
| 2,3,4 | 3882,3850,3780 | O–H stretch, free hydroxyl alcohols, phenols |
| 5 | 3421.1 | N–H stretch aliphatic primary amine O–H stretch |
| 6 | 1855.19 | C=C asymmetric stretch |
| 7 | 1614.13 | –C=C– stretch alkenes Aromatic C=C stretching N–H bend 1° amines |
| 8 | 1406.82 | C–C stretch (in–ring) aromatics S=O stretching sulfate |
| 9 | 1273.75 | C–O stretching alkaylarayl ether C–N stretch aromatic amines |
| 10 | 1119.48 | C–O alcohols, carboxylic acids, esters, ethers C–N stretch aliphatic amines |
| 11 | 930.485 | O–H bend carboxylic acids |
| 12 | 855.275 | C–Cl stretch alkyl halides |
| 13 | 612.288 | -C=C–H: C–H bend alkynes C–Br stretch alkyl halides |
| 14 | 546.72 | C–Br stretch alkyl halides |
| 15,16,17 | 444.619:411.728 | C–Cl stretch alkyl halides |
Table 3.
FTIR spectrum of bonds in the aqueous extract for C. pumilum.
| NO. of peak | Band position (cm−1) | bond and functional group |
|---|---|---|
| 1 | 3913.82 | O–H stretching of Water; N–H stretching of protein amide A |
| 2,3,4,5 | 3886.83:3720.01 | O–H stretch, free hydroxyl alcohols, phenols |
| 6.7.8 | 3697.84:3658.3 | Free OH stretch |
| 9 | 3430.74 | N–H stretch (1°, 2° amines, amides) O–H alcohol |
| 10,11 | 2973.7:2927 | C–H stretchalkanes methyl and methylene groups O–H stretch alcohol N–H stretch amine salt |
| 12 | 2075.03 | N=C=S stretching isothiocyanate |
| 13 | 1904.36 | C=C=C stretching allene |
| 14,15 | 1855.19: 1811.79 | C=O stretch acid halide |
| 16,17 | 1781.9:1758.76 | C=O stretch ester, carboxylic acid |
| 18 | 1624.73 | –C=C– stretch alkenes Aromatic C=C stretching N–H bend 1° amines |
| 19 | 1507.1 | N–O stretch nitro compounds C–C stretch (in–ring) aromatics |
| 20 | 1406.82 | S=O stretching Sulfate sulfonyl chloride |
| 21 | 1271.109 | C–O stretching Alkyi, Aryl ether C–N stretch aromatic amines |
| 22 | 1120.44 | C C–O stretching aliphatic ether secondary alcohol |
| 23 | 1049.09 | S=O stretching sulfoxide |
| 24,25,26 | 617.109:545.756 | C–Br stretch alkyl halides |
| 27,28 | 491.759:416.549 | C–Cl stretch alkyl halides |
Table 4.
FTIR spectrum of bonds in the aqueous extract for P. oleracea.
| NO. of peak | Band position (cm−1) | bond and functional group |
|---|---|---|
| 1 | 3912 | O–H stretching of Water; N–H stretching of protein amide A |
| 2,5 | 3897.84:3657.34 | O–H stretch, free hydroxyl alcohols, phenols |
| 6,7,8 | 3697.84:3685.3 | Free OH sharp |
| 9 | 3429.78 | N–H stretch (1°, 2° amines, amides) O–H alcohol |
| 10 | 2925 | C–H stretchalkanes methyl and methylene groups |
| 11 | 2075 | C ≡ C Alkynes |
| 12,13,14 | 1879.29:1855.19 | C=O Aldehydes C=O stretch non conjugated Acid halid |
| 15 | 1625.7 | –C=C– stretch alkenes Aromatic C=C stretching N–H bend 1° amines |
| 16 | 1508 | N–O stretching nitro compound |
| 17 | 1406.82 | O–H bending carboxylic acids |
| 18 | 1319.07 | O–H bending phenol |
| 19 | 1271.82 | C–O stretching Alkyi, Aryl ether |
| 20 | 1044.26 | S=O stretching sulfoxide |
| 21 | 581.433 | C–Cl stretch halocompound |
| 22 | 535.15 | C–I stretch halocompound |
| 23 | 451.261 | C–Br stretch alkyl halides |
Figure 1.
FTIR spectrum of aqueous extract for S. oleraceus. (A), C. pumilum (B) and P. oleracea (C).
4.3. Effectiveness of aqueous extract as antifungal
In generally, data presented in Table 5 showed the inhibition zone for P. oleracea extract give higher inhibition compared with S. oleraceus and C. pumilum, as well as inhibition zone increased with an increase the concentration of the extract. In case S. oleraceus extract the results indicated that the A. flavus, A. ochraceus and A. parasiticus inhibition zone was ranged (4.4 ± 0.6 to 13.7 ± 1.4 mm), (7.1 ± 0.36 to 22.8 ± 2.2 mm) and (6.6 ± 0.7 to 20.5 ± 1.8 mm), respectively. In contrast, the only case that did not show any inhibitory growth was with A. flavus at 1.5 mg/ml from C. pumilum extract. P. oleracea extract recording the highest inhibition at in all concentrations and also against three strains was (7.4 ± 1.3 to 17.1 ± 1.7 mm) with A. flavus, while with A. ochraceus and A. parasiticus strains the inhibition zone were (8.5 ± 0.5 to 26.5 ± 1.5 mm) and (9.6 ± 2.1 to 22.8 ± 2.3 mm), respectively. The analysis of variance showed higher significant differences in inhibition zone with all strains as well as concentrations of aqueous extract, also there were significant differences between plants used in this study Table 6. Anti-fungal effect for flavonoids and phenolics were through several mechanisms of action such as they influential interfere with membrane functions by changing the permeability of cellular membranes, cell division and synthesis of RNA and protein which could lead eventually to the inhibition of growth, also oxidation of lipid in cell [27, 28].
Table 5.
The inhibition zone (mm) of aqueous extract for three plants.
| Type of plant | Concentration (mg/ml) | Fungi∗ |
Mean with plant | ||
|---|---|---|---|---|---|
| A. flavus | A. ochraceus | A. parasiticus | |||
| S. oleraceus | 1.5 | 4.4 ± 0.6 | 7.1 ± 0.36 | 6.6 ± 0.7 | 6.05 ± 1.3 |
| C. pumilum | - | 3.1.±1.2 | 3.5 ± 0.51 | 2.24 ± 1.8 | |
| P. oleracea | 7.4 ± 1.3 | 8.5 ± 0.5 | 9.6 ± 2.1 | 8.5 ± 1.6 | |
| Mean with type of Fungi | 3.9 ± 3.3 | 6.2 ± 2.4 | 6.6 ± 2.8 | ||
| S. oleraceus | 2.5 | 5.5 ± 0.75 | 9.8 ± 1.9 | 11.3 ± 1.5 | 8.9 ± 2.9 |
| C. pumilum | 3.5 ± 1.5 | 4.5 ± 1.3 | 6.2 ± 0.76 | 4.7 ± 1.6 | |
| P. oleracea | 11.6 ± 1.3 | 18.7 ± 1.5 | 14.7 ± 2.5 | 14.9 ± 3.4 | |
| Mean with type of Fungi | 6.8 ± 3.2 | 10.9 ± 6.3 | 10.7 ± 4.01 | ||
| S. oleraceus | 5 | 13.7 ± 1.4 | 22.8 ± 2.2 | 20.5 ± 1.8 | 19.01 ± 4.4 |
| C. pumilum | 6.2 ± 0.77 | 9.3 ± 1.5 | 13.3 ± 0.65 | 9.6 ± 3.2 | |
| P. oleracea | 17.1 ± 1.7 | 26.5 ± 1.5 | 22.8.±2.3 | 22.1 ± 4.4 | |
| Mean with type of Fungi | 12.3 ± 4.3 | 19.5 ± 7.9 | 18.8 ± 4.5 | ||
N = 3; Mean ± SD (Std. Deviation); -: No inhibition.
Table 6.
ANOVA analysis of the effect of concentration of aqueous extract, type of plant and fungi strains on a growth.
| Source | SS | df | MS | F | P. |
|---|---|---|---|---|---|
| Intercept | 9238.414 | 1 | 9238.414 | 4504.848 | 0.00000 |
| Plant | 1282.385 | 2 | 641.1925 | 312.6591 | 0.00000 |
| Con. | 1781.679 | 2 | 890.8395 | 434.3924 | 0.00000 |
| Fungi | 356.5913 | 2 | 178.2956 | 86.94076 | 0.00000 |
| Plant ∗ Con. | 142.4493 | 4 | 35.61231 | 17.36532 | 0.00000 |
| Plant ∗ Fungi | 49.61148 | 4 | 12.40287 | 6.047904 | 0.000427 |
| Con ∗ Fungi | 62.10741 | 4 | 15.52685 | 7.571224 | 0.000064 |
| Plant ∗ Con ∗ Fungi | 59.6137 | 8 | 7.451713 | 3.633614 | 0.0019 |
| Error | 110.7417 | 54 | 2.050772 | ||
| Total | 13083.59 | 81 |
SS, sum of squares; df, degree of freedom; MS, mean square; P, probability at confidence 0_95.
4.4. Impact of aqueous extract on AFB1 and OTA produced in YES medium
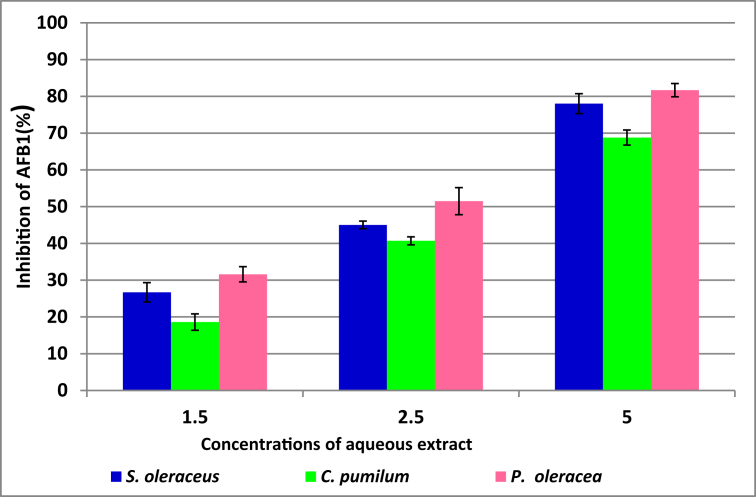
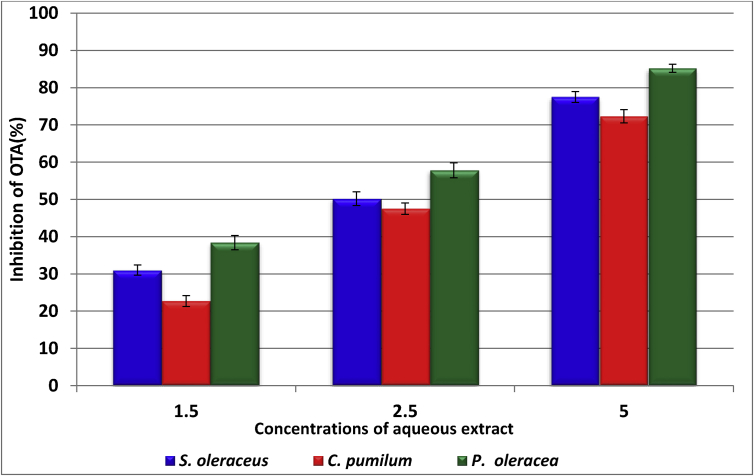
The results displayed in Figures 2 and 3 show the percentages of inhibition AFB1 and OTA produced by A. flavus and A. ochraceus in YES medium, respectively, which were treated by aqueous extract for three plants. The preliminary data reflected that the percentages of inhibition of AFB1 by aqueous extract of S. oleraceus were 26.7, 45.03 and 78.03% at 1.5, 2.5 and 5 mg concentration of extract, respectively. On the other hand at the same above concentrations extract of P. oleracea gave the highest inhibition for AFB1 was 31.6, 51.5 and 81.7%. While inhibitory effect against OTA at 5 mg/100ml was (77.5, 72.3 and 85.2%) for extract S. oleraceus, C. pumilum and P. oleracea, respectively. The obtained results showed that the extract of C. pumilum in all treatments gave the lowest values for reduce of toxin.
Figure 2.
The percentages of inhibition of AFB1 produced by A. flavus in YES media treated by aqueous extract.
Figure 3.
The percentages of inhibition of OTA produced by A. ochraceus in YES media treated by aqueous extract.
The antitoxigenic activity for these plants may be due to several of the activation compounds were identified such as 2-Pentadecanone, Luteolin-O-glucuronide, n-hexadecanoic acid and hydroxycoumarins in the aqueous extract of S. oleraceus [29, 30, 31]. As well as extract of C. pumilum has sesquiterpene, caffeic acid, cichoriin, esculin, umbelliferone, scopoletin and 6,7- ihydroxycoumarin, flavone derivatives (cichoric acid, chlorogenic acid, apigenin, quercitin) [31, 32, 33]. Many studies reported that aqueous extract of P. oleracea has some alkenals, coumarins, including hexanal, 2 hexanal and 2-Pentenal inhibited fungal growth [34, 35, 36]. The inhibitor effect of these active compounds for producer of AFB1 and OTA depend on many mechanisms including, the effect of these compounds on specific sites within the cell of the fungus, for instance effect on the expression pathways of the gene responsible for producing the toxin, in addition block the action of enzymes responsible for the biosynthesis process, as well as the induction of mitochondrial dysfunction, because of the mitochondria are responsible for providing acetyl-CoA which is a main precursor for AFB1 biosynthesis in the fungal. Aqueous extract of P. oleracea contains five flavonoids (, apigenin, myricetin, kaempferol, quercetin and luteolin) with many chemical constituents such as steroids, vitamins, minerals, fatty acids; alkaloids, saponins, etc. have been isolated from the plant. In addition to clerodane diterpene portulene, rutin, apigenin and glycolflavones. The inhibitory effect for these substances may be due to sotpping the synthesis of enzymes responsible for biosynthetic pathway for AFB1 and OTA [37, 38, 39].
5. Conclusion
The aqueous extract used in this study is a good source of many effective compounds that are easy to use with many products such as pharmaceutical products (soap or shampoo) and bakery products. This extract can be obtained in an easy, environmentally friendly, safe, and low-cost way P. oleracea extract had the greatest effect as antifungal and antitoxigenic.
Declarations
Author contribution statement
Tarek A. El-Desouky: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The author sincerely thanks to Abd El Aziz A. M for support in some analysis tools.
References
- 1.Kumar P., Mahato D.K., Kamle M., Mohanta T.K., Kang S.G. Aflatoxins: a global concern for food safety, human health and their management. Front. Microbiol. 2017;7:2170. doi: 10.3389/fmicb.2016.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varga J., Frisvad J.C., Samson R.A. A reappraisal of fungi producing aflatoxins. World Mycotoxin J. 2010;2:263–277. [Google Scholar]
- 3.Liuzzi V.C., Fanelli F., Tristezza M., Haidukowski M., Picardi E., Manzari C. Transcriptional analysis of Acinetobacter sp. neg1 capable of degrading ochratoxin A. Front. Microbiol. 2017;7:2162. doi: 10.3389/fmicb.2016.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IARC. International Agency for Research on Cancer Monograph on the evaluation of carcinogenic risk to humans IARC. 2002;82:171–300. [Google Scholar]
- 5.Fapohunda Stephen O., Adewunmi Annabella A. Climate change and mycotoxins - the African experience. Croat. J. Food Sci. Technol. 2019;11(2):283–290. [Google Scholar]
- 6.Razzaghi-Abyaneh M., Shams-Ghahfarokhi M., Rezaee M.B., Sakuda S. Natural aflatoxin inhibitors from medicinal plants. In: Rai M., Varma A., editors. Mycotoxins in Food, Feed and Bioweapons. Springer-Verlag; Berlin, Heidelberg: 2010. pp. 329–352. [Google Scholar]
- 7.Marrez Diaa A., Naguib Mohamed M., Sultan Yousef Y., Higazy Aziz M. Antimicrobial and anticancer activities of Scenedesmus obliquus metabolites. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01404. (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Desouky T.A., May M. Amer, Naguib Khayria. Effect of fenugreek seeds extracts on growth of aflatoxigenic fungus and aflatoxin B1 production. J. Appl. Sci. Res. 2013;9(7):4418–4425. http://www.aensiweb.com/jasr/jasr/201 [Google Scholar]
- 9.Salhi N., Saghir S.A.M., Terzi V., Brahmi Ghedairi N., Bissati S. Antifungal activity of aqueous extracts of some dominant Algerian medicinal plants. BioMed Res. Int. 2017:7526291. doi: 10.1155/2017/7526291. (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amer Ahmed M. Antimicrobial effects of Egyptian local chicory, Cichorium endivia subsp. Pumilum. Int. J. Microbiol. 2018:6. doi: 10.1155/2018/6475072. ID 6475072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palumbo J.D., O'Keeffe T.L., Mahoney N.E. Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia. 2007;(164):241–248. doi: 10.1007/s11046-007-9057-0. [DOI] [PubMed] [Google Scholar]
- 12.Mickymaray S., Alturaiki W. Antifungal efficacy of marine macroalgae against fungal isolates from bronchial asthmatic cases. Molecules. 2018;23(11):3032. doi: 10.3390/molecules23113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamilia A.E.H., El Seoud Abou, Bibby Michael C., Shoeib Nagwa, Wright Colin W. Evaluation of some Egyptian plant species for in vitro antimycobacterial and cytotoxic activities. Pharmaceut. Biol. 2003;41(6):463–465. [Google Scholar]
- 14.Petropoulos S.A., Fernandes Â., Tzortzakis N., Sokovic M., Ciric A., Barros L., Ferreira I.C. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019;119:859–868. doi: 10.1016/j.foodres.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Almashad A., Ibrahim Ramadan G., Abdelrazek R. Phytochemicals, antioxidant and volatile compounds evaluation of Egyptian purslane leaves. Arab Univ. J. Agric. Sci. 2019;27(5):2573–2582. [Google Scholar]
- 16.Prior R.L., Wu X., Schaich K. Standard methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 17.Yan-Hwa C., Chao-Lin C., Hsia-Fen H. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000;80:561–566. [Google Scholar]
- 18.Lee Y.H., Choo C., Watawana M.I., Jayawardena N., Waisundara V.Y. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J. Sci. Food Agric. 2015;95:2956–2964. doi: 10.1002/jsfa.7039. [DOI] [PubMed] [Google Scholar]
- 19.Perez C., Paul M., Bazerque P. Antibiotic assay by agar well diffusion method. Acta Biol. Med. Exp. 1990;15:113–115. 1990. [Google Scholar]
- 20.El-Desouky T.A., Ammar H.A.M. Honey mediated silver nanoparticles and their inhibitory effect on aflatoxins and ochratoxin A. J. Appl. Pharmaceut. Sci. 2016;6(6):83–90. [Google Scholar]
- 21.Ammar H.A.M., El-Desouky T.A. Green synthesis of nanosilver particles by Aspergillus terreus HA1N and Penicillium expansum HA2N and its antifungal activity against mycotoxigenic fungi. J. Appl. Microbiol. 2016;121:89–100. doi: 10.1111/jam.13140. [DOI] [PubMed] [Google Scholar]
- 22.Gairola S., Shariff N., Bhate A., Prakashkola C. Influence of climate change on production of secondary chemicals in high altitude medicinal plants. J. Med. Plants Res. 2010;4:1825–1829. [Google Scholar]
- 23.Kawka B., Kwiecień I., Ekiert H. Influence of culture medium composition and light conditions on the accumulation of bioactive compounds in shoot cultures of Scutellaria lateriflora L. (American Skullcap) grown in vitro. Appl. Biochem. Biotechnol. 2017;183:1414–1425. doi: 10.1007/s12010-017-2508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zargoosh Z., Ghavam M., Bacchetta G., Tavili A. Effects of ecological factors on the antioxidant potential and total phenol content of scrophularia striata boiss. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-52605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aboody M.S.A., Mickymaray S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics (Basel) 2020;9(2):45. doi: 10.3390/antibiotics9020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Yu L., Chen G. Determination of flavonoids in Portulaca oleracea L. by capillary electrophoresis with electrochemical detection. J. Pharmaceut. Biomed. Anal. 2006;41(2):493–499. doi: 10.1016/j.jpba.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.H., Yu J., Mahoney N., Chan K.L., Molyneux R.J., Varga J., Bhatnagar D., Cleveland T.E., Nierman W.C., Campbell B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008;122:49–60. doi: 10.1016/j.ijfoodmicro.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Kim J.H., Campbell B.C., Mahoney N., Chan K.L., Molyneux R.J. Chemosensitization of aflatoxigenic fungi to antimycin A and strobilurin using salicylaldehyde, a volatile natural compound targeting cellular antioxidant system. Mycopathologia. 2010 doi: 10.1007/s11046-010-9356-8. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan K.R., James F., Mohan A. Isolation and characterization of n-hexadecanoic acid from Canthium parviflorum leaves. J. Chem. Pharm. Res. 2016;8:614–617. [Google Scholar]
- 30.Banaras S., Javaid A., Khan I.H. Potential antifungal constituents of Sonchus oleraceous against Macrophomina phaseolina. Int. J. Agric. Biol. 2020;24:1376‒1382. [Google Scholar]
- 31.Mares D., Romagnoli C., Tosi B., Andreotti E., Chillemi G., Poli F. Chicory extracts from Cichorium intybus L. as potential antifungals. Mycopathologia. 2005;160(1):85–91. doi: 10.1007/s11046-004-6635-2. [DOI] [PubMed] [Google Scholar]
- 32.Rehman A., Ullah N., Ullah H., Ahmad I. Antibacterial and antifungal study of Cichorium intybus. Asian Pacific Journal of Tropical Disease. 2014;4(Suppl 2):S943–S945. [Google Scholar]
- 33.Aisa H.A., Xin X.L., Tang D. Chemical constituents and their pharmacological activities of plants from Cichorium genus. Chinese Herbal Med. 2020;12:224–236. doi: 10.1016/j.chmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taguchi T., Kozutsumi D., Nakamura R., Sato Y., Ishihara A., Nakajima H. Effects of aliphatic aldehydes on the growth and patulin production of Penicillium expansum in apple juice. Biosci. Biotechnol. Biochem. 2013;77:138–144. doi: 10.1271/bbb.120629. [DOI] [PubMed] [Google Scholar]
- 35.Prusty J.S., Kumar A. Coumarins: antifungal effectiveness and future therapeutic scope. Mol. Divers. 2020;24(4):1367–1383. doi: 10.1007/s11030-019-09992-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Yan-Xi, Xin Hai-Liang, Rahman Khalid, Wang Su-Juan, Cheng Peng, Zhang Hong. Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015;2015:11. doi: 10.1155/2015/925631. Article ID 925631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayaka H.B., Londonkar R.L., Umesh M.K., Tukappa A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014 doi: 10.1155/2014/175851. 2014, Article ID 175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Londonkar R., Nayaka H.B. Phytochemical and antimicrobial activities of Portulaca oleracea L. J. Pharm. Res. 2011;4:3553–3555. [Google Scholar]
- 39.Perlin D.S., Rautemaa-Richardson R., Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect. Dis. 2017 doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.