Abstract
Objective
Clinical trials suggest that SGLT2 inhibitors reduce the risk of cardiovascular mortality in patients with type 2 diabetes, however the mechanism is unclear. Our objective was to test the hypothesis that blood pressure reduction is one potential mechanism underlying the observed improvements in cardiovascular outcomes with SGLT2 inhibitors.
Methods
We searched MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials (inception-June 2019) for randomized controlled trials that reported the effect of SGLT2 inhibitors compared with placebo on cardiovascular outcomes in adults with type 2 diabetes. Two reviewers independently extracted data and assessed study quality. Random effects meta-analyses, stratified meta-analyses and meta-regressions were conducted to evaluate the association between blood pressure reduction in SGLT2 inhibitor treated patients and cardiovascular outcomes.
Results
Of 11,232 articles identified, 40 articles (n = 54,279 participants) were included. The relative risk of cardiovascular mortality was reduced by 18% with the use of SGLT2 inhibitors compared with placebo (RR 0.82; 95%CI 0.74, 0.91, I2 = 0.0%). Meta-regression analysis revealed no detectable difference in cardiovascular mortality (RR 0.93; 95%CI 0.88, 1.13, p = 0.483), 3-point major adverse cardiovascular events (p = 0.839) or congestive heart failure hospitalizations (p = 0.844) with change in mean systolic blood pressure.
Conclusions
Cardiovascular events are reduced in participants with type 2 diabetes treated with SGLT2 inhibitors compared with placebo. There was no significant relationship between the risk of developing adverse cardiovascular events and blood pressure reduction with SGLT2 inhibitors. There is insufficient evidence to suggest that blood pressure reduction is a significant contributor to the cardiovascular benefits observed.
Keywords: Systematic review, Meta-analysis, Diabetes, Blood pressure, Cardiac outcomes, SGLT2 inhibitors
1. Introduction
Large randomized controlled trials have demonstrated that sodium-glucose co-transporter-2 (SGLT2) inhibitors are effective in improving cardiovascular outcomes including cardiovascular mortality, stroke, and hospital admissions for congestive heart failure (CHF) in patients with type 2 diabetes and cardiovascular disease [1], [2], [3], [4], or those at very high cardiovascular risk [1]. SGLT2 inhibitors also improve renal outcomes including progression to end-stage renal disease and renal-associated mortality in patients with type 2 diabetes [4]. Accordingly, clinical practice guidelines recommend SGLT2 inhibitorsas a second line medication after metformin for management of type 2 diabetes for patients with known cardiovascular disease [5], [6].
Benefits of SGLT2 inhibitors have been demonstrated in patients with [7] and without [8] type 2 diabetes, however the mechanism by which cardiovascular and renal outcomes improve is unclear. SGLT2 inhibitors may improve cardiovascular outcomes through multiple, complimentary mechanisms including improvement in glycemic control, altered energy metabolism in the heart, blood pressure reduction, weight loss, and diuresis [9], [10]. Blood pressure reduction with SGLT2 inhibitor treatment may be due to the direct effect of these agents on arterial stiffness improvement [11], plasma volume reduction [12], and natriuresis [10], or an indirect effect of weight loss [13]. Elucidating the mechanisms by which SGLT2 inhibitors exert their beneficial effects is important as this knowledge can inform optimal use of these agents.
SGLT2 inhibitors are not currently recommended as an antihypertensive therapy. Given that these agents could plausibly affect positive cardiovascular and renal outcomes via direct antihypertensive properties, the specific role of SGLT2 inhibitors in persons with type 2 diabetes and hypertension needs to be defined. We designed this systematic review and meta-analysis to test the hypothesis that the reduction in cardiovascular outcomes in adults with type 2 diabetes is at least in part attributed to the blood pressure decrease associated with SGLT2 inhibitor use. The cardiovascular outcomes of interest included cardiovascular mortality, 3-point major adverse cardiovascular events (MACE; a composite of cardiovascular death, nonfatal stroke and nonfatal myocardial infarction) and CHF hospitalizations.
2. Methods
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14]. The protocol was registered (PROSPERO CRD42018116683).
2.1. Patient and public involvement
There was no patient or public involvement in the design, conduct, reporting or dissemination of this study.
2.2. Data sources and searches
The search strategy was developed in consultation with two experienced medical research librarians (ZP, DL). A comprehensive search was conducted from inception to 29 June 2019 in MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials using Medical Subject Heading terms and keywords related to SGLT2 inhibitors and specific SGLT2 inhibitor drug names (Table A.1). A randomized controlled trials filter was applied to searches in MEDLINE and EMBASE [15]. Reference lists of included articles and relevant reviews were hand searched. Local experts were consulted to identify additional eligible articles.
2.3. Study selection
Titles and abstracts were independently reviewed by two reviewers (JLB, JEB) for possible inclusion using the following criteria: 1) adults (≥18 years) with type 2 diabetes, 2) treatment with any SGLT2 inhibitor alone or in combination with other antidiabetic medications. Full text review was then performed by two independent reviewers (JLB, JEB) using the following inclusion criteria: 1) placebo-controlled randomized trial, 2) adults (≥18 years) with type 2 diabetes, 3) treatment with any SGLT2 inhibitor alone or in combination with other antidiabetic medications, 4) treatment duration ≥ 24 weeks, 5) report of cardiovascular outcomes (cardiovascular mortality, 3-point MACE and/or CHF hospitalizations), and 6) report of change in systolic blood pressure with SGLT2 inhibitor use. Articles were excluded for any of the following reasons: 1) no original data, 2) no published full-text article, or 3) non-English language. If data from the same trial were reported across multiple publications, the article with the longest follow-up was selected. Disagreements were resolved through consensus. A kappa statistic was calculated to quantify article selection agreement between reviewers [16].
2.4. Data extraction and quality assessment
JLB and JEB independently extracted all outcome data with subsequent discussion of any discrepancies. Data were collected on trial characteristics, baseline patient characteristics (e.g., age), SGLT2 inhibitor use (e.g., type), change in systolic blood pressure, and cardiovascular outcomes (e.g., cardiovascular death). Trial outcomes were extracted in intention-to-treat categories. The incidence of cardiovascular death was assumed to be zero if no deaths occurred during the trial period or if deaths that occurred were not cardiovascular-related.
Quality assessment was performed by extracting information on key trial validity criteria using the Cochrane Risk of Bias Tool (Modified) for Quality Assessment of Randomized Controlled Trials [17].
2.5. Data synthesis and analysis
As the cardiovascular benefits of SGLT2 inhibitors have been established [1], [2], [3], [4], [8], the primary goal of this analysis was to examine the association between blood pressure reduction among those treated and not treated with SGLT2 inhibitors and degree of cardiovascular benefit observed. Our analytic plan was structured as follows: 1) Conduct a meta-analysis to determine the pooled effect of SGLT2 inhibitors (relative to placebo) on cardiovascular outcomes; 2) Conduct a meta-regression analysis to determine if there is a significant linear association between blood pressure reduction and cardiovascular event reduction among those treated with SGLT2 inhibitors; and 3) Conduct stratified meta-analyses to determine if trials that progressively achieved lower blood pressure also had greater reported cardiovascular event reduction. Details of these analytic approaches are described herein.
Mantel-Haenszel random effects model meta-analyses were conducted to assess the effect of SGLT2 inhibitors compared with placebo on cardiovascular outcomes. Risk ratios (RR) with 95% confidence intervals (95%CI) were used as a common measure of association, and as is common in meta-analyses [18], [19], HRs were considered interchangeable with RRs. Where appropriate, experimental arms were pooled together to facilitate comparisons between SGLT2 inhibitor treatment groups and placebo. A continuity correction of 1 was used, as required, to calculate pooled estimates when there were zero event cells [20]. Statistical heterogeneity between estimates was assessed using the Cochran's Q test and I2 statistic. For the I2 statistic, heterogeneity cut-offs were: low (<25%), moderate (25–50%), and high (>50%) [21]. To interrogate heterogeneity across included trials, stratified analyses were conducted to examine the effect of SGLT2 inhibitors on cardiovascular mortality with 1) continuity correction use, 2) follow-up duration, 3) baseline hemoglobin A1C (A1C), 4) diabetes duration, 5) baseline systolic blood pressure, 6) proportion of males, and 7) SGLT2 inhibitor agent used.
A random effects model meta-regression analysis was performed to evaluate the association between degree of blood pressure lowering and cardiovascular mortality, 3-point MACE and CHF hospitalizations. Systolic blood pressure was selected for analysis because baseline and end-of-trial levels were most consistently reported for this measure compared to diastolic blood pressure. We used the weighted least-squares method [22]. The logarithm of relative risk for each cardiovascular outcome was weighted by the inverse variance of each trial and regressed against the difference in change in systolic blood pressure for participants assigned to SGLT2 inhibitors and participants assigned to placebo from baseline to end-of-intervention. Standard errors were calculated. Statistical significance was assessed using the Wald test.
Publication bias was assessed using a funnel plot and Egger’s test [23]. Data analyses were conducted using Stata 15.1 (StataCorp, College Station, Texas). A p-value of < 0.05 was used as the level of statistical significance, or 95%CI that did not enclose the null value of 1.
3. Results
3.1. Trial selection
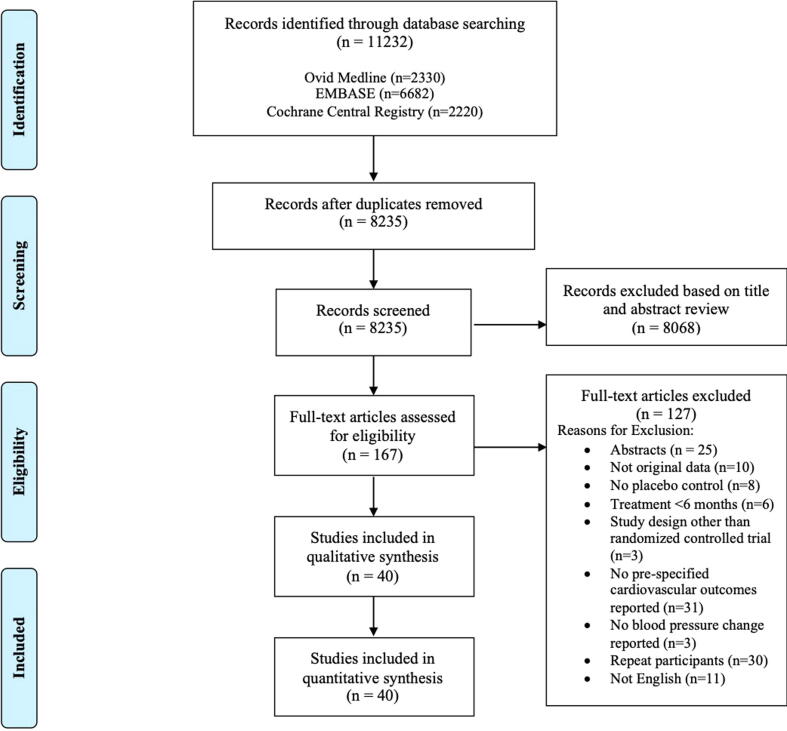
Our search strategy identified 11,232 articles, and 8,235 titles and abstracts were then screened after duplicates were removed. There were 1,867 articles that met criteria for full-text review and forty articles were included [1], [2], [3], [4], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]. Trial selection process details are presented in a PRISMA flow diagram (Fig. 1) [14]. There was moderate agreement between reviewers at the title and abstract screening stage (κ = 0.659), and strong agreement for full text screening (κ = 0.850). The majority of trials were excluded because pre-specified cardiovascular outcomes were not reported (n = 31) or they were secondary publications of trials already included in our review (n = 30).
Fig. 1.
PRISMA Flow Diagram.
3.2. Trial characteristics
Characteristics of the 40 included trials (n = 54,279 participants) are presented in Table 1. Notably, four major trials contributed 38,723 (71.3%) of all study participants [1], [2], [3], [4]. Nine trials reported the proportion of participants with hypertension [3], [4], [29], [31], [38], [41], [44], [54], [57], which ranged from 40.9% to 100%. Seven trials reported the proportion taking antihypertensive medications [1], [2], [29], [30], [44], [46], [57]. Six trials specified that antihypertensive regimens should remain stable during the intervention [29], [30], [33], [39], [44], [52]. Thirty trials reported mean diastolic blood pressure measurements, which ranged from 73.3 to 88.3 mmHg [2], [3], [4], [46], [47], [49], [50], [56], [58], [26], [27], [28], [29], [30], [31], [34], [35], [36], [38], [39], [40], [41], [42], [43], [44], [52], [53], [54].
Table 1.
Study Characteristics.
| Author, Year | Drug | SampleSize | Mean Age (y) | Primary Outcome | Treatment Duration (weeks) | Attrition (%) | Cardiovascular Mortality Events |
Mean Baseline SBP (mmHg) |
Difference in Change in SBP¦ (mmHg) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | SGLT2 | Placebo | SGLT2 | ||||||||
| Allegretti, 2019 [24] | Bexagliflozin | 312 | 69.6 | A1C | 24 | 5 | 0/155 | 0/157 | 137.6 | 135.9 | −4.00 |
| Bailey, 2012 [25] | Dapagliflozin | 282 | 53.0 | A1C | 24 | 7 | 0/68 | 0/214 | 129.3 | 128 | −4.58 |
| Bailey, 2013 [26] | Dapagliflozin | 546 | 53.9 | A1C | 102 | 38 | 0/137 | 2/409 | 128 | 127 | −1.73 |
| Bode, 2015 [27] | Canagliflozin | 714 | 63.6 | A1C | 104 | 24 | 0/237 | 1/477 | 131.4 | 131 | −6.59 |
| Bolinder, 2014 [28] | Dapagliflozin | 182 | 60.7 | Weight† | 102 | 23 | 0/91 | 0/91 | 133.3 | 135 | −2.40 |
| Cefalu, 2015 [29] | Dapagliflozin | 922 | 62.4 | Composite§ | 52 | 25 | 1/459 | 4/455 | 133 | 132 | −3.58 |
| Dagogo-Jack, 2018 [30] | Ertugliflozin | 462 | 59.1 | A1C | 52 | 13 | 0/153 | 0/309 | 130.2 | 131 | −4.95 |
| Ferdinand, 2019 [31] | Empagliflozin | 150 | 56.8 | A1C | 24 | 18 | 0/72 | 0/78 | 148.3 | 148.9 | −7.43 |
| Ferrannini, 2010 [32] | Dapagliflozin | 274 | 52.2 | A1C | 24 | 15 | 0/75 | 0/199 | NR | NR | −2.61 |
| Fioretto, 2018 [33] | Dapagliflozin | 321 | 65.8 | A1C | 24 | 3 | 0/161 | 0/160 | 135 | 135 | −3.10 |
| Forst, 2014 [34] | Canagliflozin | 342 | 57.3 | A1C | 26 | 23 | 0/115 | 0/227 | 128.2 | 127 | −3.80 |
| Gallo, 2019 [35] | Ertugliflozin | 621 | 56.7 | A1C | 104 | 15 | 2/207 | 1/411 | 129.3 | 130.4 | −3.42 |
| Haering, 2015 [36] | Empagliflozin | 666 | 57.1 | A1C | 72 | 9 | 0/225 | 1/441 | 128.8 | 129 | −2.15 |
| Ji, 2019 [37] | Ertugliflozin | 506 | 56.4 | A1C | 26 | 5 | 0/167 | 0/339 | NR | NR | −4.70 |
| Ji, 2014 [38] | Dapagliflozin | 393 | 51.3 | A1C | 24 | 13 | 0/132 | 0/261 | 123.5 | 124 | −2.56 |
| Kaku, 2014 [39] | Tofogliflozin | 230 | 57.0 | A1C | 24 | 8 | 0/56 | 0/173 | 128.3 | 129 | −4.74 |
| Kashiwagi, 2015 [41] | Ipragliflozin | 168 | 56.7 | A1C | 24 | 21 | 1/57 | 0/112 | 125.8 | 126 | −3.60 |
| Kashiwagi, 2015 [40] | Ipragliflozin | 240 | 59.7 | A1C | 24 | 46 | 0/75 | 0/165 | 129.2 | 130 | −4.20 |
| Kohan, 2014 [42] | Dapagliflozin | 252 | 67.0 | A1C | 104 | 45 | 3/84 | 4/168 | 130.7 | 132 | −5.53 |
| Kovacs, 2015 [43] | Empagliflozin | 498 | 54.5 | Composite§ | 76 | 47 | 1/165 | 3/433 | 125.7 | 126 | −2.86 |
| Leiter, 2014 [44] | Dapagliflozin | 964 | 63.6 | A1C | 52 | 22 | 1/482 | 2/480 | 134.6 | 135 | −3.00 |
| Mathieu, 2015 [45] | Dapagliflozin | 320 | 55.1 | A1C | 24 | 6 | 0/160 | 0/160 | NR | NR | −3.90 |
| Matthaei, 2015 [46] | Dapagliflozin | 216 | 61.0 | A1C | 52 | 13 | 0/108 | 0/108 | 136.4 | 136 | −2.10 |
| Merker, 2015 [47] | Empagliflozin | 637 | 55.7 | A1C | 76 | 34 | 0/207 | 0/430 | 128.6 | 129 | −4.05 |
| Neal, 2017 [3] | Canagliflozin | 10,142 | 63.3 | 3-pt MACE‡ | 188.2 | 4 | 12.8* | 11.6* | 136.9 | 137 | −3.93 |
| Perkovic, 2019 [4] | Canagliflozin | 4401 | 63.0 | Composite# | 31.4 | 1 | 140/2059 | 110/2092 | 140.2 | 139.8 | −2.38 |
| Rodbard, 2016 [48] | Canagliflozin | 213 | 57.4 | A1C | 26 | 17 | 0/106 | 0/107 | 128.7 | 130 | −5.90 |
| Rosenstock, 2015 [49] | Dapagliflozin | 355 | 54.0 | A1C | 24 | 8 | 0/176 | 0/179 | 128 | 129 | −2.20 |
| Rosenstock, 2014 [50] | Empagliflozin | 563 | 56.7 | A1C | 52 | 16 | 0/188 | 0/375 | 132.6 | 133 | −0.70 |
| Rosenstock, 2012 [51] | Dapagliflozin | 420 | 53.5 | A1C | 48 | 19 | 0/139 | 0/281 | NR | NR | −3.60 |
| Seino, 2014 [52] | Luseogliflozin | 158 | 59.3 | A1C | 24 | 6 | 0/79 | 0/79 | 128.9 | 129 | −5.70 |
| Stenlof, 2013 [53] | Canagliflozin | 584 | 55.4 | A1C | 26 | 13 | 1/192 | 0/392 | 127.7 | 128 | −4.55 |
| Strojek, 2014 [54] | Dapagliflozin | 592 | 59.8 | A1C | 48 | 13 | 0/145 | 3/447 | 133.3 | 133 | −5.06 |
| Terra, 2017 [55] | Ertugliflozin | 461 | 56.4 | A1C | 26 | 10 | 0/153 | 0/308 | NR | NR | −1.71 |
| Wilding, 2013 [56] | Canagliflozin | 469 | 56.8 | A1C | 52 | 34 | 0/156 | 0/313 | 130.1 | 130 | −3.40 |
| Wilding, 2014 [57] | Dapagliflozin | 807 | 58.8 | A1C | 104 | 36 | 0/197 | 3/610 | NR | NR | −2.80 |
| Wiviott, 2018 [1] | Dapagliflozin | 17,160 | 63.9 | 3-pt MACE‡ | 218 | 31 | 249/3578 | 245/8582 | 134.8 | 135 | −2.70 |
| Yang, 2016 [58] | Dapagliflozin | 444 | 53.7 | A1C | 24 | 8 | 0/145 | 0/299 | 126.3 | 128 | −5.09 |
| Yang, 2018 [59] | Dapagliflozin | 272 | 57.5 | A1C | 24 | 5 | 0/133 | 0/139 | 131.3 | 132 | −5.00 |
| Zinman, 2015 [2] | Empagliflozin | 7020 | 63.1 | 3-pt MACE‡ | 133 | 3 | 137/2283 | 172/4687 | 135.8 | 136 | −3.80 |
SBP systolic blood pressure; NR not reported; * events per 1000 patient years; † change in total body weight from baseline to end of treatment period; ‡ 3-pt MACE is a composite of cardiovascular mortality, nonfatal stroke and nonfatal myocardial infarction; § co-primary outcome measures were mean change in baseline A1c and proportions of participants achieving a three-outcome measure of combined clinical benefit: simultaneous A1c decrease of 0.5% or greater, total body weight reduction of 3% or greater, and systolic blood pressure reduction of 3 mmHg or greater from baseline; # composite of end-stage kidney disease, serum creatinine doubling from baseline for ≥ 30 days, or death from cardiovascular or renal disease; ¦ the difference in the change in mean systolic blood pressure from baseline to end-of-intervention between the intervention and placebo group
3.3. Quality assessment
The results of our quality assessment are presented in Table A.2. Overall, the risk of bias was determined to be “low” for 15 trials [2], [3], [4], [24], [25], [33], [35], [36], [39], [45], [49], [52], [55], [58], [59], “unclear” for one trial [37], and “high” for 24 trials [1], [34], [38], [50], [51], [53], [54], [56], [27], [28], [29], [30], [31], [32], [40], [41], [42], [43], [44], [46], [47], [48]. All included trials received an assessment of “low” risk of bias for the categories of selective outcome reporting and participant and assessor blinding. A minority of trials did not report on sequence generation [32], [41], [42], or allocation concealment [32], [41], [42]. Some trials received a rating of “high” risk of bias due to reporting attrition > 10% [1], [26], [27], [28], [29], [30], [31], [32], [34], [38], [40], [41], [42], [43], [44], [46], [47], [48], [50], [51], [53], [54], [56], [57].
3.4. Cardiovascular outcomes
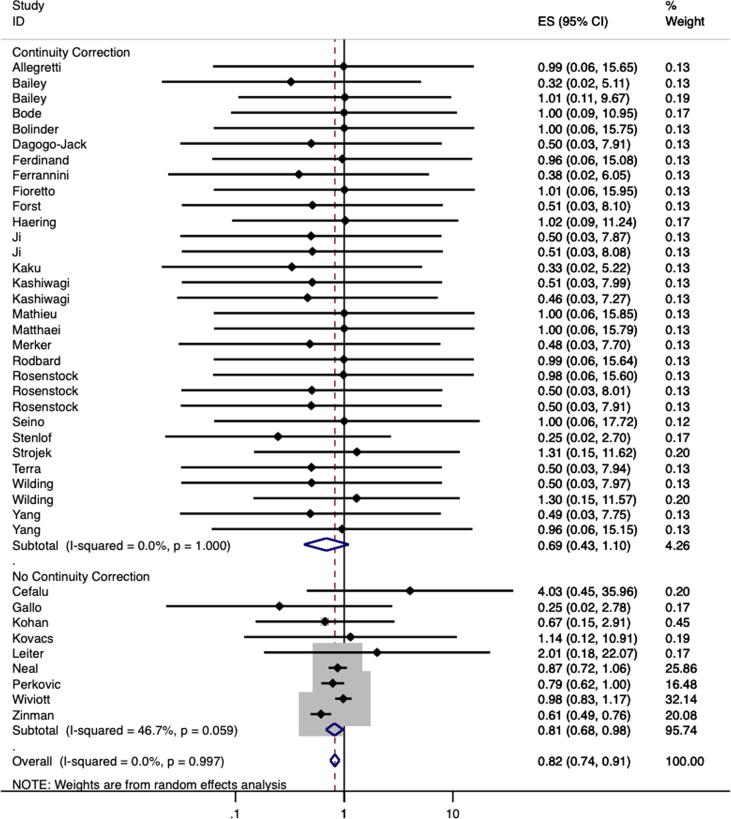
For trials that reported event rates (n = 39), a total of 551 deaths from cardiovascular causes were reported among the 25,458 participants who were treated with a SGLT2 inhibitor compared to 536 events among 18,719 controls [1], [2], [4], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59] (Table 1). Individually, only one trial reported a statistically significant reduction in cardiovascular mortality risk in those treated with a SGLT2 inhibitor compared to placebo [2]. The pooled estimate indicated that treatment with a SGLT2 inhibitor led to an 18% reduction in cardiovascular death (RR 0.82, 95%CI 0.74, 0.91, I2 = 0.0%; Fig. 2).
Fig. 2.
Meta-Analysis of Risk Ratio for Cardiovascular Mortality Stratified by Requirement of Continuity Correction to Calculate Risk Ratio Secondary to Zero Cells.
The pooled estimates from the four largest trials [1], [2], [3], [4], which were designed to assess cardiovascular events, indicated that treatment with a SGLT2 inhibitor relative to placebo led to a 19% reduction in cardiovascular mortality (RR 0.81, 95%CI 0.66, 0.98, I2 = 73.7%), 12% reduction in 3-point MACE (RR 0.88, 95%CI 0.82, 0.94, I2 = 0.0%) and 32% reduction in CHF hospitalizations (RR 0.68, 95%CI 0.60, 0.76, I2 = 0.0%).
Most trials (n = 31) reported zero cardiovascular deaths in the placebo group (n = 5) [26], [27], [36], [54], [57], intervention group (n = 2) [41], [53], or both (n = 24) [24], [25], [28], [55], [56], [58], [31], [32], [33], [34], [37], [38], [39], [40], [45], [46], [47], [48], [49], [50], [51], [52] and therefore a continuity correction was required to calculate relative risk. None of these trials were designed to primarily assess cardiovascular mortality. Within these 31 trials, the cardiovascular death event rate was 10/8039 among those randomized to SGLT2 inhibitors and 2/4260 among those randomized to placebo. The pooled point estimate suggested a 31% reduction in cardiovascular mortality for SGLT2 inhibitor use compared with placebo (RR 0.69, 95%CI 0.43, 1.10, I2 = 0.0%; Fig. 2) [24], [25], [26], [27], [28], [30], [31], [32], [33], [34], [36], [37], [38], [39], [40], [41], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59].
Only nine trials reported cardiovascular death in both the placebo and experimental groups (and therefore a continuity correction was not required) [29], [35], [1], [2], [3], [4], [42], [43], [44]. For these trials, the risk of cardiovascular mortality was reduced by 19% for individuals who received SGLT2 inhibitor treatment compared to placebo (RR 0.81, 95%CI 0.68, 0.98, I2 = 46.7%; Fig. 2).
We conducted stratified analyses based on baseline characteristics, trial characteristics, and treatment effects in order to explore the heterogeneity in cardiovascular mortality (Table A.3). Differences were non-significant when strata were compared for baseline A1C (p = 0.624), baseline systolic blood pressure (p = 0.421), diabetes duration (p = 0.208), biologic sex (p = 0.666), and follow-up duration (p = 0.377). We performed a stratified analysis by drug type when more than two estimates were available. The pooled estimates for relative risk of cardiovascular mortality with a SGLT2 inhibitor compared with placebo were statistically significant for canagliflozin (RR 0.83, 95%CI 0.71, 0.96) and empagliflozin (RR 0.62, 95%CI 0.50, 0.77) but not for dapagliflozin, ertugliflozin or ipragliflozin.
3.5. Blood pressure reduction and cardiovascular outcomes
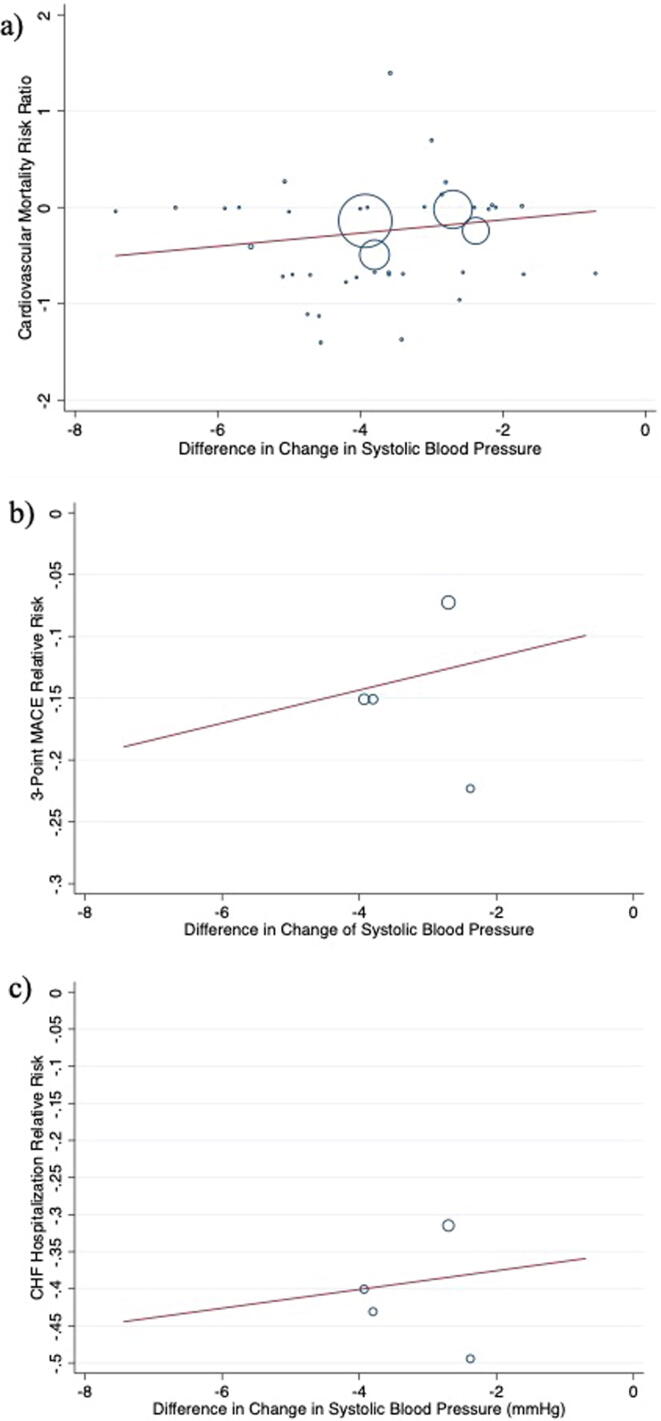
For every 1 mmHg reduction in systolic blood pressure, there was a statistically non-significant 7% relative risk reduction in cardiovascular mortality (RR 0.93, 95%CI 0.88, 1.3, p = 0.483; Fig. 3). To account for the possibility that the relationship between systolic blood pressure lowering and cardiovascular mortality was non-linear, we re-examined the magnitude of within-group systolic blood pressure reduction from baseline in three distinct strata separately according to trials where patients achieved a mean blood pressure reduction of < 2 mmHg [26], [28], [38], [41], [42], [45], [46], [49], [51] (RR 0.68; 95%CI 0.15, 2.98, I2 = 0.0%); 2–4 mmHg [1], [2], [4], [25], [27], [29], [32], [35], [36], [43], [44], [50], [54], [55], [56], [57], [58] (RR 0.83; 95%CI 0.75, 0.91, I2 = 0.0%); and > 4 mmHg [3], [24], [30], [31], [33], [34], [37], [39], [40], [47], [48], [52], [53], [59] (RR 0.63; 95%CI 0.33, 1.18, I2 = 0.0%). Differences between strata were non-significant (p = 0.543).
Fig. 3.
Meta-regression by mean change in systolic blood pressure for a) cardiovascular mortality risk ratio, b) 3-point MACE risk ratio, and c) CHF hospitalizations risk ratio.
Further, four trials [1], [2], [3], [4] reported on 3-point MACE and CHF hospitalizations. When examined there was no apparent association between blood pressure reduction and 3-point MACE (p = 0.839; Fig. 3) or CHF hospitalizations (p = 0.844; Fig. 3).
3.6. Assessment for publication bias
There was no evidence of publication bias with a visually symmetric funnel plot for the outcome of cardiovascular mortality (Figure A.1). This was formally evaluated with Egger’s linear regression test, which showed a statistically non-significant beta-coefficient of bias estimate of −0.15 (95%CI −0.40, 0.11, p = 0.245).
4. Discussion
This systematic review and meta-analysis of 40 randomized controlled trials explored the relationship between the magnitude of reduction in systolic blood pressure and cardiovascular outcomes including cardiovascular mortality, 3-point MACE, and CHF hospitalizations using meta-regression. There appeared to be linear relationships between these cardiovascular outcomes and difference in change in systolic blood pressure based on the line of best fit. Our analysis was underpowered to make definitive conclusions, so there is insufficient evidence to suggest that SGLT2 inhibitors reduce cardiovascular mortality through significant blood pressure reduction. We found that the relative risk of cardiovascular mortality is reduced by 18% (RR 0.82, 95%CI 0.74, 0.91) in patients with type 2 diabetes treated with SGLT2 inhibitors compared with placebo.
Our findings are consistent with other published meta-analyses and confirm that SGLT2 inhibitors are protective against cardiovascular events [7], [60], [61]. The observed reductions in cardiovascular events related to SGLT2 inhibitor therapy are primarily based on four large trials [1], [2], [3], [4], which were powered to assess for 3-point MACE [1], [2], [3] or a composite including death from renal or cardiovascular disease [4]. These trials were substantially larger than the other included reports, and therefore contributed heavily in weight to this meta-analysis.
The overall pooled risk estimate for cardiovascular mortality should be interpreted with caution because cardiovascular mortality was a rare occurrence, with most trials reporting zero events. Notably, a continuity correction was applied to 31 trials in order to calculate risk ratio estimates. The low event rates in the included trials may have been due to inclusion of lower-risk patients (e.g., participants with no recent history of cardiovascular events), small sample sizes, and short trial follow-up periods, which were inadequate to detect cardiovascular death.
When our analysis was limited to trials that reported cardiovascular deaths in both groups, the point estimate was consistent with the overall pooled estimate calculated when all trials were included; however, we observed high heterogeneity between trials that reported cardiovascular deaths in both groups. A possible explanation for this heterogeneity could be that individuals at high-risk including those with poor glycemic control, hypertension, and known cardiovascular disease may benefit most from treatment with SLGT2 inhibitors. Indeed, the trends from our stratified analyses examining the impact of cardiovascular risk factors suggest that individuals at high-risk due to poor glycemic control and/or hypertension experience more cardiovascular protection with SGLT2 inhibitors than those without these risk factors. To this end, a meta-analysis found that SGLT2 inhibitors may have variable effects depending on the baseline characteristics of the population with type 2 diabetes to which they are prescribed [7]. Specifically, reductions in MACE were demonstrated in patients with known cardiovascular disease, while SGLT2 inhibitors had no significant effect compared with placebo in those without known atherosclerotic disease [7]. When taken together, above evidence suggests that individuals with type 2 diabetes at high-risk may benefit most from the use of SGLT2 inhibitors. This is biologically plausible given the combination of vascular, metabolic and natriuretic effects demonstrated by SGLT2 inhibitors.
We were not able to include the DAPA-HF trial [8] in our meta-analysis as it was a trial conducted in a heart failure population and while there was a substantial diabetes sub-group, blood pressure reduction within this group was not reported. This trial found that SGLT2 inhibitors are beneficial in individuals with heart failure and a reduced ejection fraction, with or without type 2 diabetes, suggesting that the mechanism for improved cardiovascular outcomes is not primarily related to glycemic control improvements.
Blood pressure reductions with SGLT2 inhibitors have been observed in animal and clinical studies [63]. Theorized mechanisms by which SGLT2 inhibitors affect blood pressure include plasma volume reduction through osmotic diuresis and natriuresis [12], [64], arterial stiffness improvement [11] and weight loss [13]. Several complementary mechanisms have been postulated to explain how SGLT2 inhibitors improve cardiovascular outcomes including glycemic control, altered energy metabolism in the heart, blood pressure reduction, weight loss, and diuresis [9], [10]. Animal models have shown that vascular dysfunction associated with type 2 diabetes is reduced with SGLT2 inhibitor administration secondary to a reduction in oxidative stress, glucotoxicity, and inflammation [62].
With their diuretic-like effect, SGLT2 inhibitors exhibit a similar response to thiazide and loop diuretics, which reduce blood pressure through natriuresis leading to decreased plasma volume [65]. While no additive blood pressure reduction has been observed when SGLT2 inhibitors were combined with thiazide [65] or loop diuretics [66], additive blood pressure lowering effects have been observed when SGLT2 inhibitors were combined with renin-angiotensin-aldosterone system inhibitors [67], beta blockers, and calcium channel blockers [68]. The additive effects of these pharmacologic agents should be considered in the management of hypertension in the setting of type 2 diabetes.
This systematic review and meta-analysis has notable strengths. It is the largest meta-analysis to date examining the relative risk of cardiovascular disease with SGLT2 inhibitors compared with placebo, including 54,279 participants from 40 clinical trials. We were able to explore the potential relationship between the cardioprotective effects of SGLT2 inhibitors and the magnitude of blood pressure reduction.
Our study has certain limitations. First, the mortality reduction demonstrated by SGLT2 inhibitors is driven largely by a reduction in heart failure deaths. Therefore, natriuresis is likely a major contributing factor in the observed improvements in cardiovascular outcomes. The reported trial data limits our ability to test the mechanism hypothesis and further trial level data including daily body weight, fluid intake and urinary output would help to further explore if natriuresis is the mechanism, or a contributing mechanism, by which cardiovascular outcomes are improved with SGLT2 inhibitor use. Second, the meta-regression was underpowered to detect a significant association between blood pressure reduction and cardiovascular outcomes due to a diluted event rate. The results were driven by a few large cardiovascular outcome trials [1], [2], [3], [4], however it was necessary to include all trials that reported on cardiovascular mortality to obtain an adequate distribution of blood pressures and conduct a robust meta-regression. Third, a HR was used for one included trial [3] instead of a RR. While pooling HRs and RRs together is commonly performed in meta-analyses [18], [19], the approach may introduce methodological heterogeneity. Finally, a greater distribution of baseline characteristic variables (e.g., A1C, blood pressure) is needed to better understand their relationship with cardiovascular outcomes.
In conclusion, our findings confirm the beneficial effect of SGLT2 inhibitors in reducing cardiovascular events in individuals with type 2 diabetes. Our study lacked the necessary statistical power to definitively answer our research question, thus we do not know with certainty if the cardioprotective effect of SGLT2 inhibitors, though believed to be pleotropic [7], is mediated through blood pressure reduction. Further investigations are needed to explore potential mechanisms so that clinical management can be targeted to those who would receive the most benefit, while minimizing side-effects. While it is unclear if the blood pressure lowering observed with SGLT2 inhibitors contributes to the cardiovascular benefits observed, using SGLT2 inhibitors as an adjunct to recommended antihypertensive agents in adults with diabetes is sensible, particularly in the presence of early renal disease or atherosclerotic cardiovascular disease, to assist in achieving glycemic and blood pressure targets.
Declaration of Competing Interest
There is no conflict of interest.
Acknowledgments
Acknowledgements
We would like to acknowledge the medical research librarians at the University of Calgary who provided critical review of our search strategy, Drs. Zahra Premji and Diane Lorenzetti.
Author Contributions: Study conception and design was performed by JLB, JEB, AAL and DMR. JLB and JEB performed the literature search, data extraction, and data analysis. The initial manuscript draft was written by JLB and JEB. JLB, JEB, RJS, SSD, AAL, and DMR all participated in the critical revision of the manuscript for important intellectual content and approved the final version. JLB is the guarantor of this work, had full access to all the study data and takes responsibility for the integrity of the data. JLB, JEB and DMR take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflicts of Interest and Sources of Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Jamie L. Benham is supported by the Dr. Fernand Labrie Fellowship Grant from the Canadian Society of Endocrinology and Metabolism. Alexander A. Leung is supported by the Hypertension Canada New Investigator Award. Stella S. Daskalopoulou is a Chercheur-Boursier Clinicien Senior supported by the Fonds de recherche du Québec-Santé. For the remaining authors, none were declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100725.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wiviott S.D., Raz I., Bonaca M.P. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 2.Zinman B., Wanner C., Lachin J.M. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 3.Neal B., Perkovic V., Mahaffey K.W. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V., Jardine M.J., Neal B. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 5.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41(12):2669-701. doi: 10.2337/dci18-0033 [published Online First: 2018/10/07] [DOI] [PMC free article] [PubMed]
- 6.Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2018;42(Suppl 1):S1-S325. [DOI] [PubMed]
- 7.Zelniker T.A., Wiviott S.D., Raz I. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 8.McMurray J.J.V., Solomon S.D., Inzucchi S.E. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. New England Journal of Medicine. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 9.Heerspink H.J., Perkins B.A., Fitchett D.H. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 10.Verma S., Jüni P., Mazer C.D. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? The Lancet. 2019;393(10166):3–5. doi: 10.1016/s0140-6736(18)32824-1. [DOI] [PubMed] [Google Scholar]
- 11.Chilton R., Tikkanen I., Cannon C.P. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambers Heerspink H.J., de Zeeuw D., Wie L. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cefalu W.T., Stenlof K., Leiter L.A. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183–1187. doi: 10.1007/s00125-015-3547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J, Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]: The Cochrane Collaboration. Available from http://handbook.cochrane.org/. 2011.
- 16.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G., Hemmelgarn B., Alhaider S. Meta-analysis of adverse cardiovascular outcomes associated with antecedent hypertension after myocardial infarction. Am J Cardiol. 2009;104(1):141–147. doi: 10.1016/j.amjcard.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud A.N., Mentias A., Elgendy A.Y. Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman DS, GD & Egger, M. Systematic reviews in health care : meta analysis in context (2nd ed). London: BMJ 2001. [DOI] [PMC free article] [PubMed]
- 21.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson S.G., Higgins J.P. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 23.Egger M., Davey Smith G., Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allegretti A.S., Zhang W., Zhou W. Safety and Effectiveness of Bexagliflozin in Patients With Type 2 Diabetes Mellitus and Stage 3a/3b CKD. Am J Kidney Dis. 2019;74(3):328–337. doi: 10.1053/j.ajkd.2019.03.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey C.J., Iqbal N., T'Joen C. Dapagliflozin monotherapy in drug-naive patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obes Metab. 2012;14(10):951–959. doi: 10.1111/j.1463-1326.2012.01659.x. [DOI] [PubMed] [Google Scholar]
- 26.Bailey C.J., Gross J.L., Hennicken D. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11(101190723):43. doi: 10.1186/1741-7015-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bode B., Stenlof K., Harris S. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17(3):294–303. doi: 10.1111/dom.12428. [DOI] [PubMed] [Google Scholar]
- 28.Bolinder J., Ljunggren O., Johansson L. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16(2):159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 29.Cefalu W.T., Leiter L.A., de Bruin T.W. Dapagliflozin's Effects on Glycemia and Cardiovascular Risk Factors in High-Risk Patients With Type 2 Diabetes: A 24-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study With a 28-Week Extension. Diabetes Care. 2015;38(7):1218–1227. doi: 10.2337/dc14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dagogo-Jack S., Liu J., Eldor R. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: The VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metab. 2018;20(3):530–540. doi: 10.1111/dom.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferdinand K.C., Izzo J.L., Lee J. Antihyperglycemic and Blood Pressure Effects of Empagliflozin in Black Patients With Type 2 Diabetes Mellitus and Hypertension. Circulation. 2019;139(18):2098–2109. doi: 10.1161/CIRCULATIONAHA.118.036568. [DOI] [PubMed] [Google Scholar]
- 32.Ferrannini E., Ramos S.J., Salsali A. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fioretto P., Del Prato S., Buse J.B. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): The DERIVE Study. Diabetes Obes Metab. 2018;20(11):2532–2540. doi: 10.1111/dom.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forst T., Guthrie R., Goldenberg R. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16(5):467–477. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallo S., Charbonnel B., Goldman A. Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial. Diabetes Obes Metab. 2019;21(4):1027–1036. doi: 10.1111/dom.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haering H.U., Merker L., Christiansen A.V. Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;110(1):82–90. doi: 10.1016/j.diabres.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 37.Ji L., Liu Y., Miao H. Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia. Diabetes Obes Metab. 2019;21(6):1474–1482. doi: 10.1111/dom.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji L, Ma J, Li H, et al. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther 2014;36(1):84-100 e9. doi: 10.1016/j.clinthera.2013.11.002 [published Online First: 2014/01/01] [DOI] [PubMed]
- 39.Kaku K., Watada H., Iwamoto Y. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13(101147637):65. doi: 10.1186/1475-2840-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kashiwagi A., Akiyama N., Shiga T. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetology International. 2014;6(2):125–138. doi: 10.1007/s13340-014-0184-9. [DOI] [Google Scholar]
- 41.Kashiwagi A., Kazuta K., Goto K. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17(3):304–308. doi: 10.1111/dom.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohan D.E., Fioretto P., Tang W. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as Add-on Therapy to Pioglitazone With or Without Metformin in Patients With Type 2 Diabetes Mellitus. Clin Ther 2015;37(8):1773-88 e1. doi: 10.1016/j.clinthera.2015.05.511 [published Online First: 2015/07/04] [DOI] [PubMed]
- 44.Leiter L.A., Cefalu W.T., de Bruin T.W. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;62(7):1252–1262. doi: 10.1111/jgs.12881. [DOI] [PubMed] [Google Scholar]
- 45.Mathieu C., Ranetti A.E., Li D. Randomized, Double-Blind, Phase 3 Trial of Triple Therapy With Dapagliflozin Add-on to Saxagliptin Plus Metformin in Type 2 Diabetes. Diabetes Care. 2015;38(11):2009–2017. doi: 10.2337/dc15-0779. [DOI] [PubMed] [Google Scholar]
- 46.Matthaei S., Bowering K., Rohwedder K. Durability and tolerability of dapagliflozin over 52 weeks as add-on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab. 2015;17(11):1075–1084. doi: 10.1111/dom.12543. [DOI] [PubMed] [Google Scholar]
- 47.Merker L., Haring H.U., Christiansen A.V. Empagliflozin as add-on to metformin in people with Type 2 diabetes. Diabet Med. 2015;32(12):1555–1567. doi: 10.1111/dme.12814. [DOI] [PubMed] [Google Scholar]
- 48.Rodbard H.W., Seufert J., Aggarwal N. Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes Obes Metab. 2016;18(8):812–819. doi: 10.1111/dom.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenstock J., Hansen L., Zee P. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376–383. doi: 10.2337/dc14-1142. [DOI] [PubMed] [Google Scholar]
- 50.Rosenstock J., Jelaska A., Frappin G. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(7):1815–1823. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 51.Rosenstock J., Vico M., Wei L. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35(7):1473–1478. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seino Y., Sasaki T., Fukatsu A. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30(7):1245–1255. doi: 10.1185/03007995.2014.912983. [DOI] [PubMed] [Google Scholar]
- 53.Stenlof K., Cefalu W.T., Kim K.A. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15(4):372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strojek K., Yoon K.H., Hruba V. Dapagliflozin added to glimepiride in patients with type 2 diabetes mellitus sustains glycemic control and weight loss over 48 weeks: a randomized, double-blind, parallel-group, placebo-controlled trial. Diabetes Ther. 2014;5(1):267–283. doi: 10.1007/s13300-014-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terra S.G., Focht K., Davies M. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19(5):721–728. doi: 10.1111/dom.12888. [DOI] [PubMed] [Google Scholar]
- 56.Wilding J.P., Charpentier G., Hollander P. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67(12):1267–1282. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilding J.P., Woo V., Rohwedder K. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16(2):124–136. doi: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 58.Yang W., Han P., Min K.W. Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: A randomized controlled trial. J Diabetes. 2016;8(6):796–808. doi: 10.1111/1753-0407.12357. [DOI] [PubMed] [Google Scholar]
- 59.Yang W., Ma J., Li Y. Dapagliflozin as add-on therapy in Asian patients with type 2 diabetes inadequately controlled on insulin with or without oral antihyperglycemic drugs: A randomized controlled trial. J Diabetes. 2018;10(7):589–599. doi: 10.1111/1753-0407.12634. [DOI] [PubMed] [Google Scholar]
- 60.Monami M., Dicembrini I., Mannucci E. Effects of SGLT-2 inhibitors on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Acta Diabetol. 2017;54(1):19–36. doi: 10.1007/s00592-016-0892-7. [DOI] [PubMed] [Google Scholar]
- 61.Wu J.H., Foote C., Blomster J. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4(5):411–419. doi: 10.1016/S2213-8587(16)00052-8. [DOI] [PubMed] [Google Scholar]
- 62.Oelze M., Kroller-Schon S., Welschof P. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michel M.C., Mayoux E., Vallon V. A comprehensive review of the pharmacodynamics of the SGLT2 inhibitor empagliflozin in animals and humans. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(8):801–816. doi: 10.1007/s00210-015-1134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawasoe S., Maruguchi Y., Kajiya S. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol. 2017;18(1):23. doi: 10.1186/s40360-017-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devineni D., Vaccaro N., Polidori D. Effects of hydrochlorothiazide on the pharmacokinetics, pharmacodynamics, and tolerability of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Clin Ther. 2014;36(5):698–710. doi: 10.1016/j.clinthera.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 66.Mordi N.A., Mordi I.R., Singh J.S. Renal and Cardiovascular Effects of SGLT2 Inhibition in Combination With Loop Diuretics in Patients With Type 2 Diabetes and Chronic Heart Failure: The RECEDE-CHF Trial. Circulation. 2020;142(18):1713–1724. doi: 10.1161/CIRCULATIONAHA.120.048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weber M.A., Mansfield T.A., Alessi F. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin-angiotensin system blockade. Blood Press. 2016;25(2):93–103. doi: 10.3109/08037051.2015.1116258. [DOI] [PubMed] [Google Scholar]
- 68.Weber M.A., Mansfield T.A., Cain V.A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4(3):211–220. doi: 10.1016/S2213-8587(15)00417-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.