Abstract
Polysaccharides have long been recognized as the anticancer agent with low toxicity and slight side effects. Okra (Abelmoschus esculentus L.), a flowering plant from the Malvaceae family that is found in tropical, subtropical, and warm temperate regions around the world. Hence, no in vivo studies have addressed the anticancer and immunomodulatory potential of polysaccharides from okra pods grown in Indonesia. This study aims to investigate the effect of okra raw polysaccharide extract (ORPE) to immune cells and cytokines of mice with hepatocarcinogenic conditions induced by diethylnitrosamine (DEN). Thirty-six male mice (BALB/C, 3–4 months old) were divided into six groups: the normal control group (CN), negative control (C-), positive control giving doxorubicin (C+), and three groups of ORPE treatment given the dose of 50 (P1), 100 (P2) and 200 (P3) mg/kg body weight. The administration of ORPE directly suppressed the regulatory T cells accumulations, suppressed macrophages activations, and balanced the number of effector T cells. However, it promoted CD8+ T cell activation at a low dose and increased interleukin-2 levels at all doses. These results suggest that ORPE has unique dual-functions as immunosuppressant and immunostimulant which can be a foundation for the application of the ORPE in the nutraceutical and pharmaceutical industries.
Keywords: Okra, Raw polysaccharide extract, Diethylnitrosamine, Immunomodulator, Liver injury
Okra, Raw polysaccharide extract, Diethylnitrosamine, Immunomodulator, Liver injury.
1. Introduction
Liver cancer is the fifth most common cause of cancer mortality in the world. Hepatocellular carcinoma (HCC) accounts for about 80%–90% of all liver cancers (Yang et al., 2019a, Yang et al., 2019b). The Indonesian Heart Research Association states that 80% of HCC cases in the world are in developing countries such as Central Africa, East Asia, and Southeast Asia, one of which is in Indonesia (Hayaza et al., 2019b). Hepatitis infection, food additives, alcohol consumption, toxic exposure, environmental and industrial toxic chemicals are several risk factors that can cause HCC.
Diethylnitrosamine (DEN) is a well-known potent hepatocarcinogen agent present in tobacco smoke, water, cured and fried meals, cheddar cheese, agricultural chemicals, cosmetics, and pharmaceutical products (Al-Rejaie et al., 2009). Cytotoxicity induced by DEN is critical for malignant transformation. The administration of the smallest quantities of DEN results in severe liver damage. It can also lead to hepatocellular accumulation of reactive oxygen species (ROS), oxidative damage to DNA, and create a hepatocarcinogenesis condition (Tolba et al., 2015).
Heretofore, the most common way to treat cancer is by chemotherapy. It targets tumors, but it is often accompanied by side effects including damaging healthy organs (Purnamasari et al., 2019). Immunomodulators, also known as biological response modifiers (BRMs), are a variety of materials, both recombinant, synthetic, and natural, that are capable of altering the immune response. Immunomodulators can be endogenous and exogenous and can enhance the immune response or suppress it. The function of this suppression is to prevent the body's response from becoming excessive and harming other cells outside the target cells. Giving natural ingredients as immunomodulators is a mild but effective therapeutic approach because the side effects are often less severe than the side effects of synthetic drugs and rarely cause resistance to the body. In the past few years, the researcher has shifted the traditional chemotherapy to natural products, such as plants. Plant polysaccharides, a biological macromolecule commonly found in traditional medicinal herbs, have attracted growing scientific interest for their ability on exerting marked effects on immune system function, inflammation and cancers. Immunomodulatory activity performed by plants' active compounds can be used as preventive strategies, boosting or suppressing the host defense response. A recent study has proved that Polygonatum sibiricum polysaccharide can enhance T cell and B cell proliferation responses and restore the levels of interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), and interleukin-10 (IL-10) in the serum of the Cyclophosphamide (CTX)-treated mice (Liu et al., 2018). Another study also demonstrated that Ligustrum vicaryi L. fruit polysaccharide significantly increased spleen and thymus indexes, enhanced the phagocytic function of neutrophils, activated B and T lymphocytes, and upregulated serum levels of IL-10 and TNF-α (Liu et al., 2020). Plants polysaccharides have also been recognized as inexpensive and less toxic.
Okra (Abelmoschus esculentus L.), also known as slime nuts in Indonesia, is a plant that is rich in polysaccharides, vitamin C, and secondary metabolite compounds. The flavonoid compounds in the form of oligomers catechins, quercetin, and vitamin C play role as an antioxidant (Husen et al., 2019; Hayaza et al., 2019a). Its polysaccharides contain rhamnose and galactose (Chen et al., 2016). Besides being consumed as food, okra is also used as a traditional medicine to treat dysentery and diarrhea (Hayaza et al., 2019b).
In our previous studies, administration of okra raw polysaccharides at a dose of 600 μg/mL was proven to be able to cause 9.13% of Huh7it cells to undergo apoptosis with most of the cells were arrested on G0/G1 phase and it significantly activated 87.55% of natural killer cells (Hayaza et al., 2019b). Another study also found that okra polysaccharides could enhance B-lymphocytes proliferation and spleen weight through the activation of the NFкB transcription factor in mice with bacterial infection (Wahyuningsih et al., 2018). However, the immunomodulatory functions of okra polysaccharide are still not fully understood especially on hepatocarcinogenesis conditions. This study is the first report on the immunomodulatory effect of okra raw polysaccharide extracts (ORPE) on the population and the functional activity of immune cells in liver injury induced by DEN. All okra pods used in this study were grown locally in Surabaya, Indonesia.
2. Materials and methods
2.1. Preparation of polysaccharides raw extract
The okra fruits were obtained from traditional markets in Surabaya, Indonesia. Fresh okra pods (500 g) were cleaned with water, cut into pieces, smoothen, and macerated with 500 ml of distilled water overnight three times. Supernatants were collected and centrifuged at 4300 rpm for 5 min. The supernatant was precipitated in absolute ethanol 1X sample volume, incubated for 24 h at 4 °C, and centrifuged. The pellet was dissolved in distilled water and centrifuged. The supernatant was collected and lyophilized (Chen et al., 2016; Hayaza et al., 2019b). This powder of crude polysaccharide from okra pods is called Okra Raw Polysaccharide Extract (ORPE).
2.2. Analysis of okra polysaccharide content using the phenol sulfuric acid method
Sample solution of crude okra polysaccharides was made from the stock of crude okra polysaccharides (10 μL) and aquadest (90 μL). Then, 50 μL of phenol 5% was added. After being homogenized for 1 min, 2 mL of sulphuric acid was added to the solution and incubated for 10 min at room temperature. The blank solution was made from 50 μL of phenol 5% and 100 μL of aquadest. The absorbance was measured at 490 nm (Wahyuningsih et al., 2018).
2.3. Ethics statement
Animals were treated humanely and maintained in a pathogen-free chamber for acclimation. All procedures involving animal care were approved by the Animal Care and Use Committee (ACUC) of Veterinary Faculty, Universitas Airlangga, Surabaya, Indonesia (No.701-KE).
2.4. Experimental design
The used samples were adult male mice, strain BALB/C, 3–4 months old, weight ranged from 30 to 40 g. The study samples consisted of 36 male mice. Diethylnitrosamine (DEN) was injected to cause severe liver damage and promote tumor growth (Tolba et al., 2015). After 14 days of acclimatization, mice were randomly divided into six groups: the normal control group without any treatment (CN), negative control induced by DEN with no ORPE administration (C-), positive control induced by DEN with 10 mg/kg BW doxorubicin administration (C+), and three groups of ORPE treatment induced by DEN and given the dose of 50 mg/kg BW (P1), 100 mg/kg BW (P2) and 200 mg/kg BW (P3). The injection of 100 mg/kg BW DEN was performed intraperitoneally for three days in the first week. Okra raw polysaccharides extracts were administrated by gavage for four weeks. After four weeks, blood samples were collected from the heart, and then all mice were sacrificed. Spleen was collected and placed in cold PBS for splenocytes isolation.
2.5. Toxicity test of okra raw polysaccharide extract
The Lethal Dose 50 (LD50) value was used to measure the toxicity of ORPE. The LD50 value is a measure that states a single dose of a compound that is estimated to be deadly or cause a toxic effect in 50% of experimental animals after treatment. The doses of ORPE given were 50, 100, 200, 400, 800, and 1600 mg/kg BW. The extract was given orally using a 0.3 mL cannula needle. Mice were observed every 6 h to 24 h. The number of mice that died was recorded and the LD50 value was determined. The LD50 > 15 g/kg BW is considered nontoxic.
2.6. Splenocytes isolation from spleen
The excised spleen was washed with sterile PBS 2x and put on a petri dish containing sterile PBS. The spleen organ was crushed in PBS by using a syringe holder. The single-cell solution was filtered with a sterile wire and put into a microtube. Homogenates were centrifuged at 2500 rpm, 10 °C, for 5 min. The supernatant was discarded and the obtained pellet was resuspended in 1 mL of PBS. The suspension then proceeded for flow cytometry analysis.
2.7. Serum isolation and cytokine assay
Whole blood was collected and centrifuged at 3000 rpm and 4 °C for 10 min, while the upper layer contained the serum. Blood serum collected from mice was centrifuged at 2000–3000 rpm for 20 min. Cytokine analysis of IL-2 and IL-12 was performed using an ELISA kit (Bioassay Technology, Shanghai, China) according to the manufacturer's protocol. Briefly, 40μl blood serum was placed on wells. Then, 10 μl antibody (anti-IL-2; anti-IL-12) and 50 μl streptavidin-HRP were added. After 60 min of incubation at 37 °C, the plate was washed 5 times with wash buffer. Around 50 μl substrate solutions A and B were added to each well. The plate was then incubated for 10 min at 37 °C in the dark. Within 10 min after adding a 50 μl stop solution to each well, the optical density was measured using a microplate reader (λ = 450 nm).
2.8. Alpha-fetoprotein tumor marker test
Alpha-Fetoprotein is a major mammalian embryo-specific and tumor-associated protein that is also present in small quantities under normal conditions (Tolba et al., 2015). Therefore, the measurement of the serum AFP level was used as a marker of an initial liver tumor. The analysis was performed using an ELISA kit (Bioassay Technology, Shanghai, China) according to the manufacturer's protocol. After adding 40μl blood serum to wells, around 10 μl of anti-AFP-antibody and 50 μl streptavidin-HRP was added. Plates were incubated for 60 min at 37 °C and washed 5 times with wash buffer. Then, 50 μl substrate solution A and B were added to each well and incubated for 10 min at 37 °C in the dark. Lastly, a 50 μl stop solution was added to each well. The optical density was measured using a microplate reader (λ = 450 nm).
2.9. Flowcytometry analysis of immune cells
As much as 2 × 106 cells were taken as and transferred into microtube then 500 μL PBS was added. The microtube was centrifuged at 2500 rpm, 10 °C for 5 min. The pellet was then stained with 100 μL extracellular antibody at a final concentration of 0.005 mg/100 μL, then incubated at 4 °C for 30 min.14-15 The suspension was added by 200 μL of a fixative solution (cytofix) and incubated in an icebox of 4 °C for 20 min. The fixative solution was washed with 500 μL washperm solution and centrifugated at 2500 rpm, 10 °C for 5 min. The supernatant was aspirated, the pellet was added by 100 μL intracellular antibody at a final concentration of 0.005 mg/100 μL and then incubated at 4 °C for 20 min. The cells were then resuspended and added with 400 μL PBS then transferred to a cuvette and analyzed using BD FACSCalibur Flow Cytometer (BD Bioscience, CA, USA) with BD CellQuest™ Pro software. The combination of antibodies and fluorochromes that used in this study were the FITC-conjugated rat anti-mouse CD11b, PE-conjugated rat anti-mouse CD8, FITC-conjugated rat anti-mouse CD8, PE-conjugated rat anti-mouse B220, FITC-conjugated rat anti-mouse CD4, FITC-conjugated rat anti-mouse CD4, PE-conjugated rat anti-mouse CD25, PE-conjugated rat anti-mouse FoxP3 (BioLegend, San Diego, USA).
2.10. Statistical analysis
All data obtained were analyzed by statistical tests using the GraphPad Prism version 8 (GraphPad Software, La Jolla, CA, USA) The differences between the experimental and control groups were compared by One-Way ANOVA test followed by Dunnett test. The results were presented as mean ± standard deviation (SD) of five independent experiments. P-values less than 0.05 (p < 0.05) considered a statistically significant difference.
3. Result
3.1. Polysaccharide content in okra pods
The regression equation of the polysaccharide was y = 0,008x + 0,079 (Wahyuningsih et al., 2018; Hayaza et al., 2019a, Hayaza et al., 2019b). Using the polysaccharide standard regression equation, the polysaccharides content in the stock solution was 35.62 mg/mL for dose of 50 mg/kg BW, 71.25 mg/mL for dose of 100 mg/kg BW, and 142.50 mg/mL for dose of 200 mg/kg BW.
3.2. The toxicity level of ORPE
Based on the toxicity test performed, the group given ORPE of dose between 50-1600 mg/kg BW did not cause any mice mortality. The results of the toxicity test are influenced by several factors such as species, strain, individual diversity, sex, age, animal health, and housing conditions. These factors are made uniform so that the resulting response is only influenced by treatment. Experimental animals that do not die when given several substances in high doses is considered that all dangerous acute toxicities can be eliminated (Lu and Kacew, 2002). Therefore, it proved that ORPE is not toxic. However, during the test, each experimental animal reacted differently at a certain dose. The different reactions are caused by differences in the sensitivity level of each animal.
3.3. The effect of ORPE on alpha-fetoprotein and cytokine level of liver cancer mice
Normal AFP level for 3–4 months old male BALB/C mice is 0.42–1.33 μg/ml (Olson et al., 1977). The highest AFP level is shown by C-. All groups, except CN, have AFP levels ranging from 4-5 μg/ml. This is an indicator that the injection of 100 mg/kg BB DEN intraperitoneally had significantly cause liver damages and initiate a cancerous effect. P2 and P3 show significant differences compared to normal control groups in IL-2 levels (Table 1). The highest IL-2 level is shown by P3 which has the highest dose of ORPE administration. On the other hand, IL-12 levels of all groups do not show significant differences compared to the normal and negative control group (Table 1).
Table 1.
ORPE changes the level of AFP, Interleukin-2, and interleukin-12 on DEN-induced mice blood serum.
| Groups | AFP (μg/ml) | IL-2 (μg/ml) | IL-12 (μg/ml) |
|---|---|---|---|
| CN | 1.145 ± 0.5620### | 1.625 ± 0.3095 | 0.330 ± 0.01261 |
| C- | 5.169 ± 0.3848∗∗∗ | 2.058 ± 0.5968 | 0.391 ± 0.0687 |
| C+ | 4.578 ± 0.1293∗∗ | 2.223 ± 0.1984 | 0.339 ± 0.0330 |
| P1 | 3.986 ± 0.7143# | 2.242 ± 0.1643 | 0.369 ± 0.0397 |
| P2 | 4.026 ± 0.3268# | 2.665 ± 0.5817∗ | 0.388 ± 0.0546 |
| P3 | 4.160 ± 0.7468 | 3.064 ± 0.4319∗∗∗# | 0.364 ± 0.0110 |
Values are mean ± standard deviation of five independent experiments. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 vs. control. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. negative control.
3.4. The effect of ORPE on CD8+ and macrophage
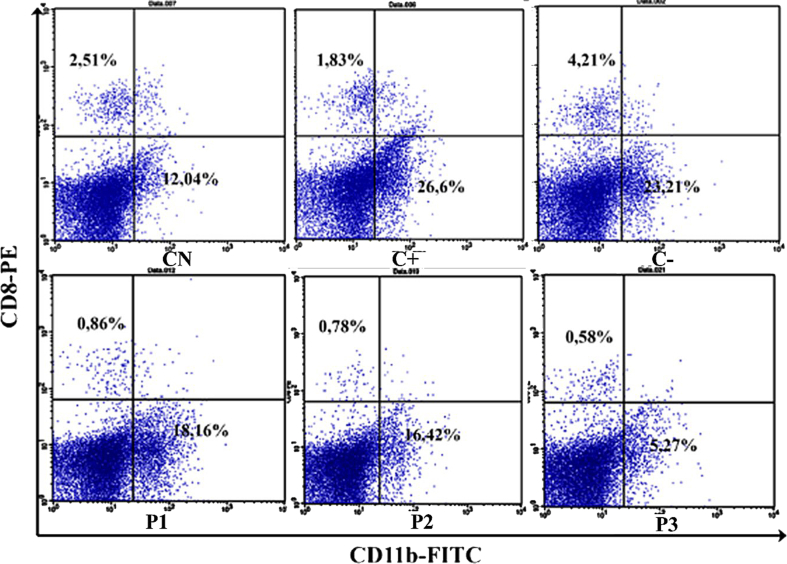
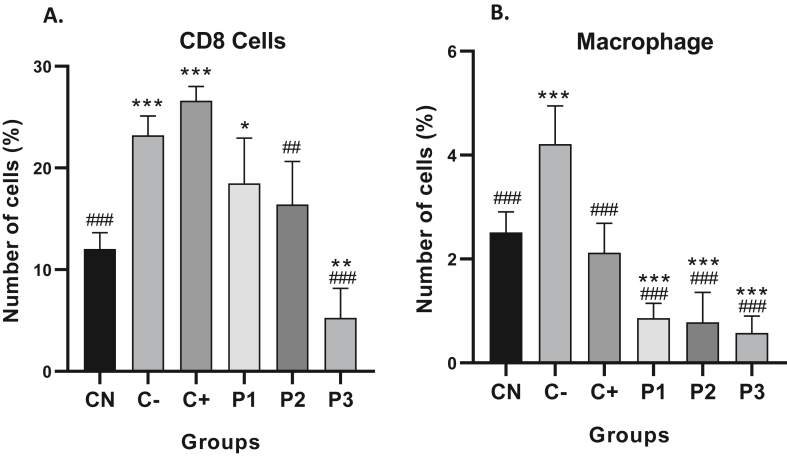
Splenocyte was isolated and stained using CD8+ and CD11b + extracellular antibodies to understand the integration of innate immune cells represented by macrophage cell expression and adaptive immune cells by CD8+ cytotoxic T cells. The normal control group has 12.04% of CD8 cells while the percentage of CD11b + cells (macrophages) is only 2.51% (Figure 1). The administration of 50 mg/kg BW of ORPE shows a significant increase in CD8+ T cells compared to untreated control (CN). However, the highest dose of ORPE has the lowest percentage of CD8+ T cells of 5.27% (Figure 2). All groups of ORPE significantly lowered the percentage of macrophages compared to CN and C- groups (Figure 2). The highest dose of ORPE also caused the lowest macrophage percentage of 0.57%.
Figure 1.
Flow cytometry analysis on CD8 cells and CD11b cells using PE and FITC staining reagent. The upper left quadrant represents the number of CD8 cells while the lower right represents the number of CD11b cells or macrophages.
Figure 2.
ORPE affected the percentage of CD8 cells and macrophages in spleens. (A) The percentage of CD8 cells in the spleen was detected by flow cytometric analysis. (B) The percentage of macrophages in the spleen was detected by flow cytometric analysis. Values are mean ± SD of five independent experiments. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 vs. control. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. negative control.
3.5. The effect of ORPE on CD4 T-cells, T-effs, and T-reg cells
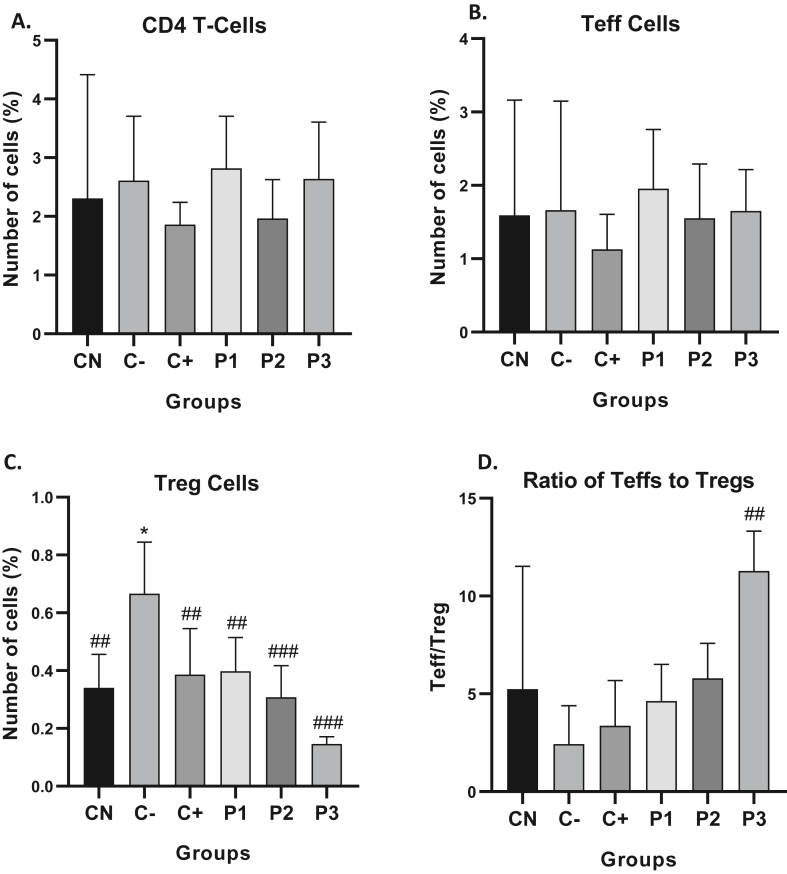
To explore the mechanism of ORPE inhibiting tumor growth in the hepatoma-bearing mice, we investigated the impact of ORPE on the balance of Treg (distinguished as CD4 + CD25+) and Teff (distinguished as CD4 + CD25-) in the spleen. After injection of ORPE for 4 weeks, the ratio of Tregs was dose-dependently decreased but that of Teffs was increased, which increased the ratio of Teffs to Tregs (Figure 2A and B). FoxP3 is a core protein that plays a role as a major regulator of the development and the suppressive function of Tregs in maintaining self-tolerance (Li et al., 2015). FoxP3 is used as a specific marker that differentiates T regulators from conventional T cells. Flow cytometry results showed that the highest Treg accumulations were shown in the C- group, namely 66.57%. Whereas in the CN, C++, P1 and P2 groups, the percentage of Treg is almost the same, namely, 34.01%; 38.46%; 39.59%; and 30.70%. The P3 group has the lowest Treg percentage of 14.31% and the highest ratio of Teff/Treg cells (Figure 3C and Figure 3D). Statistically, all ORPE treatment groups have significantly decreased the percentage of Treg cells compared to the negative control (Figure 3C). On the other hand, the percentage of CD4+ and Teff cells are relatively the same in all groups (Figure 3A and B).
Figure 3.
ORPE shifts the regulatory T cell (Treg) to effector T cell (Teff) balance. (A) The percentage of CD4+ T cells in the spleen was detected by flow cytometric analysis. (B) The population of effector T cells in the spleen of DEN-induced mice was measured with flow cytometric analysis. (C) The population of regulatory T cells in the peripheral blood of hepatoma-bearing mice was measured with flow cytometric analysis in the spleen of DEN-induced mice was measured by flow cytometric analysis. (D) The ratio of Teff to Treg was calculated. Values are mean ± SD of five independent experiments. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 vs. control. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. negative control.
4. Discussion
Developing new immunomodulatory agents is one of the most effective methods for the prevention and treatment of immunosuppressive diseases. Immunomodulatory agents combined with chemotherapy drugs appear to be useful in cancer therapy (Schlom et al., 2007; Wu et al., 2019). Several reports have indicated positive correlations between the immunomodulatory actions and polysaccharides (Li et al., 2016; Purwaningsari et al., 2019; Hayaza et al., 2019b). Polysaccharides are a kind of natural polymer linked by aldose or ketose through glycosidic bonds (Maji, 2019). In this study, a crude polysaccharide was extracted from the okra pods and its immunomodulatory activities were assessed.
Mice were injected intraperitoneally with diethylnitrosamine (DEN) 100 mg/kg BB for three consecutive days. The application of DEN in rodents and mice has become an experimental model for studies aimed to create a carcinogenesis condition in the liver (Tolba et al., 2015). Diethylnitrosamine induces hepatocarcinogenesis, increasing mitotic hepatocytes and leading to chronic inflammation (Arboatti et al., 2018). The injury-associated alterations are correlated to increased activities of serum aminotransferase, and sometimes to elevated levels of tumor-associated biomarkers such as serum a-fetoprotein (AFP). The elevation of AFP level indicates certain tumors including HCC (Jalanko et al., 1978). After four weeks, injection of DEN was able to increase the AFP levels to 5.169 ± 0.3848 μg/ml while the normal AFP level for 3–4 months old male BALB/C mice is 0.42–1.33 μg/ml (Olson et al., 1977).
At the level of immune cells, it was observed that the ORPE caused CD8+ T cells activation at doses 50 and 100 mg/kg BW. However, all doses of ORPE did not significantly stimulate CD4+ T cells and macrophages activities. They even lowered the number of macrophages compared to normal and negative control which described the immunosuppressant activity. These data indicate that the administration of ORPE acted as both immunostimulant and immunosuppressant on adaptive immunity and innate immunity in mice with hepatocarcinogenesis conditions. T lymphocytes are one of the most important immune cells in the body. T cells can be divided into different subsets based on different surface antigens and antibodies marked on the T lymphocytes. CD4+ T cell represents a helper T cell that helps lymphocytes differentiate and produce antibodies. CD8+ T cell represents the cytotoxic T cell or CTL (Ostroumov et al., 2018). Therefore, the ability of ORPE to increase CTL activation will help kill malignant cells and caused higher cancer cell apoptosis. Our result is supported by the findings of Yang et al., 2019a, Yang et al., 2019b that the administration of Weikangfu polysaccharide at a low and high dose significantly increased CTL activities and lymphocyte count.
In previous research, it has been proven that giving okra polysaccharide extract can activate the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells or what we know as NF-κB (Wahyuningsih et al., 2018). The activation of transcription factor NF-κB has an impact on stimulating the production of cytokines in the body. In this study, immune-related cytokines in the serum were also examined, and the results indicated that the ORPE increased IL-2 levels significantly compared to the control group. According to Smith et al. (2003), immunostimulants are substances that can increase immune system activity and resistance to pathogens. The ELISA test results in this study proved that giving ORPE at doses of 100 and 200 mg/kg BW was able to significantly increase IL-2 production up to three times higher than the normal control group. Meanwhile, the 200 mg/kg dose group was also able to significantly increase IL-2 production compared to the negative control group. This condition explains the role of ORPE as an effective immunostimulant agent against IL-2 production. This is in line with research conducted by Xia et al. (2012) that used the polysaccharide extract of Dendrobium officinale in an in vitro study. Xia et al. (2012) revealed that administration of the polysaccharide Dendrobium officinale at a dose of 50 μg/mL was able to increase the release of cytokines IL-2 by 5.4 times compared to controls.
Cytokines are small-molecule polypeptides secreted by activated immune cells. Cytokines can regulate immune response, participate in the development of immune cell differentiation, mediate inflammatory reaction, and stimulate hematopoietic function (Li et al., 2016). Interleukin-2, a major host defense, regulates communication between APCs, lymphocytes, and other host cells during an immune response. It induces interferon production and involves inflammatory processes or autoimmune reactions. Studies have demonstrated that plant polysaccharides can affect T lymphocyte activations and cytokine production. A study by Ayeka et al. (2017) demonstrated that polysaccharides extracted from Glycyrrhiza uralensis Fisch. could activate the CD4+ and CD8+ immune cell populations and increase the production of various cytokines, such as IL-2, IL-6, and IL-7. Lycium ruthenicum Murr. the polysaccharide was also able to restore the levels of IL-2 and IL-6 in the serum of cyclophosphamide (CTX)-treated mice (Gong et al., 2015).
On the other hand, the increase in IL-2 production in this study suppressed the activity of CD8 Tc cells and Treg cells, which means that ORPE gave an immunosuppressant effect on CD8 Tc cells and Treg cells. The function of Treg cells in the body is to provide negative feedback where the activated Treg cells will synthesize the anti-inflammatory cytokine IL-10 which will suppress the activation of the pro-inflammatory cytokine IL-2. This is in line with research conducted by Li et al. (2012) where polysaccharide extract from Astragalus membranaceus was shown to restore the balance of cytokines and reduce FOXp3 (Treg) expression. According to Whiteside (2015), in cancer patients, the high accumulation of Treg cells results in a low prognosis. Therefore, the effect of ORPE administration in increasing IL-2 production and suppressing the number of activated Treg cells is a beneficial role and is expected to improve healing in cancer patients. Our study provided a direct demonstration that ORPE treatment may interfere with Treg accumulation in vivo. However, the percentage of Teff was relatively the same in all groups and had no significant difference with control.
5. Conclusion
Taken together, the administration of okra raw polysaccharide extract effectively modulated the immune system by suppressing the T-regulator level and stimulating the production of interleukin-2, in mice with hepatocarcinogenic conditions. Therefore, we suggested that ORPE is a promising nutraceutical for a patient with a liver carcinogenic condition for daily consumption to help modulate the immune system.
Declarations
Author contribution statement
Suhailah Hayaza: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sri Puji Astuti Wahyuningsih: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Raden Joko Kuncoroningrat Susilo: Performed the experiments; Analyzed and interpreted the data.
Saikhu Akhmad Husen; Dwi Winarni; Ruey-an Doong: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Win Darmanto: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the DRPM research grant of Universitas Airlangga and the Ministry of Research and Technology of the Republic of Indonesia [893/UN3/2019].
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Dr. Junairiah from the department of biology for providing taxonomic identification of okra.
References
- Al-Rejaie S.S., Aleisa A.M., Al-Yahya A.A., Bakheet S.A., Alsheikh A., Fatani A.G., Al-Shabanah O.A., Sayed-Ahmed M.M. Progression of diethylnitrosamine-induced hepatic carcinogenesis in carnitine-depleted rats. World J. Gastroenterol. 2009;15(11):1373–1380. doi: 10.3748/wjg.15.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboatti A.S., Lambertucci F., Sedlmeier M.G., Pisani G., Monti J., Álvarez M.L., Francés D., Ronco M.T., Carnovale C.E. Diethylnitrosamine increases proliferation in early stages of hepatic carcinogenesis in insulin-treated type 1 diabetic mice. BioMed Res. Int. 2018;9472939 doi: 10.1155/2018/9472939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayeka P.A., Bian Y., Githaiga P.M., Zhao Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Compl. Alternative Med. 2017;17(1):536. doi: 10.1186/s12906-017-2030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Jiao H., Cheng Y., Xu K., Jia X., Shi Q., Guo S., Wang M., Du L., Wang F. In vitro and in vivo immunomodulatory activity of okra (Abelmoschus esculentus L.) polysaccharides. J. Med. Food. 2016;19(3):253–265. doi: 10.1089/jmf.2015.3513. [DOI] [PubMed] [Google Scholar]
- Gong Y., Wu J., Li S.T. Immuno-enhancement effects of Lycium ruthenicum Murr. polysaccharide on cyclophosphamide-induced immunosuppression in mice. Int. J. Clin. Exp. Med. 2015;8(11):20631–20637. [PMC free article] [PubMed] [Google Scholar]
- Hayaza S., Istiqomah S., Susilo R.J.K., Inayatillah B., Ansori A.N.M., Winarni D., Husen S.A., Darmanto W. Antidiabetic activity of ketapang (Terminalia catappa L.) leaves extract in streptozotocin-induced diabetic mice. Indian Vet. J. 2019;96(12):11–13. [Google Scholar]
- Hayaza S., Wahyuningsih S.P.A., Susilo R.J.K., Permanasari A.A., Husen S.A., Winarni D., Punnapayak H., Darmanto W. Anticancer activity of okra raw polysaccharides extracts against human liver cancer cells. Trop. J. Pharmaceut. Res. 2019;18(8):1667–1672. [Google Scholar]
- Husen S.A., Ansori A.N.M., Hayaza S., Susilo R.J.K., Zuraidah A.A., Winarni D., Punnapayak H., Darmanto W. Therapeutic effect of okra (Abelmoschus esculentus Moench) pods extract on streptozotocin-induced type-2 diabetic mice. Res. J. Pharm. Technol. 2019;12(8):3703–3708. [Google Scholar]
- Jalanko H., Virtanen I., Engvall E., Ruoslahti E. Early increase of serum alpha-fetoprotein in spontaneous hepatocarcinogenesis in mice. Int. J. Cancer. 1978;21(4):453–459. doi: 10.1002/ijc.2910210409. [DOI] [PubMed] [Google Scholar]
- Liu N., Dong Z., Zhu X., Xu H., Zhao Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018;107(Pt A):796–802. doi: 10.1016/j.ijbiomac.2017.09.051. [DOI] [PubMed] [Google Scholar]
- Liu S., Wang L., Ren Q., Wang J., Li Y., Wang G., Gao H., Du R., Qin W. Immunomodulatory and antioxidant activities of a polysaccharide from Ligustrum vicaryi L. fruit. Evid. Based Complementary Altern. Med. 2020;5431350 doi: 10.1155/2020/5431350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Li D., Tsun A., Li B. FOXP3+ regulatory T cells and their functional regulation. Cell. Mol. Immunol. 2015;12(5):558–565. doi: 10.1038/cmi.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Gu L., Zhong Y., Chen Y., Zhang L., Zhang A.R., Sobol R.W., Chen T., Li J. Administration of polysaccharide from Panax notoginseng prolonged the survival of H22 tumor-bearing mice. OncoTargets Ther. 2016;9:3433–3441. doi: 10.2147/OTT.S79427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Bao J.M., Li X.L., Zhang T., Shen X.H. Inhibiting effect of Astragalus polysaccharides on the functions of CD4+CD25 highTreg cells in the tumor microenvironment of human hepatocellular carcinoma. Chin. Med. J. 2012;125(5):786–793. [PubMed] [Google Scholar]
- Lu Frank C, Kacew Sam. Lu’s Basic Toxicology: Fundamentals, Target Organs, and Risk Assessment. 4th. Taylor & Francis; 2002. 2002. Conventional toxicity studies; pp. 75–81.http://www.ssu.ac.ir/cms/fileadmin/user_upload/Moavenatha/MBehdashti/TebKar/PDFs/Lu%E2%80%99s_Basic_Toxicology.pdf In press. [Google Scholar]
- Maji B. Introduction to natural polysaccharides. Functional Polysaccharides for Biomedical Applications. 2019;1:1–31. [Google Scholar]
- Olsson M., Lindahl G., Ruoslahti E. Genetic control of alpha-fetoprotein synthesis in the mouse. J. Exp. Med. 1977;145(4):819–827. doi: 10.1084/jem.145.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov D., Fekete-Drimusz N., Saborowski M., Kühnel F., Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 2018;75(4):689–713. doi: 10.1007/s00018-017-2686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnamasari R., Winarni D., Permanasari A.A., Agustina E., Hayaza S., Darmanto W. Anticancer activity of methanol extract of Ficus carica leaves and fruits against proliferation, apoptosis, and necrosis in Huh7it cells. Cancer Inf. 2019;18:1–7. doi: 10.1177/1176935119842576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwaningsari D., Nugraha J., Wahyuningsih S.P.A., Hayaza S., Susilo R.J.K., Darmanto W. Effect of polysaccharide krestin on MMP3 expression and foot diameter in rheumatoid arthritis in rat. Indian Vet. J. 2019;97(1):24–26. [Google Scholar]
- Schlom J., Arlen P.M., Gulley J.L. Cancer vaccines: moving beyond current paradigms. Clin. Cancer Res. 2007;13(13):3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V.J., Brown J.H., Hauton C. Immunostimulation in crustaceans: does it really protect against infection? Fish Shellfish Immunol. 2003;15(1):71–90. doi: 10.1016/s1050-4648(02)00140-7. [DOI] [PubMed] [Google Scholar]
- Tolba R., Kraus T., Liedtke C., Schwarz M., Weiskirchen R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim. 2015;49(S1):59–69. doi: 10.1177/0023677215570086. [DOI] [PubMed] [Google Scholar]
- Wahyuningsih S.P.A., Pramudya M., Putri I.P., Winarni D., Savira N.I.I., Darmanto W. Crude polysaccharides from okra pods (Abelmoschus esculentus) grown in Indonesia enhance the immune response due to bacterial infection. Adv. Pharmacol. Sci. 2018 doi: 10.1155/2018/8505383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T.L. The role of regulatory T cells in cancer immunology. ImmunoTargets Ther. 2015;4:159–171. doi: 10.2147/ITT.S55415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Su Z., Fan S. Effects of macrofungal polysaccharides combined with vemurafenib on melanoma and its associated mechanism. Int. J. Polym. Sci. 2019 [Google Scholar]
- Xia L., Liu X., Guo H., Zhang H., Zhu J., Rena F. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro. J. Funct. Foods. 2012;4:294–301. [Google Scholar]
- Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Fadhil R., Yang B., Good D., Liu W., Ni G., Kaur J., Song X., Liu Mosaiab T., Jessop-Neggo C., Hu C., Wei M.Q. The effects of traditional Chinese medicine on immunomodulation for cancer therapy. Adv. Complement. Alt. Med. 2019;4(2) ACAM.000581. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.