Abstract
Medicinal plants are essential aspects of readily available primary healthcare remedies. Phytochemical constituents of medicinal plants cover a broad variety of chemical fields to explore medicines. This review highlights selected empirical data on traditional uses, phytochemistry, and pharmacological properties of Taunggyi medicinal plants, Andrographis paniculata, Physalis peruviana, and Cassia fistula. Historically, these plants have been used for many infections and diseases in Taunggyi. More than 361 chemical compounds have been isolated and identified from the selected plants. Some of the chemical constituents have substantial pharmacological properties. It is clear that these herbs have significant potential for useful natural supplements in many contemporary diseases. Thus, the aim of this review compiles an ethnobotanical survey and documentation of medicinal plants in Taunggyi (Myanmar). This review will also inspire Myanmar researcher's to further investigate the potential of these plants in their future work into new compound and new drugs.
Keywords: Taunggyi, Traditional medicine, Medicinal plants, Bioactive compounds
Taunggyi; Traditional medicine; Medicinal plants; Bioactive compounds
1. Introduction
Throughout the history of human civilization, medicinal plants also played a significant part. Medicinal plants are used as a source of medication, and many modern drugs are made of these plants as a filled supply of traditional products [1, 2, 3, 4]. Natural ingredients derived from medicinal plants, which have their therapeutic potential, natural product-based medicines, and the application to the healthcare companies [5, 6, 7]. Such compounds have adjusted numerous physiological transforms in humans, and have furnished to the development of health. Natural products are an essential source in pharmaceutical enlargement, and are much more triumphant than artificially designed compounds [5, 8, 9, 10]. A lot of research attempted with advanced bioassays, and bioassay guided fractionation to find the biologically active compounds of medicinal plants. Because of the researchers' enthusiastic efforts, many effective medicines have been produced from herbal plants [11].
Shan State is largely rural, with only three cities of significant size: Lashio, Kengtung, and the capital Taunggyi. It expands to China to the north, Laos to the east, and Thailand to the south, and five administrative divisions of Myanmar in the west. Taunggyi is 150.7 km north east of the nation's capital Naypyitaw. Taunggyi is the fifth largest city of Myanmar. Taunggyi has a humid subtropical climate. The climate usually comprises three seasons: the hot summer, the rainy monsoon, and the cold winter. There are 8 ethnic groups in Taunggyi, and almost every group has its own traditional medical knowledge and experiences. A lot of medicinal plants found in Taunggyi. Thus, this paper discusses the traditional uses and experimental studies of some plants, including Andrographis paniculata, Physalis peruviana, and Cassia fistula, used for natural remedies in Taunggyi.
2. Andrographis paniculata (Burm.f.) Nees
Kingdom Plantae
Order Lamiales, Family Acanthaceae, Genus Andrographis
Species A. paniculator
English name King of bitter
Myanmar name Say-khar
A. paniculata grows as an erect herb about 30–110 cm in height, with the dark green slender stem and the small flower. The flowers produce from September to December. The fruit is about 2 cm long and few millimeters broad. It is mainly found in America, south western Nigeria, Bangladesh, Pakistan, India, China, Hong Kong, Malaysia, Indonesia, Thailand, Brunei, Taunggyi (Myanmar), and the Philippines [12, 13]. In Taunggyi, dried powder of this plant used for diabetes, malaria, cough, and hypertension.
2.1. Chemical constituents
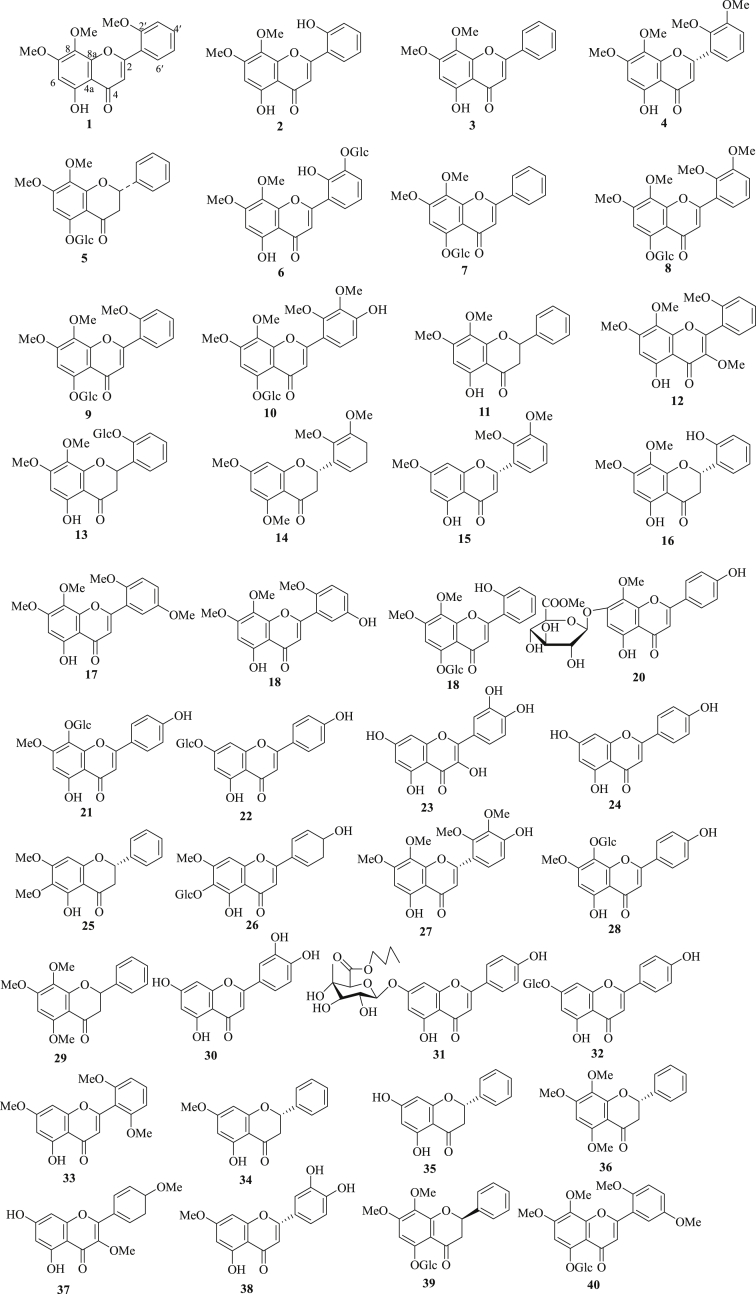
A total of 135 compounds, including 40 flavonoids, 82 terpenes (diterpene glucoside, diterpenoids, diterpenes dimer, and triterpenoid), 3 steroids, and 10 other compounds were isolated and identified from A. paniculata by chromatographic methods. Most have been studies for different biological activities [14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49]. The chemical structures of the isolated compounds from A. paniculata are shown in Figures 1, 2, and 3 and Figure S1. The list of compounds name and their biological activities are presented in Table S1, as well as their structure classification.
Figure 1.
The structure of flavonoids (1–40) isolated from A. paniculata.
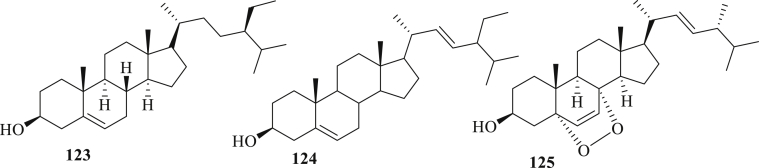
Figure 2.
The structure of steroids (123–125) isolated from A. paniculata.
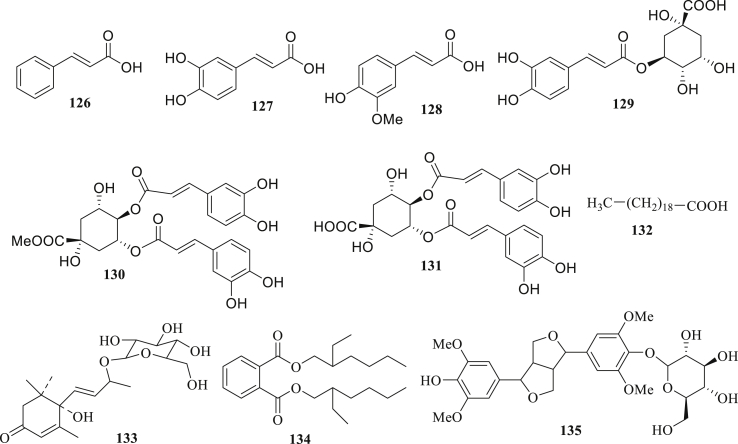
Figure 3.
The structure of other compounds (126–135) isolated from A. paniculata.
2.1.1. Flavonoids
Forty flavonoids (1–40) were identified from the roots, aerial parts, and the whole plant of A. paniculata (Figure 1) [14, 17, 18, 20, 21, 22, 26, 28, 29, 31, 32, 37, 41, 44, 45, 48]. Some isolated compounds were evaluated for their biological activities. For example, anti-HIV and cytotoxic activities of compounds 3 and 11 were reported by Reddy et al., 2005 [22]. In addition, compounds 3, 24, and 25 displayed antiplatele aggregation activities [29]. Chao et al., 2010 [31], reported anti-inflammatory effects not only on NF-kB-dependent luciferase activity but also on its downstream inflammatory mediators (TNF-α, IL-6, MIP-2, and NO). Compounds 3 and 11 showed the ability to inhibit NF-kB transcriptional activity with IC50 values of 6.1, and 6.7 μg/mL. Chel et al., 2014 [41], evaluated antiproliferative activity of compounds 1, 3, 5, 7, 8, 11, 19, 24, and 28–32, using human lekaemia HL-60 cell with adriamycin as a positive control. The result revealed that compound 30 displayed significant activity with IC50 value of 3.5 μM. Compounds 1, 3, 24, and 31 exhibited moderate activities with the IC50 values in the range of 10–20 μM. Moreover, compounds 1 (IC50 0.10 mM), 3 (IC50 0.05 mM), 11 (IC50 0.15 mM), and 16 (IC50 0.10 mM) demonstrated moderate cytotoxic activity against Jurkat cell line. Preferential cytotoxicity revealed that compound 17 (IC50 74.9 and 74.1 μM) displayed weak activity on PANC-1 and PSN-1 (Human pancreatic cancer) cell lines [44].
2.1.2. Terpenes
Eighty-two terpenes (41–122), including diterpenes glycoside, terpenoids, diterpenes dimer, and triterpenoids have been isolated from the leaves, roots, aerial parts, and the whole plant of A. paniculata (Figure S1) 15, 16, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 33, 34, 35, 36, 37, 38, 42, 43, 44, 45, 46, 47, 48, 49. Some of these were evaluated for their biological activities. For example, Matsuda et al., 1994 [19], evaluated phagocytosis activity of compounds 41–50, and 52–58, using M1 (mouse myeloid leukemia) cells. According to the results, compounds 42, 44, and 45 have phagocytosis ratio of more than 30 % at the concentration of 5 × 10−6 M. The diterpene dimmers (54–56) have more potent phagocytosis activity than that of diterpenes (42, and 44–50). Moreover, diterpene glucosides (41, 43, and 52–54) have low activities of phagocytosis inducing and growth inhibition. Reddy et al., 2005 [22], evaluated anti-HIV and cytotoxic activity of compound 41, 51, and 60, using azidothymidine (AZT, as a positive control for anti-HIV, EC50 = 0.02 5 μg/mL) and etoposide (as a positive control for cytotocicity, LD50 = 5 μg/mL). Compounds 41 and 51 have potential anti-HIV activity with EC50 values of 49.0 and 56.8 μg/mL, but compound 60 has no activity. However, compound 60 was very cytotoxic than the other compounds with LD50 = 4.63 μg/mL. Shen et al., 2006 [23], evaluated antibacterial activity of compounds 42, 43, 45–53, 56, 57, 61, 62, and 64–71 against Bacillus subtils, Staphylococcus aureus, Escherichia coli, Micrococcus luteus, Sarcina lutes, Candida albicans, Candida sake, and Aspergillus niger using a disk diffusion assay. Compound 45, 50, 51, and 61 inhibited the activities with clear zones inhibition with a diameter of 7–8 mm at the minimal concentration (10 μg/mL) used. Li et al., 2007 [26], evaluated cytotoxic activity of compounds 42, 45, 51, 53, and 72 against the oral epidermoid carcinoma KB cell lines. Compounds 42 and 45 displayed cytotoxic activities with ED50 values of 6.5 and 5.1 μg/mL. The others compounds have no significant cytotoxicity with both ED50 values over 20 mg/mL. Chen et al., 2008 [27], evaluated antiproliferative activity of compounds 42, 43, 45, 46, 51, 53, 73–78, and 82–87, using human leukemia HL-60 cells with Adriamycin as a positive control. The most active compounds with GI50 values of 9.33 and 6.30 μM were 42 and 45, while compounds 83 (GI50 26.36 μM), 84 (GI50 20.41 μM), 85 (GI50 22.42 μM), 86 (GI50 28.81 μM), and 87 (GI50 24.95 μM) displayed weak cytotoxic activities. Wu et al., 2008 [29], also reported antiplatelet aggregatory and vasorelaxing activities of compounds 41, 45, 47, 51 and 53. Geethangili et al., 2008 [28], evaluated the cytotoxicity of compounds 42, 45, 51, and 113, using MTT assay, with Jurkat (human lymphocytic cancer cell line), PC-3 (human prostate cancer cell line), HepG2 (human hepatoma cancer line), Colon 205 (human colonic cancer line), and normal cell PBMCs (peripheral blood mononuclear cells). The results revealed that compounds 113 (IC50 0.05, 0.07, and 0.05 mM) and 45 (IC50 0.10, 0.15, and 0.15 mM) displayed moderate cytotoxic activity on Jurkat, PC3 and Colon 205, while compound 51 (IC50 0.10 and 0.15 mM) showed moderate activity on Jurkat and Colon 205. Compound 42 (IC50 0.05 mM) possess only moderate activity on Colon 205 (IC50 0.05mM). Moreover, compound 113 inhibited exclusively cell cycle progression at G0/G1, while compounds 45 and 51 inhibited G2/M phase of the Jurkat cell line. Chao et al., 2010 [31], noted that compounds 113 and 114 displayed significant anti-inflammatory activities with IC50 values of 2.0 and 4.4 μg/mL. In another study, Xu et al., 2012 [39], reported that compounds 96–100 exhibited no significant antibacterial activity using microtitre plate both dilution method. Compounds 43, 46, 49, 51, and 53 have no significant cardiovascular effects [31]. Moreover, Wang et al., 2014 [43], documented that the weak antiviral activity of compounds 43 (IC50 1.5 μg/mL) and 103 (IC50 30 μg/mL), using respiratory syncytial virus (RSV) assay with ribavirin (IC50 1.5 μg/mL) as a positive control. Additionally, Lee et al., 2015 [44], noted that compound 51(IC50 10.0 and 9.27 μM) exhibited potent preferential cytotoxic activity against PANC-1 and PSN-1 cells, whereas compound 61 (IC50 46.0 and 43.9 μM) has weak activity. The mechanism of cell death induced by compound 51 in PANC-1 and PSN-1 cell using microscopical observation, EB/AO double staining, and flow cytometry were also studied in the same report. Compounds 108 and 109 displayed potential anti-inflammatory activities in vitro and in vivo [47]. Furthermore, Hanh et al., 2020 [48], documented that compound 63 (IC50 31.8, 43.5, 37.9, 45.9, and 42.1 μM) showed not only the cytotoxic activities toward five human cancer cell lines (LNCaP, HepG2, KB, MCF7, and SK-Mel2), but also inhibited the overproduction of NO (nitric oxide) in lipolysaccharide (LPS)-stimulated RAW264.7 macrophages (IC50 13.4 mM). Additionally, compounds 119 (IC50 40.4 and 58.6 μM) has weak cytotoxic activity against PANC-1 and PSN1- cell. Wen et al., 2020 [49], found that the anticomplement activity of compounds 1, 43, 46, 50–54, 67–68, 70–71, 80, 94, and 115–118 with the CH50 and AP50 values of 23.1–638.3 μg/mL and 54.2–603.9 μg/mL.
2.1.3. Steriods
Three steroids (123–125) were isolated from the whole plant, aerial part, and leaves of A. paniculata (Figure 2). Compounds (123 + 124) and 125 displayed the significant activity to inhibit NF-κB transcriptional activity with IC50 values of 5.2 and 4.7 μg/mL. Compounds 125 (IC50 60.5 and 79.2 μM) has weak cytotoxic activity against PANC-1 and PSN1- cell [21, 31, 44].
2.1.4. Other compounds
Other compounds (126–135) were isolated from the leaves, aerial parts, and whole part of A. paniculata (Figure 3) [21,29,[37], [38],[44], [45]].
3. Physalis peruviana (L.)
Kingdom Plantae
Order Solanales
Family Solanaceae
Genus Physalis
Species P. peruviana
English name Cape gooseberry, goldenberry, and physalis
Myanmar name Bout-tee
P. peruviana is a perennial evergreen plant that produces a group of branched stems. It can expand by 1.0–1.5 m without guidance, but it may reach 2.0 m high with trimmed and guided. Leaves are soft, simple, alternate, and heart-shaped with lengths between 5 and 15 cm and widths from 4 and 10 cm. Leaves turn yellow and collapse after the fruit maturation. The fruit is a globose berry, yellowish in color, around 12.5–25.0 mm, with several yellowish seeds [50, 51, 52]. In Taunggyi, this plant is locally used for antidiabetic and antihypertension.
3.1. Chemical constituents
A total of 106 compounds, including 76 withanolides, 5 flavonoids, 2 alkaloids, 14 sucrose ester, and 9 other compounds were isolated and identified from P. peruviana by chromatographic methods. Most have been studies for different biological activities [53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83]. The chemical structures of the isolated compounds from A. paniculata are shown in Figures 4, 5, 6, and 7 and Figure S2. The list of compounds name and their biological activities are presented in Table S2, as well as their structure classification.
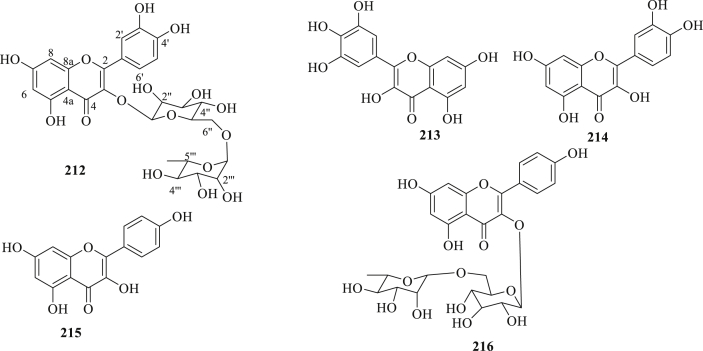
Figure 4.
The structure of flavonoids (212–216) isolated from P. peruviana.
Figure 5.
The structure of alkaloids (217–218) isolated from P. peruviana.
Figure 6.
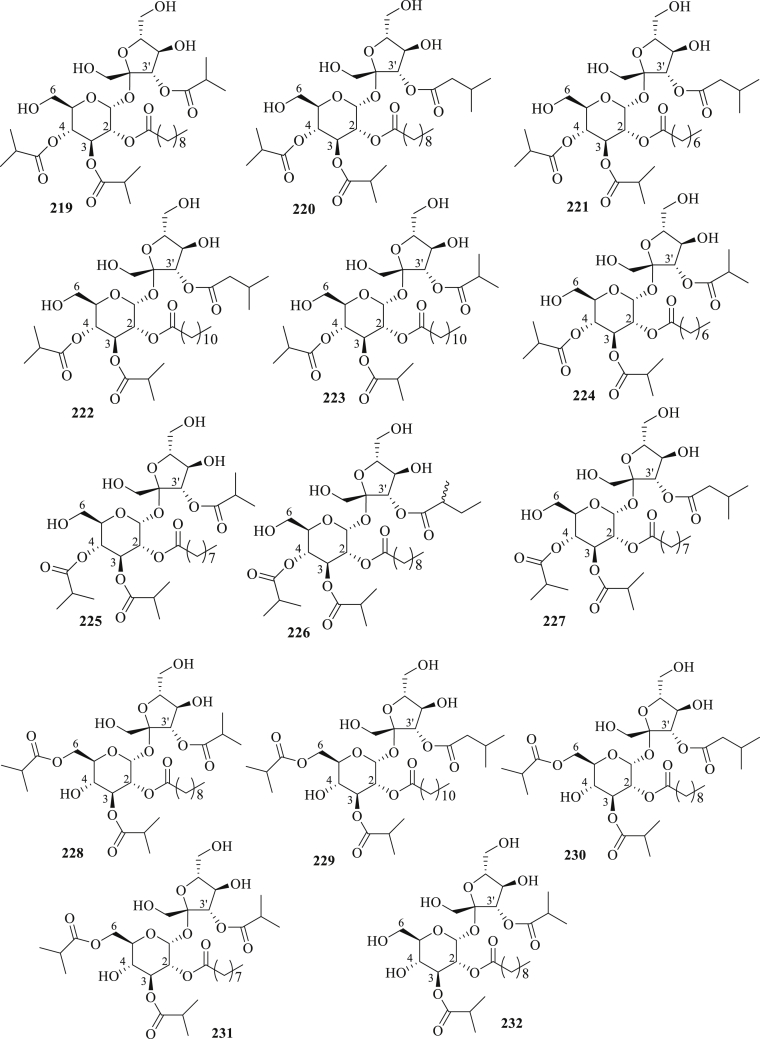
The structure of sucrose esters (219–232) isolated from P. peruviana.
Figure 7.
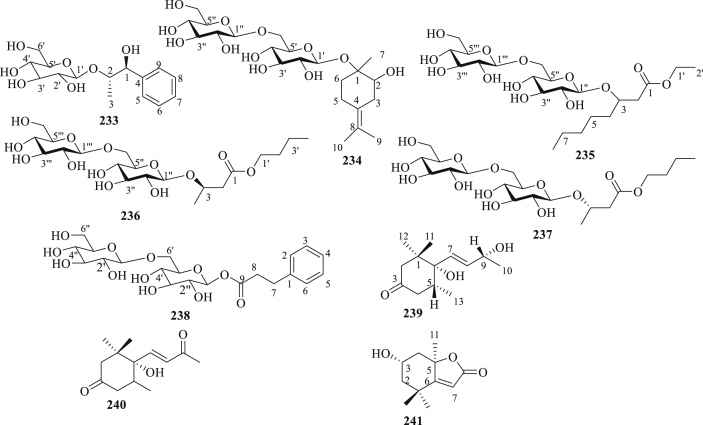
The structure of other compounds (233–241) isolated from P. peruviana.
3.1.1. Withanolides
In the plant of P. peruviana, withanolides are prominent components. A total of 76 withanolides and a triterpene (136–211) have been isolated from the roots, leaves, calyces, whole plant, fruits, and aerial parts of P. peruviana (Figure S2) [[54], [55], [56], [57], [58], [59], [60], [61],[63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73],78,80,83]. Some isolated compounds displayed significant biological activities. For example, compounds 142, 172, 174, 176, and 191 (IC50 0.04, 2.1, 1.1, 2.1, and 0.06 μM) displayed the NF-kB activity with stably-transfected NF-kB Luc-293 human embryonic kidney cells induced by TNF-α. Compounds 142, 154, and 191 also possessed NO inhibitory activity against lipopolysaccharide (LPS)-induced NO (nitric oxide) release with IC50 values of 0.32, 2.4, and 2.3 μM. In addition, no significant cytotoxicity has been observed at the concentration of 50 μM. Antiproliferative activity of compound 142 was also observed with the HT-29 human colorectal cancer cell line [78, 79]. Ergostanoid lactone, perulactone 3-O-β-D-glucopyranoside (205) reduces the growth of Helicoverpazea larvae to 50% of control size at a diretary concentration of 150 mg/kg [61]. Moreover, compounds 141 and 142 were obviously cytotoxic at concentration >10−5 M, but there were no specific agonistic or antagonistic effects of either compound [63]. Cirigliano et al., 2008 [69], studied the biological effects of P. peruvianacurde extract and its major withanolides, 4β-hydroxywithanolide E (140) and withanolide E (142) against Ceratitiscapitata Wiedemann (Diptera: Tephritidae). According to the results, P. peruviana could not only provide edible fruits but could be a source of natural insecticides. Lan et al., 2009 [71], evaluated cytotoxic activity of fractions and compounds 139, 140, 142, 152, 156, 167–171, 191, 192, 198, 201, and 227 isolated from the aerial parts of P. peruviana, using MTT assay against lung (A549), breast (MEA-MB-231 and MCF7), and liver (hepG2 and 3B) cancer cell lines, with Doxorubincin as a positive control. The results revealed that the MeOH/water fraction displayed inhibitory activity against A549, MDA-MB-231, and HepG2 cells (IC50 < 20 mg/mL). Compounds 139, 140, 142, and 167 exhibited potent cytotoxic activity against lung (A549), breast (MEA-MB-231 and MCF7), and liver (HepG2 and Hep 3B) cancer cell lines. Xu et al., 2017 [80], studied the cytotoxic activity of compounds (140, 142–151, 162, 164, 167, 173, 175, 181–185, 192, 194, and 199–200) isolated from aerial parts of P. peruviana against a panel of tumor cell lines, androgen-sensitivehuman prostate adenocarcinoma (LNCaP), androgen-resistant human prostate adenocarcinoma (22Rv1), human renal adenocarcinoma (ACHN), human melanoma (M14), human melanoma (SK-MEL-28), and normal human foreskin fibroblast cells. According to the results, compounds 142–144, 146–147, 149, 162, 164, 167, and 199–200 demonstrated cytotoxicity against LNCaP and 22Rv1, whereas compounds 140 and 200 showed cytotoxicity against ACHN cell lines. The other compounds have no potential cytotoxicity at the concentration of 5 μM. In another study, compounds 187–190 inhibited NO production with IC50 values below 8 μM. Compounds 187–190 also showed strong interaction with iNOS protein [83].
3.1.2. Flavonoids
The fruits extract of P. peruviana reported to contain flavonoids, like rutin (212), myricetin (213), quercetin (214), and kaempferol (215) (Figure 4) [[76], [77]]. Toro et al., 2014 [76], evaluated the antioxidant and anti-inflammatory activity of crude extracts, fractions, and compounds, rutin (212) and nicotoflorin (216) obtained from the bioassay guided isolation and identification of calyces from P. peruviana. Compound 212 showed not only potent superoxide anion scavenging activity (88.8 %), comparable to positive control, n-propylgallate (92.9 %), but also showed higher NO inhibition (66.7 %) than the positive control, gallic acid (43.9 %). In addition, compound 216 showed lower superoxide anion scavenging activity, but displayed an important NO scavenging effect (49.6 %), comparable to that of gallic acid.
3.1.3. Alkaloid
The roots of extract of P. peruviana contain alkaloids, like (+)-physoperruvine (217) and cuscohygrine (218) (Figure 5) [53,74].
3.1.4. Sucrose esters
A series of sucrose esters, peruvioses A-M (219–231), and nicandrose D (232) were isolated from the fruits and calyces of P. peruviana (Figure 6) [75,81,82]. Bernal et al., 2018 [81], studied the α-amylase inhibition activity of compounds 219–224 at the concentration of 640 μg/mL. The study revealed that compound 222 displayed the highest activity, with an inhibition value of 84.8 %. It was the first research to identify the ability of sucrose esters for alpha-amylase inhibitors and explain the hypoglycemic impact of gooseberry fruit. Moreover, a mixture of compounds (219 + 220) has potent anti-inflammatory activity [75].
3.1.5. Other compounds
Nine other compounds; such as hydroxyester disaccharides (233–235), glycosidically bound compounds (236–237), carbohydrate ester of cinamic acid (238), blumenol A (239), (+)-(S)-dehydrovomifoliol (240), and loliolide (241) have been isolated from the roots, fruits, and aerial parts of P. peruviana (Figure 7) [62, 67, 68, 70, 71].
4. Plant description of Cassia fistula (Linn.)
Kingdom Plantae
Order Fabales
Family Fabaceae
Genus Cassia
Species C. fistula
English name Golden Shower
Myanmar name Ngu-wah
C. fistula is a medium-sized tree up to 24 m in height. It is a deciduous tree with greenish grey bark, composites with leaves; leaf lets are each 5–12 cm long pairs. The fruit is a legume, 40–70 cm long with a pugent smell and containing many seeds. It is grows throughout in Bangladesh India, China, Philippines, Malaysia, Indonesia, Thailand, and Taunggyi (Myanmar) [84, 85]. In Taunggyi, the bark of this plant locally used in anti-hepatic C.
4.1. Chemical constituents
A total of 120 compounds, including 39 flavonoids, 19 anthraquinones, 22 chromones, 3 coumarins, 10 alkaloids, 11 phenolic and other compounds, 4 phytosterols and a triterpene, and 11 long-chain hydrocarbons were isolated and identified from C. fistula by chromatographic methods [86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112]. Most have been studies for different biological activities. The chemical structures of the isolated compounds from C. fistula are shown in Figures 8, 9, 10, 11, 12, 13, and 14 and S3. The list of compounds name and their biological activities are presented in Table S3, as well as their structure classification.
Figure 8.
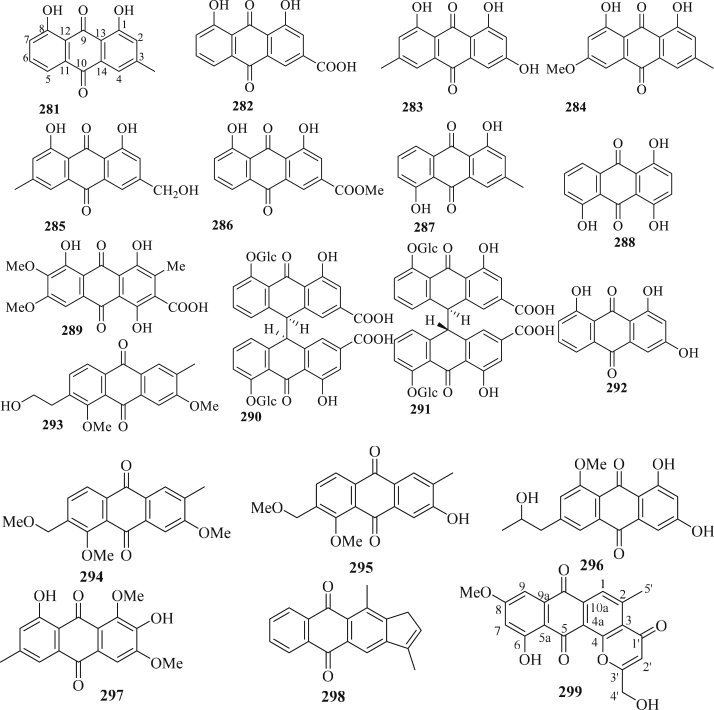
The structure of anthraquinones (281–299) isolated from C. fistula.
Figure 9.
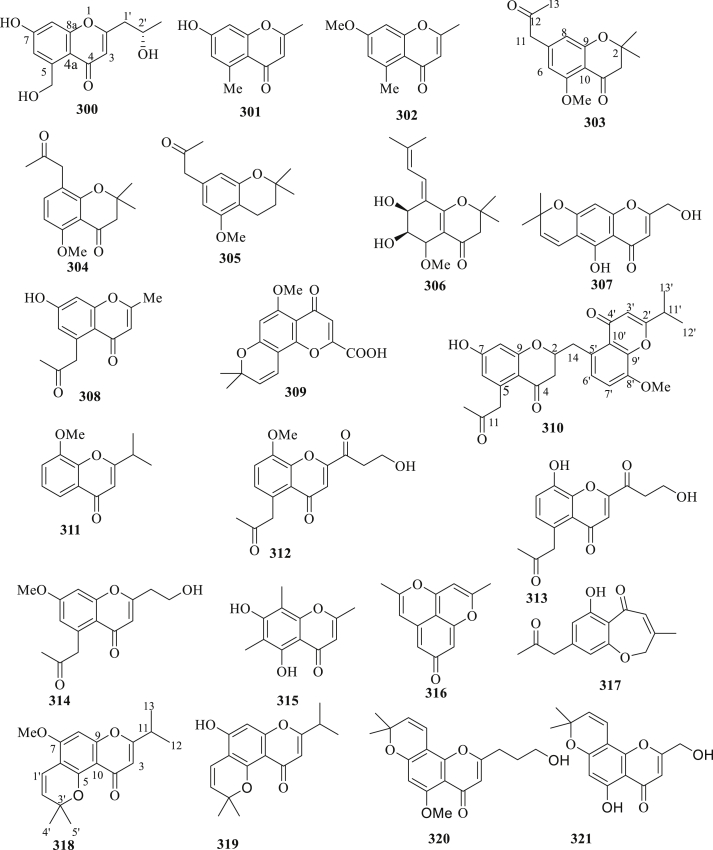
The structure of chromones (300–321) isolated from C. fistula.
Figure 10.
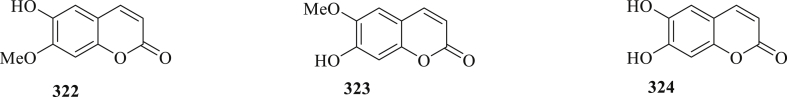
The structure of coumarins (322–324) isolated from C. fistula.
Figure 11.
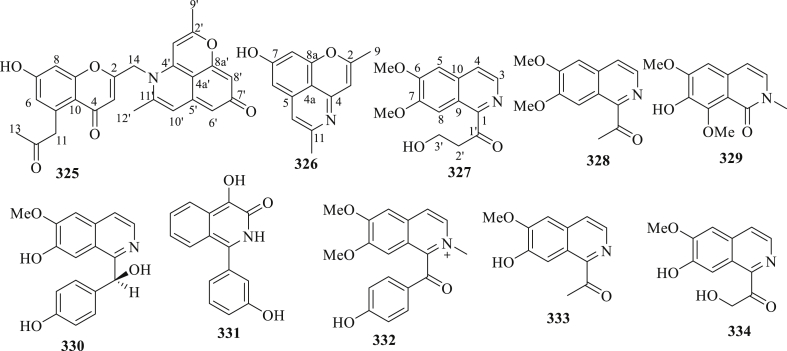
The structure of alkaloids (325–334) isolated from C. fistula.
Figure 12.
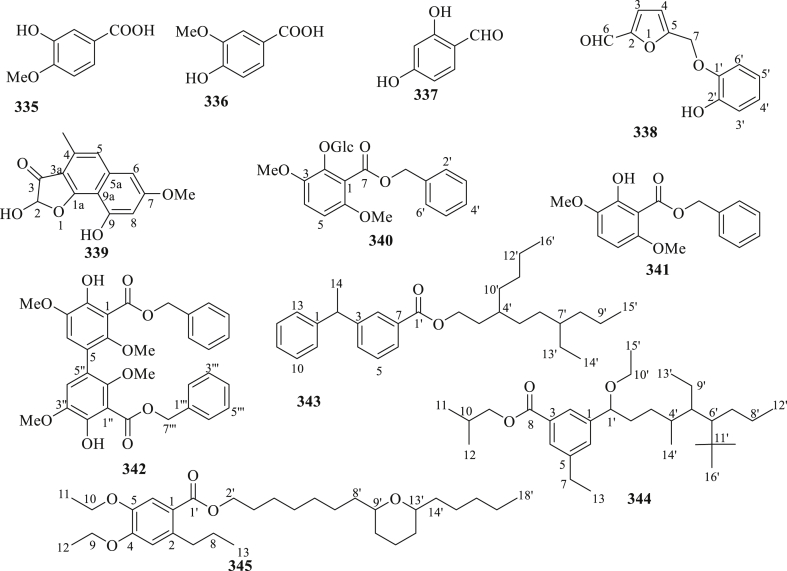
The structure of phenolic and other compounds (335–345) isolated from C. fistula.
Figure 13.
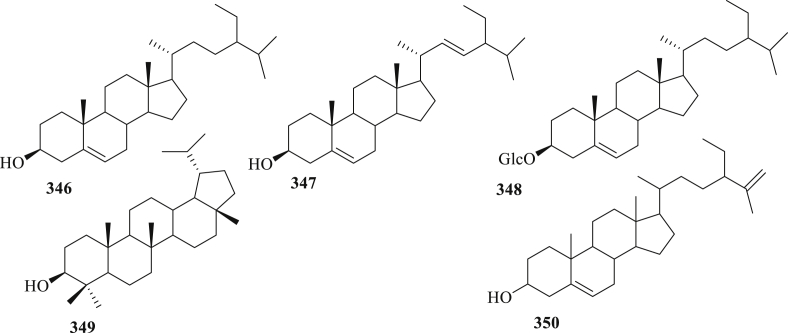
The structure of phytosterols and a triterpene (346–350) isolated from C. fistula.
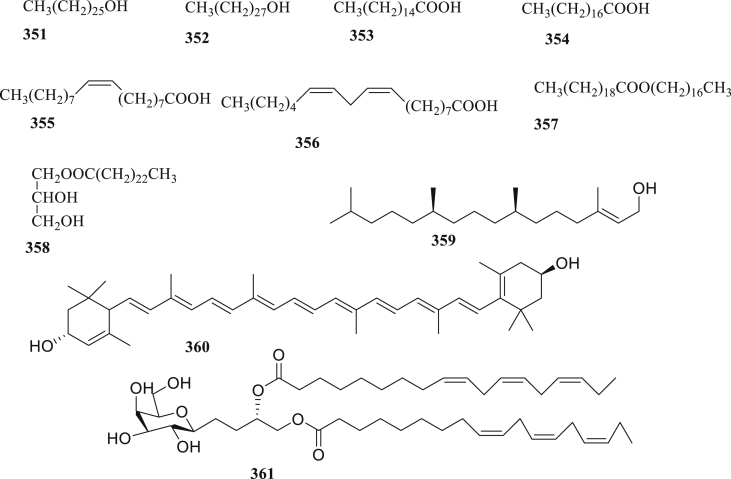
Figure 14.
The structure of long-chain hydrocarbons (351–361) isolated from C. fistula.
4.1.1. Flavonoids
A total of 39 flavonoids (242–280) have been isolated and identified from the fruits, stems, leaves, roots, and bark of C. fistula (Figure S3) [86, 87, 88, 91, 93, 97, 98, 99, 100, 105]. Some isolated flavonoids have significant biological activities. For example, Sartorelli et al., 2009 [93], evaluated the antileishmanial, antitrypanosomal, cytotoxic, and hemolytic activity of compound 260 isolated from the fruits of C. fistula. Compound 260 (EC50 18.32 μg/mL) showed the best activity (2.5-fold more effective) against the trypomastigotes form of T. cruzi as compared to the standard drug, benznidazole (EC50 44.86 μg/mL), and cytotoxic (EC50 42.85 μg/mL) against rhesus monkey kidney cell (LLC-MK2-ATCC) after 48 h incubation. The findings of this analysis suggest that compound 260 could be a new therapeutic drug for Chagas' disease. Gao et al., 2013 [97], evaluated the cytotoxicity of compounds 272–279 against five human tumor cell lines (NB4, A549, SHSY5Y, PC3, and MCF7). The results revealed that compound 274 displayed significant activity against SHSY5Y and MCF7 cell lines with IC50 values of 2.7 and 2.6 μmol/L. Compounds 273, 276, 277, and 279 showed no activity against all selected cell lines (IC50 > 10 μmol/L), while compounds 272, 275, and 278 displayed moderate activity against some selected cell lines (IC50 < 10 μmol/L). Zeng et al., 2013 [99], evaluated the antitobacco mosaic virus activity (anti-TMV) of compound 261 isolated from the roots of C. fistula. Compound 261 displayed moderate activity with inhibition rate of 18.2% at a concentration of 20 mM. In another study, Zhao et al., 2013 [100], also reported the anti-TMV activity of compounds 262–267 and 271 isolated from the bark and stems of C. fistula, using half-leaf method with Ningnanmycin (24.7%, a commercial product for plant disease in China) as a positive control. According to the results, compounds 262 and 263 demonstrated high activity with inhibition rate of 28.5% and 31.3% at a concentration of 20 μM. Compounds 265–267 and 271 exhibited moderate activity with inhibition rate of 18.5%, 22.7%, 16.4%, and 15.3% at the same concentration. Additionally, Srividhya et al., 2017 [105], evaluated antioxidant and cytotoxic activity of compound 280 isolated from the leaves of C. fistula using DPPH and MTT assays. Compound 280 was presumably dose-dependent antioxidant and cytotoxic activities with IC50 values of 29.7 and 25.3 μg/mL.
4.1.2. Anthraquinone
Nineteen anthraquinones (281–299) were reported from the arils, leaves, fruits, and flower of C. fistula (Figure 8) [87, 89, 91, 95, 98, 101, 108, 110]. A study by Duraipandiyan et al., 2012 [95], on compound 282 isolated from C. fistula flower exhibited cytotoxicity toward COLO 320 DM (human colon cancer cells) and it also induced apotosis at 6.25 mg/mL. Compound 282 also has anti-inflammatory activity. Cytotoxicity of compound 300 isolated from the fruit of C. fistula was evaluated by using five human tumor cell lines (NB4, A549, SHSY5Y, PC3, and MCF7), and the results revealed that compound 299 displayed significant cytotoxicity against NB4 and PC3 cell lines with IC50 value of 6.3 and 5.8 μM. Zhou et al., 2017 [108], evaluated the anti-TMV activity of compounds 293–298 isolated from the twigs of C. fistula using half-leaf method with Ningnanmycin (28.8%, a commercial product for plant disease in China) as a positive control. The results showed that compound 295 displayed potential anti-TMV activity with inhibition rate of 35%. The other compounds displayed weak anti-TMV activity with inhibition rates in the ranges of 18.2–26.3%. Cytotoxicity of compounds 293–298 was also evaluated against NB4 (human leukemia), A549 (carcinomic human alveolar basal epithelial), SHSY5Y (human neuroblastoma), PC3 (human prostate cancer), and MCF7 (human breast adenocarcinoma) cell lines, using MTT assay with taxol as a positive control. According to the analysis, all compounds displayed moderate cytotoxic activity for some selected human tumor cell lines with IC50 values in the ranges of 2.8–9.4 μM.
4.1.3. Chromones
Twenty-two chromones (300–321) were isolated from the arils, seeds, and stems of C. fistula (Figure 9) [89, 90, 94, 102, 103, 104, 109]. Chromones from C. fistula have reported for their insightful biological activity. For example, compound 300 has the highest antifungal activity against Sacromycescerevisiae and lowest activity against Penicillium chrysogenum. The MIC values of compound 300 against used fungal strains range from 18-27 μg/mL. Moreover, compound 300 also has the highest antibacterial activity against Staphylococcus aureus and lowest activity against Klebseilla pneumonia. The MIC values of compound 300 against Gram-positive and Gram-negative ranged from 22-38 μg/mL [94]. Anti-TMV activity of compounds 303–309 isolated from the stem of C. fistula was evaluated by using half-leaf method with Ningnanmycin (34.8%, a commercial product for plant disease in China) as a positive control. The results demonstrated that compound 307 exhibited high activity with inhibition rate of 30.8% at a concentration of 20 μM, and the other compounds 303–306, 308, and 309 also showed potential activity with inhibition rates in the ranges of 15.6–22.1% at the same concentration [102]. In another study, compounds 310 and 311 isolated from the bark of C. fistula were evaluated for their biological activities, including antidiabetic, antimicrobial, anti-TMV, and cytotoxic activities. Compounds 310 and 311 showed no significant antidiabetic and antimicrobial activity, while weak activity on anti-TMV and cytotoxic activity was detected [103]. Moreover, Hu et al., 2015 [104], also documented the anti-TMV activity of compounds 312–314 isolated from the twigs of C. fistula, using half-leaf method with Ningnanmycin (30.8%, a commercial product for plant disease in China) as a positive control. The results showed that compounds 312–314 exhibited high activity with inhibition rates of 26.6, 28.2, and 29.7% at the concentration of 20 μM. Hu et al., 2017 [109], also evaluated anti-TMV activity of compounds 318 and 319 isolated from the twigs of C. fistula, using half-leaf method with Ningnanmycin (32.8%, a commercial product for plant disease in China) as a positive control, and the result showed that both compounds exhibited high activity with inhibition rates of 27.5 and 30.8% at the concentration of 20 μM.
4.1.4. Coumarins
Three coumarins, isoscopoletin (322), scopoletin (323), and esculetin (324), were isolate from the arils and leaves, of C. fistula (Figure 10) [89,98].
4.1.5. Alkaloids
Ten alkaloids (325–334) were recorded on the bark and twigs of C. fistula (Figure 11) [103, 106, 107]. Alkaloids from C. fistula have an important biological activity. For example, compound 325 showed not only significant anti-TMV activity with an IC50 value of 43.8 μM as compared to the positive control Ningnamycin (IC50 52.4 μM), but also weak cytotoxic activity (IC50 < 10 μM) against NB4, A459, and MCF7 cell lines [103]. In another study, Wu et al, 2016 [106], reported the anti-TMV activity of compounds 327–332 isolated from the twigs of C. fistula, using half-leaf method with Ningnanmycin (30.8%, a commercial product for plant disease in China) as a positive control. The results showed that compounds 327–332 exhibited weak activity with inhibition rates in the range of 15.4–23.5% at the concentration of 20 μM. Similarly, Zhou et al, 2017 [107], noted that the anti-TMV activity of compounds 333 and 334 isolated from the bark of C. fistula, using half-leaf method with Ningnanmycin (30.8%, a commercial product for plant disease in China) as a positive control. Compounds 333 and 334 displayed weak activity with inhibition rates of 18.9 and 22.6%.
4.1.6. Phenolic and other compounds
Eleven phenolic and other compounds (335–345) were reported from the arils, seeds, and leaves of C. fistula (Figure 12) [89, 90, 94, 96, 98, 111]. The results of the analysis by Nagpal et al., 2011 [94], exhibited the highest antifungal activity against Sacromyces cerevisiae and lowest activity against Aspergillus niger by compound 340. The MIC values of compound 340 against used fungal strains ranged from 22 to 28 μg/mL. In addition, compound 340 also showed highest antibacterial activity against Bacillus subtilis and lowest activity against Pseudomonas aeruginosa. The MIC values of compound 341 against Gram-positive and Gram negative ranged from 41 to 50 μg/mL. Sartorelliet al., 2012 [96], found that no antifungal activity on compounds 341 and 342 against Cladosporium cladosporioides and Cladosporiums phaerospermum. Aftab et al., 2019 [111], demostrated the antioxidant properties of compounds 343–345 isolated from the EtOAc soluble fraction of C. fistula, using DPPH, ABTS, and super oxide anion radical scavenging assays with ascorbic acid and trolox as positive controls. The results showed that C. Fistula includes active antioxidant compounds that can help cure oxidative stress disorder and other diseases.
4.1.7. Phytosterols and a triterpene
Four phytosterol like β-sitosterol (346), stigmasterol (347), β-sitosterol-D-glucopyranoside (348), clerosterol (349) and a triterpene, lupeol (350), were isolated from the arils, fruits, and leaves of C. fistula (Figure 13) [89, 92, 98]. Compound 351 displayed significant antileishmanial activity against axenic promastigotes and intercellular amastigotes of Leishmania (L.) chagai in vitro. According to the mammalian cytotoxic activity, compound 351 has 3.6-fold less toxic than the standard drug pentamidine [92].
4.1.8. LONG-CHAIN hydrocarbons
Eleven long-chain hydrocarbons, including 1-hexacosanol (351), 1-octacosanol (352), palmitic acid (353), steric acid (354), oleic acid (355), linoleic acid (356), heptacosyleicosanate (357), glyceryl-1-tetraeicosanoate (358), E-phytol (359), lutein (360), and DLGG (361) were isolated and identified from the arils and leaves of C. fistula (Figure 14) [89, 98, 112]. Grace et al., 2012 [112] noted that compounds 359–361 displayed antiplasmodial activity with IC50 values of 18.9, 12.5, and 12.8 μM. Furthermore, compound 361 showed very low toxicity against Chinese Hamster Ovarian (CHO) cell lines, although compounds 359 and 360 were nontoxic, expect at the highest doses.
5. Conclusions
This review finds the description of the plants, different types of chemical constituents, and their biological activities from the selected Taunggyi medicinal plants. Over 361 chemical compounds were isolated and identified from three selected Taunggyi medicinal plants. Among then, flavonoids and terpenes are the main bioactive components in Andrographis paniculata, withanolides and sucrose ester are the principle bioactive constituents in Physalis peruviana, and the main bioactive constituents in Cassia fistula are flavonoids, chromones, anthraquinones, and alkaloids. A total of 140 compounds were evaluated for their biological activity. Some of the biological findings on the selected plants provide scientific data to support the traditional use of Taunggyi medicinal plants. However, the mechanism of action of the selected plants is weakly implicit and further analysis is required.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by a research group grant from Universitas Airlangga (343/UN3.14/PT/2020)
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Amalraj A., Gopi S. Medicinal properties of Terminalia arjuna (Roxb.) wight & arn: a review. J. Tradit Compl. Med. 2017;7:65–78. doi: 10.1016/j.jtcme.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur P., Dhull S.B., Sandhu K.S., Salar R.K., Purewal S.S. Tulsi (Ocimum tenuiflorum) seeds: in vitro DNA damage protection, bioactive compounds and antioxidant potential. J. Food Meas. Charact. 2018;12(3):1530–1538. [Google Scholar]
- 3.Dhull S.B., Kaur P., Purewal S.S. Phytochemical analysis, phenolic compounds, condensed tannin content and antioxidant potential in Marwa (Origanum majorana) seed extracts. Resource-Efficient Technol. 2016;2(4):168–174. [Google Scholar]
- 4.Dhull S.B., Kaur M., Sandhu K.S. Antioxidant characterization and in vitro DNA damage protection potential of some Indian fenugreek (Trigonella foenum-graecum) cultivars: effect of solvents. J. Food Sci. Technol. 2020;57:3457–3466. doi: 10.1007/s13197-020-04380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogunwandea I.A., Walkerb T.M., Setzerb W.N. A review of aromatic herbal plants of medicinal importance from Nigeria. Nat. Prod. Commun. 2007;2(12):1311–1316. [Google Scholar]
- 6.Dhull S.B., Punia S., Sandhu K.S., Chawla P., Kaur R., Singh A. Effect of debittered fenugreek (Trigonella foenum-graecum L.) flour addition on physical, nutritional, antioxidant, and sensory properties of wheat flour rusk. Legume Sci. 2019:e21. [Google Scholar]
- 7.Dhull S.B., Sandhu K.S. Wheat-Fenugreek composite flour noodles: effect on functional, pasting, cooking and sensory properties. Curr. Res. Nutr. Food Sci. 2018;6(1):174–182. [Google Scholar]
- 8.Dhull S.B., Punia S., Kidwai M.K., Kaur M., Chawla P., Purewal S.S., Sangwan M., Palthania S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020:e37. [Google Scholar]
- 9.Dhull S.B., Punia S., Kumar R., Kumar M., Nain K.B., Jangra K., Chudamani C. Solid state fermentation of fenugreek (Trigonella foenum-graecum): implications on bioactive compounds, mineral content and in vitro bioavailability. J. Food Sci. Technol. 2020 doi: 10.1007/s13197-020-04704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhull S.B., Sandhu K.S., Punia S., Kaur M., Chawla P., Malik A. Functional, thermal and rheological behavior of fenugreek (Trigonella foenum–graecum L.) gums from different cultivars: a comparative study. Int. J. Biol. Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.05.094. [DOI] [PubMed] [Google Scholar]
- 11.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra S.K., Sangwan N.S., Sangwan R.S. Andrographis paniculata (Kalmegh): a review. Pharmacog. Rev. 2007;1:283–298. [Google Scholar]
- 13.Hossain S., Urbi Z., Sule A., Rahman K.M.H. Andrographis paniculata (Burm. F.) wall. Ex nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014;2014:274905. doi: 10.1155/2014/274905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jalai M.A.F., Overton K.H., Rycroft D.S. Formation of three new flavones by differentiating callus cultures of Andrographis paniculata. Phytochemistry. 1979;18:149–151. [Google Scholar]
- 15.Weiming C., Xiaotian L. Deoxyandrographolide-19β-D-glucoside from the leaves of Andrographis paniculata. Planta Med. 1982;45:245–246. doi: 10.1055/s-2007-971383. [DOI] [PubMed] [Google Scholar]
- 16.Fujita T., Fujitani R., Takeda Y., Takaishi Y., Yamada T., Jido M., Miura I. On the diterpenoids of Androgaphis paniculata: X-ray crystallographic analysis of andrographolide and structure determination of new minor diterpenoids. Chem. Pharm. Bull. 1984;32(6):2117–2125. [Google Scholar]
- 17.Gupta K.K., Taneja S.C., Dhar K.L., Atal C.K. Flavonoids of Andrographis paniculata. Phytochemistry. 1983;22:314–315. [Google Scholar]
- 18.Kuroyanagi M., Sata M., Ueno A., Nishi K. Flavonoids from Andrographic paniculata. Chem. Pharm. Bull. 1987;35(11):4429–4435. [Google Scholar]
- 19.Mastuda T., Kuroyanagi M., Sugiyama S., Umehara K., Ueno A., Nishi K. Cell differentiation-inducing diterpenes from Andrographic paniculata Nees. Chem. Pharm. Bull. 1994;42(6):1216–1225. doi: 10.1248/cpb.42.1216. [DOI] [PubMed] [Google Scholar]
- 20.Reddy M.K., Reddy M.V.B., Gunasekar D., Murthy M.M., Caux C., Bodo Bernard. A flavone and an unsual 23-carbon terpenoids from Andrographis paniculata. Phytochemistry. 2003;62:1271–1275. doi: 10.1016/s0031-9422(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 21.Rao Y.K., Vimalamma G., Rao C.V., Tzeng Y.M. Flavonoids and andrographolides from Andrographic paniculata. Phytochemistry. 2004;65:2317–2321. doi: 10.1016/j.phytochem.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Reddy V.L., Reddy S.M., Ravikanth V., Krishnaiah P., Goud T.V., Rao T.P., Ram T.S., Gonnade R.G., Bhadbhade M., Venkateswarlu Y. A new bis-andropholide ether from Andrographis paniculata Nees and evaluation of anti-HIV activity. Nat. Prod. Res. 2005;19(3):223–230. doi: 10.1080/14786410410001709197. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y.H., Li R.T., Xiao W.L., Xu G., Lin Z.W., Zhao Q.S., Sun H.D. ent-Labdane diterpenoids from Andrographis paniculata. J. Nat. Prod. 2006;69(3):312–322. doi: 10.1021/np050160u. [DOI] [PubMed] [Google Scholar]
- 24.Chen L.X., Qui H., Qu G.X., Yao X.S. Nine new ent-Labdane diterpenoids from the aerial parts of Andrographis paniculata. Helv. Chim. Acta. 2006;89(11):2654–2664. [Google Scholar]
- 25.Pramanick S., Banerjee S., Achari B., Das B., Sen A.K.Sr., Mukhopadhyay S., Neuman A., Prangé T. Andropanolide and isoandrographolide, minor diterpenoids from Andrographis paniculata: structure and X-ray crystallographic analysis. J. Nat. Prod. 2006;69(3):403–405. doi: 10.1021/np050211n. [DOI] [PubMed] [Google Scholar]
- 26.Li W., Xu X., Zhang H., Ma C., Fong H., Breemen R.V., Fitzloff J. Secondary metabolites from Andrographis paniculata. Chem. Pharm.Bull. 2007;55(3):455–458. doi: 10.1248/cpb.55.455. [DOI] [PubMed] [Google Scholar]
- 27.Chen L., Zhu H., Wang R., Zhou K., Jing Y., Qiu F. ent-Labdane diterpenoid lactone stereoisomers from Andrographis paniculata. J. Nat. Prod. 2008;71(5):852–855. doi: 10.1021/np0704452. [DOI] [PubMed] [Google Scholar]
- 28.Geethangili M., Rao Y.K., Fang S.H., Tzeng Y.M. Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytother Res. 2008;22(10):1336–1341. doi: 10.1002/ptr.2493. [DOI] [PubMed] [Google Scholar]
- 29.Wu T.S., Chern H.J., Damu A.G., Kuo P.C., Su C.R., Lee E.J., Teng C.M. Flavonoids and ent-labdane diterpenoids from Andrographis paniculata and their antiplatelet aggregatory and vasorelaxing effects. J. Asian Nat. Prod. Res. 2008;10(1-2):17–24. doi: 10.1080/10286020701273627. [DOI] [PubMed] [Google Scholar]
- 30.Zhou K.L., Chen L.X., Zhuang Y.L., Wang N.L., Yao X.S., Qiu F. Two new ent-labdane diterpenoid glycosides from the aerial parts of Andrographis paniculata. J. Asian Nat. Prod. Res. 2008;10(9-10):939–943. doi: 10.1080/10286020802217432. [DOI] [PubMed] [Google Scholar]
- 31.Chao W.W., Kuo Y.H., Lin B.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-kappa B transactivation inhibition. J. Agric. Food Chem. 2010;58(4):2505–2512. doi: 10.1021/jf903629j. [DOI] [PubMed] [Google Scholar]
- 32.Radhika P., Prasad Y.R., Lakshmi K.R. Flavones from the stem of Andrographis paniculata nees. Nat. Prod. Commun. 2010;5(1):59–60. [PubMed] [Google Scholar]
- 33.Pfisterer P.H., Rollinger J.M., Schyschka L., Rudy A., Vollmar A.M., Stuppner H. Neoandrographolide from Andrographis paniculata as a potential natural chemosensitizer. Planta Med. 2010;76(15):1698–1700. doi: 10.1055/s-0030-1249876. [DOI] [PubMed] [Google Scholar]
- 34.Xu C., Chou G.X., Wang Z.T. A new diterpene from the leaves of Andrographis paniculata Nees. Fitoterapia. 2010;81(6):610–613. doi: 10.1016/j.fitote.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Zou Q.Y., Li N., Dan C., Deng W.L., Peng S.L., Ding L.S. A new ent-labdane diterpenoid from Andrographis paniculata. Chin. Chem. Lett. 2010;21(9):1091–1093. [Google Scholar]
- 36.Awang K., Abdullah N.H., Hadi A.H., Fong Y.S. Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. J. Biomed. Biotechnol. 2012;2012:876458. doi: 10.1155/2012/876458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hapuarachchi S.D., Ali Z., Abe N., Sugandhika S.T., Sandun S.T., Khan I.A., Andrographidine G. A new flavone glucoside from Andrographis paniculata. Nat. Prod. Commun. 2013;8(3):333–334. [PubMed] [Google Scholar]
- 38.Radhika P., Prasad Y.R., Sowjanya K. A new diterpene from the leaves of Andrographis paniculata Nees. Nat. Prod. Commun. 2012;7(4):485–486. [PubMed] [Google Scholar]
- 39.Xu C., Chou G.X., Wang C.H., Wang Z.T. Rare noriridoids from the roots of Andrographis paniculata. Phytochemistry. 2012;77:275–279. doi: 10.1016/j.phytochem.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Pholphana N., Rangkadilok N., Saehun J., Ritruechai S., Satayavivad J. Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian) Chin. Med. 2013;8(1):2. doi: 10.1186/1749-8546-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L.X., He H., Xia G.Y., Zhou K.L., Qiu F. A new flavonoid from the aerial parts of Andrographis paniculata. Nat. Prod. Res. 2014;28(3):138–143. doi: 10.1080/14786419.2013.856907. [DOI] [PubMed] [Google Scholar]
- 42.Suriyo T., Pholphana N., Rangkadilok N., Thiantanawat A., Watcharasit P., Satayavivad J. Andrographis paniculata extracts and major constituent diterpenoids inhibit growth of intrahepatic cholangiocarcinoma cells by inducing cell cycle arrest and apoptosis. Planta Med. 2014;80(7):533–543. doi: 10.1055/s-0034-1368399. [DOI] [PubMed] [Google Scholar]
- 43.Wang C.H., Li W., Qiu R.X., Jiang M.M., Li G.Q. A new diterpenoid from the aerial parts of Andrographis paniculata. Nat. Prod. Commun. 2014;9(1):13–14. [PubMed] [Google Scholar]
- 44.Lee S., Morita H., Tezuka Y. Preferentially cytotoxic constituents of Andrographis paniculata and their preferential cytotoxicity against human pancreatic cancer cell lines. Nat. Prod. Commun. 2015;10(7):1153–1158. [PubMed] [Google Scholar]
- 45.Zhang L., Liu Q., Yu J., Zeng H., Jiang S., Chen X. Separation of five compounds from leaves of Andrographis paniculata (Burm. f.) Nees by off-line two-dimensional high-speed counter-current chromatography combined with gradient and recycling elution. J. Sep. Sci. 2015;38(9):1476–1483. doi: 10.1002/jssc.201401458. [DOI] [PubMed] [Google Scholar]
- 46.Wang G.Y., Wen T., Liu F.F., Tian H.Y., Chun-Lin F., Huang X.J., Ye W.C., Wang Y. Two new diterpenoid lactones isolated from Andrographis paniculata. Chin. J. Nat. Med. 2017;15(6):458–462. doi: 10.1016/S1875-5364(17)30068-7. [DOI] [PubMed] [Google Scholar]
- 47.Gan L., Zheng Y., Deng L., Sun P., Ye J., Wei X., Liu F., Yu L., Ye W., Fan C., Liu J., Zhang W. Diterpenoid lactones with anti-inflammatory effects from the aerial parts of Andrographis paniculata. Molecules. 2019;24(15):2726. doi: 10.3390/molecules24152726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanh T., My N., Cham P.T., Quang T.H., Cuong N.X., Huong T.T., Nam N.H., Minh C.V. Diterpenoids and flavonoids from Andrographis paniculata. Chem. Pharm. Bull. 2020;68(1):96–99. doi: 10.1248/cpb.c19-00662. [DOI] [PubMed] [Google Scholar]
- 49.Wen Q., Jin X., Lu Y., Chen D.F. Anticomplement ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Fitoterapia. 2020;142:104528. doi: 10.1016/j.fitote.2020.104528. [DOI] [PubMed] [Google Scholar]
- 50.Puente L.A., Pinto-Muñoz C.A., Castro E.S., Cortés M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: a review. Food Res. Int. 2011;44(7):1733–1740. [Google Scholar]
- 51.Muniz J., Kretzschmar A.A., Rufato L., Pelizza T.R., Rufato A.D.R., Macedo T.A.D. General aspects of physalis cultivation, Ciência Rural. St. Maria Times. 2014;44(6):964–970. [Google Scholar]
- 52.Singh N., Singh S., Maurya P., Arya M., Khan F., Dwivedi D.H., Saraf S.A. An updated review on Physalis peruviana fruit: cultivational, nutraceutical and pharmaceutical aspects. Indian J. Nat. Prod. Resour. 2019;10(2):97–110. [Google Scholar]
- 53.Sahai M., Ray A.B. Secotropane alkaloids of Physalis peruviana. J. Org. Chem. 1980;45(16):3265–3268. [Google Scholar]
- 54.Frolow F., Ray A.B., Sahai M., Glotter E., Gottlieb H.E., Kirson I. Withaperuvin and 4-deoxyphysalolactone, two new ergostane-type steroids from Physalis peruviana(Solanaceae) J. Chem. Soc., Perkin Trans. 1981;1:1029. [Google Scholar]
- 55.Sahai M., Neogi P., Ray A.B. Structures of withaperwin B and C withanolides of Physalis perwiana roots. Heterocycles. 1982;19(1):37–40. [Google Scholar]
- 56.Bagchi A., Neogi P., Sahai M., Ray A.B., Oshima Y., Hikino H. Withaperuvin E and nicandrin B, withanolides from Physalis peruviana and Nicandra physaloides. Phytochemistry. 1984;23(4):853–855. [Google Scholar]
- 57.Ali A., Sahai M., Ray A.B., Slatkin D.J. Physalolactone C, a new withanolide from Physalis peruviana. J. Nat. Prod. 1984;47(4):648–651. [Google Scholar]
- 58.Neogi P., Sahai M., Ray A.B. Withaperuvins F and G, two withanolides of Physalis peruviana roots. Phytochemistry. 1987;26(1):243–247. [Google Scholar]
- 59.Eguchi T., Fujimoto Y., Kakinuma K., Ikekawa N., Sahai M., Verma M.P., Gupta Y.K. 23-Hydroxyphysalolactone, a new withanolide with a 23-hydroxyl group from Physalis peruviana (Solanaceae) Chem. Pharm. Bull. 1988;36(8):2897–2901. [Google Scholar]
- 60.Oshima Y., Hikino H., Sahai M., Ray A.B. Withaperuvin H, a withanolide of Physalis peruviana roots. J. Chem. Soc. Chem. Commun. 1989:628–629. [Google Scholar]
- 61.Waiss A.C., Elliger C.A., Benson M. Insect inhibitory lactone glucosides of Physalis peruvian. Nat. Prod. Lett. 1993;2(2):115–118. [Google Scholar]
- 62.Latza S., Ganßer D., Berger R.G. Carbohydrate esters of cinnamic acid from fruits of Physalis peruviana, Psidium guajava and Vaccinium vitis-idaea. Phytochemistry. 1996;43(2):481–485. [Google Scholar]
- 63.Dinan L.N., Sarker S.D., Šik V. 28-Hydroxywithanolide E from Physalis peruviana. Phytochemistry. 1997;44(3):509–512. [Google Scholar]
- 64.Ahmad S., Malik A., Afza N., Yasmin R. A new withanolide glycoside from physalis peruviana. J. Nat. Prod. 1999;62(3):493–494. doi: 10.1021/np980228o. [DOI] [PubMed] [Google Scholar]
- 65.Ahmad S., Malik A., Yasmin R., Ullah N., Gul W., Khan P.M., Nawaz H.R., Afza N. Withanolides from Physalis peruviana. Phytochemistry. 1999;50(4):647–651. [Google Scholar]
- 66.Ahmad S., Yasmin R., Malik A. New withanolide glycosides from Physalis peruviana L. Chem. Pharm. Bull. 1999;47(4):477–480. doi: 10.1021/np980228o. [DOI] [PubMed] [Google Scholar]
- 67.Mayorga H., Knapp H., Winterhalter P., Duque C. Glycosidically bound flavor compounds of cape gooseberry (Physalis peruviana L.) J. Agric. Food Chem. 2001;49(4):1904–1908. doi: 10.1021/jf0011743. [DOI] [PubMed] [Google Scholar]
- 68.Mayorga H., Duque C., Knapp H., Winterhalter P. Hydroxyester disaccharides from fruits of cape gooseberry (Physalis peruviana) Phytochemistry. 2002;59(4):439–445. doi: 10.1016/s0031-9422(01)00467-8. [DOI] [PubMed] [Google Scholar]
- 69.Cirigliano A., Colamarino I., Mareggiani G., Bado S. Biological effects of Physalis peruviana L. (Solanaceae) crude extracts and its major withanolides on Ceratitiscapitata Wiedemann (Diptera: Tephritidae) Boletin de Sanidadd Vegetal Plagas. 2008;34:509–515. [Google Scholar]
- 70.Fang S.T., Li B., Liu J.K. Two new withanolides from Physalis peruviana. Helv. Chim. Acta. 2009;92(7):1304–1308. [Google Scholar]
- 71.Lan Y.H., Chang F.R., Pan M.J., Wu C.C., Wu S.J., Chen S.L., Wang S.S., Wu M.J., Wu Y.C. New cytotoxic withanolides from Physalis peruviana. Food Chem. 2009;116(2):462–469. [Google Scholar]
- 72.Fang S.T., Liu J.K., Li B. A novel 1,10-seco withanolide from Physalis peruviana. J. Asian Nat. Prod. Res. 2010;12(7):618–622. doi: 10.1080/10286020.2010.482523. [DOI] [PubMed] [Google Scholar]
- 73.Fang S.T., Liu J.K., Li B. Ten new withanolides from Physalis peruviana. Steroids. 2012;77(1-2):36–44. doi: 10.1016/j.steroids.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 74.El-Gengaihi S.E., Hassan E.E., Hamed M.A., Zahran H.G., Mohammed M.A. Chemical composition and biological evaluation of Physalis peruviana root as hepato-renal protective agent. J. Diet. Suppl. 2013;10(1):39–53. doi: 10.3109/19390211.2012.760509. [DOI] [PubMed] [Google Scholar]
- 75.Franco L.A., Ocampo Y.C., Gómez H.A., De la Puerta R., Espartero J.L., Ospina L.F. Sucrose esters from Physalis peruviana calyces with anti-inflammatory activity. Planta Med. 2014;80(17):1605–1614. doi: 10.1055/s-0034-1383192. [DOI] [PubMed] [Google Scholar]
- 76.Toro R.M., Aragón D.M., Ospina L.F., Ramos F.A., Castellanos L. Phytochemical analysis, antioxidant and anti-inflammatory activity of calyces from Physalis peruviana. Nat. Prod. Commun. 2014;9(11):1573–1575. [PubMed] [Google Scholar]
- 77.Sathyadevi M., Subramanian S. Extraction, isolation and characterization of bioactive flavonoids from the fruits of Physalis peruvianalinn extract. Asian J. Pharm. Clin. Res. 2015;8(1):152–157. [Google Scholar]
- 78.Chang L.C., Sang-Ngern M., Pezzuto J.M., Ma C. The Daniel K. Inouye college of pharmacy scripts: poha berry (Physalis peruviana) with potential anti-inflammatory and cancer prevention activities. Hawai‘i J. Med. Public Health J. Asia Pac. Med. Public Health. 2016;75(11):353–359. [PMC free article] [PubMed] [Google Scholar]
- 79.Sang-Ngern M., Youn U.J., Park E.J., Kondratyuk T.P., Simmons C.J., Wall M.M., Ruf M., Lorch S.E., Leong E., Pezzuto J.M., Chang L.C. Withanolides derived from Physalis peruviana (Poha) with potential anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2016;26(12):2755–2759. doi: 10.1016/j.bmcl.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 80.Xu Y.M., Wijeratne E., Babyak A.L., Marks H.R., Brooks A.D., Tewary P., Xuan L.J., Wang W.Q., Sayers T.J., Gunatilaka A. Withanolides from Aeroponically Grown Physalis peruviana and their selective cytotoxicity to prostate cancer and renal carcinoma cells. J. Nat. Prod. 2017;80(7):1981–1991. doi: 10.1021/acs.jnatprod.6b01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernal C.A., Castellanos L., Aragón D.M., Martínez-Matamoros D., Jiménez C., Baena Y., Ramos F.A. Peruvioses A to F, sucrose esters from the exudate of Physalis peruviana fruit as α-amylase inhibitors. Carbohydr. Res. 2018;461:4–10. doi: 10.1016/j.carres.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Cicchetti E., Duroure L., Le Borgne E., Laville R. Upregulation of skin-aging biomarkers in aged NHDF cells by a sucrose ester extract from the Agroindustrial Waste of Physalis peruviana Calyces. J. Nat. Prod. 2018;81(9):1946–1955. doi: 10.1021/acs.jnatprod.7b01069. [DOI] [PubMed] [Google Scholar]
- 83.Dong B., An L., Yang X., Zhang X., Zhang J., Tuerhong M., Jin D.Q., Ohizumi Y., Lee D., Xu J., Guo Y. Withanolides from Physalis peruviana showing nitric oxide inhibitory effects and affinities with iNOS. Bioorg. Chem. 2019;87:585–593. doi: 10.1016/j.bioorg.2019.03.051. [DOI] [PubMed] [Google Scholar]
- 84.Ali M.A. Cassia fistula Linn: a review of phytochemical and pharmacological studies. Int. J. Pharm. Sci. Res. 2014;5(6):2125–2130. [Google Scholar]
- 85.Rahmani A.H. Cassia fistula Linn: potential candidate in the health management. Pharmacogn. Res. 2015;7(3):217–224. doi: 10.4103/0974-8490.157956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morimoto S., Nonaka G.I., Chen R.F., Nishioka I. Tannins and related compounds. LXI. Isolation and structures of novel bi- and triflavanoids from the leaves of Cassia fistula L, Chem. Pharm. Bull. 1988;36(1):39–47. [Google Scholar]
- 87.Mahesh V.K., Sharma R., Singh R.S., Upadhya S.K. Anthraquinones and kaempferol from Cassia species section fistula. J. Nat. Prod. 1984;47(4) 733–733. [Google Scholar]
- 88.Kashiwada Y., Iizuka H., Yoshioka K., Chen R.F., Nonaka G., Nishioka I. Tannins and related compounds. XCIII. Occurrence of enantiomeric proanthocyanidins in the leguminosae plants, Cassia fistula L. and C. javanica L. Chem. Pharm. Bull. 1990;38(4):888–889. [Google Scholar]
- 89.Lee C.K., Lee P.H., Kuo Y.H. The chemical constituents from the aril of Cassia Fistula L. J. Chin. Chem. Soc. 2001;48(6A):1053–1058. [Google Scholar]
- 90.Kuo Y.H., Lee P.H., Wein Y.S. Four new compounds from the seeds of Cassia fistula. J. Nat. Prod. 2002;65(8):1165–1167. doi: 10.1021/np020003k. [DOI] [PubMed] [Google Scholar]
- 91.Bahorun T., Neergheen V., Aruoma O. Phytochemical constituents of Cassia fistula. Afr. J. Biotechnol. 2006;4(13):1530–1540. [Google Scholar]
- 92.Sartorelli P., Andrade S.P., Melhem M.S., Prado F.O., Tempone A.G. Isolation of antileishmanial sterol from the fruits of Cassia fistula using bioguided fractionation. Phytother Res. 2007;21(7):644–647. doi: 10.1002/ptr.2131. [DOI] [PubMed] [Google Scholar]
- 93.Sartorelli P., Carvalho C.S., Reimão J.Q., Ferreira M.J., Tempone A.G. Antiparasitic activity of biochanin A, an isolated isoflavone from fruits of Cassia fistula (Leguminosae) Parasitol. Res. 2009;104(2):311–314. doi: 10.1007/s00436-008-1193-z. [DOI] [PubMed] [Google Scholar]
- 94.Nagpal M.A., Nagpal N., Rahar S., Shah G., Swami G., Kapoor R. Phytochemical investigation of methanolic extract of Cassia fistula leaves. Pharmacogn. J. 2011;3(26):61–69. [Google Scholar]
- 95.Duraipandiyan V., Baskar A.A., Ignacimuthu S., Muthukumar C., Al-Harbi N. Anticancer activity of rhein isolated from Cassia fistula L. flower. Asian Pac. J. Trop. Dis. 2012;2:S517–S523. [Google Scholar]
- 96.Sartorelli P., Lago J.H., Cunha R.L., Kitamura R.O., Young M.C. A new minor dimmeric ester from seeds of Cassia fistula L. (Leguminosae) Nat. Prod. Res. 2012;26(1):36–41. doi: 10.1080/14786419.2010.532128. [DOI] [PubMed] [Google Scholar]
- 97.Gao X.M., Shen Y.Q., Huang X.Z., Yang L.Y., Shu L.D., Hu Q.F., Li G.P. 2"-Ethyl-furanoflavone derivatives from the stems of Cassia fistula and their cytotoxicity. J. Brazilian Chem. Soc. 2013;24(4):685–689. [Google Scholar]
- 98.Wang L.Q., Tang Z.R., Mu W.H., Kou J.F., He D.Y. A new natural naphtho[1,2-b]furan from the leaves of Cassia fistula. J. Asian Nat. Prod. Res. 2013;15(11):1210–1213. doi: 10.1080/10286020.2013.812077. [DOI] [PubMed] [Google Scholar]
- 99.Zeng X., Zhao W., Zhang T., Wang L., Yang G., Chen Y.K., Hu Q., Miao M. A new flavonoid from the roots of Cassia fistula and its anti-tobacco mosaic virus activity. Asian J. Chem. 2013;25(12):6622–6624. [Google Scholar]
- 100.Zhao W., Zeng T., Zhang T., Wang L., Yang G., Chen Y.K., Hu Q., Miao M. Flavonoids from the bark and stems of Cassia fistula and their anti-tobacco mosaic virus activities. Phytochem. Lett. 2013;6(2):179–182. [Google Scholar]
- 101.Yang J., Wang H., Liu G., Lou J., Li L., Hu Q., Ye Y. A new anthraquinone from the fruit of Cassia fistula and its cytotoxicity. Asian J. Chem. 2014;26(14):4519–4520. [Google Scholar]
- 102.Li Y., Meng Y., Yang Y., Qin Y., Xia C., Ye Y., Goa X., Hu Q. Chromones from the stems of Cassia fistula and their anti-tobacco mosaic virus activities. Phytochem. Lett. 2014;10:46–49. [Google Scholar]
- 103.Zhou M., Zhou K., Gao X.M., Jiang Z.Y., Lv J.J., Liu Z.H., Yang G.Y., Miao M.M., Che C.T., Hu Q.F., Fistulains A. and B. New bischromones from the bark of Cassia fistula, and their activities. Org. Lett. 2015;17(11):2638–2641. doi: 10.1021/acs.orglett.5b01007. [DOI] [PubMed] [Google Scholar]
- 104.Hu Q.F., Li L.M., Zhu D.L., Yu Z.H., Zhan J.B., Lou J., Wang Y.D., Zhou M., Li Y.K., Gao X.M. Chromones from the twigs of Cassia fistula and their anti-tobacco mosaic virus activities. Heterocycles. 2015;91(10):1980–1985. [Google Scholar]
- 105.Srividhya M., Hridya H., Shanthi V., Ramanathan K. Bioactive Amento flavone isolated from Cassia fistula L. leaves exhibits therapeutic efficacy. 3 Biotech. 2017;7(1):33. doi: 10.1007/s13205-017-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu H.Y., Liu Q., Yu Z.H., Hu W.Y., Yan K.L., Wang Y.D., Zhou K., Dong W., Zhou M., Hu Q.F. Isoquinoline alkaloids from the twigs of Cassia fistula and their anti-tobacco mosaic virus activity. Heterocycles. 2016;92(1):114. [Google Scholar]
- 107.Zhou L., Liu L.X., Yang G.R., Xing H.H., Ma H.Y., Yang Y., Wang Y.D., Zhou K., Wei-Dong, Zhou M., Ye Y.Q., Hu Q.F. Two new isoquinoline alkaloids from the barks of Cassia fistula and their anti-tobacco mosaic virus activity. Chem. Nat. Compd. 2017;53(3):508–511. [Google Scholar]
- 108.Zhou M., Xing H.H., Yang Y., Wang Y.D., Zhou K., Dong W., Li G.P., Hu W.Y., Liu Q., Li X.M., Hu Q.F. Three new anthraquinones from the twigs of Cassia fistula and their bioactivities. J. Asian Nat. Prod. Res. 2017;19(11):1073–1078. doi: 10.1080/10286020.2017.1285911. [DOI] [PubMed] [Google Scholar]
- 109.Hu Q.F., Wang Y.D., Yu Z.H., Zhou K., Dong W., Zhou M., Li Y.K., Gao X.M., Zhu D.L., Ye Y.Q. Anti-tobacco mosaic virus chromones from the twigs of Cassia fistula. Chem. Nat. Compd. 2017;53(3):453–456. [Google Scholar]
- 110.Antonisamy P., Agastian P., Kang C.W., Kim N.S., Kim J.H. Anti-inflammatory activity of rhein isolated from the flowers of Cassia fistula L. and possible underlying mechanisms. Saudi J. Biol. Sci. 2019;26(1):96–104. doi: 10.1016/j.sjbs.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aftab Z., Afzal M., Bushra, Khan H., Badshah S., Khan D., Ullah H., Khan S., Fistuloates A.–C. New antioxidative aromatic compounds isolated from Cassia fistula. J. Chem. Res. 2019:1–7. [Google Scholar]
- 112.Grace M.H., Lategan C., Graziose R., Smith P.J., Raskin I., Lila M.A. Antiplasmodial activity of the ethnobotanical plant Cassia fistula. Nat. Prod. Commun. 2012;7(10):1263–1266. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.