Abstract
Purpose
Chitosan is a natural polymer that has excellent properties include biocompatibility, biodegradability, no cytotoxicity, high charge density, low cost, mucoadhesive, permeation enhancing (ability to cross tight junction), and immunomodulating ability that makes the spectrum of its applicability much broader. This study was conducted to investigate the stabilizing, preservative and immunogenicity properties of N-trimethyl chitosan nanospheres (N-TMCNS).
Materials and Methods
The tetanus toxoid (TT) was encapsulated into N-TMCNS and then characterized by scanning electron microscope, atomic force microscope, and dynamic light scattering. For stabilizer assay of N-TMCNS after storage of TT-N-TMCNS at different temperatures for 3 weeks, they were used for immunization of mice and different temperatures groups' anti-TT-N-TMCNS production compared with other groups. Finally, the immunized mice were challenged with tetanus toxin. The preservation activity of TT-N-TMCNS against Escherichia coli was compared with thimerosal formulated TT.
Results
Our results revealed that heat-treated TT-N-TMCNS could induce higher titer of neutralizing immunoglobulin G in compared to TT vaccine and was able to protect the mice better than TT vaccine in challenge test. Furthermore, N-TMCNS as a preservative inhibited the growth of E. coli more effective than thimerosal.
Conclusion
Overall, the obtained results indicated that the N-TMCNS is one of the best stabilizer and preservative agent that can be used in the formulation of TT vaccine.
Keywords: N-trimethyl chitosan chloride, Nanospheres, Tetanus toxoid, Stabilizer, Preservative
Introduction
Tetanus is an infectious disease caused by Clostridium tetani toxin that involves the nervous system [1,2]. The World Health Organization estimates that only in 2013, about 49,000 newborns were killed by tetanus [3]. Vaccination has been one of the most effective approaches for controlling various infectious diseases. Tetanus toxoid (TT) is used to make vaccines to develop immunity against tetanus [4]. Beside the immunogenicity, there are two particularly important factors that can increase the efficiency of TT vaccines: (1) the choice of stabilizers for vaccine formulation, such as alum and sucrose and (2) the preservative compounds such as thimerosal [5]. Nowadays, about 80% of the vaccination cost is incurred due to cold chain requirements for protecting the vaccines. Therefore, using stabilizers that can remove the cold chain requirements and protect the vaccine formulation in high ambient temperatures is a major concern for vaccine manufacturers [6]. In addition, the current stabilizers approved for use in human vaccines are expensive. Thus, the vaccine developers are looking for low-cost stabilizers that are capable of protecting the vaccine against ambient temperatures [7].
Chitosan is a linear polysaccharide made of D-glucosamine and N-acetyl-D-glucosamine units. After cellulose, chitosan is the most abundant natural polymer [8]. Chitosan is a conveniently suitable biopolymer for various biomedical applications because of its rigid linear molecular structure, biocompatibility, biodegradability, non-toxicity, and antibacterial activity [9,10]. So, the focus of this study is the assessment of chitosan stabilizing capability resulting from its polycationic and conformational nature [9].
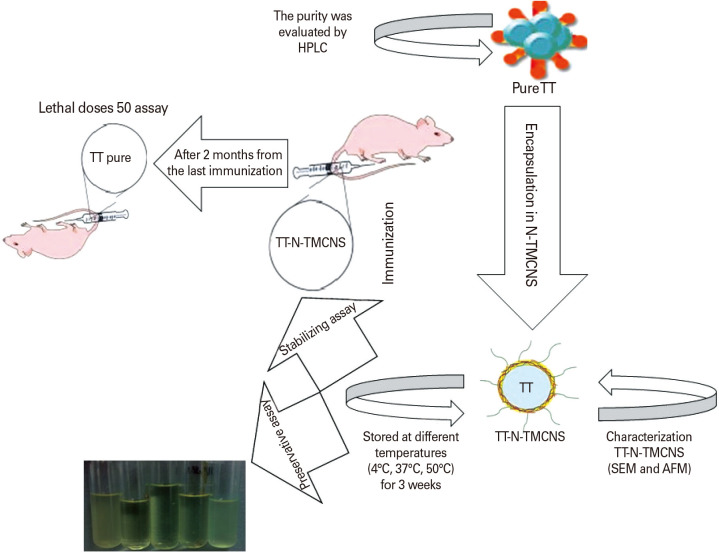
In this regard, adding preservative agents to vaccine formulation, especially in multi-dose formulations is necessary to prevent the growth of microorganisms [11]. However, some of these preservative compounds may cause toxicological side effects, especially in childhood vaccines [12]. For example, thimerosal is a mercury-based organometallic preservative that is used in TT vaccine formulation as an antiseptic and antifungal agent [13]. Despite these useful properties, there are reports about the toxic side effects of this compound, including causing central nervous system diseases such as autism. A literature review in this field reveals that chitosan and chitosan nanoparticles have antibacterial properties that make them suitable for use as preservative agents in vaccine formulation [14,15]. Despite the above-mentioned advantages for chitosan, the low aqueous solubility and possible aggregation in serum under the normal physiological pH are the major challenges in the exploitation of chitosan as a preservative agent [16]. To address this issue in this research, we used N-trimethyl chitosan (N-TMC) with improved solubility at physiological pH of 7.4 for preparation of N-TMC nanospheres (N-TMCNS) by ionic gelation technique. In order to evaluate the specified characteristics of N-TMC, we encapsulated TT in the N-TMCNS and investigated their stabilizing property in various temperatures (i.e., low, medium, and high) as well as its preserving properties (Appendix 1: graphical abstract).
Materials and Methods
Chemicals and reagents
Tetanus vaccine, toxin, and toxoid were obtained from Razi Vaccine and Serum Research Institute (Karaj, Iran). All reagents and N-trimethyl chitosan chloride were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Characterization of tetanus toxoid samples
The purity of TT samples was evaluated by a Cecil high-performance liquid chromatography (HPLC) system (Cecil Instruments Ltd., Cambridge, UK) equipped with an ultraviolet-visible detector. These measurements were performed in the mobile phase by injecting the liquid mixture of methanol (0.005 M acetate buffer pH 4.5, 4:6 volume/volume) into a stream of liquid with the flow rate of 1.0 cm3/min created by an HPLC pump (model HLC-803A; Toyo Soda Manufacturing Co. Ltd., Tokyo, Japan) at room temperature. A Kromasil 100-10-C18 column (4.6 mm×250 mm) was used and fractions were collected from absorbance peaks at 280 nm. Also, to confirm the HPLC results, the purity of TT protein samples was also evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) 12% with constant current 25 mA.
Encapsulation of tetanus toxoid in N-TMCNS
N-TMCNS were prepared by ionic gelation technique. The aqueous N-TMC solution with the concentration of 1% weight per volume (w/v) was prepared by dissolving 10 mg N-TMC powder in 10 mL HEPES buffer solution (50 mM, pH 7.4) using magnetic stirring. Then, 60 µL of aqueous pentasodium tripolyphosphate solution (10 mg/mL) with desired TT protein concentration was added to the N-TMC solution dropwise. Each of these steps was separated by 15-minute time intervals. The final solution was centrifuged at 14,000 g for about 30 minutes to separate the produced N-TMCNS. In order to estimate the amount of TT loaded in the N-TMCNS, the concentration of remaining TT in the supernatant was determined spectrophotometrically using Bradford protein assay and the encapsulation efficiency (EE%) was calculated as follows [17]:
Where AFP is the concentration of remaining TT in the supernatant after centrifugation and ATP is the initial total concentration of TT.
Characterization of N-TMCNS
The surface morphology of the N-TMCNS in the prepared samples was characterized by scanning electron microscope (SEM, KYKY-EM3200; KYKY Technology Development Ltd., Beijing, China) operated at 26 kV. Also, their surface topography and roughness were analyzed by atomic force microscopy (AFM, model DS 95-200/50; DME, Herlev, Denmark), in contact mode with a Si3N4 tip and under atmospheric condition. The hydrodynamic size distribution of the nanoparticles was determined by dynamic light scattering (DLS) using a Zetasizer system (Nano ZS; Malvern Instruments, Malvern, UK). The same apparatus was used to measure the zeta potentials of the nanoparticles, which can help provide an insight into the surface charges of these particles [18].
Assessment of stabilizing property of TT-N-TMCNS
In order to study the stabilization behavior of N-TMCNS, the TT-N-TMCNS samples were stored at different temperatures, i.e., cold (4℃), incubation (37℃), and high (50℃), for 3 weeks. Samples of pure TT, encapsulation TT in N-TMCNS, and TT with N-TMC solutions and the conventional TT vaccine (containing alum) were used as controls. Afterward, the immunogenicity of encapsulation TT-N-TMCNS samples was assayed in an animal model. The Balb/c mice (8 weeks old) were purchased from Razi Vaccine and Serum Research Institute and maintained under standard conditions. All experiments were conducted in accordance with the Guideline from the Animal Care Committee of Imam Hossein University, which is in agreement with the Guide for the Care and Use of Laboratory Animals (DHEW publication no. [NIH] 85-23, revised 1985). The animals were randomly divided into five groups (seven mice each group) and immunized by different TT formulations and phosphate-buffered saline (PBS; control) according to Table 1. Prime immunizations were performed on day 1st and 14th, and boost immunizations on day 28th and 42nd. Blood samples were taken 20 days after the last immunization.
Table 1. The maximum values of LD50 (mg/mL) for different groups of mice challenged with tetanus toxin after 3 months from last immunization (only for formulations which store in 50℃ for 3 weeks).
| Mice groups | Encapsulation TT-N-TMC | Alum TT | TT-N-TMC soluble | Pure TT |
|---|---|---|---|---|
| Survival at LD50 | 106 | 104 | 102 | 102 |
TT, tetanus toxoid; N-TMC, N-trimethyl chitosan.
Determination of antibody titer by indirect enzyme-linked immunosorbent assay
The amount of anti-TT antibodies in the serum samples of mice groups was determined by indirect enzyme-linked immunosorbent assay (ELISA) [19]. The microplate was coated by TT solution (2 µg/100 µL/well) and incubated at 37℃ for 2 hours. between the steps, the plates were washed with PBS-Tween (PBST; PBS, 0.05%, Tween 20). Next, the blocking step was performed by applying the blocking buffer (2% bovine serum albumin in PBST w/v) and then 100 µL of the serially diluted serum (1:100 to 1:12,800) was administered into each well. After that, 100 µL horseradish peroxidase-conjugated anti-mouse immunoglobulins (1/2,000, Sigma-Aldrich) was added to each well and the plates were incubated at 37℃ for about 1 hour. Next, 3, 3′, 5, 5′-Tetramethylbenzidine dihydrochloride hydrate (N-TMB) substrate was added to the plates and then the plates were incubated in the dark room temperature. Finally, the reaction was stopped by adding 2 M H2SO4 solution and the plates were read at 495 nm in a BioTek EL×800 Absorbance Microplate Readers (BioTek Instruments, Winooski, VT, USA).
Challenge experiment
After 2 months from the last immunization, the immunized and control mice were challenged with up to 106 lethal doses of tetanus toxin. Briefly, the mice were injected subcutaneously with TT toxin in PBS buffer and their mortality was monitored for up to 72-hour post-injection [20].
Antibacterial activity assay
For studying the preservative and antibacterial activity of N-TMC, the thimerosal in TT vaccine was replaced by N-TMC solution (0.1% w/v). TT vaccines with and without thimerosal were used as positive and negative controls, respectively. Then, 200 µL of Escherichia coli solution (1.2×109 colony-forming unit/mL) and 100 µL of different formulations of TT vaccine, explained above, were added to 4 mL of nutrient broth medium and incubated at 37℃ for 24 hours. The bacterial growth was analyzed by measuring the solution turbidity by a spectrophotometer (CE-1100; Cecil Instruments Ltd.) according to the McFarland standard [21].
Results
Characterization of tetanus toxoid protein and N-TMCNS
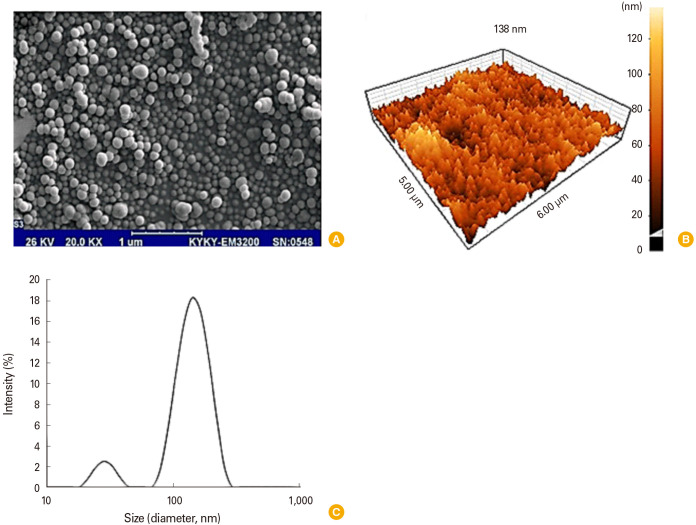
HPLC chromatography technique was used to determine the purity of the TT protein samples. As shown in Fig. 1, only one clear peak can be seen in the HPLC chromatogram that occurs at the retention time of 12 minutes, which confirms the purity of the TT protein sample. Moreover, the SDS-PAGE results (Fig. 2) confirmed that the TT sample contained pure protein due to observing no other bands. Since the tested TT sample was integral protein, it was completely retained by formaldehyde in the gel. Fig. 3A shows an SEM image of the N-TMCNS containing TT protein. Analyzing SEM images, the average diameter of the produced TT loaded N-TMCNS, which are monodispersed spherical nanoparticles, was found to be about 90 nm. The surface roughness and porosity of the TT loaded N-TMCNS can also be seen clearly in the three-dimensional (3D) AFM image of these particles shown in Fig. 3B. Such surface features are important for enhancing the TT loading efficiency. Hydrodynamic particle size distribution profile of the TT loaded N-TMCNS was measured by DLS as well, and the obtained results were used for crosschecking the SEM and AFM data. The DLS measurement results are demonstrated in Fig. 3C. As can be seen, there are two particle sizes of 30 nm and 140 nm in the aqueous phase. The zeta potential measurement showed that the zeta potential value of pure N-TMCNS is 34.3 mV, while it drops down to 18.8 mV for TT loaded N-TMCNS. According to the results of Bradford protein assay, the EE% of loading the TMCNS sample with TT is 92%.
Fig. 1. Analysis of tetanus toxoid purity by HPLC method. One clear peak can be seen in the HPLC chromatogram that occurs at the retention time of 12 minutes, which confirms the purity of the tetanus toxoid protein sample. HPLC, high-performance liquid chromatography; AU, absorbance units.
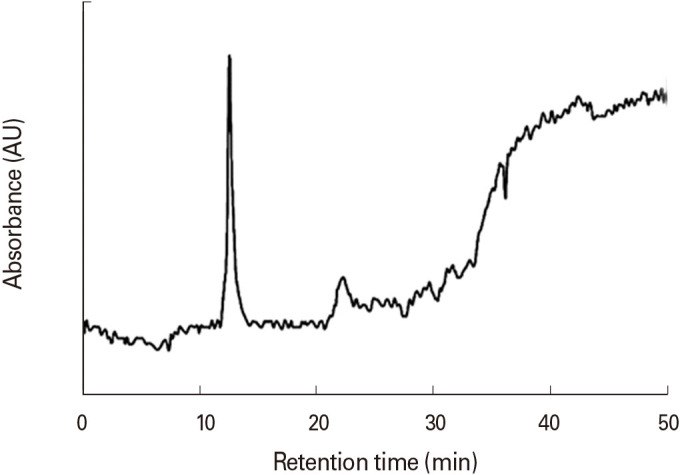
Fig. 2. Analysis of tetanus toxoid purity by SDS-PAGE method. SDS-PAGE analysis showed only a 150 kDa band in the electrophoresis gel. SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; TT, tetanus toxoid.
Fig. 3. Analysis size and shape of N-TMCNS loaded with tetanus toxoid with different methods. (A) Scanning electron microscope image of TT-loaded N-TMCNS, (B) AFM, and (C) hydrodynamic particle size distribution images of N-TMCNS containing TT. (A) The average diameter of the produced TT loaded N-TMCNS, which are monodispersed spherical nanoparticles, was found to be about 90 nm. (B) AFM result showed that nanospheres was about 90 nm in the dynamic light scattering method, and (C) two types of nanospheres with a diameter of 40 and 150 nm were observed. N-TMCNS, N-trimethyl chitosan nanospheres; TT, tetanus toxoid; AFM, atomic force microscope.
Anti-tetanus toxin immunoglobulin G antibody measurement
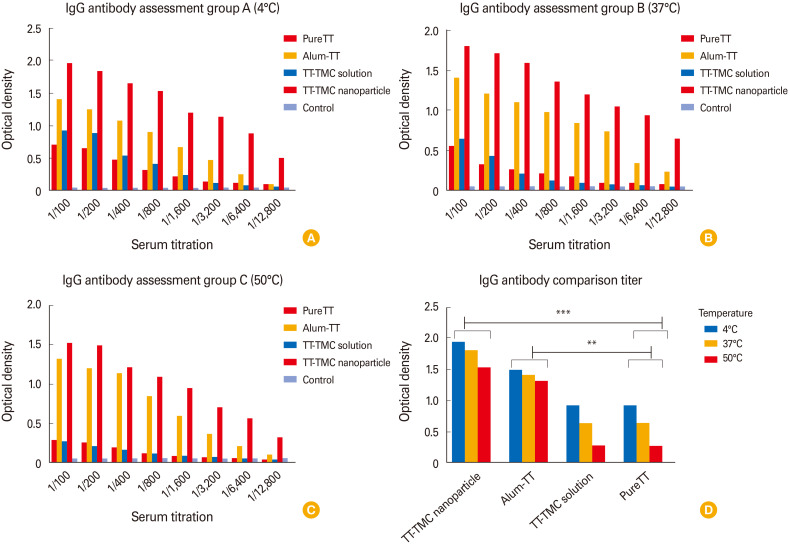
After incubating the TT samples with different formulations (alum, TT-N-TMC soluble, and encapsulation TT in N-TMC) at 4℃, 37℃, and 50℃ for 3 weeks, the samples were used for immunization of different mice groups. Then, blood samples were collected from each group of mice to be used in the relevant immunoassay (ELISA). The results showed that the N-TMC capsules can stabilize TT protein as same as an alum and can be used as a protective compound (stabilizer). Indeed, these nanoparticles could provide even better protection for TT protein (although not statically significant) under low, medium, and high-temperature conditions (Fig. 3A, B). The samples with N-TMC solution offered a weak stabilizing effect at 4℃ for 3 weeks, but it could not protect TT protein exposed to temperatures of 37℃ and 50℃. As expected, the TT protein in all of the formulations was protected more strongly at 4℃ than at 37℃ and 50℃. So, the antibody titers of the mice that were immunized by the TT formulations kept at 4℃ were higher than those kept at 37℃ and 50℃. The Fig. 4A, B, and C represent antibody assay results for different groups of mice after incubating the TT samples with different formulations at 4℃ (Fig. 4A), 37℃ (Fig. 4B), 50℃ (Fig. 4C) for 3 weeks.
Fig. 4. Antibody assay results for different groups of mice after incubating the TT samples with different formulations at (A) 4℃, (B) 37℃, (C) 50℃ for 3 weeks, and (D) antibody comparison. IgG, immunoglobulin G; TT, tetanus toxoid; TMC, tri-methyl chitosan. **p<0.01 and ***p<0.001 revealed statistically significant.
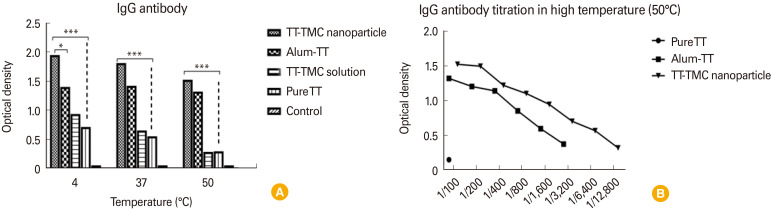
The N-TMCNS samples stored at 4℃ can protect TT protein more effectively in comparison with the samples kept at 37℃ and 50℃. In other words, the optical density of the serum collected from those mice immunized by N-TMCNS samples at 4℃ was higher than 37℃ and 50℃ (a 20% reduction in 50℃ and 10% in 37℃). The same result was obtained for TT and N-TMC solution and pure TT samples. Moreover, the TT samples with alum (conventional TT vaccine) showed the same level of antibody titer regardless of the storage temperature. Among various formulations used in this experiment and for all storage temperatures (i.e., 50℃, 37℃, and 4℃), the serum samples from the mice immunized by TT-N-TMCNS formulations had the highest antibody titer, except for the TT formulation with alum that resulted in the same antibody titer in the mice blood for various storage temperatures. The formulation with N-TMC solution as well as pure TT could not protect TT protein against temperature tension and, consequently, the antibody titer of the respective mice group was lower than other groups. The Fig. 5 represented as mean value±standard deviation all groups antibody titer compared with control group.
Fig. 5. (A) Various TT formulations incubated at different temperatures. Then, they were used for immunization of different groups of mice. As shown in figure, TT-N-TMC formulation could protect the tetanus toxin in low, medium and high temperature during 3 weeks and can stimulate antibody response. However, a 10% and 20% decrease in 37℃ and 50℃, respectively, are seen in stimulation of immune system. Conventional vaccine (TT-alum) could tolerate the heat stress and stimulate immune system almost similar to TT-N-TMC formulation but free chitosan with TT and TT-alone dramatically degraded in high temperature and could not effectively stimulate immune responses. (B) Antibody titration showed that TT-N-TMC can protect the TT against heat stress better than TT-alum. TT, tetanus toxoid; TMC, tri-methyl chitosan; N-TMC, N-trimethyl chitosan. Values are presented as mean±standard deviation; *p<0.05 and ***p<0.001 revealed statistically significant different groups compared to the control group.
Challenge experiment
The mice immunized by the formulations kept at 50℃ were selected for the challenge against tetanus toxin. As shown in Table 1 and Fig. 6, the LD50 of the control group (TT alone) was 102, while the mice that were immunized by the TT-N-TMCNS formulation survived in the LD50 of 106. The mice in the group immunized by the TT formulation containing alum (TT vaccine) survived at the toxin doses with LD50 of 104. The LD50 of the control groups in which the mice were immunized by the N-TMC solution and pure TT formulations was 102.
Fig. 6. (A, B) Mice after treated with LD50 of different formulation of tetanus toxoid.
Antibacterial activity measurement
The results of antibacterial activity test are summarized in Table 2. In this experiment, the standard sample was the commercial TT vaccine with thimerosal as a preservative and thimerosal was replaced by N-TMC in the test samples. Results showed that there is no E. coli growth in the presence of N-TMC in the nutrient broth medium. The same antibacterial effect was observed in the standard sample with thimerosal. In other words, N-TMC was found to have high preservative activity in TT vaccines (Fig. 7).
Table 2. The preservative activity of trimethyl chitosan in comparison with thimerosal.
| Sample group no. | Content | E. coli growth |
|---|---|---|
| 1 | NB + E. coli | Yes |
| 2 | NB + E. coli + TT vaccine with thimerosal | No |
| 3 | NB + E. coli + encapsulation TT in N-TMC | No |
| 4 | NB + E. coli + TT with N-TMC solution | No |
| 5 | NB + E. coli + TT vaccine without thimerosal | Yes |
E. coli, Escherichia coli; NB, nutrient broth; TT, tetanus toxoid; N-TMC, N-trimethyl chitosan.
Fig. 7. Escherichia coli growth in the presence different treatments in the nutrient broth medium. The numbers on the tubes represent the treatment group.

Discussion
Cold chain storage, transportation into distant tropical areas, and administration are among the important parts of vaccine production that affect vaccine efficacy and cost. The stabilizers in the vaccine formulation can prevent the inactivation of immunogens against high temperature. Currently, many vaccines such as TT toxoid still require the cold chain. Therefore, many attempts have been made to develop a novel thermostabilizing system that can keep the vaccine immunogens against high temperature.
The present study investigates the stabilized and preservative role of chitosan in TT toxoid model. First, the purity of TT toxoid was examined by HPLC and SDS-PAGE. The results showed no extra proteins or components in TT toxoid. So, observing any stabilizing or preservative activity can be specifically referred to chitosan. Pirouzmand et al. and Walke et al. did not mention the purity of their TT protein or the existence of other components in their study [17,18].
The size of the N-TMCNS nanoparticles was measured using different methods. The SEM observations showed that the mean diameter of these nanospheres was about 90 nm, which was confirmed by the 3D AFM method. However, in the DLS method, two types of nanospheres with a diameter of 40 and 150 nm were observed. The smooth surface of the nanoscale indicated the complete removal of excess proteins during washing and not remaining on the surface of the nanospheres. A positive Zeta potential level suggests that the nanospheres can be easily bound to the immune cell membrane with the negative charge [22]. Also, Harde et al. [23] reported the TT-nanosphere size between 100 and 200 nm by SEM and TEM methods and showed that APC cells are phagocytes, which better and more than free toxoid induce immune responses. Benne et al. [24] reported that the optimum size for nanoparticles is less than 100 nm, because the antigens are easily transferred from the injection site to the lymph nodes, and the small particles are better captured by the APCs. On the other hand, the large particles transfer a larger amount of antigen into the APCs. In the next step, particles smaller than 200 easily are introduced to APCs, processed, and presented into major histocompatibility complex class II. Very small particles (<40) more induce cell-mediated immunity and TH1 cells. Reversely, 100-nanometer nanoparticles stimulate humoral immunity and antibody. In the case of TT, humoral immunity and antibody response are essential for protection, which is well induced by 100 nm nanoparticles. As a result, the size, shape, and elasticity of the particle affect the immunogenicity of the particle [25]. Moreover, Abkar et al. [26] reported that TMC/omp19 nanospheres with 200 nm size were able to stimulate both T helper (Th)2 and/or Th1/Th17 immune responses because of their route administration [26].
In the present study, the amount of protein found in nanospheres was 92%, which contained a high protein content and a small amount of protein was wasted in the process of encapsulation. Other researchers have also reported the amount of encapsulation from 80% to over 90% [23]. ELISA results showed that TT alone and/or in combination with free chitosan in solution was not able to resist temperatures above 4℃. In other words, until toxoid is not encapsulated, the existing free chitosan in the solution cannot protect it against high temperatures. So, toxoid quickly degraded and could not stimulate the immune system efficiently [27,28,29].
The comparison between storage of the chitosan nanospheres (TT-N-TMCNS) and the alum vaccine at various temperatures showed that they both are capable to tolerate the high temperature, suggesting that nanochitosan is able to play a similar role in the stability of the TT protein. Also, chitosan has some advantages such as lower cost compared to alum vaccine, bioavailability, biodegradability, biocompatibility, and non-toxicity. In addition, chitosan was approved as a “generally recognized as safe (GRAS)” material according to United States Food and Drug Administration [30].
The antibody titer in immunized mice with alum vaccine and chitosan nanospheres was slightly different in favor of the nanosphere. Another property of N-TMCNS is an immunostimulatory or adjuvant effect. The adjuvanticity effect of N-TMC has been associated to its 40%–50% degree of quaternization (DQ). A 40% DQ makes N-TMC transport and deliver a variety of antigens and generate an optimum immune response. However, N-TMC by itself has no antigenic property and the immune system does not response against chitosan [31].
Although the antibody titer in TT-N-TMC mice group and alum vaccine group was similar, in the challenge test with tetanus toxin, TT-N-TMC mice had tolerated the toxin up to 100 times more than alum vaccine mice group. Considering the similarity of the antibody titer in both groups, only the level of neutralizing antibody induction and antibody affinity could be proposed. In this regard, one of the major issues that affect the induction of neutralizing antibody is an adjuvant role. The high stimulation of immune cells such as B cells can induce and produce more neutralizing antibodies with a greater affinity [32,33].
In the TT vaccine, the thimerosal as a preservative is an ethyl mercury derivative and prevents the vaccine against bacterial contamination. However, some adverse reactions due to thimerosal are reported in vaccine administration [34,35]. In the present study, we showed that chitosan in addition to its antigen delivery and adjuvant has a preservative property. Therefore, chitosan as a safe molecule (GRAS) prevents E. coli growth in the vaccine. So, N-TMCNS could be used as an alternative preservative agent for the currently toxic one in the vaccines.
In conclusion, according to results of this research, the immunization by the TT-N-TMCNS formulation (stored at different temperature levels) resulted in higher neutralizing immunoglobulin G titers than immunization by the TT-alum formulation. Besides, there is no E. coli growth in the TT-N-TMCNS samples. This study is the first to offer results that demonstrate stronger stabilizing and preserving properties of N-TMCN in comparison with alum. Based on these findings, we propose the application of N-TMCNS as a promising adjuvant compound in TT vaccine formulations. Further studies are underway to assess the stabilizing and preserving behavior of N-TMCNS in other vaccines.
Appendix 1
Experiment design in graphical abstract
Footnotes
No potential conflict of interest relevant to this article was reported.
Authors wish to thank all staffs of Student Research Committee, Department of Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; Applied Virology Research Center, Baqiyatallah University of Medical Science, Tehran, Iran; Biology Research Center, Faculty of Basic Sciences, Imam Hossein University, Tehran, Iran; Razi Vaccine and Serum Research Institute, Karaj, Iran; Department of Polymer Science and Engineering, University of Bonab, Bonab, Iran; Molecular Biology Research Center, Systems Biology and Poisonings Institute, Baqiyatallah University of Medical Science, Tehran, Iran; Chemical Injuries Research Center, Systems Biology and Poisonings Institute, Baqiyatallah University of Medical Science, Tehran, Iran, for their cooperation in implementing experimental procedures and analysis of data.
References
- 1.Candel FJ, Rico CM, Diaz de la Torre I, et al. Update in infectious diseases 2019. Rev Esp Quimioter. 2019;32 Suppl 2:1–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Surana S, Tosolini AP, Meyer IFG, Fellows AD, Novoselov SS, Schiavo G. The travel diaries of tetanus and botulinum neurotoxins. Toxicon. 2018;147:58–67. doi: 10.1016/j.toxicon.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Blencowe H, Cousens S, Mullany LC, et al. Clean birth and postnatal care practices to reduce neonatal deaths from sepsis and tetanus: a systematic review and Delphi estimation of mortality effect. BMC Public Health. 2011;11(Suppl 3):S11. doi: 10.1186/1471-2458-11-S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezaie E, Nekoie H, Miri A, et al. Different frequencies of memory B-cells induced by tetanus, botulinum, and heat-labile toxin binding domains. Microb Pathog. 2019;127:225–232. doi: 10.1016/j.micpath.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Sanina N. Vaccine adjuvants derived from marine organisms. Biomolecules. 2019;9:340. doi: 10.3390/biom9080340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Pasquale A, Preiss S, Tavares Da Silva F, Garcon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel) 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31 Suppl 2(Suppl 2):B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biao L, Tan S, Wang Y, et al. Synthesis, characterization and antibacterial study on the chitosan-functionalized Ag nanoparticles. Mater Sci Eng C Mater Biol Appl. 2017;76:73–80. doi: 10.1016/j.msec.2017.02.154. [DOI] [PubMed] [Google Scholar]
- 9.Islam N, Dmour I, Taha MO. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon. 2019;5:e01684. doi: 10.1016/j.heliyon.2019.e01684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motiei M, Kashanian S, Lucia LA, Khazaei M. Intrinsic parameters for the synthesis and tuned properties of amphiphilic chitosan drug delivery nanocarriers. J Control Release. 2017;260:213–225. doi: 10.1016/j.jconrel.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Meyer BK, Ni A, Hu B, Shi L. Antimicrobial preservative use in parenteral products: past and present. J Pharm Sci. 2007;96:3155–3167. doi: 10.1002/jps.20976. [DOI] [PubMed] [Google Scholar]
- 12.Geier DA, Jordan SK, Geier MR. The relative toxicity of compounds used as preservatives in vaccines and biologics. Med Sci Monit. 2010;16:SR21–SR27. [PubMed] [Google Scholar]
- 13.Hurley AM, Tadrous M, Miller ES. Thimerosal-containing vaccines and autism: a review of recent epidemiologic studies. J Pediatr Pharmacol Ther. 2010;15:173–181. [PMC free article] [PubMed] [Google Scholar]
- 14.Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339:2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Benhabiles MS, Salah R, Lounici H, Drouiche N, Goosen MF, Mameri N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012;29:48–56. [Google Scholar]
- 16.Tafaghodi M, Saluja V, Kersten GF, et al. Hepatitis B surface antigen nanoparticles coated with chitosan and trimethyl chitosan: impact of formulation on physicochemical and immunological characteristics. Vaccine. 2012;30:5341–5348. doi: 10.1016/j.vaccine.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 17.Pirouzmand H, Khameneh B, Tafaghodi M. Immunoadjuvant potential of cross-linked dextran microspheres mixed with chitosan nanospheres encapsulated with tetanus toxoid. Pharm Biol. 2017;55:212–217. doi: 10.1080/13880209.2016.1257032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walke S, Srivastava G, Routaray CB, et al. Preparation and characterization of microencapsulated DwPT trivalent vaccine using water soluble chitosan and its in-vitro and in-vivo immunological properties. Int J Biol Macromol. 2018;107(Pt B):2044–2056. doi: 10.1016/j.ijbiomac.2017.10.073. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Pan Y, Tao Y, et al. Development of a sensitive monoclonal antibody–based indirect competitive enzyme-linked immunosorbent assay for the determination of monensin in edible chicken tissues. Food Anal Methods. 2019;12:1479–1486. [Google Scholar]
- 20.Jneid B, Moreau K, Plaisance M, Rouaix A, Dano J, Simon S. Role of T3SS-1 SipD protein in protecting mice against non-typhoidal Salmonella typhimurium. PLoS Negl Trop Dis. 2016;10:e0005207. doi: 10.1371/journal.pntd.0005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khanifar J, Hosseini RH, Kazemi R, Ramandi MF, Amani J, Salmanian AH. Prevention of EHEC infection by chitosan nano-structure coupled with synthetic recombinant antigen. J Microbiol Methods. 2019;157:100–107. doi: 10.1016/j.mimet.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Ha HK, Rankin SA, Lee MR, Lee WJ. Development and characterization of whey protein-based nano-delivery systems: a review. Molecules. 2019;24:3254. doi: 10.3390/molecules24183254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harde H, Agrawal AK, Jain S. Tetanus toxoids loaded glucomannosylated chitosan based nanohoming vaccine adjuvant with improved oral stability and immunostimulatory response. Pharm Res. 2015;32:122–134. doi: 10.1007/s11095-014-1449-5. [DOI] [PubMed] [Google Scholar]
- 24.Benne N, van Duijn J, Kuiper J, Jiskoot W, Slutter B. Orchestrating immune responses: how size, shape and rigidity affect the immunogenicity of particulate vaccines. J Control Release. 2016;234:124–134. doi: 10.1016/j.jconrel.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Jaganathan KS, Rao YU, Singh P, et al. Development of a single dose tetanus toxoid formulation based on polymeric microspheres: a comparative study of poly(D,L-lactic-co-glycolic acid) versus chitosan microspheres. Int J Pharm. 2005;294:23–32. doi: 10.1016/j.ijpharm.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Abkar M, Lotfi AS, Amani J, et al. Survey of Omp19 immunogenicity against Brucella abortus and Brucella melitensis: influence of nanoparticulation versus traditional immunization. Vet Res Commun. 2015;39:217–228. doi: 10.1007/s11259-015-9645-2. [DOI] [PubMed] [Google Scholar]
- 27.Premaletha K, Licy CD, Jose S, Saraladevi A, Shirwaikar A, Shirwaikar A. Formulation, characterization and optimization of hepatitis B surface antigen (HBsAg)-loaded chitosan microspheres for oral delivery. Pharm Dev Technol. 2012;17:251–258. doi: 10.3109/10837450.2010.535824. [DOI] [PubMed] [Google Scholar]
- 28.Esmaili Gourvarchin Galeh H, Meysam Abtahi, Afzale Ahangaran N, Hadai SN. Effects of educated monocytes with xenogeneic mesenchymal stem cell-derived conditioned medium in a mouse model of chronic asthma. Immunol Invest. 2018;47:504–520. doi: 10.1080/08820139.2018.1458108. [DOI] [PubMed] [Google Scholar]
- 29.Shariatinia Z. Pharmaceutical applications of chitosan. Adv Colloid Interface Sci. 2019;263:131–194. doi: 10.1016/j.cis.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Malik A, Gupta M, Gupta V, Gogoi H, Bhatnagar R. Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int J Nanomedicine. 2018;13:7959–7970. doi: 10.2147/IJN.S165876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Yu R, Fang T, et al. Tetanus neurotoxin neutralizing antibodies screened from a human immune scFv antibody phage display library. Toxins (Basel) 2016;8:266. doi: 10.3390/toxins8090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ Health Perspect. 2005;113:1015–1021. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurley AM, Tadrous M, Miller ES. Thimerosal-containing vaccines and autism: a review of recent epidemiologic studies. J Pediatr Pharmacol Ther. 2010;15:173–181. [PMC free article] [PubMed] [Google Scholar]
- 34.Jneid B, Moreau K, Plaisance M, Rouaix A, Dano J, Simon S. Role of T3SS-1 SipD protein in protecting mice against non-typhoidal salmonella typhimurium. PLoS Negl Trop Dis. 2016;10:e0005207. doi: 10.1371/journal.pntd.0005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zapata A, Ramirez-Arcos S. A comparative study of McFarland turbidity standards and the Densimat photometer to determine bacterial cell density. Curr Microbiol. 2015;70:907–909. doi: 10.1007/s00284-015-0801-2. [DOI] [PubMed] [Google Scholar]