Abstract
Background
Coronavirus-Disease-2019 (COVID-19) caused by Severe-Acute-Respiratory-Syndrome-Coronavirus-2 (SARS-CoV-2) is rapidly spreading worldwide causing a pandemic. To control the pandemic, the One Health approach (https://www.who.int/news-room/q-a-detail/one-health) is very important. We herein provide a real-world example of efficient COVID-19 control in Anhui Province, China with outbreak originating from imported cases through implementation of a series of measures as part of the One Health approach and describe the stratified cases features.
Methods
Since the identification of the first imported COVID-19 case on Jan 22, 2020, Anhui immediately initiated a sequence of systematic and forceful interventions. We detailed the control measures and analyzed the effects as demonstrated by the corresponding temporal changes of overall epidemiology data on confirmed, cured, and hospitalized cases and contacts. An accumulated number of 991 cases were confirmed, with a total number of 29,399 contacts traced. We further retrieved individual-level data of confirmed cases and compared them across stratifications by sex, age group, linkage to Wuhan, and period of diagnosis.
Results
With a series of interventions including active field investigation, case tracing, quarantine, centralization, education, closed management, and boundary control implemented, number of hospitalized COVID-19 cases peaked, new case disappeared, and all cases were discharged 21, 36, and 46 days after the identification of the initial case, respectively. Male patients were younger, more often had linkage to Wuhan, and received timelier care, but less often had infected cohabitants. Patients aged 25–44 years most often had linkage to Wuhan, while such frequency was lowest in those ≥65 years. Cases <25 years most often had a known contact with COVID-19 patients and any infected family member and cohabitant and were beforehand quarantined, and received fastest management. Patients with linkage to Wuhan were younger, less often had infected family member, had longer incubation period, and received earlier quarantine and timelier care. With more recent periods, the proportion of cases with linkage to Wuhan markedly decreased while the proportion of cases with known contact with COVID-19 cases dramatically increased; the proportions of patients with any infected family member or cohabitant, those beforehand quarantined, and those taking drugs before admission increased; incubation period lengthened, and patients received timelier professional care. Nonspecific systemic symptoms were most common, whose proportion decreased in more recent periods.
Conclusions
Timely and powerful measures as part of the One Health approach (https://www.who.int/news-room/q-a-detail/one-health) effectively and efficiently controlled the COVID-19 outbreak in Anhui, which can be a good real-world example strongly demonstrating the usefulness of such measures in places with outbreaks originating from imported cases. Precise and dynamic prevention and control measures should be implemented and based on features including sex, age group, exposure history, and phase of outbreak.
Keywords: COVID-19, SARS-CoV-2, Case importation, Outbreak, Contact tracing, Control, Precise and dynamic interventions
Highlights
-
•
Anhui initiated a sequence of systematic and powerful measures against COVID-19, which effectively controlled the epidemic in about 1-month time.
-
•
We detailed the effects as shown by the temporal changes of epidemiology data on confirmed, cured, and hospitalized cases and contacts.
-
•
We further described and compared the case characteristics by sex, age group, and linkage to Wuhan, and across periods of diagnosis.
-
•
Our report suggests that precise and dynamic prevention and control measures should be implemented and based on several features.
1. Introduction
Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is rapidly spreading causing a pandemic [1]. It has affected almost all countries around the world. As of June 18, 2020, about 8,337,000 COVID-19 cases have been accumulatively confirmed, and more than 448,000 cases have died from COVID-19 with an overall fatality rate of over 5% [1]. Currently no specific therapies or vaccines targeting SARS-CoV-2 exist [2], and humans are generally susceptible to the virus. The interpersonal transmission of COVID-19 is efficient, and the disease can even be transmitted during the incubation period or asymptomatically [3,4]. While SARS-CoV-2 often causes mild disease, severe conditions even death can occur [5]. Management of COVID-19 cases can cause a great burden to healthcare system [6]. It is therefore particularly important to spare no efforts to protect the general public from contracting the disease.
Case importations play a vital role in local COVID-19 outbreak [7,8]. Several modelling studies have suggested that active, strict, timely, substantial, continued, and large-scale interventions at both the population and individual levels including enhanced isolation, quarantine, surveillance, contact tracing, border and travel control, movement and activity restrictions, school closures, and population education can effectively and substantially contribute to curbing the community transmission, blocking the regional and international spread, and finally controlling the COVID-19 outbreaks [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18]].
Anhui Province has 63.7 million residents in 2019, has 16 prefecture-level cities, and locates northeast to Wuhan. Every year near the China Spring Festival, Anhui residents working in Wuhan return to their hometown for reunion with their family. The first COVID-19 cases were reported in Wuhan in Dec 2019 [19], and the human-to-human transmission was known to public in Jan 2020 [20]. Case importation occurred in Anhui especially before the lockdown of Wuhan on Jan 23, 2020. The total number of COVID-19 cases ranked fifth among all provinces outside Wuhan in mainland China. Since the identification of the first imported COVID-19 case in Anhui on Jan 22, 2020, Anhui quickly initiated a series of systematic and powerful measures, which effectively controlled the epidemic in about one-month time, with the total number of infected cases not exceeding 1000. Afterwards the situation remained well controlled without rebounding.
In this report, we detailed the control measures as part of the One Health approach (https://www.who.int/news-room/q-a-detail/one-health) against COVID-19 applied in Anhui and the effects as demonstrated by epidemiology data. We further described the case characteristics by sex, age group, and linkage to Wuhan, and across periods of diagnosis. The Anhui experience can be a good example of efficient COVID-19 control strongly supporting the use of timely and forceful interventions.
2. Methods
2.1. Cases
Following the COVID-19 emergence in Wuhan, once a suspected case, defined as one with a recent history of travel to Wuhan or contact with those from Wuhan, and with relevant symptoms and chest imaging suggesting COVID-19, was identified in Anhui, detailed field investigations and contact tracing were immediately initiated. COVID-19 case was confirmed by at least two positive real-time reverse-transcriptase-polymerase-chain-reaction (rRT-PCR) assays of respiratory (throat-swabs and sputum) specimens for SARS-CoV-2 RNA performed ≥24 h apart based on the criteria by the World Health Organization (WHO) [[21], [22], [23]]. Sample-collection, procession, viral RNA extraction, primers and probes design, and laboratory testing followed the WHO recommendations [[24], [25], [26], [27], [28]]. The diagnostic criteria were based on the recommendation by the China National Institute for Viral Disease Control and Prevention [29]. All COVID-19 cases and contacts were immediately reported to the local municipal, prefecture, city, and Anhui Province CDCs. Repeated tests for SARS-CoV-2 were performed in patients confirmed to have COVID-19 to show viral clearance before discharge from hospital or discontinuation of isolation.
The management of confirmed COVID-19 cases followed the WHO [30] and the China National Health Commission [31]. Fitness for discharge was based on abatement of fever for ≥3 days and resolved respiratory and other major relevant symptoms and signs, with substantial improvement of chest radiographic evidence and viral clearance in respiratory samples as demonstrated by ≥2 consecutively negative rRT-PCR tests for SARS-CoV-2 separated by ≥24 h [31]. Discharged patients and contacts continued to be closely monitored and isolated for at least two weeks, followed by reexamination to exclude relapse of infection.
The first confirmed COVID-19 case in Anhui was a 30-year-old male resident of Hefei, the capital city of Anhui, who went back to Hefei from his workplace in Wuhan on Jan 17, 2020, six days before the lockdown of Wuhan, and who had illness onset on Jan 18, 2020. He had his first clinical visit on Jan 20, 2020, and was diagnosed with COVID-19 on Jan 22, 2020. Since the identification of this initial case, Anhui immediately activated fully the COVID-19 prevention and control work, and implemented a sequence of timely and efficient interventions [32] (Table 1) including: Immediate and strict public transport control, timely centralization and immediate and precise management of COVID-19 cases in designated institutions with centralized resources, careful protection of medical staff, postponing school opening time with online education in place, full use of the internet for fast and professional medical assistance and education, strong guarantee of information transparency, reimbursement of costs for patients with COVID-19, strict control of entrance and exit of Anhui, careful quarantine of people crossing the provincial boundaries, closed management strategy, quarantine of all close contacts in centralized fixed places and in single room, thorough, careful, and active tracing, monitoring, and quarantine of contacts and people returning to Anhui, strict de-isolation criteria, full motivation of grassroots medical workers, appropriate disposal of medical wastes, best support of enterprises to ensure medical material supply, etc. Special attention was paid to places with majorly older populations, and multidisciplinary joint efforts were made.
Table 1.
Selected major measures against COVID-19 undertaken in Anhui Province, China [32].
| Date | Measure |
|---|---|
| Jan 21, 2020 |
|
| Jan 22, 2020 |
|
| Jan 23, 2020 |
|
|
|
| Jan 24, 2020 |
|
|
|
|
|
|
|
| Jan 25, 2020 |
|
|
|
|
|
| Jan 29, 2020 |
|
| Feb 2, 2020 |
|
| Feb 7, 2020 |
|
|
|
|
|
| Feb 8, 2020 |
|
|
|
| Feb 9, 2020 |
|
|
|
|
|
|
|
|
|
|
|
| Feb 12, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Feb 17, 2020 |
|
|
|
| Feb 18, 2020 |
|
|
|
|
|
| Feb 20, 2020 |
|
| Feb 22, 2020 |
|
|
|
| Feb 25, 2020 |
|
| Feb 28, 2020 |
|
| Mar 14, 2020 |
|
| Mar 15, 2020 |
|
|
|
| Mar 18, 2020 |
|
| Apr 10, 2020 |
|
| Apr 11, 2020 |
|
| Apr 14, 2020 |
|
| Apr 21, 2020 |
|
All dates were in 2020. COVID-19, Coronavirus Disease 2019.
As part of these efforts, the overall epidemiology data including numbers of all new and accumulated confirmed cases, new and accumulated cured cases, hospitalized cases, and new and accumulated contacts were released daily by the Health Commission of Anhui Province [32], and anonymous data on individual case confirmed with COVID-19 were collected by local CDCs and reported by the health commission of each prefecture and city, where information including patient sex, age, occupation, places of residence, exposure, quarantine, and diagnosis, dates of exposure, quarantine, illness onset, first medical visit, hospital admission, diagnosis, first and second transfers, and report to public, symptoms (respiratory, digestive, and systemic) on admission, histories of travel to Wuhan and of contact with people from Wuhan and with symptomatic and asymptomatic patients with COVID-19 within two weeks before illness onset, numbers of infected family members or relatives and of infected cohabitants, any or family clustering, drug intake before admission, hospital transfer, and disease severity on report to public was retrieved. Data on comorbidities and date of death were further retrieved for deceased cases. Both authors extracted data using a standardized customized form and crosschecked them. The study period was from Jan 22, 2020 through Jun 18, 2020. This study was approved by the local institutional review board, and informed consent was waived.
Date of diagnosis was categorized into four periods: Jan 22, 2020 through Jan 30, 2020 (Period 1), Jan 31, 2020 through Feb 6, 2020 (Period 2; the week ahead the date with peak daily increase), Feb 7, 2020 through Feb 13, 2020 (Period 3; the week after the peak), and Feb 14, 2020 and later (Period 4). A cluster included ≥3 relevant patients including the index. Incubation period was the interval between dates of exposure and illness onset, and was calculated among symptomatic patients with an exact date of exposure. Disease severity was according to the China National Health Commission [31].
2.2. Statistics
Categorical data were summarized as count (percentage, %), and continuous data as median (interquartile range) if not otherwise specified. Besides overall analysis, subgroup analysis according to sex, age group (<25, 25–44, 45–64, and ≥ 65 years), linkage to Wuhan (with and without a history of travel to Wuhan or contact with people from Wuhan), and period of report were performed. Categorical data were compared using the χ2 test or Fisher's exact test where appropriate, and continuous data using the Wilcoxon rank-sum or Kruskal-Wallis nonparametric test where appropriate. We used the Kaplan-Meier method to plot the temporal changes of the probability of severe-to-critical disease. Analyses were performed using the R 3.6.2 software (https://www.r-project.org/), and statistical significance was indicated by a two-sided p value <0.05.
3. Results
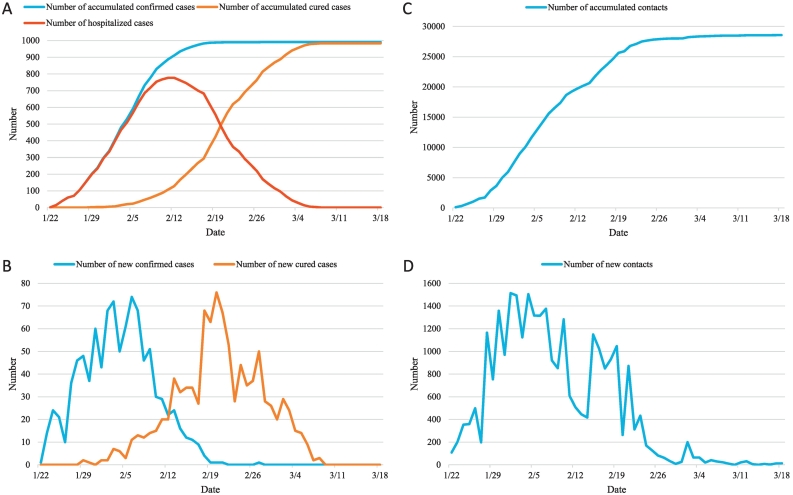
Since the identification of the first confirmed COVID-19 case in Anhui on Jan 22, 2020, the number of new confirmed cases quickly increased and peaked at 74 with a number of accumulated confirmed cases of 665 on Feb 6, 2020 (Fig. 1). Then the number of daily increase rapidly decreased, and during the study period the last confirmed case was identified on Feb 27, 2020, when the number of accumulated confirmed cases totaled 991. Two patients were first cured on Jan 29, 2020, and then the number of cured cases gradually increased and peaked at 76 on Feb 20, 2020. The number of hospitalized cases peaked at 777 on Feb 12, 2020. During the study period three patients were last discharged on Mar 8, 2020, making the number of hospitalized cases reduce to zero. On Jan 22, 2020 108 contacts of COVID-19 cases were identified, and the number of daily identified contacts quickly increased and peaked at 1514 on Feb 1, 2020. As of Jun 18, 2020, a total number of 29,399 contacts were traced.
Fig. 1.
Temporal changes of numbers of accumulated and new confirmed cases, accumulated and new cured cases, hospitalized cases, and accumulated and new contacts. All dates were in 2020.
Characteristics of overall and stratified confirmed cases are shown in Table 2, Table 3, and description of overall cases is presented in Supplementary Results. Male patients were on average two years younger than females, and there was a larger proportion of patients aged 18–24 years (9% vs 3%). City of residence and city of exposure were less often the same as city of diagnosis in males (73% vs 81% and 50% vs 66%, respectively). Male patients more often had a history of travel to Wuhan (36% vs 26%) and contact with people from Wuhan (9% vs 7%). Female patients had more often ≥1 other cohabitant patient (17% vs 12%). The durations from illness onset to hospital admission, diagnosis, and report were all on average one day shorter for male patients.
Table 2.
Demographic, baseline, and epidemiological characteristics of patients with confirmed COVID-19 in Anhui Province, China, overall and stratified by sex, age group, exposure history, and period of diagnosis (for brevity, the descriptive results in stratification analyses are only shown if the intergroup comparison has a p value < 0.1).a
| Characteristics |
Sex |
Age group (yr) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Category (n) | All (917) | Male (496) | Female (421) | p | < 25 (106) | 25–44 (367) | 45–64 (362) | ≥ 65 (82) | p |
| Male sex | 496 (54) | 496 (100) | 0 (0) | <0.001 | 72 (68) | 198 (54) | 184 (51) | 42 (51) | 0.019 |
| Age (yr) | 44 (32–53) | 43 (31–53) | 45 (34–54) | 0.024 | 19 (12−22) | 36 (31–41) | 52 (48–56) | 70 (67–75) | <0.001 |
| ≤ 1 | 6 (1) | 1 (< 1) | 5 (1) | 0.021 | 6 (6) | 0 (0) | 0 (0) | 0 (0) | <0.001 |
| 2–17 | 39 (4) | 24 (5) | 15 (4) | 39 (37) | 0 (0) | 0 (0) | 0 (0) | ||
| 18–24 | 61 (7) | 47 (9) | 14 (3) | 61 (58) | 0 (0) | 0 (0) | 0 (0) | ||
| 25–34 | 152 (17) | 80 (16) | 72 (17) | 0 (0) | 152 (41) | 0 (0) | 0 (0) | ||
| 35–44 | 215 (23) | 118 (24) | 97 (23) | 0 (0) | 215 (59) | 0 (0) | 0 (0) | ||
| 45–54 | 233 (25) | 118 (24) | 115 (27) | 0 (0) | 0 (0) | 233 (64) | 0 (0) | ||
| 55–64 | 129 (14) | 66 (13) | 63 (15) | 0 (0) | 0 (0) | 129 (36) | 0 (0) | ||
| 65–74 | 59 (6) | 30 (6) | 29 (7) | 0 (0) | 0 (0) | 0 (0) | 59 (72) | ||
| 75–84 | 18 (2) | 10 (2) | 8 (2) | 0 (0) | 0 (0) | 0 (0) | 18 (22) | ||
| ≥ 85 | 5 (1) | 2 (< 1) | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 5 (6) | ||
| Period of diagnosis | 0.013 | 0.147 | |||||||
| Jan 22-Jan 30, 2020 | 209 (23) | 131 (26) | 78 (19) | ||||||
| Jan 31-Feb 6, 2020 | 417 (45) | 215 (43) | 202 (48) | ||||||
| Feb 7-Feb 13, 2020 | 234 (26) | 115 (23) | 119 (28) | ||||||
| Feb 14-Jun 18, 2020 | 57 (6) | 35 (7) | 22 (5) | ||||||
| Medical workers | 11 (1) | 0.523 | 0.222 | ||||||
| City of residency was the same as city of diagnosis | 702 (77) | 362 (73) | 340 (81) | 0.006 | 89 (84) | 244 (66) | 292 (81) | 77 (94) | <0.001 |
| City of exposure was the same as city of diagnosis | 527 (57) | 249 (50) | 278 (66) | <0.001 | 64 (60) | 166 (45) | 227 (63) | 70 (85) | <0.001 |
| Linkage to Wuhan | 0.001 | <0.001 | |||||||
| No direct linkage to Wuhan | 550 (60) | 270 (54) | 280 (67) | 58 (55) | 195 (53) | 231 (64) | 66 (80) | ||
| Travel to Wuhan | 290 (32) | 180 (36) | 110 (26) | 34 (32) | 148 (40) | 100 (28) | 8 (10) | ||
| Contact with people from Wuhan, without travel to Wuhan | 77 (8) | 46 (9) | 31 (7) | 14 (13) | 24 (7) | 31 (9) | 8 (10) | ||
| Contact with patients with COVID-19 | 0.519 | <0.001 | |||||||
| No known contact with patients | 542 (59) | 41 (39) | 241 (66) | 223 (62) | 37 (45) | ||||
| Contact with symptomatic patients | 371 (40) | 65 (61) | 124 (34) | 138 (38) | 44 (54) | ||||
| Contact with asymptomatic patients | 4 (< 1) | 0 (0) | 2 (1) | 1 (< 1) | 1 (1) | ||||
| ≥1 other family member/relative patient | 259 (28) | 0.234 | 48 (45) | 82 (22) | 98 (27) | 31 (38) | <0.001 | ||
| ≥1 other cohabitant patient | 129 (14) | 58 (12) | 71 (17) | 0.025 | 28 (26) | 42 (11) | 42 (12) | 17 (21) | <0.001 |
| Cluster onset | 113 (12) | 0.529 | 34 (32) | 26 (7) | 45 (12) | 8 (10) | <0.001 | ||
| Family cluster onset | 85 (9) | 38 (8) | 47 (11) | 0.068 | 27 (25) | 24 (7) | 29 (8) | 5 (6) | <0.001 |
| Places of quarantine before illness onset | 0.446 | 0.031 | |||||||
| Not beforehand quarantined | 802 (87) | 82 (77) | 323 (88) | 323 (89) | 74 (90) | ||||
| Home/hotel | 87 (9) | 18 (17) | 33 (9) | 28 (8) | 8 (10) | ||||
| Hospital | 28 (3) | 6 (6) | 11 (3) | 11 (3) | 0 (0) | ||||
| Change of quarantine place before hospitalizationb | 24/115 (21) | 0.886 | 0.615 | ||||||
| Drug intake before medical visit | 85 (9) | 0.172 | 0.608 | ||||||
| Asymptomatic | 18 (2) | 0.725 | 4 (4) | 3 (1) | 7 (2) | 4 (5) | 0.030 | ||
| Interval of diagnosis between source of infection and patientc (d) | 4 (2–6) | 0.244 | 0.105 | ||||||
| Incubation periodd (d) | 5 (3–9) | 0.342 | 0.548 | ||||||
| Days from quarantine to illness onsetb | 4 (2–8) | 0.378 | 0.385 | ||||||
| Days from illness onset to first medical visit | 2 (0–4) | 0.829 | 1 (0–3) | 2 (0–4) | 2 (0–5) | 2 (0–4) | 0.013 | ||
| Days from illness onset to admission to designated hospital | 3 (1–6) | 3 (1–5) | 4 (1–7) | 0.004 | 2 (1–4) | 3 (1–6) | 4 (2–6) | 4 (1–8) | 0.002 |
| Days from illness onset to diagnosis | 6 (3–9) | 5 (3–8) | 6 (3–9) | 0.027 | 4 (3–7) | 6 (3–8) | 6 (4–9) | 6 (3−10) | 0.001 |
| Days from illness onset to report to public | 7 (4–10) | 6 (4–9) | 7 (4–10) | 0.020 | 5 (4–8) | 7 (4–9) | 7 (5–10) | 7 (4–11) | 0.002 |
| Times of transfer | 0.065 | 0.735 | |||||||
| 0 | 628 (68) | 353 (71) | 275 (65) | ||||||
| 1 | 242 (26) | 124 (25) | 118 (28) | ||||||
| ≥ 2 | 47 (5) | 19 (4) | 28 (7) | ||||||
| Days from illness onset to first transfer | 5 (3–8) | 4 (2–7) | 6 (3–8) | 0.051 | 3 (1–7) | 5 (3–8) | 5 (3–7) | 8 (3−11) | 0.042 |
| Days from illness onset to second transfer | 9 (6–11) | 0.509 | 4 (3–5) | 9 (7–11) | 8 (5–10) | 12 (9–16) | 0.056 | ||
| Severe condition of disease on report to public | 15 (2) | 0.324 | 0.315 | ||||||
Note: COVID-19, coronavirus disease 2019.
Continuous variables are shown as median (interquartile range), and categorical variables as count or count/total number of patients with available or applicable data (percentage [%]), respectively. The denominators of patients are provided if they differed from the overall numbers in the group. p values <0.05 are shown in bold, and p values ≥0.05 and < 0.10 are shown in both bold and italic.
For 115 patients (12%) quarantined before illness onset.
For 229 pairs (50%) of sources of transmission and transmitted patients. Stratifications were based on the transmitted patients.
For 429 patients (47%) with an exact exposure date.
Table 3.
Demographic, baseline, and epidemiological characteristics of patients with confirmed COVID-19 in Anhui Province, China, overall and stratified by sex, age group, exposure history, and period of diagnosis (for brevity, the descriptive results in stratification analyses are only shown if the intergroup comparison has a p value < 0.1).a
| Characteristics |
Linkage to Wuhan |
Period of diagnosis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Category (n) | All (917) | Yes (367) | No (550) | p | Jan 22-Jan 30, 2020 (209) | Jan 31-Feb 6, 2020 (417) | Feb 7-Feb 13, 2020 (234) | Feb 14-Jun 18, 2020 (57) | p |
| Male sex | 496 (54) | 226 (62) | 270 (49) | <0.001 | 131 (63) | 215 (52) | 115 (49) | 35 (61) | 0.013 |
| Age (yr) | 44 (32–53) | 41 (30–49) | 46 (35–56) | <0.001 | 0.111 | ||||
| ≤ 1 | 6 (1) | 2 (1) | 4 (1) | <0.001 | 0.139 | ||||
| 2–17 | 39 (4) | 11 (3) | 28 (5) | ||||||
| 18–24 | 61 (7) | 35 (10) | 26 (5) | ||||||
| 25–34 | 152 (17) | 76 (21) | 76 (14) | ||||||
| 35–44 | 215 (23) | 96 (26) | 119 (22) | ||||||
| 45–54 | 233 (25) | 92 (25) | 141 (26) | ||||||
| 55–64 | 129 (14) | 39 (11) | 90 (16) | ||||||
| 65–74 | 59 (6) | 15 (4) | 44 (8) | ||||||
| 75–84 | 18 (2) | 1 (<1) | 17 (3) | ||||||
| ≥ 85 | 5 (1) | 0 (0) | 5 (1) | ||||||
| Period of report | <0.001 | <0.001 | |||||||
| Jan 22-Jan 30, 2020 | 209 (23) | 165 (45) | 44 (8) | 209 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| Jan 31-Feb 6, 2020 | 417 (45) | 159 (43) | 258 (47) | 0 (0) | 417 (100) | 0 (0) | 0 (0) | ||
| Feb 7-Feb 13, 2020 | 234 (26) | 37 (10) | 197 (36) | 0 (0) | 0 (0) | 234 (100) | 0 (0) | ||
| Feb 14-Jun 18, 2020 | 57 (6) | 6 (2) | 51 (9) | 0 (0) | 0 (0) | 0 (0) | 57 (100) | ||
| Medical workers | 11 (1) | 0.215 | 0.734 | ||||||
| City of residency was the same as city of diagnosis | 702 (77) | 203 (55) | 499 (91) | <0.001 | 114 (55) | 331 (79) | 205 (88) | 52 (91) | <0.001 |
| City of exposure was the same as city of diagnosis | 527 (57) | 55 (15) | 472 (86) | <0.001 | 54 (26) | 240 (58) | 187 (80) | 46 (81) | <0.001 |
| Linkage to Wuhan | <0.001 | <0.001 | |||||||
| No direct linkage to Wuhan | 550 (60) | 0 (0) | 550 (100) | 44 (21) | 258 (62) | 197 (84) | 51 (89) | ||
| Travel to Wuhan | 290 (32) | 290 (79) | 0 (0) | 143 (68) | 124 (30) | 19 (8) | 4 (7) | ||
| Contact with people from Wuhan, without travel to Wuhan | 77 (8) | 77 (21) | 0 (0) | 22 (11) | 35 (8) | 18 (8) | 2 (4) | ||
| Contact with patients with COVID-19 | <0.001 | <0.001 | |||||||
| No known contact with patients | 542 (59) | 276 (75) | 266 (48) | 179 (86) | 271 (65) | 75 (32) | 17 (30) | ||
| Contact with symptomatic patients | 371 (40) | 89 (24) | 282 (51) | 30 (14) | 146 (35) | 155 (66) | 40 (70) | ||
| Contact with asymptomatic patients | 4 (< 1) | 2 (1) | 2 (< 1) | 0 (0) | 0 (0) | 4 (2) | 0 (0) | ||
| ≥1 other family member/relative patient | 259 (28) | 80 (22) | 179 (33) | <0.001 | 21 (10) | 112 (27) | 103 (44) | 23 (40) | <0.001 |
| ≥1 other cohabitant patient | 129 (14) | 0.790 | 15 (7) | 61 (15) | 42 (18) | 11 (19) | 0.006 | ||
| Cluster onset | 113 (12) | 0.284 | 13 (6) | 48 (12) | 45 (19) | 7 (12) | 0.001 | ||
| Family cluster onset | 85 (9) | 0.483 | 8 (4) | 36 (9) | 39 (17) | 2 (4) | <0.001 | ||
| Places of quarantine before illness onset | 0.445 | <0.001 | |||||||
| Not beforehand quarantined | 802 (87) | 202 (97) | 384 (92) | 181 (77) | 35 (61) | ||||
| Home/hotel | 87 (9) | 4 (2) | 26 (6) | 41 (18) | 16 (28) | ||||
| Hospital | 28 (3) | 3 (1) | 7 (2) | 12 (5) | 6 (11) | ||||
| Change of quarantine place before illness onsetb | 24/115 (21) | 0.391 | 0.276 | ||||||
| Drug intake before medical visit | 85 (9) | 25 (7) | 60 (11) | 0.036 | 6 (3) | 36 (9) | 39 (17) | 4 (7) | <0.001 |
| Asymptomatic | 18 (2) | 3 (1) | 15 (3) | 0.041 | 1 (<1) | 7 (2) | 7 (3) | 3 (5) | 0.055 |
| Interval of diagnosis between source of infection and patientc (d) | 4 (2–6) | 4 (2−11) | 3 (2–6) | 0.018 | 3 (0–4) | 3 (2–5) | 5 (2–8) | 6 (3–9) | 0.001 |
| Incubation periodd (d) | 5 (3–9) | 5 (3–9) | 7 (3–10) | 0.009 | 3 (2–5) | 7 (4–9) | 9 (6–13) | 11 (2–25) | <0.001 |
| Days from quarantine to illness onsetb | 4 (2–8) | 6 (3–9) | 3 (1–5) | 0.005 | 0.710 | ||||
| Days from illness onset to first medical visit | 2 (0–4) | 0.124 | 2 (1–4) | 2 (0–4) | 2 (0–5) | 0 (0–2) | 0.011 | ||
| Days from illness onset to admission to designated hospital | 3 (1–6) | 2 (1–5) | 4 (1–6) | <0.001 | 3 (1–5) | 4 (1–6) | 4 (1–7) | 1 (0–6) | 0.009 |
| Days from illness onset to diagnosis | 6 (3–9) | 5 (3–8) | 7 (4–9) | <0.001 | 5 (3–7) | 6 (4–8) | 6 (4–10) | 4 (2–9) | 0.017 |
| Days from illness onset to report to public | 7 (4–10) | 6 (4–9) | 8 (5–10) | 0.001 | 6 (5–9) | 7 (5–9) | 7 (5–11) | 5 (3–10) | 0.078 |
| Times of transfer | 0.004 | 0.001 | |||||||
| 0 | 628 (68) | 272 (74) | 356 (65) | 171 (82) | 272 (65) | 149 (64) | 36 (63) | ||
| 1 | 242 (26) | 84 (23) | 158 (29) | 34 (16) | 120 (29) | 70 (30) | 18 (32) | ||
| ≥ 2 | 47 (5) | 11 (3) | 36 (7) | 4 (2) | 25 (6) | 15 (6) | 3 (5) | ||
| Days from illness onset to first transfer | 5 (3–8) | 4 (2–7) | 5 (3–8) | 0.047 | 0.751 | ||||
| Days from illness onset to second transfer | 9 (6–11) | 0.100 | 0.242 | ||||||
| Severe condition of disease on report to public | 15 (2) | 0.287 | 9 (4) | 1 (< 1) | 3 (1) | 2 (4) | 0.001 | ||
Note: COVID-19, coronavirus disease 2019.
Continuous variables are shown as median (interquartile range), and categorical variables as count or count/total number of patients with available or applicable data (percentage [%]), respectively. The denominators of patients are provided if they differed from the overall numbers in the group. p values <0.05 are shown in bold, and p values ≥0.05 and < 0.10 are shown in both bold and italic.
For 115 patients (12%) quarantined before illness onset.
For 229 pairs (50%) of sources of transmission and transmitted patients. Stratifications were based on the transmitted patients.
For 429 patients (47%) with an exact exposure date.
Male proportion decreased from 68% in patients <25 years to 51% in those ≥45 years. City of residence and city of exposure were least often the same as city of diagnosis in patients aged 25–44 years (66% and 45%, respectively), and most often in those ≥65 years (94% and 85%). Patients aged 25–44 years most often had a history of travel to Wuhan or contact with people from Wuhan (47%), followed by those <25 years (45%); those ≥65 years had least often such exposure histories (20%). Cases <25 years had most often a known contact with COVID-19 patients (61%), followed by those ≥65 years (55%), and such frequency was lowest in those aged 25–44 years (34%). Cases <25 years had most often ≥1 other infected family member or relative (45%) and ≥ 1 other cohabitant patient (26%), followed by those ≥65 years (38% and 21%, respectively), and such frequencies were lowest in those aged 25–44 years (22% and 11%, respectively). Patients <25 years were most often of cluster onset (32%) and most frequently belonged to a family cluster (25%). Patients <25 years were most often identified during quarantine before illness onset (23%), while those ≥65 years were least often beforehand quarantined (10%). Patients ≥65 years were most often asymptomatic (5%), followed by those <25 years (4%); those aged 25–44 years were least often asymptomatic (1%). Duration from illness onset to first medical visit, hospital admission, first transfer, diagnosis, and report were all on average 1–2 days shorter for cases <25 years than others.
Patients with a linkage to Wuhan were on average five years younger, and had less often a known contact with COVID-19 patients (25% vs 52%). They less often had any other infected family member or relative (22% vs 33%), less often took drugs before medical visit (7% vs 11%), and were less often asymptomatic (1% vs 3%). The interval between diagnosis of infection source and patient was on average one day longer for them, while they had on average a one-day shorter incubation period. Among beforehand quarantined cases, duration from quarantine to illness onset was on average three days longer for those with an association with Wuhan. The duration from illness onset to hospital admission, diagnosis, and report were all on average two days shorter for those with a linkage to Wuhan. They were more often initially admitted to a designated hospital (74% to 65%), and among transferred cases, duration from illness onset to first transfer was on average one day shorter for those with a linkage.
Male proportion was larger in Periods 1 (63%) and 4 (61%) than Periods 2 (52%) and 3 (49%). From Period 1 to 4, the frequency of city of residence and city of exposure being the same as city of diagnosis increased from 55% to 91% and from 26% to 81%, respectively; the proportion of cases with a history of travel to Wuhan and of contact with people from Wuhan decreased from 68% to 7% and from 11% to 4%, respectively; the proportions of patients with a known contact with COVID-19 cases increased from 14% to 70%. The proportion of patients with any other infected family member or relative increased from 10% in Period 1 to 44% in Period 3 and 40% in Period 4, and the proportion of those with any cohabitant patient increased from 7% in Period 1 to 18% in Period 3 and 19% in Period 4. Cases were most often of any (19%) or family cluster onset (17%) in Period 3. The proportion of beforehand quarantined patients increased markedly from Period 1 (home or hotel, 2%; hospital, 1%) to Period 4 (home or hotel, 28%; hospital, 11%). Patients most often took drugs by themselves in Period 3 (17%), and least often in Period 1 (3%). The interval between diagnosis of infection source and patient increased from three days in Periods 1 and 2 to six days in Period 4, and incubation period sequentially increased from three days in Period 1 to 11 days in Period 4. The durations from illness onset to first medical visit, hospital admission, and diagnosis were on average 2–3 days shorter in Period 4 than in previous periods. Patients were most often initially admitted to a designated hospital in Period 1 (82%) than in other periods (63%–65%). The proportion of severe-to-critical cases were highest in Periods 1 and 4 (both 4%).
Symptoms before or on hospitalization of overall and stratified cases are shown in Table S1, and description of overall symptoms is presented in Supplementary Results. Stratified symptoms before or on admission were mostly similar across subgroups by sex, age group, linkage to Wuhan, and period of diagnosis with a few exceptions. Male patients more often had fever (85% vs 76%) and shiver (4% vs 1%), while all patients experiencing dizziness were females (3%). Overall, male patients had more often any systemic symptom (89% vs 79%). All patients having sneezing were < 25 years (4%). Patients with linkage to Wuhan had more often dizziness (3% vs 1%). The proportion of patients having fever was highest in Period 2 (85%) and lowest in Period 4 (70%), and the proportion of patients experiencing fatigue was highest in Period 1 (19%) and lowest in Period 4 (0%). Patients most often had expectoration, hemoptysis, rhinorrhea, shortness of breath, and chest discomfort all in Period 3 (13%, 9%, 7%, 9%, and 12%, respectively). The proportion of patients having any systemic symptom decreased from 86% in Period 1 and 90% in Period 2 to 76% in Period 3 and 70% in Period 4.
Disease severity and characteristics of deceases cases are shown in Fig. S1 and Table S2, respectively, and both are described in Supplementary Results.
4. Discussion
This report summarized the data on outbreak through control of COVID-19 in Anhui, a province with about 64 million people and with number of accumulated confirmed cases ranking fifth outside Wuhan in mainland China. Since outbreak, COVID-19 was quickly controlled by strict measures in only about one month, and the situation remained well controlled afterwards. Features stratified by sex, age group, linkage to Wuhan, and period of diagnosis were further provided. Various differences across groups can provide important hints for timely and efficient stratified management.
To combat, control, and contain the COVID-19 pandemic, the One Health approach is of great importance. One Health is an approach to designing and implementing programs, policies, legislation, and research in which multiple sectors communicate and work together to achieve better public health outcomes (https://www.who.int/news-room/q-a-detail/one-health). Efforts by just one sector cannot prevent or eliminate the COVID-19 pandemic. For instance, SARS-CoV-2 can infect and spread between animals and humans [19]; to effectively prevent and contain SARS-CoV-2 infection in humans, it is also necessary to target and control the animal source of the virus, and a well-coordinated approach in humans and in animals is required. Professionals with a range of expertise who are active in different sectors, such as public health, animal health, and the environment, should join forces to support the One Health approach against COVID-19. To effectively prevent, detect, and respond to outbreaks of COVID-19, epidemiological data and laboratory information should be shared across sectors. Government officials, researchers, and workers across multiple sectors at the local, national, regional, and global levels should implement joint responses to COVID-19. As part of the One Health approach against COVID-19, in this report we showed the measures undertaken in Anhui Province, China and the effects.
The first case in Anhui was diagnosed one day before the lockdown of Wuhan and three days before the China New Year. Then the accumulated number of confirmed cases quickly increased with increasing speed and peaked in about two weeks, well representing the early outbreak phase of an epidemic [33,34]. Strict isolation measures immediately initiated efficiently made the number of new cases start to decrease two weeks after the initial case. Active contact tracing was started immediately after the initial diagnosis, and a total of more than 29 thousand contacts have been quarantined. The last case during the study period was diagnosed about one month after confirmation of the initial case, and the number of new cases remained zero for three weeks afterwards. The number of new cases after the date with largest daily increase was only about half of the number before the date. The date of first cure was one week later than that of initial diagnosis, and the peak number of cure occurred two weeks after the peak number of diagnosis. The largest number of hospitalized cases occurred three weeks after the initial diagnosis, and the last cure occurred more than three weeks later. These data nicely showed the rapid control of COVID-19 under timely and efficient measures.
Various measures played key roles in controlling the outbreak in Anhui (Table 1). Public transport with relatively confined space and a relatively high density of passengers can be an important medium for efficient disease spread, and corresponding strict control measures were immediately implemented. Being highly contagious and potentially severe [4], COVID-19 cases were quickly centralized and precisely managed in designated institutions with centralized resources where medical staff were carefully protected. This effectively reduced the number of nosocomial infections and infections of medical workers, and ensured that patients received best possible care. COVID-19 has strong infectivity even during the incubation period especially in young patients [4], and school opening time was postponed with online education in place. The function of the internet was fully exerted, and people could obtain fast and professional feedbacks regarding their conditions and timely updates and educational information regarding the epidemic at home. This prevented irrelevant individuals from contracting the disease, and alleviated the burden of hospitals. Information transparency effectively increased the public awareness and motivated the whole society to battle the epidemic. Costs for patients with COVID-19 could all be reimbursed, and this encouraged the efficient identification and timely management of relevant cases. Imported cases play a vital role in local outbreak [35]. Entrance and exit of Anhui were strictly controlled, and people crossing the provincial boundaries were carefully quarantined, which effectively reduced case importation and exportation. Many COVID-19 cases were community acquired. The closed management strategy could effectively cut off transmission route, protect the susceptible populations, and promote the precision management of individuals. While home isolation could alleviate hospital burdens, it increases the risks of infecting family members. Close contacts were all quarantined in centralized fixed places and in single room. Thorough and careful contact tracing, monitoring, and quarantine ensured that all possible cases were identified. Strict de-isolation criteria ensured the absolute wellbeing of cases. Grassroots medical workers played vital roles as first-line power battling the epidemic. Their motivation effectively contributed to epidemic control. Disposal of medical wastes was appropriately done. Economic development could hardly be possible without epidemic control. During the epidemic, enterprises were best supported to ensure medical material supply. Upon identifying nearly all potential cases, work resumption gradually and orderly started under strict regulations. Older people are more vulnerable to COVID-19 [36]. Special attention was paid to places with majorly older populations. Cases were managed at the earliest possible time, to avoid the further expansion of the epidemic. Multidisciplinary joint efforts were vital in the epidemic control. After a series of timely and efficient measures, COVID-19 cases quickly disappeared and emergency response level was accordingly lowered with closed management lifted. Now the work focus has been shifted to prevent imported cases from abroad and places outside Anhui, and people returning to Anhui are required to be carefully quarantined. Close monitoring of indigenous cases continues.
Our subgroup analyses call for stratified management strategy. Male patients were younger than female patients, and male proportion decreased by 1/4 from <25 to ≥65 years. A larger proportion of males than of females were found in patients aged 18–24 years, and young males may be more socially active. Quarantine of these patients can be particularly important, since while COVID-19 less often causes severe disease in them, they can efficiently spread the disease and may rapidly endanger people with disadvantaged features [4]. For males city of residence or exposure was more often different from city of diagnosis, possibly due to the greater mobility of males. This may increase the difficulty of tracing contacts of male patients, and intercity cooperation is important. Women patients were less often linked to Wuhan, and might more often contract the infection locally or from their partners. While male patients received more timely management, the 1-day difference was unremarkable.
Patients aged 25–44 years appeared most mobile and active with most frequent intercity activities, and had most frequent association with Wuhan, while those ≥65 years showed lowest mobility. This may be due to work reasons, and older patients are more often retired. On the contrary and interestingly, patients aged 25–44 years had least often a known history of contact with COVID-19 cases and might least likely contract the infection from their family members, while those <25 years most often had such exposure histories. Accordingly, patients <25 years most often belonged to any or a family cluster. The frequency of having any other infected family member is also high for those ≥65 years. This highlight the important of quarantine of those aged 25–44 years with exposure to places where COVID-19 has been known to be occurring even without a clear contact with a sick person. During home isolation of a patient, it would be necessary to closely monitor both the patient and his/her family members, especially older ones who may be more vulnerable to the disease [5]. Notably, only 12% of patients were beforehand quarantined, and patients <25 years were most often identified during quarantine, while those ≥65 years underwent least often quarantine beforehand. There could be a significant proportion of patients only identified after illness onset but not beforehand quarantined, who may increase the risk of disease spread. Patients with milder symptoms especially younger ones may have been missed in the symptom-based quarantine strategies. It would be important to increase the capability to identify those at an increased risk of infection in a cost-effective manner, and to improve the efficiency of capturing likely contacts. Patients<25 or ≥ 65 years were more often asymptomatic, and thorough contact identification and tracing would be especially useful in identifying these asymptomatic patients. While with milder conditions [4], younger patients received earlier management. It is important to ensure that older patients are identified and quarantined beforehand as timely too.
Patients without a linkage to Wuhan more often took drugs before clinical visit and were more frequently asymptomatic. While this could be partly associated with the time shift of diagnosis (the proportion of patients with a linkage decreased in more recent periods), it could challenge the identification of such patients. Nevertheless, those without a linkage had more often a known contact with COVID-19 cases or infected family members. Both history of travel and of sick contact may be equally important in screening potential cases. Patients with association with Wuhan had shorter incubation period, while the difference was unremarkable. They were quarantine earlier possibly due to a clear exposure history, and managed in a designated institute timelier. Fortunately, with the passage of the virus, manifestations of patients may weaken [37]. However, it is still necessary to identify and manage particularly the disadvantaged populations without clear exposure histories earlier to avoid development of serious outcomes.
With the implementation of strict control measures, city of residence and of exposure became more often the same as city of diagnosis. The proportion of patients with a linkage to Wuhan markedly decreased, while the proportion of those with a clear sick contact increased. Family members became increasingly important as a source of infection. An increasing proportion of patients were identified during quarantine. The proportion of patients with drug intake beforehand also increased, possibly due to a better understanding of the disease. However, this may need to be discouraged to avoid a biased professional assessment. Notably, the interval between diagnosis of consecutive patients and incubation period both profoundly increased. This may however partly reflect the more efficient tracing and greater efficiency of recalling previous exposures due to a greater attention to the disease. The distribution of incubation period in Period 4 was relatively large, and to avoid missing cases, quarantine period for contacts may need to be lengthened with the ongoing of an outbreak. However, the cost-effectiveness should also be carefully considered. With increasing awareness, patients received medical care earlier, especially in Period 4 after the total number of hospitalized cases started to decrease. With increasing burden of care, however, the proportion of patients initially managed in a designated hospital decreased.
Patients in Anhui mostly had milder conditions compared to Wuhan [5,38], and the proportion of severe-to-critical disease or fatal case was rather small. Fatal cases had inferior features consistent with previous reports [5,39]. Any nonspecific systemic symptom was most common before or on admission, followed by any respiratory symptom. Symptoms were mostly similar across stratifications by sex, age group, and exposure history. These highlight the importance of quarantining cases with any suspicious presentations in this pandemic era. Women had less often fever or any systemic symptom, but more often dizziness. The sex difference in thermoregulation might render female patients more easily missed by the widely used temperature-based screening strategy. The proportion of patients with specific respiratory symptoms was highest in Period 3. The proportion of patients with fever, fatigue, or any systemic symptom showed a decreasing trend, suggesting the disease more insidious in recent periods. While this can indicate the alleviating clinical conditions, it could also be associated with the more efficient and widespread identification of any potentially infected cases.
Our study has some limitations. First, individual level data were not available for all patients, and variable kinds were limited. We did not have further treatment or outcome data. Nevertheless, the available variables could already well address the major research questions. Second, missing values existed for some variables, especially incubation period. However, it could be common that patients were unaware of the exact exposure, and SARS-CoV-2 could be efficiently transmitted through a variety of ways [40]. Third, due to the insidious nature, asymptomatic cases could have been under-identified despite great efforts. Given the increasing awareness of the significance of asymptomatic cases [4], further efforts to screen for asymptomatic cases have been strengthened (e.g., a population-based screening strategy to test both RNA and antibodies).
5. Conclusions
Timely and strong measures effectively and efficiently controlled the COVID-19 outbreak in Anhui, which can be a good example demonstrating the usefulness of measures such as isolation, centralization, patient education, and active contact tracing. Precise and dynamic prevention and control measures should be implemented and based on features including sex, age group, exposure history, and phase of epidemic outbreak.
Author statement
LH and XYZ had the idea for the study and full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. LH, XYZ, and AMX played roles in the literature search, study conception and design, data collection, analysis, and interpretation, and writing of the report, and reviewed and approved the final version of the manuscript.
Ethical approval and consent to participate
This study was approved by the local Institutional Review Board, and informed consent was waived for this observational, population-based study. The presented secondary data are anonymous without any risk of identification, and no individual patient data were reported.
Consent for publication
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from the corresponding authors on reasonable request. Anonymous participant data without names and identifiers can be provided after approval by the corresponding authors and healthcare authorities. After publication of study findings, the data will be available for others to request. The proposal with detailed description of research objectives and analysis plan will be needed for evaluation of the reasonability to request for our data. The corresponding authors and healthcare authorities will make a decision based on these materials regarding whether to share the data or not. Additional materials may also be required during the process.
Funding
None.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
We would like to thank all the patients and contacts and all the people involved in the One Health approach (https://www.who.int/news-room/q-a-detail/one-health) against COVID-19, including all healthcare workers and hospitals who provide care for patients with COVID-19 and are involved in the diagnosis and management of COVID-19 patients and those who trace and quarantine the contacts; staff at the local, city, and province healthcare departments; members of the COVID-19 response teams at the local, city, province, national, international, and global levels; Anhui Province CDC, all city, prefecture, county, and local CDCs, and medical institutions in Anhui for assistance with field investigation administration, data collection, and coordinating data collection for patients with COVID-19.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100224.
Contributor Information
Lei Huang, Email: huangleizhenting@126.com.
Aman Xu, Email: amanxu@163.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.WHO Coronavirus disease (COVID-19) Pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Cao B., Wang Y., Wen D. N Engl J Med. 2020. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L., Zhang X., Zhang X. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: A prospective contact-tracing study. J. Infect. 2020;80(6):e1–e13. doi: 10.1016/j.jinf.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. Jama. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert M., Pullano G., Pinotti F. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020;395(10227):871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling B.J., Ali S.T., Ng T.W.Y. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5(5):e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan A., Liu L., Wang C. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. Jama. 2020;323(19):1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395(10233):1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayham J., Fenichel E.P. Impact of school closures for COVID-19 on the US health-care workforce and net mortality: a modelling study. Lancet Public Health. 2020;5(5):e271–e278. doi: 10.1016/S2468-2667(20)30082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J., Litvinova M., Wang W. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect. Dis. 2020;20(7):793–802. doi: 10.1016/S1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prem K., Liu Y., Russell T.W. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5(5):e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo J.R., Cook A.R., Park M. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect. Dis. 2020;20(6):678–688. doi: 10.1016/S1473-3099(20)30162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucharski A.J., Russell T.W., Diamond C. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect. Dis. 2020;20(5):553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pung R., Chiew C.J., Young B.E. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395(10229):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellewell J., Abbott S., Gimma A. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health. 2020;8(4) doi: 10.1016/S2214-109X(20)30074-7. (e488-e96) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan J.F.-W., Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (London, England) 2020;S0140-6736(20):30154–30159. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Coronavirus Disease (COVID-19) Technical Guidance: Laboratory Testing for 2019-nCoV in Humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance
- 23.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention, Respiratory Viruses Branch . 2019-Novel Coronavirus (2019-nCoV) Real-Time rRT-PCR Panel Primers and Probes. 2020. Division of Viral Diseases.https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf [Google Scholar]
- 25.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. (S0140-6736(20)30183–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases. Interim Guidance. Jan 17, 2020. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 (accessed Feb 4, 2020.
- 29.China-National-Institute-for-Viral-Disease-Control-and-Prevention Specific Primers and Probes for Detection 2019 Novel Coronavirus. 2020. http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html
- 30.Organization WH Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance. 2020. https://www.who.int/publications-detail/clinical-managementof-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected
- 31.China-National-Health-Commission Prevention and Control of COVID-19 in China. 2020. http://www.nhc.gov.cn/xcs/xxgzbd/gzbd_index.shtml [DOI] [PMC free article] [PubMed]
- 32.Health Commission of Anhui Province Prevention and Control of COVID-19 in Anhui Province, China. 2021. http://wjw.ah.gov.cn/ztzl/xxgzbdfyyqfk/index.html
- 33.Laxminarayan R., Wahl B., Dudala S.R. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370(6517):691–697. doi: 10.1126/science.abd7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. Jama. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 35.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. Jama. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMichael T.M., Currie D.W., Clark S. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N. Engl. J. Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. Bmj. 2020;m606:368. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;m1091:368. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on reasonable request. Anonymous participant data without names and identifiers can be provided after approval by the corresponding authors and healthcare authorities. After publication of study findings, the data will be available for others to request. The proposal with detailed description of research objectives and analysis plan will be needed for evaluation of the reasonability to request for our data. The corresponding authors and healthcare authorities will make a decision based on these materials regarding whether to share the data or not. Additional materials may also be required during the process.