Abstract
Many cancer therapies aim to trigger apoptosis in cancer cells. Nevertheless, the presence of oncogenic alterations in these cells and distorted composition of tumour microenvironment largely limit the clinical efficacy of this type of therapy. Luckily, scientific consensus describes about 10 different cell death subroutines with different regulatory pathways and cancer cells are probably not able to avoid all of cell death types at once. Therefore, a focused and individualised therapy is needed to address the specific advantages and disadvantages of individual tumours. Although much is known about apoptosis, therapeutic opportunities of other cell death pathways are often neglected. Molecular heterogeneity of head and neck squamous cell carcinomas (HNSCC) causing unpredictability of the clinical response represents a grave challenge for oncologists and seems to be a critical component of treatment response. The large proportion of this clinical heterogeneity probably lies in alterations of cell death pathways. How exactly cells die is very important because the predominant type of cell death can have multiple impacts on the therapeutic response as cell death itself acts as a second messenger. In this review, we discuss the different types of programmed cell death (PCD), their connection with HNSCC pathogenesis and possible therapeutic windows that result from specific sensitivity to some form of PCD in some clinically relevant subgroups of HNSCC.
Subject terms: Oral cancer, Cell death, Oncogenesis
FACTS
How cancer cells die is very important because the specific type of cell death has different immunomodulatory impact crucial for the therapeutic response.
Scientific consensus describes many different cell death subroutines with different regulatory pathways and cancer cells are not able to avoid all of cell death types at once.
Resistance to one type of cell death can confer sensitivity to another type.
Specific sensitivity of cancer cells to some form of programmed cell death provides an interesting therapeutic window.
Human papillomavirus (HPV) can interfere with some cell death regulatory pathways.
OPEN QUESTIONS
How do different cell death regulatory pathways interact with each other and with secretory and/or endocytic pathways?
Is a certain genetic background in cancer cells tightly related to specific resistance and sensitivity to different kinds of cell death?
Can be different types of programmed cell death modulated and switched?
Introduction
Head and neck squamous cell carcinomas (HNSCC) are the fifth most common cancer globally. They emerge in the upper aero-digestive tract (including the oral cavity, pharynx and larynx). HNSCC exhibit high levels of heterogeneity and deep differences in therapeutic response1. Conventional HNSCC classification and clinical management are mainly based on clinical staging and grading, and anatomic location. However, for most of the advanced HNSCC, clinical staging does not correlate with treatment responses or prognosis and pre-operative clinical assessment of tumour and nodal involvement is often in disagreement with pathological T and N stage2. Variability in prognosis and molecular profiles of different head and neck tumours have attracted much attention in recent years as tumour heterogeneity represents a grave challenge for oncologists and seems to be a critical component of therapeutic response, cancer recurrence and patient survival. HPV infection probably covers most of the HNSCC heterogeneity. Beyond the role of HPV, current molecular classification categorises HNSCC into classical (CL), basal (BA) and mesenchymal (MS) subtype. HPV + tumours are not gathered into one group but fall into the MS and CL subgroup3. Genomic and proteomic data obtained from many HNSCC patients have demonstrated that HPV‐positive (HPV + ) and HPV‐negative (HPV−) HNSCC are different clinical entities4; (Box 1). Although the role of HPV in dysregulation of DNA damage response (DDR) is well established5, the influence of HPV on triggering of different kinds of cell death in HNSCC is discussed in a lesser extent, which is unfortunate because many viruses, including HPV, have developed numerous strategies to modulate host cell death to persist in the host for a long time without being eliminated. Accordingly, a growing spectrum of evidence suggests that the HPV-derived oncoproteins, such as E6 and E7 or E5, can inhibit death receptor signalling6. Nevertheless, the effect of HPV infection on other cell death types is not often discussed.
Deep understanding of the sensitivity or resistance to a specific cell death type given by certain genetic background and/or microenvironment that occur during the HNSCC pathogenesis may reveal targets for novel therapeutic approaches. We suggest that different approaches should be employed for therapy of different subgroups of HNSCC patients as the predominant type of cell death can have multiple impacts on the therapeutic response as cell death acts as a second messenger that guides both immune system and tissue microenvironment to ensure tissue repair and homoeostasis7. In this review, we discuss the different types of cell death, their connection with HNSCC pathogenesis and possible therapeutic windows that result from specific cell death sensitivity in some subgroups of HNSCC.
Box 1 HPV: the resource of heterogeneity in HNSCC.
HPV positivity is detected in about 25% of HNSCCs207. HPV‐positive (HPV + ) and HPV‐negative (HPV-) HNSCCs are derived from different anatomical locations (HPV‐negative cases are particularly located within the oral cavity, hypopharynx and larynx) and also have different mutation profiles, molecular characteristics, immune landscapes and clinical prognosis208,209. The immunologic profile of HPV + HNSCC was associated with a better outcome24. Accordingly, many studies demonstrated better response to therapy in HPV + HNSCC patients4. HPV infection also influences the genetic landscape of HNSCCs tumours and their predisposition to cell death. Despite the p53 tumour suppressor is the best-known target of HPV protein E6, some active p53 may still occur in HPV + HNSCC because these tumours usually harbour the wild-type form of the TP53 gene. On the other hand, HPV-unrelated HNSCCs often have p53 mutations14,15. In HPV-negative HNSCC, deletion of 9p21–22 occurs early in cancerogenesis and the function of the tumour suppressor p16 is lost. On the contrary, when HPV protein E7 inactivates the Rb protein, p16 is overexpressed210. Proteins p16 and p53 are deeply involved in senescence and apoptosis.
Intrinsic apoptosis
Apoptosis is a form of regulated cell death demarcated by the mitochondrial outer membrane permeabilization (MOMP) and accelerated by executioner caspases, mainly caspase-3. Once activated, caspase-3 cleaves target substrates such as poly(ADP-ribose) polymerase (PARP) and Lamin B which leads to the demise of the cell. While MOMP is essential for intrinsic cell death, the same is not true for caspases8. Nevertheless, the activity or inactivity of caspases provides a mechanism, which determines whether mitochondria initiate an immunologically silent or a pro-inflammatory type of cell death. If caspase activity is blocked following MOMP, cell death is accompanied by a type I interferon (IFN) response that alerts the immune system9,10. This response can be managed by mitochondria-dependent activation of the cGAS/STING pathway. Engagement of caspase-independent cell death displays potent anti-tumourigenic effects, often leading to complete tumour regression in the organism with intact immunity9. However, HPV + HNSCC cells respond poorly to activators of the cGAS-STING pathway. The attenuation of IFN responses results from the direct blockade of STING by viral protein E711. Despite this fact, HPV + cancer cells can be still more easily recognisable by the immune system as HPV antigens could be presented together with common danger signals from dying cancer cells12.
HPV replication occurs in terminally differentiating epithelium and requires the activation of cellular DNA replication proteins. However, unplanned DNA replication can result in apoptosis and therefore the viral E6 protein induces the degradation of tumour suppressor p53 and the pore-forming protein BAK to prevent apoptosis13. Nevertheless, active p53 may still occur in HPV + HNSCC because these tumours usually harbour the wild-type form of the TP53 gene. On the other hand, in HPV- HNSCC, p53 is mostly mutated14,15. Despite the wild-type form of the TP53 gene, persistent infection with high-risk HPV presents a major risk factor in HPV-associated cancers. It was shown that NF-κB activity is involved in the establishment of persistent HPV infection as activation of NF-κB by HPV proteins limits viral replication through degradation of protein E116. Unfortunately, NF-κB signalling can contribute to cisplatin resistance and cancer cells survival. Chemoresistant HNSCC cells with active NF-κB signalling respond to chemotherapy by promoting histone deacetylation and generation of heterochromatin. Therefore, targeted inhibition of histone deacetylases may be used as a possible therapeutic strategy for disrupting tumour resistance caused by NFκB17. NF-κB-mediated stabilisation of SNAI2 (Slug) can also underlie the inflammation-induced epithelial–mesenchymal transition (EMT) and metastasis in HNSCC18. On the other hand, NF-κB activation leads to reduced levels of nuclear BRCA1, impaired and prolonged DNA damage repair, prolonged accumulation of γH2AX foci and increased genomic instability17. As NF-κB induces the expression of various pro-inflammatory genes and participates in inflammasome activation19,20 and activation of the DDR has been linked to the increased presentation of major histocompatibility complex I (MHC I) molecules on the cell surface, this can result in increased recognition of cancer cells by cytotoxic T-lymphocytes21. Accordingly, HPV-positivity was correlated with increased T-cell infiltration, increased immune cytolytic activity, T-cell-inflamed immune microenvironment, the higher diversity of T-cell receptors, immune effector cell activation and improved response to anti-PD-1 therapy22. Infiltration of tumours with CD8 + cytotoxic T-lymphocytes has been associated with a favourable prognosis in several tumour types and may represent a predictive biomarker for cancer immunotherapy23. Consequently, the immunologic profile of HPV-positive HNSCC was associated with a significantly better outcome24. The HPV-specific immune response is also suggested to play a role in the significantly better response of HPV-positive patients to radiotherapy25. This higher sensitivity does not probably result from increased apoptosis or permanent G1-arrest but is rather associated with high levels of residual double-strand breaks and extensive G2-arrest26 and can be connected with a radiation-induced loss of cell surface CD47 enhancing the immune-mediated clearance of HPV + cancer cells27. Further evidence of the easier immune recognition is the fact that DNA damage generally induces higher expression of multiple ligands activating receptors of NK cells, such as NKG2D or DNAM121,28,29. The DDR-mediated activation of NK cells may importantly contribute to the recognition and removal of pre-malignant and malignant cells30.
E1∧E4 is a viral protein profusely expressed in HPV-infected epithelia. It binds to the cytokeratin networks and in some cases induces their collapse. When cytokeratin is not present in the cell, E1∧E4 associates with mitochondria soon after its synthesis and induces the detachment of mitochondria from microtubules. This is followed by a severe reduction in the mitochondrial membrane potential and an induction of MOMP31. Interestingly, multiple studies have shown that HPV-positive oropharyngeal carcinomas are more likely to present reduced keratinization32 and more favourable outcome33. This can be connected to fast induction of MOMP in cells with low keratinization31. MOMP sensitivity can be especially supposed in HPV + mesenchymal subtypes (MS) of HNSCC as MS-signature contains downregulation of markers for epithelial differentiation and keratinization3. On the other hand, BA HNSCC subgroup exhibits high levels of epithelial keratinization and differentiation34,35. It is also possible that high cellular keratin content can confer some protection against MOMP triggered by mitochondria-binding chemotherapeutics as it was shown that high levels of keratin 6 cause chemoresistance to platinum drugs36.
Furthermore, it has been demonstrated that HPV-positive HNSCC cells use mitochondrial respiration and produce high levels of cytochrome c oxidase (COX), the key enzyme in the mitochondrial respiratory pathway. E6 oncoproteins also increased mitochondrial mass, protein levels of mitochondrial complexes (I to IV), ATP synthase and the voltage-dependent anion channel (VDAC). On the other hand, in HPV-negative HNSCC the mitochondrial OXPHOS is decreased and glycolysis is preferred due to non-functional p5337–40. Consequently, HPV-positive HNSCC may be more sensitive to mitochondria-targeted treatments, such as mitocans. Accordingly, HPV oncoprotein E7 enhances ceramide-mediated mitochondrial fission and lethal mitophagy in response to chemotherapy-induced mitochondria damage41.
The higher expression of VDAC, high mitochondria mass, and sensitivity to MOMP can also facilitate the therapeutic effect of cisplatin in HNSCC as cisplatin preferentially binds mitochondrial DNA and VDAC in the mitochondrial membrane42. TP53 mutations in HPV-negative HNSCC cells correlates with a metabolic shift toward glycolysis suggesting some beneficial effects of glycolytic inhibition during anticancer treatment. In contrast, wtTP53 expressing HPV-positive cells probably require inhibition of both, the mitochondrial respiration and glycolysis, to become sensitised to treatment40.
Extrinsic apoptosis
Typically, extrinsic apoptosis is induced by the binding of the death ligands to death receptors, which then recruit adaptor molecules and initiator caspases to form the death-inducing signalling complex (DISC). Initiator caspases are then activated by proximity-induced cleavage at the DISC and in turn activate executioner caspases, which leads to the demise of the cell. In cancer cells, DISC formation is often weak and amplification of the death signal via the mitochondrial pathway is necessary for apoptosis.
From the view of death ligands and receptors, TNFSF10 (encoding TRAIL) is the only gene found to be significantly altered in HNSCC. The most common alterations in components of the DISC found in HNSCC are amplifications of FADD gene (25% of HNSCC) and mutations of gene CASP8 encoding procaspase-8 (10% of HNSCC). Interestingly, except HNSCC, there are no other tumour types with >10% incidence of CASP8 mutations. The alterations in CASP8 and FADD genes are significantly mutually exclusive, suggesting that they can be synthetically lethal43 as FADD and caspase-8 are needed for TRAIL-induced activation of NF-κB44.
In HPV-positive HNSCC, HPV proteins modulate apoptosis to prevent cell demise at early stages of viral infection. HPV16 E5 protein inhibits TRAIL signalling by interfering with the formation of DISC and subsequent cleavage of procaspases-8 and -3, as well as PARP. E5 also decreases the cell surface expression of the FAS receptor45. Similarly, it has been reported that E6 can inhibit apoptosis induced by TNF, FAS and TRAIL through the accelerated degradation of pro-apoptotic proteins such as FADD and/or procaspase-8 or through interactions with proteins that form DISC. Although E6 suppresses activation of both caspase-3 and caspase-8, it does not affect apoptotic signalling through the mitochondrial pathway fundamentally46–49. HPV-16 E6 and E7 oncoproteins upregulate BIRC3 (C-IAP2) expression conferring the resistance to apoptosis50. In primary human keratinocytes, the HPV16 E7 protein alone increased both spontaneous and TNF-α-induced apoptosis but co-expression of E7 and E6 has cancelled the E7-mediated apoptosis51. The HPV E2 protein represses the expression of the viral oncogenes and activates viral DNA replication. An intact E2 gene is common in HPV16 positive oropharyngeal carcinomas but rare in HPV18-associated carcinomas. Cells expressing HPV18 E2 had perinuclear clustering of the mitochondria, higher ROS production, and loss of the cristae structure52. The presence of an intact E2 gene is associated with higher HPV viral load and improved clinical outcome53. The E2 protein induces apoptosis in both normal and HPV-transformed cells through activation of caspase-854,55.
The finding that HPV16 E6 protein stimulates the degradation of the c-Myc oncoprotein seems rather surprising56. Nevertheless, c-Myc promotes oncogene-induced senescence (OIS) which is a critical tumour-suppressor mechanism preventing the transformation of cells. c-Myc promotes OIS through the transcriptional activation of p14/Arf/INK4B followed by p53 activation57. p14/Arf/INK4B is encoded by CDKN2A. HPV-positive HNSCCs rarely have TP53 and CDKN2A mutations58 and overexpression of c-Myc can activate OIS in these cells. HPV16 can remain infectious for 2 weeks on senescent cells but require cell cycle re-activation for successful HPV infection59. Consequently, downregulation of c-Myc may be beneficial for HPV infection because of reduction of the senescent phenotype. On the contrary, most of the smoking-related HNSCCs demonstrate TP53 and CDKN2A inactivation34 and therefore do not force HPV to c-Myc downregulation. These tumours can exploit the oncogenic force of c-Myc without OIS induction. Accordingly, it was demonstrated that HPV + oral SCC patients with a history of tobacco use have a significantly poorer prognosis even compared to HPV − patients60. Nevertheless, CDKN2A copy number loss predicted poor survival independently of other clinical and treatment factors61.
Crosstalk between apoptosis and autophagy
Caspases play a critical role in the crosstalk between autophagy and apoptosis as many autophagy-related proteins are recognised and cleaved by caspases (for example ATG3, ATG5, ATG16L1 or Beclin-1). In most cases, ATG proteins are degraded by caspases and the autophagic response is shut off62. In some special cases, the pro-autophagic proteins can be cleaved by caspases and converted into pro-apoptotic ones. For example, caspase-mediated cleavage of Beclin-1 inactivates autophagy and enhances apoptosis by promoting the release of pro-apoptotic factors from mitochondria63 and enhancing caspase-9 activity64.
The inhibition of essential apoptotic proteins during cancerogenesis can switch a cellular stress response from the default apoptotic pathway to autophagy. Accordingly, the role of autophagy regarding cell death should be rather protective. Nevertheless, the autophagy machinery can also interact with apoptosis in another way. As mentioned above, caspase-8 is activated by DISC. Another way of caspase-8 activation is managed by autophagosomes. This is executed by binding to the autophagic cargo receptor p6265. In the case that procaspase-8 is not activated in DISC, for example under high levels of c-FLIPL66, which is a frequent event in HNSCC67, this mechanism can rescue caspase-8 activity and enables cell death. This mechanism involves autophagosomes but not necessarily autophagic flux and degradation of cargo in autolysosomes. Consequently, late-stage autophagy inhibitors such as chloroquine or hydroxychloroquine can preserve this pro-apoptotic effect but can weaken some protective effects of autophagy. Thus, autophagic proteins either directly or through their caspase-cleaved fragments can effectively influence apoptosis. In addition to the above mentioned, ATG12 interacts with Bcl-2 and Mcl-1 and promotes apoptosis by acting upstream of mitochondria68. ATG12 also forms a complex with ATG3 regulating mitochondrial integrity during mitophagy69. Furthermore, cells undergoing BAX/BAK-mediated apoptosis show signs of activation of unc-51 like autophagy activating kinase 1 (ULK1) and marks of autophagy. This autophagic flux is triggered early in the apoptotic signalling and therefore an activation of the apoptosome or caspases are not necessary. This BAX/BAK-mediated autophagy inhibits the secretion of the pro-inflammatory IFN-β produced in response to mitochondrial damage which may be important for keeping immunological silence during apoptosis70.
Necroptosis
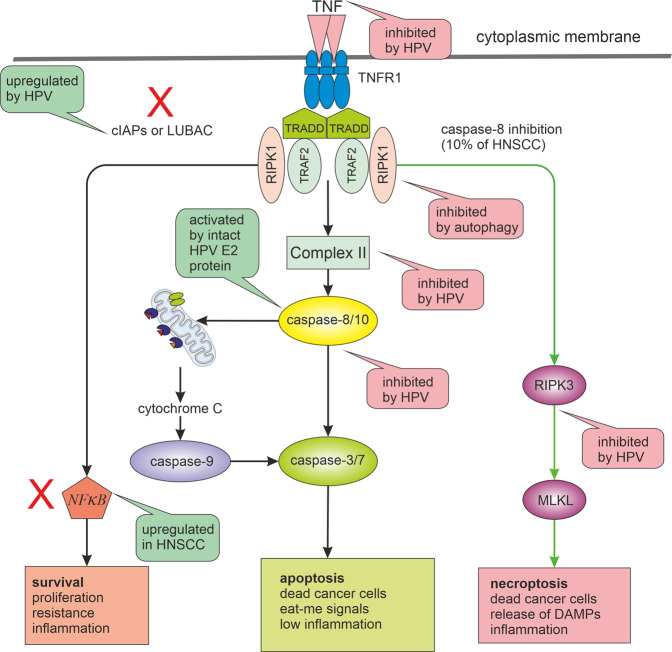
Necroptosis is an alternative mode of regulated cell death displaying features of apoptosis and necrosis. Necroptosis is characterised by the formation of a complex called the necrosome that consists of the proteins RIPK1, RIPK3 (receptor-interacting serine/threonine–protein kinases 1 and 3) and mixed lineage kinase domain-like pseudokinase (MLKL). MLKL translocating towards the plasma membrane results in the formation of pores, causing an inflammatory response. Loss of RIPK1 and RIPK3 function by promoter hypermethylation strongly correlated with metastatic disease and poor prognosis in HNSCC patients71,72.
In normal circumstances, caspase-8 blocks necroptosis by cleaving RIPK1 and CYLD73. Inactivation of caspase-8 activity was not shown in HPV + HNSCC74. On the contrary, the stimulation of caspase-8 activity and concomitant re-localisation to the nucleus due to the interaction between viral E6 protein and caspase-8 was shown in both high and low-risk HPV types. E6 appears able to stimulate caspase-8 activity without apoptosis triggering75,76. This nuclear accumulation of caspase-8 was reported also in HPV-positive tumour cell lines and cervical cancer77. Protein E6 most likely recruits caspase-8 to the nucleus to perform functions that are beneficial either for the viral life cycle or in the maintenance of cell proliferation in E6 transformed cells. For example, active caspase-8 suppresses necroptotic cell death mediated by RIPK3 and MLKL and cleavage of RIPK1 by caspase-8 is a mechanism for dismounting death-inducing complexes, which is essential for limiting cell death in response to tumour necrosis factor α (TNFα)78. Moreover, HPV oncoproteins downregulate the expression of IFITM1 and RIPK3 to escape from IFNγ- and TNFα-mediated antiproliferative effects and necroptosis79.
The gene CASP8 encoding caspase-8 is mutated in 10% of HNSCC tumours analysed by The Cancer Genome Atlas34. Caspase-8 plays an important role in the apoptotic response of HNSCC to cisplatin and knockdown of caspase-8 substantially decreased apoptosis and cisplatin sensitivity80. But it is possible that HNSCC subtypes with caspase-8 mutations can be more sensitive to necroptosis triggering (e.g. by TNF-α) (see Fig. 1). Accordingly, a subgroup of oral cavity tumours with good clinical outcomes displayed inactivating mutations of caspase-834. Consequently, targeting the necroptotic pathway seems to be a relevant therapeutic approach with compromised caspase-8 activity. However, the triggering of necroptosis should be done with caution. Necroptotic cells were shown to promote the migration and invasion of HNSCC cells in vitro through releasing DAMPs and RIPK1 can activate the NF-κB pathway in tumour cells which can lead to increased migration, invasion and proliferation81,82. Accordingly, TNF-α inhibits the growth of non-malignant cervical keratinocytes but stimulates proliferation of HPV-immortalised and cervical carcinoma-derived cell lines when mitogens such as epidermal growth factor (EGF) or serum are depleted83. Therefore, the triggering of necroptosis should be accompanied with suitable synergic treatment securing inactivation of the NF-κB pathway (see Fig. 1). It was shown that loss of caspase-8 function in combination with SMAC mimetic treatment sensitises HNSCC to radiation through induction of necroptosis, in case that RIPK3 function is maintained84. SMAC mimetics stimulate degradation of cIAP1 and cIAP2 and disrupt the activation of NF-κB85,86. Furthermore, the linear ubiquitin chain assembly complex (LUBAC) can also mediate NF-κB signalling and induce cell death resistance87,88. Inhibition of NF-κB activation by LUBAC inhibitors sensitised lung squamous cell carcinoma to cisplatin, suggesting a possible utilisation of these inhibitors and other NF-κB pathway inhibitors (e.g. curcumin) also in HNSCC patients89–92.
Fig. 1. Alternative pathways of TNF signalling and their alterations in HNSCC.
TNF signalling can lead to different results. It depends on the post-translational modification and activation of key molecules such as caspase-8, RIPK1 and NF-κB. While active caspase-8 triggers apoptosis and suppresses necroptosis, its inactivity (e.g. by mutations) leads to necroptosis through RIPK3 activation. If the RIPK1 signal is modified by the addition of ubiquitin, cell death can be attenuated, and the cell receives a signal for survival and proliferation by the transcription factor NF-κB. NF-κB pathway inhibitors such as LUBAC inhibitors or SMAC mimetics can sensitise HNSCC cells to necroptosis or apoptosis. For successful anticancer treatment response, the pathway marked by red exes should be inhibited (inhibition of autophagy should be also beneficial for necroptosis triggering). The TNF signalling pathways are also significantly influenced by HPV infection and by the genetic background of HNSCC. Green bubbles indicate activation and pink inhibition of the process. LUBAC linear ubiquitin chain assembly complex, c-IAPs inhibitors of apoptosis, RIPK receptor-interacting serine/threonine–protein kinases, MLKL mixed lineage kinase domain-like pseudokinase.
The important role in necroptosis induction may also play autophagy as autophagy inhibition or specifically ULK1 inhibition can enhance necroptosis by tumour necrosis factor (TNF) and toll-like receptor (TLR) ligands93–95. This pro-survival function of ULK1 is mediated via the phosphorylation of RIPK1 at Ser35796. The pro-survival function of autophagy is also supported by COP9 (Constitutive Photomorphogenesis 9) signalosome function. COP9 signalosome suppresses RIPK1-RIPK3–mediated necroptosis by regulating autophagosome maturation. Impaired autophagosome maturation causes necroptosis97,98. Proteins of COP9 signalosome are often overexpressed in cancer99. However, the crosstalk between autophagy and cell death can be more complex as the death-triggering function of ULK1 by the enhancement of PARP1 activity100, the scaffolding role of the autophagy proteins in balancing necroptosis and apoptosis (via the SQSTM1/p62-dependent recruitment of RIPK1)101, or the anti-autophagic function of RIPK1 (via the control of transcription factor EB102) were also described.
Necroptosis has dual effects as promoter or reducer of tumour growth in different types of cancer. As a backup form of cell death in cells with apoptosis failure, necroptosis can prevent tumorigenesis. Nevertheless, it can also trigger metastasis and immunosuppression. This can be caused by the release of IL-33 during necroptosis103. IL-33 seems to be involved in the shaping of the immunosuppressive environment during cancerogenesis104,105 and high expression of IL-33 in cancer-associated fibroblasts (CAFs) and tumour cells was associated with poor prognosis106,107. While these data suggest that IL-33 blockade may be beneficial for HNSCC patients, further investigations are needed to define all downstream signalling targets dependent on IL-33. Furthermore, the necrosome can promote oncogenesis via CXCL1 and Mincle-induced immune suppression108. On the other hand, necroptotic cells can provide both antigens and inflammatory cytokines to dendritic cells for antigen cross-priming which activates cytotoxic CD8 + T-lymphocytes demonstrating cytolytic effects and defence against tumorigenesis. RIPK1 expression and NF-κB activation are essential for this tumour suppressive mechanism109. Induction of necroptosis was also shown to induce anti-tumour immunity in an HMGB1-, nucleotide- and T-cell-dependent manner110.
Pyroptosis
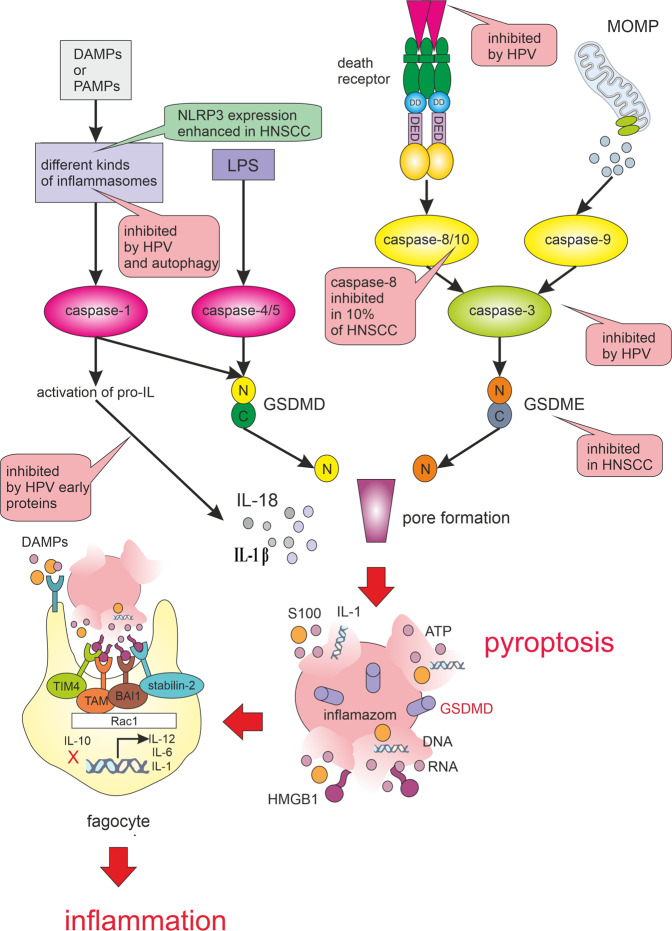
Pyroptosis is initiated by inflammatory caspases (1, 4 and 5) upon activation of the canonical or non-canonical inflammasome pathways. In the canonical inflammasome pathway, caspase-1 mediates the cleavage of gasdermin D (GSDMD) and the maturation of pro-inflammatory interleukins (IL-1β and IL-18). GSDMD pores then induce cell lysis, cell death and the leakage of intracellular components into the extracellular space. The non-canonical inflammasome pathway can be initiated by the direct binding of caspase-4 and -5 to lipopolysaccharide from Gram-negative bacteria8. Another way to activate pyroptosis is caspase-3/Gasdermin E (GSDME) pathway. Caspase-3 can be activated by mitochondrial intrinsic and death receptor pathway. The activated caspase-3 then cleaves GSDME. Cleaved GSDME N-fragments form pores in the plasma membrane, causing pyroptosis111 (see Fig. 2). This activation of GSDME may divert TNF-induced apoptosis to pyroptosis. Because GSDME expression is often silenced in tumour cells, which is not the case of healthy cells, this caspase-3 activity may be responsible for serious side effects of many chemotherapeutic regimens112. Pyroptosis can also be activated by caspase-8 and subsequent cleavage of GSDMD113. In the absence of GSDMD, caspase-1 can activate caspase-8, caspase-3, and caspase-7 and induce apoptosis, making apoptosis a backup programme for dysfunctional pyroptosis. Accordingly, tissue samples of HNSCC without the presence of lymph node metastasis showed high expression of caspase-1114. During apoptosis, caspase-3 and -7 specifically block cleavage of GSDMD115. In contrast, expression of inactive caspase-8 or pan-caspase inhibition induces the formation and subsequent activation of caspase-1. This mechanism triggers pyroptosis in cells with dysfunctional apoptosis116. Caspase-1 is also involved in the facilitation of cytoprotective autophagy during hypoxia-induced mitochondrial stress by activating LC3 and Beclin-1 and favouring clearance of damaged mitochondria117. In the feedback loop, autophagy downregulates inflammasomes as activation of autophagy by inflammatory signals limits IL-1β production by targeting inflammasomes for destruction. Both the AIM2 and NLRP3 inflammasomes can be recruited to p62 and engulfed by autophagosomes118. On the other hand, inhibition of autophagy enhances inflammasome activity118. Many studies have suggested that mitochondrial defects may promote inflammasome activation through excessive ROS production, mitochondrial cardiolipin exposure or release of mitochondrial DNA119.
Fig. 2. Pyroptosis pathways. Pyroptosis is triggered when damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) activate the inflammasomes.
Active inflammasomes lead to the cleavage and activation of caspase-1. The activated caspase-1 cleaves Gasdermin D (GSDMD). The N-fragment of GSDMD form cell membrane pores resulting in pyroptosis. Activated caspase-1 also promotes the maturation and secretion of interleukins IL-1β and IL-18. When pathogen-derived lipopolysaccharide (LPS) binds to the precursor of caspase-4/5, it can also cause GSDMD cleavage and induction of pyroptosis. Another way to activate pyroptosis is caspase-3/Gasdermin E (GSDME) pathway. Caspase-3 can also be activated by mitochondrial outer membrane permeabilization (MOMP) and death receptor pathways. The activated caspase-3 then cleaves GSDME and produces GSDME N-fragments, The N-fragment of GSDME form cell membrane pores resulting in pyroptosis. Pyroptosis results in membrane rupture and the release of DAMPs that are detected by phagocyte receptors, such as TIM 4 (T cell immunoglobulin mucin receptor 4), BAI 1 (brain-specific angiogenesis inhibitor 1), stabilin-2 and TAM (Tryo3-Axl-Mer receptor). DAMPs recognition leads to the production of pro-inflammatory interleukins (e.g. IL-1, IL-6) and IL-12, which activates NK cells and induces the differentiation of naive CD4 T cells. The pyroptotic signalling pathways are significantly influenced by HPV infection and by the genetic background of HNSCC. Green bubbles indicate activation and pink inhibition of the process.
Inflammation belongs among major causes of HNSCC cancerogenesis. Accordingly, inflammasome NLRP3 expression was enhanced in human HNSCC tissues and the IL-1β concentration was increased in the peripheral blood of these patients120. The increased expression of NLRP3 was also associated with tumour growth, invasiveness, metastasis, development of cancer stem cells (CSCs) and their self-renewal in HNSCC121–123. Furthermore, it has been reported that high NLRP3 expression is associated with poor clinical outcome in 5-FU-treated oral squamous cell carcinoma (OSCC) patients and NLRP3 knockdown increased 5-FU-induced apoptosis in OSCC cells124. Blockage of the NLRP3 inflammasome/IL-1β pathway by MCC950 improved anti-tumour immune responses in an HNSCC mouse model120 and blockade of the IL-1β pathway by biopharmaceutical drug anakinra has overcome erlotinib resistance in HNSCC xenografts125. On the other hand, alcohol has been shown to promote both the release of IL-1β and pyroptosis126. Infection of human keratinocytes with HPV16 also induced the secretion of IL-1β. Yet, upon expression of the viral early genes, IL-1β transcription is blocked, because HPV16 derived E6 protein can antagonise IL-1β production by inhibiting IRF6 transcription and upregulation of sirtuin 1 (SIRT1)127,128. Knockdown of SIRT1 upregulates AIM2 expression and triggers pyroptosis128. SIRT1 expression was associated with good prognosis in HNSCC patients129. Nevertheless, cigarette smoke impairs SIRT1 activity and promotes pro-inflammatory responses in epithelial cells130. HPV E7 may also inhibit pyroptosis by promoting TRIM21-mediated degradation and ubiquitination of the IFI16 inflammasome131.
Although pyroptosis seems to have rather tumour-promoting effects in HNSCC, the exogenous activation of pyroptosis has recently been shown to trigger powerful anti-tumour effect112. As many tumour cells have an innate resistance to apoptosis, the induction of pyroptosis may provide an efficient cancer therapy strategy. Indeed, interventions with some chemotherapeutic agents cause a switch from caspase 3-dependent apoptosis to pyroptosis by activation of GSDME112,132 and unleashing of inflammasome activation augments the efficacy of some immune checkpoint inhibitors133. GSDME also enhances the number and activity of tumour-infiltrating natural-killer (NK) and CD8 + T-lymphocytes, as well as phagocytosis of tumour cells by tumour-associated macrophages. Moreover, granzyme B produced by NK cells also activates pyroptosis in target cells by cleaving GSDME at the same site as caspase-3, thereby establishing a positive feedback loop. However, this mechanism of tumour suppression is abrogated in perforin-deficient mice or mice without killer lymphocytes134. Caspase-3 activation and cytochrome c release in response to apoptotic stimuli are significantly reduced in GSDME-deficient cells135. Uncleavable or pore-defective GSDME proteins are also not tumour suppressive and many cancer-associated GSDME mutations reduce GSDME function, suggesting that GSDME inactivation is a strategy developed by cancer cells to reach the immune evasion134. A decrease in GSDME expression and function was shown in radioresistant HNSCC136.
Ferroptosis
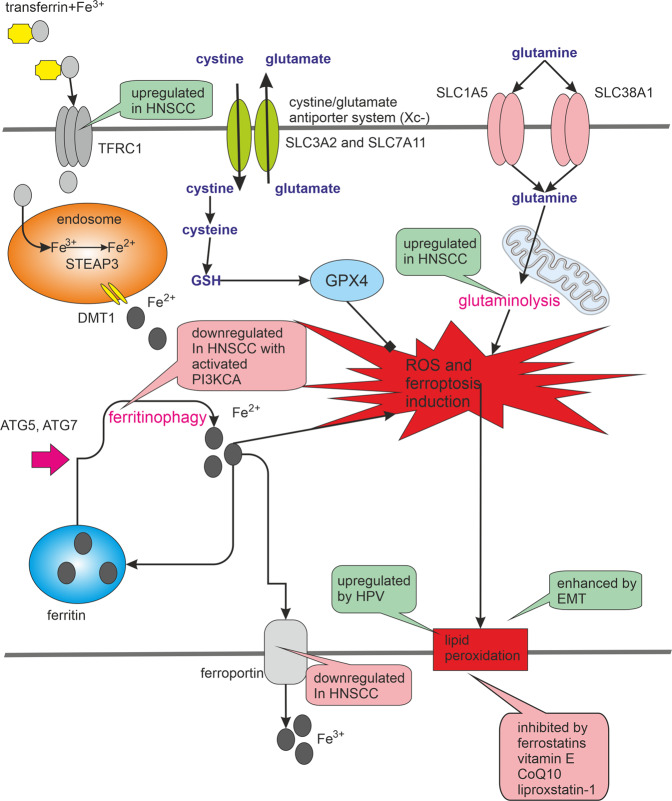
Ferroptosis is independent of caspase activity. Instead, ferroptotic cells die following iron-dependent membrane lipid peroxidation. Importantly, tumour cells capable of evading other types of cell death probably maintain or acquire a sensitivity to ferroptosis. Given that HNSCC cells often manifest an increased intracellular iron concentration due to a high level of TFRC1 (transferrin receptor 1 responsible for cellular iron uptake)137 and a low abundance of ferroportin (responsible for iron efflux)138, ferroptosis-inducing therapy can be expected to effectively induce cell death in HNSCC cells without affecting normal tissue; see Fig. 3. Interestingly, the TFRC gene is located in the genomic region frequently amplified in HNSCC (3q29)139.
Fig. 3. Ferroptosis. Ferroptosis is characterised by iron-induced lipid peroxidation.
The intracellular concentration of iron can be affected by the activity of transferrin, transferrin receptor and ferroportin, or by the release of iron from ferritin, which is often managed by ferritinophagy. HNSCC cells often manifest an increased intracellular iron concentration due to a high level of TFRC1 (transferrin receptor 1 responsible for cellular iron uptake) and a low abundance of ferroportin (responsible for iron efflux). The non-physiological degree of lipid peroxidation and the initiation of ferroptosis is prevented by glutathione and glutathione peroxidase 4 (GPX4) activity. Ferroptosis could be also prevented with chelating agents or vitamin E, which casts a bad light on the benefits of antioxidants in the treatment of some ferroptosis-prone cancers. Glutamine and glutaminolysis also play a crucial role in the activation of ferroptosis. PI3KCA is among the most frequently mutated and activated genes in HNSCC. PIK3CA activation can lead to increased mTOR activity and decreased autophagy. Consequently, the presence of PI3KCA activation may predispose these cancer cells to avoid autophagy, ferritinophagy and ferroptosis. The ferroptotic signalling pathways are significantly influenced by HPV infection and by the genetic background of HNSCC. Green bubbles indicate activation and pink inhibition of the process.
The induction of ferroptosis can be reversed by glutathione peroxidase 4 (GPX4) and ferroptosis suppressor protein 1 (FSP1). The blockade of cystine-glutamate antiporter (Xc–) system and cystine import is important for the induction of ferroptosis as inhibitors of (Xc–) system including sulfasalazine and erastin have been shown to induce ferroptosis140. The system (Xc–) consists of two subunits, SLC3A2 and SLC7A11. The expression of the SLC7A11 subunit is suppressed by protein p53 that inhibits cystine uptake and sensitises cells to ferroptosis. Even some mutated forms of p53, which are no longer able to induce apoptosis or senescence, do not lose their effect on the induction of ferroptosis141.
Tumour cells having mesenchymal features (tumour cell lines of mesenchymal origin, epithelial tumour cell lines that have undergone EMT and tumour cells exhibiting mesenchymal state-mediated resistance to anti-tumour therapy) appear to be particularly sensitive to ferroptosis. These cells usually have a higher activity of enzymes that promote the synthesis and storage of long-chain polyunsaturated fatty acids (PUFAs), which are a source of lipid peroxidation during oxidative stress. As a result, such cells are highly sensitive to GPX4 inhibition and ferroptosis. The interrelationship of mesenchymal phenotype and sensitivity to lipid peroxidation appears to be a result of the high expression of the protein ZEB1 (Zinc finger E-box-binding homeobox 1), which functions in both EMT and lipogenic processes. Deletion of ZEB1 was able to remove sensitivity to GPX4 inhibition in these cells142. ZEB1 was identified as a key player in the inflammation-induced promotion of EMT in HNSCC143. Accordingly, the MS subgroup of HNSCC characterised as having an elevated expression of EMT-associated genes could be the most sensitive to ferroptosis. In epithelial cells, interactions mediated by E-cadherin suppress ferroptosis by activating the intracellular NF2 and Hippo signalling pathway144. Furthermore, HPV16-derived E6 and E7 oncoproteins induce expression of the EMT-activating transcriptional factors Slug, Twist, ZEB1 and ZEB2145.
Another mechanism underlying ferroptosis was observed after erastin treatment. Erastin can bind with VDAC2 on the mitochondrial outer membrane, where it alters membrane permeability and the ion selectivity of the channels (allows only cations to move into mitochondria), causing mitochondrial dysfunction and ROS release that ultimately leads to glutathione (GSH) depletion and ferroptosis146. Recently, it was also found that activation of ferroptosis by erastin increases the level of lysosomal-associated membrane protein 2a (LAMP2), thereby promoting chaperone-mediated autophagy, which in turn promotes the degradation of GPX4147. Ferroptosis seems to be also tightly associated with ferritinophagy as inhibition of ferritinophagy by blockage of autophagy or knockdown of NCOA4, which mediates the selective autophagic degradation of ferritin, abrogated the accumulation of ROS and cellular labile iron148. HPV16 oncoproteins mute the host autophagic response at different levels of the autophagic pathway. E5 interferes with the phagophore assembly, while E6 and E7 inhibit autophagosome/lysosome fusion149,150. This autophagy inhibition may provide some resistance against ferroptosis in HPV-positive HNSCC, but the exact influence of HPV on ferritinophagy is currently not clear because it was also shown that HPV16 E6/E7 oncoproteins can activate autophagy via accelerating autophagosome formation and degradation151.
PI3KCA is among the most frequently mutated and activated genes in HNSCC in both HPV-positive and negative diseases (56 and 34%, respectively)34. Interestingly, PIK3CA activation in HPV-positive HNSCC can lead to increased mTOR activity and decreased autophagy152. Consequently, the presence of PI3KCA activation may predispose these cancer cells to avoid autophagy, ferritinophagy and ferroptosis. It was shown that aspirin or its active metabolite salicylate induce autophagy by inhibiting the acetyltransferase activity of EP300153,154. This effect of aspirin may be especially beneficial for HNSCC patients with PI3KCA over-activation. Accordingly, the use of nonsteroidal anti-inflammatory drugs (NSAID), such as aspirin, caused improved survival among HNSCC patients with PIK3CA mutations. Among subjects with PIK3CA mutations or amplification, regular NSAID use (≥6 months) conferred markedly prolonged disease-specific survival and overall survival compared to non-regular NSAID users155. In our opinion, this could be partly a consequence of autophagy and ferroptosis reactivation. Autophagy induction by rapamycin also showed the synergistic effects with irradiation in oral squamous cell carcinoma cells156.
In the absence of glutamine or inhibition of glutaminolysis, blocked cystine import cannot induce ferroptosis. Since many types of tumours, including HNSCC, are dependent on glutaminolysis157 and glutaminolysis is necessary to induce ferroptosis, can these tumour cells be more sensitive to induction of ferroptosis? Glutamine is synthesized by the enzyme glutamine synthetase (GS) from glutamate and ammonia. Glutamate is generated from glutamine during glutaminolysis, which is a series of biochemical reactions degrading the amino acid glutamine to glutamate, aspartate, CO2, pyruvate, lactate, alanine and citrate157. Both glutaminase-1 (GLS1) and glutaminase-2 (GLS2) are involved in glutaminolysis, but only GLS2 mediates ferroptosis and is a transcriptional target of the p53 protein. Accordingly, GLS2 has been recognised as a tumour suppressor and GLS1 more as an oncoprotein158. GLS1 expression was correlated with a poor survival rate in HNSCC patients159. It was also demonstrated that high expression of system (Xc–) components and glutamine transporter ASCT2 is correlated with undifferentiated status in HNSCC160 and ASCT2-dependent glutamine uptake is involved in the progression of HNSCC161. Sulfasalazine is a specific inhibitor of (Xc–)-mediated cystine transport and inhibits the growth of HNSCC cells162. It was shown that the cytotoxicity of sulfasalazine relies on ASCT2‐dependent glutamine uptake and glutamate dehydrogenase (GLUD)‐mediated α‐ketoglutarate production. Therefore, the activity of glutaminolysis-related proteins, such as ASCT2 and GLUD, can be probably used as a biomarker to predict the efficacy of sulfasalazine therapy in HNSCC160. On the other hand, sulfasalazine resistant HNSCC cells were found to highly express aldehyde dehydrogenase ALDH3A1 which plays a key role in the protection of cells from lipid peroxidation. This resistance was reversed by knockdown of ALDH3A1 or by ALDH inhibitor dyclonine163. Several studies have also revealed that transporters ASCT2 and (Xc–) mediate resistance in HNSCC cells after cetuximab, cisplatin and AG1478 treatment164–166.
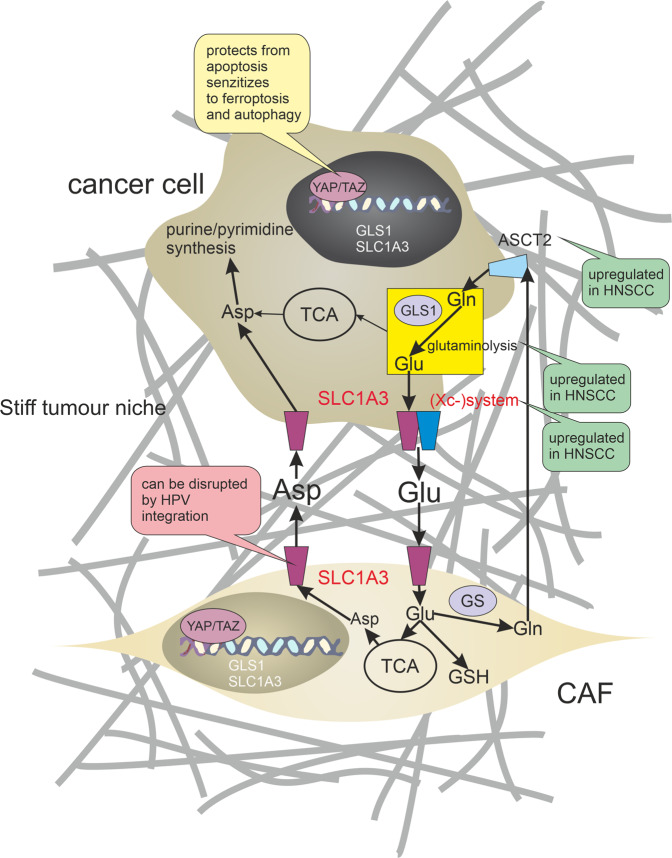
High concentrations of extracellular glutamate can block cystine uptake by the (Xc–) system and induce ferroptosis167,168. As cancer cells secrete high concentrations of glutamate through the activity of the cystine/glutamate antiporter (Xc–) system, they can be sensitive to ferroptosis when they have no support of other cells in the tumour microenvironment (TME). Nevertheless, increased stiffness of extracellular matrix during tumour progression induces CAFs to import glutamate (through SLC1A3) and release aspartate and glutamine supporting cancer cell proliferation; see Fig. 4. Glutamine synthetase (GS/GLUL) is often overexpressed in these CAFs169. In some cases, SLC1A3 gene can be interrupted by HPV integration170,171. The metabolic symbiosis enables cancer cells to get rid of ferroptosis-promoting glutamate and to gain glutamine. This metabolic exchange can take place also between cancer cells and M2-macrophages172. Consequently, blocking the transport of glutamate into the CAFs or M2-macrophages may lead to enhanced sensitivity of glutamine-addicted cancer cells to ferroptosis. Nevertheless, this approach should be used with caution because increased levels of extracellular glutamate have been associated with the progression of cancer-induced pain173.
Fig. 4. Metabolic symbiosis between cancer-associated fibroblasts and HNSCC cells.
HNSCC cells undergo numerous metabolic changes including increased glutaminolysis. Glutaminolysis produces large pools of intracellular glutamate. Upregulation of the cystine/glutamate antiporter ((Xc–) system) and excitatory amino acid transporter (SLC1A3) promotes aberrant glutamate (Glu) release from cancer cells. Increased stiffness of extracellular matrix during tumour progression induces cancer-associated fibroblasts (CAFs) to import glutamate (through SLC1A3) and release aspartate (Asp) and glutamine (Gln) supporting cancer cell purine/pyrimidine synthesis. Glutamine synthetase (GS) is often overexpressed in these CAFs. CAFs can also promote chemoresistance through the production of glutathione (GSH). Establishing of this metabolic symbiosis is coordinated by a YAP/TAZ-dependent mechanotransduction pathway. Green bubbles indicate activation and pink inhibition of the process.
Establishing of glutamate metabolic symbiosis by metabolic reprogramming is coordinated by a YAP/TAZ-dependent mechanotransduction pathway174; see Fig. 4. Accordingly, levels of nuclear YAP/TAZ in fibroblasts associated with perineural invasion of HNSCC were higher than those in the stroma of normal mucosa175. YAP expression was elevated at the invasive front of HNSCC tumours176 and also as a consequence of PIK3CA overexpression177. Although YAP activation in tumour stroma can lead to avoiding ferroptosis caused by a high concentration of glutamate in TME, YAP itself can promote ferroptosis under some circumstances by upregulating ferroptosis modulators ACSL4 and transferrin receptor TFRC144. It seems possible that YAP/TAZ activity balances the tendency of cancer cells to undergo a distinct form of cell death as nuclear accumulation and activation of YAP/TAZ protect cells from apoptosis but sensitise cells to ferroptosis and autophagy144,178.
Some cells in TME, such as CD8 + T-cells, can induce ferroptosis in tumour cells and the insensitivity to PD-L1 inhibitors is often accompanied by resistance to ferroptosis179. One of the key factors causing the immunogenicity of ferroptotic cancer cells may be HMGB1180. Cancer cells undergoing ferroptosis release HMGB1 in an autophagy-mediated manner, when autophagy promotes HMGB1 acetylation, resulting in HMGB1 release181. Although HMGB1 serum and tissue levels were found to be elevated in HNSCC, they are associated rather with chemoattraction of regulatory T cells (Treg) and promoting of their immunosuppressive functions182. Ferroptosis was also associated with an increased expression of cyclooxygenase-2 and the release of prostaglandin E2 (PGE2) which facilitates immune evasion of tumour cells183.
Targeted therapy of non-apoptotic programmed cell deaths in cancer
The development of new antineoplastic drugs targeting programmed cell death suitable for clinical use is a demanding and time-consuming process. Therefore, the effects of previously approved drugs on any type of programmed cell death should be intensively studied. The potential for such type of clinical use has been attributed to some cholesterol-lowering drugs, muscle relaxants, antimalarials or anti-rheumatic drugs such as sulfasalazine, lanperisone, statins, artesunate or aspirin.
Sulfasalazine (Azulfidine) has been repurposed to induce ferroptosis via inhibition of (Xc–) system. Cytotoxicity of sulfasalazine relies on glutamine uptake and α‐ketoglutarate production162. However, HNSCC cancer cells are capable of developing resistance. Resistance to ferroptosis induced by sulfasalazine may be overcome by ALDH inhibitor dyclonine or inhibition of CISD2163,184. Both pharmacological and genetic inhibition of SLC7A11 induce ferroptotic cell death and increase the cytotoxicity of cisplatin in HNSCC cells, which were resistant to cisplatin before this inhibition185. Activation of ferroptosis also appears to contribute to the efficacy of radiotherapy186 or some novel anticancer drugs with potential in HNSCC treatment, such as dihydroartemisinin187. (Xc–)-targeted therapy may also kill undifferentiated HNSCC cells expressing variant isoforms of CD44 (CD44v) and concurrently may sensitise the remaining HNSCC cells to available treatments including EGFR-targeted therapy165.
Lanperisone is a modified form of muscle relaxant tolperisone and selectively kills K-Ras-mutant cells through the induction of ROS, which is mediated through iron and ferroptosis pathways188. Ferroptosis may also be induced by statins as statins target the mevalonate pathway, which is crucial for GPX4 maturation189. This fact opens novel prospects of statins as therapeutic agents in cancer190. Another inductor of ferroptosis is probably artesunate and its derivatives. Artesunate is a medication used to treat malaria and can also produce ROS and cause oxidative stress in cancer cells. In pancreatic ductal adenocarcinoma, HNSCC and ovarian cancer cells, the anti-tumour effect of artesunate was mediated by induction of ferroptosis185.
Many proven anticancer drugs show a profound effect on programmed cell death. Dabrafenib, a B-RAF inhibitor approved for the treatment of melanoma, has also been shown to be a potent, high-affinity RIPK3 inhibitor that blocks TNF-α-induced necroptosis191. Other clinically approved drugs that may inhibit necroptosis include vemurafenib, sorafenib, pazopanib and ponatinib192. In contrast, induction of necroptosis has been identified as an important effector mechanism of oxaliplatin, mitoxantrone or 5-fluorouracil in anti-tumour activity193,194. In some tumour cells, inhibition of caspases is essential for the induction of necroptosis. Inhibitors of caspase activity can block caspase-8-mediated cleavage of RIPK1 and stabilise RIPK1-containing protein complexes. Inhibitors of caspase activity (e.g. IDN-7314, Z-VAD) are being tested as a novel approach in adjuvant chemotherapy of colorectal cancer showing resistance to 5-fluorouracil194. Induction of necroptosis also appears to be beneficial in the treatment of leukaemias and neuroblastomas195,196. Some results indicate that cisplatin and Val-boroPro (Talabostat) induce pyroptosis in cancer cells suggesting that they may provide additional advantages in the treatment of cancers with high levels of GSDME expression197,198. Ferroptosis can be induced by altretamine and sorafenib. Altretamine is an orally administered alkylating agent which is currently used as a secondary therapy for advanced ovarian carcinoma. It directly binds and inactivates GPX4199. Sorafenib is a kinase inhibitor approved for the treatment of renal cell carcinoma, hepatocellular carcinoma and thyroid carcinoma. Sorafenib induces ferroptosis independent from the oncogenic status of Ras, RAF, PIK3CA and p53 in cancer cell lines originating from different solid tumours200. Sorafenib increased the antiproliferative effect of cisplatin without affecting apoptosis in HNSCC cells, also enhanced HNSCC radiosensitivity201. Cisplatin alone was found to be a potent inducer of ferroptosis202.
The only clinically approved autophagy inhibitors nowadays are chloroquine (CQ) and hydroxychloroquine (HCQ)203. HCQ is currently in various stages of clinical trials as monotherapy or as part of combination therapy for solid tumours, however, pharmacodynamic studies suggest that the maximum permitted dose of HCQ (1200 mg/day) shows only slight inhibition of autophagy in vivo. This may be due to the reduced absorption of the drug into cells in an acidic environment (pH around 6.5), which is unfortunately typical of the tumour microenvironment204. Many CQ analogues with promising metabolic and antineoplastic effects are also in clinical trials205. Furthermore, some otherwise used compounds may have a profound effect on autophagy. For example, pro-apoptotic BH3-mimetic compounds such as ABT737, competitively disrupt the interaction between Beclin-1 and BCL2 or BCL-XL liberating Beclin-1 from an inhibitory complex and thus induce autophagy206. Aspirin induces autophagy via inhibition of the acetyltransferase EP300 and recapitulates features of caloric restriction153,154.
Conclusion
Many HNSCC cancer therapies aim to induce apoptosis to suspend tumour growth. However, avoiding apoptosis is one of the key hallmarks of cancer and the presence of genetic heterogeneity and supporting TME severely limits the clinical efficacy of these approaches. Nevertheless, scientific consensus describes many different cell death subroutines with different regulatory pathways and cancer cells are probably not able to avoid all of cell death types at once. Therefore, a more focused and individualised therapeutic approach is needed to address the specific advantages and disadvantages of individual tumours. A full understanding of the sensitivity or resistance to specific cell death type given by certain genetic background and/or microenvironment that occurs during the HNSCC pathogenesis may reveal specific and effective targets for novel tailored therapeutic approaches. The genetic fingerprint of individual tumours can direct the development of novel agents to selectively hit the tumour cells while sparing the healthy ones. For example, HPV-positive HNSCC may be more sensitive to mitochondria-targeted treatments, such as mitocans, and the MS subgroup of HNSCC, having an elevated expression of EMT-associated genes, could be the most sensitive to ferroptosis. Consequently, the future development of agents that directly target cell death pathways could lead to disease regression even in patients with poor prognosis.
Author contributions
M.R. and J.B. developed the rationale of the review. M.R., J.B. and M.M. wrote the paper.
Ethics statement
No ethics approvals were required for this paper.
Funding statement
This work was supported by funds from the Faculty of Medicine, Masaryk University to Junior researcher (Jan Balvan), by Grant Agency of the Czech Republic (GACR - 18-03978S), by the Ministry of Health of the Czech Republic (NU20J-08-00018), by funds from Specific University Research Grant, as provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2021 (MUNI/A/1698/2020 and MUNI/A/1246/2020), and by the “Center for Tumour Ecology—Research of the Cancer Microenvironment Supporting Cancer Growth and Spread” (reg. no. CZ.02.1.01/0.0/0.0/16_019/0000785) supported by the Operational Programme Research, Development and Education.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by F. Pentimalli
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lemaire F, et al. Differential expression profiling of head and neck squamous cell carcinoma (HNSCC) Br. J. Cancer. 2003;89:1940–1949. doi: 10.1038/sj.bjc.6601373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch WM, Ridge JA, Forastiere A, Manola J. Comparison of clinical and pathological staging in head and neck squamous cell carcinoma: results from Intergroup Study ECOG 4393/RTOG 9614. Arch. Otolaryngol. Head. Neck Surg. 2009;135:851–858. doi: 10.1001/archoto.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keck MK, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin. Cancer Res. 2015;21:870–881. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 4.Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat. Rev. Cancer. 2018;18:269–282. doi: 10.1038/nrc.2018.11. [DOI] [PubMed] [Google Scholar]
- 5.Wallace NA, Galloway DA. Manipulation of cellular DNA damage repair machinery facilitates propagation of human papillomaviruses. Semin. cancer Biol. 2014;26:30–42. doi: 10.1016/j.semcancer.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garnett TO, Duerksen-Hughes PJ. Modulation of apoptosis by human papillomavirus (HPV) oncoproteins. Arch. Virol. 2006;151:2321–2335. doi: 10.1007/s00705-006-0821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legrand AJ, Konstantinou M, Goode EF, Meier P. The diversification of cell death and immunity: memento mori. Mol. Cell. 2019;76:232–242. doi: 10.1016/j.molcel.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giampazolias E, et al. Mitochondrial permeabilization engages NF-kappaB-dependent anti-tumour activity under caspase deficiency. Nat. Cell Biol. 2017;19:1116–1129. doi: 10.1038/ncb3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rongvaux A, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaikh MH, Bortnik V, McMillan NAJ, Idris A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb. Pathog. 2019;132:162–165. doi: 10.1016/j.micpath.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Andersen AS, Koldjaer Sølling AS, Ovesen T, Rusan M. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int. J. Cancer. 2014;134:2755–2763. doi: 10.1002/ijc.28411. [DOI] [PubMed] [Google Scholar]
- 13.Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J. Gen. Virol. 1999;80:1513–1517. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- 14.Kimple RJ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–4800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruyama H, et al. Human papillomavirus and p53 mutations in head and neck squamous cell carcinoma among Japanese population. Cancer Sci. 2014;105:409–417. doi: 10.1111/cas.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakahara T, et al. Activation of NF-κB by human papillomavirus 16 E1 limits E1-dependent viral replication through degradation of E1. J. Virol. 2015;89:5040–5059. doi: 10.1128/JVI.00389-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida L., et al. NF kappa B mediates cisplatin resistance through histone modifications in head and neck squamous cell carcinoma (HNSCC). FEBS Open Bio.4, (2013) [DOI] [PMC free article] [PubMed]
- 18.Liu S, et al. Stabilization of slug by NF-kappaB is essential for TNF-alpha -induced migration and epithelial-mesenchymal transition in head and neck squamous cell carcinoma cells. Cell Physiol. Biochem. 2018;47:567–578. doi: 10.1159/000489990. [DOI] [PubMed] [Google Scholar]
- 19.Boaru S. et al. NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem. Biophys. Res. Commun. 458, 700–706 (2015). [DOI] [PubMed]
- 20.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19:731–745. doi: 10.1038/s41580-018-0068-0. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Rep. 2019;9:13404. doi: 10.1038/s41598-019-49771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 24.Badoual C, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 25.Park JW, et al. Human papillomavirus type 16 E7 oncoprotein causes a delay in repair of DNA damage. Radiother. Oncol. 2014;113:337–344. doi: 10.1016/j.radonc.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rieckmann T, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother. Oncol. 2013;107:242–246. doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Vermeer DW, et al. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int. J. Cancer. 2013;133:120–129. doi: 10.1002/ijc.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayitoglu E. C. et al. Boosting natural killer cell-mediated targeting of sarcoma through DNAM-1 and NKG2D. Front. Immunol.11, 40 (2020). [DOI] [PMC free article] [PubMed]
- 30.Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32:135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Raj K, Berguerand S, Southern S, Doorbar J, Beard P. E1 empty set E4 protein of human papillomavirus type 16 associates with mitochondria. J. Virol. 2004;78:7199–7207. doi: 10.1128/JVI.78.13.7199-7207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckley L, Jackett L, Clark J, Gupta R. HPV-related oropharyngeal carcinoma: a review of clinical and pathologic features with emphasis on updates in clinical and pathologic staging. Adv. Anat. Pathol. 2018;25:180–188. doi: 10.1097/PAP.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 33.Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol. Ital. 2014;34:299–309. [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence MS, et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter V, et al. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PloS ONE. 2013;8:e56823. doi: 10.1371/journal.pone.0056823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim SC, Parajuli KR, Han SI. Keratin 6, induced by chronic cisplatin exposure, confers chemoresistance in human gastric carcinoma cells. Oncol. Rep. 2019;42:797–804. doi: 10.3892/or.2019.7201. [DOI] [PubMed] [Google Scholar]
- 37.Jung YS, et al. HPV-associated differential regulation of tumor metabolism in oropharyngeal head and neck cancer. Oncotarget. 2017;8:51530–51541. doi: 10.18632/oncotarget.17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puzio-Kuter AM. The role of p53 in metabolic regulation. Genes Cancer. 2011;2:385–391. doi: 10.1177/1947601911409738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz-Gregorio A, et al. E6 oncoproteins from high-risk human papillomavirus induce mitochondrial metabolism in a head and neck squamous cell carcinoma model. Biomolecules. 2019;9:351. doi: 10.3390/biom9080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandulache VC, et al. Individualizing antimetabolic treatment strategies for head and neck squamous cell carcinoma based on TP53 mutational status. Cancer. 2012;118:711–721. doi: 10.1002/cncr.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas RJ, et al. HPV/E7 induces chemotherapy-mediated tumor suppression by ceramide-dependent mitophagy. EMBO Mol. Med. 2017;9:1030–1051. doi: 10.15252/emmm.201607088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z, et al. Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin. Cancer Res. 2006;12:5817–5825. doi: 10.1158/1078-0432.CCR-06-1037. [DOI] [PubMed] [Google Scholar]
- 43.Leonard BC, Johnson DE. Signaling by cell surface death receptors: alterations in head and neck cancer. Adv. Biol. Regul. 2018;67:170–178. doi: 10.1016/j.jbior.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grunert M, et al. The adaptor protein FADD and the initiator caspase-8 mediate activation of NF-κB by TRAIL. Cell Death Dis. 2012;3:e414–e414. doi: 10.1038/cddis.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabsch K, Alonso A. The human papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated apoptosis in HaCaT cells by different mechanisms. J. Virol. 2002;76:12162–12172. doi: 10.1128/JVI.76.23.12162-12172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filippova M, Parkhurst L, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to Fas-associated death domain and protects cells from Fas-triggered apoptosis. J. Biol. Chem. 2004;279:25729–25744. doi: 10.1074/jbc.M401172200. [DOI] [PubMed] [Google Scholar]
- 47.Duerksen-Hughes PJ, Yang J, Schwartz SB. HPV 16 E6 blocks TNF-mediated apoptosis in mouse fibroblast LM cells. Virology. 1999;264:55–65. doi: 10.1006/viro.1999.9977. [DOI] [PubMed] [Google Scholar]
- 48.Lagunas-Martínez A, Madrid-Marina V, Gariglio P. Modulation of apoptosis by early human papillomavirus proteins in cervical cancer. Biochimica et Biophysica Acta. Cancer. 2010;1805:6–16. doi: 10.1016/j.bbcan.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Garnett TO, Filippova M, Duerksen-Hughes PJ. Accelerated degradation of FADD and procaspase 8 in cells expressing human papilloma virus 16 E6 impairs TRAIL-mediated apoptosis. Cell Death Differ. 2006;13:1915–1926. doi: 10.1038/sj.cdd.4401886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan H, et al. Human papillomavirus type 16 E6 and E7 oncoproteins upregulate c-IAP2 gene expression and confer resistance to apoptosis. Oncogene. 2005;24:5069–5078. doi: 10.1038/sj.onc.1208691. [DOI] [PubMed] [Google Scholar]
- 51.Stöppler H, et al. The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene. 1998;17:1207–1214. doi: 10.1038/sj.onc.1202053. [DOI] [PubMed] [Google Scholar]
- 52.Lai D, et al. Localization of HPV-18 E2 at mitochondrial membranes induces ROS release and modulates host cell metabolism. PloS ONE. 2013;8:e75625. doi: 10.1371/journal.pone.0075625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anayannis NV, et al. Association of an intact E2 gene with higher HPV viral load, higher viral oncogene expression, and improved clinical outcome in HPV16 positive head and neck squamous cell carcinoma. PloS ONE. 2018;13:e0191581–e0191581. doi: 10.1371/journal.pone.0191581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demeret C, Garcia-Carranca A, Thierry F. Transcription-independent triggering of the extrinsic pathway of apoptosis by human papillomavirus 18 E2 protein. Oncogene. 2003;22:168–175. doi: 10.1038/sj.onc.1206108. [DOI] [PubMed] [Google Scholar]
- 55.Webster K, et al. The human papillomavirus (HPV) 16 E2 protein induces apoptosis in the absence of other HPV proteins and via a p53-dependent pathway. J. Biol. Chem. 2000;275:87–94. doi: 10.1074/jbc.275.1.87. [DOI] [PubMed] [Google Scholar]
- 56.Gross-Mesilaty S, et al. Basal and human papillomavirus E6 oncoprotein-induced degradation of Myc proteins by the ubiquitin pathway. Proc. Natl Acad. Sci. USA. 1998;95:8058–8063. doi: 10.1073/pnas.95.14.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ko A, et al. Oncogene-induced senescence mediated by c-Myc requires USP10 dependent deubiquitination and stabilization of p14ARF. Cell Death. Differ. 2018;25:1050–1062. doi: 10.1038/s41418-018-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broniarczyk J, Ring N, Massimi P, Giacca M, Banks L. HPV-16 virions can remain infectious for 2 weeks on senescent cells but require cell cycle re-activation to allow virus entry. Sci. Rep. 2018;8:811. doi: 10.1038/s41598-017-18809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duray A, et al. Human papillomavirus DNA strongly correlates with a poorer prognosis in oral cavity carcinoma. Laryngoscope. 2012;122:1558–1565. doi: 10.1002/lary.23298. [DOI] [PubMed] [Google Scholar]
- 61.Chen WS, et al. CDKN2A copy number loss is an independent prognostic factor in HPV-negative head and neck squamous cell carcinoma. Front. Oncol. 2018;8:95. doi: 10.3389/fonc.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsapras P, Nezis IP. Caspase involvement in autophagy. Cell Death Differ. 2017;24:1369–1379. doi: 10.1038/cdd.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wirawan E, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furuya D, Tsuji N, Yagihashi A, Watanabe N. Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp. Cell Res. 2005;307:26–40. doi: 10.1016/j.yexcr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 65.Young MM, et al. Autophagosomal membrane serves as platform for intracellular death-inducing signaling complex (iDISC)-mediated caspase-8 activation and apoptosis. J. Biol. Chem. 2012;287:12455–12468. doi: 10.1074/jbc.M111.309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes MA, et al. Co-operative and hierarchical binding of c-FLIP and caspase-8: a unified model defines how c-FLIP isoforms differentially control cell fate. Mol. Cell. 2016;61:834–849. doi: 10.1016/j.molcel.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, et al. Overexpression of cFLIP in head and neck squamous cell carcinoma and its clinicopathologic correlations. J. Cancer Res. Clin. Oncol. 2008;134:609–615. doi: 10.1007/s00432-007-0325-7. [DOI] [PubMed] [Google Scholar]
- 68.Rubinstein AD, Eisenstein M, Ber Y, Bialik S, Kimchi A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol. Cell. 2011;44:698–709. doi: 10.1016/j.molcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Radoshevich L, et al. ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell. 2010;142:590–600. doi: 10.1016/j.cell.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindqvist LM, et al. Autophagy induced during apoptosis degrades mitochondria and inhibits type I interferon secretion. Cell Death Differ. 2018;25:784–796. doi: 10.1038/s41418-017-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCormick KD, et al. Innate immune signaling through differential RIPK1 expression promote tumor progression in head and neck squamous cell carcinoma. Carcinogenesis. 2016;37:522–529. doi: 10.1093/carcin/bgw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi F, et al. EBV(LMP1)-induced metabolic reprogramming inhibits necroptosis through the hypermethylation of the RIP3 promoter. Theranostics. 2019;9:2424–2438. doi: 10.7150/thno.30941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Donnell MA, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beck T. N. & Golemis E. A. Genomic insights into head and neck cancer. Cancers Head Neck. 1, 1 (2016). [DOI] [PMC free article] [PubMed]
- 75.Moody CA, Fradet-Turcotte A, Archambault J, Laimins LA. Human papillomaviruses activate caspases upon epithelial differentiation to induce viral genome amplification. Proc. Natl Acad. Sci. USA. 2007;104:19541–19546. doi: 10.1073/pnas.0707947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manzo-Merino J, Massimi P, Lizano M, Banks L. The human papillomavirus (HPV) E6 oncoproteins promotes nuclear localization of active caspase 8. Virology. 2014;450–451:146–152. doi: 10.1016/j.virol.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 77.Aréchaga-Ocampo E, et al. HPV+ cervical carcinomas and cell lines display altered expression of caspases. Gynecologic Oncol. 2008;108:10–18. doi: 10.1016/j.ygyno.2007.08.070. [DOI] [PubMed] [Google Scholar]
- 78.Newton K, et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574:428–431. doi: 10.1038/s41586-019-1548-x. [DOI] [PubMed] [Google Scholar]
- 79.Ma W, et al. Human papillomavirus downregulates the expression of IFITM1 and RIPK3 to escape from IFNγ- and TNFα-mediated antiproliferative effects and necroptosis. Front. Immunol. 2016;7:496. doi: 10.3389/fimmu.2016.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hammon RJ, Michaud WA, Rocco JW. Status of the intrinsic and extrinsic apoptotic pathways in HNSCC and impact on sensitivity to etoposide-, TRAIL-, and Cisplatin-induced cell death: molecular biology and therapeutics. Int. J. Radiat. Oncol., Biol., Phys. 2014;88:515. doi: 10.1016/j.ijrobp.2013.11.163. [DOI] [Google Scholar]
- 81.Jackson-Bernitsas DG, et al. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappaB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene. 2007;26:1385–1397. doi: 10.1038/sj.onc.1209945. [DOI] [PubMed] [Google Scholar]
- 82.Li J, et al. Necroptosis in head and neck squamous cell carcinoma: characterization of clinicopathological relevance and in vitro cell model. Cell Death Dis. 2020;11:391. doi: 10.1038/s41419-020-2538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaiotti D, et al. Tumor necrosis factor-alpha promotes human papillomavirus (HPV) E6/E7 RNA expression and cyclin-dependent kinase activity in HPV-immortalized keratinocytes by a ras-dependent pathway. Mol. Carcinog. 2000;27:97–109. doi: 10.1002/(SICI)1098-2744(200002)27:2<97::AID-MC5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 84.Uzunparmak, B. et al. Caspase-8 loss radiosensitizes head and neck squamous cell carcinoma to SMAC mimetic-induced necroptosis. JCI Insight.5, e139837 (2020). [DOI] [PMC free article] [PubMed]
- 85.Safferthal C, Rohde K, Fulda S. Therapeutic targeting of necroptosis by Smac mimetic bypasses apoptosis resistance in acute myeloid leukemia cells. Oncogene. 2017;36:1487–1502. doi: 10.1038/onc.2016.310. [DOI] [PubMed] [Google Scholar]
- 86.Mahoney DJ, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc. Natl Acad. Sci. USA. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taraborrelli L, et al. LUBAC prevents lethal dermatitis by inhibiting cell death induced by TNF, TRAIL and CD95L. Nat. Commun. 2018;9:3910. doi: 10.1038/s41467-018-06155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amin P, et al. Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFα-mediated apoptosis. Proc. Natl Acad. Sci. USA. 2018;115:E5944–E5953. doi: 10.1073/pnas.1806973115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruiz EJ, et al. LUBAC determines chemotherapy resistance in squamous cell lung cancer. J. Exp. Med. 2019;216:450–465. doi: 10.1084/jem.20180742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lun M, et al. Nuclear factor-kappaB pathway as a therapeutic target in head and neck squamous cell carcinoma: pharmaceutical and molecular validation in human cell lines using Velcade and siRNA/NF-kappaB. Ann. Clin. Lab Sci. 2005;35:251–258. [PubMed] [Google Scholar]
- 91.Duarte VM, et al. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKβ protein of the NFκB pathway. Mol. Cancer Ther. 2010;9:2665–2675. doi: 10.1158/1535-7163.MCT-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duffey DC, et al. Inhibition of transcription factor nuclear factor-kappaB by a mutant inhibitor-kappaBalpha attenuates resistance of human head and neck squamous cell carcinoma to TNF-alpha caspase-mediated cell death. Br. J. Cancer. 2000;83:1367–1374. doi: 10.1054/bjoc.2000.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lim J. et al. Autophagy regulates inflammatory programmed cell death via turnover of RHIM-domain proteins. Elife. 8, e44452 (2019). [DOI] [PMC free article] [PubMed]
- 94.Wu W, Stork B. Regulating RIPK1: another way in which ULK1 contributes to survival. Autophagy. 2020;16:1544–1546. doi: 10.1080/15548627.2020.1783110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bray K, et al. Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PloS ONE. 2012;7:e41831–e41831. doi: 10.1371/journal.pone.0041831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu W, et al. The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep. 2020;31:107547. doi: 10.1016/j.celrep.2020.107547. [DOI] [PubMed] [Google Scholar]
- 97.Su H, Li F, Ranek MJ, Wei N, Wang X. COP9 signalosome regulates autophagosome maturation. Circulation. 2011;124:2117–2128. doi: 10.1161/CIRCULATIONAHA.111.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao P, et al. COP9 signalosome suppresses RIPK1-RIPK3–mediated cardiomyocyte necroptosis in mice. Circ. Heart Fail. 2020;13:e006996. doi: 10.1161/CIRCHEARTFAILURE.120.006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee M-H, Zhao R, Phan L, Yeung S-CJ. Roles of COP9 signalosome in cancer. Cell Cycle. 2011;10:3057–3066. doi: 10.4161/cc.10.18.17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joshi A, et al. Nuclear ULK1 promotes cell death in response to oxidative stress through PARP1. Cell Death Differ. 2016;23:216–230. doi: 10.1038/cdd.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goodall ML, et al. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev. Cell. 2016;37:337–349. doi: 10.1016/j.devcel.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yonekawa T. et al. RIP1 negatively regulates basal autophagic flux through TFEB to control sensitivity to apoptosis. EMBO Rep. 16, 700–708 (2015). [DOI] [PMC free article] [PubMed]
- 103.Shlomovitz I, et al. Necroptosis directly induces the release of full-length biologically active IL-33 in vitro and in an inflammatory disease model. FEBS J. 2019;286:507–522. doi: 10.1111/febs.14738. [DOI] [PubMed] [Google Scholar]
- 104.Pastille E, et al. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol. 2019;12:990–1003. doi: 10.1038/s41385-019-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schiering C, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen SF, et al. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. J. Pathol. 2013;231:180–189. doi: 10.1002/path.4226. [DOI] [PubMed] [Google Scholar]
- 107.Ishikawa K, et al. Expression of interleukin-33 is correlated with poor prognosis of patients with squamous cell carcinoma of the tongue. Auris Nasus Larynx. 2014;41:552–557. doi: 10.1016/j.anl.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Seifert L. et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532:245–249. doi: 10.1038/nature17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yatim N. et al. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science. 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Werthmöller N, Frey B, Wunderlich R, Fietkau R, Gaipl US. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. 2015;6:e1761. doi: 10.1038/cddis.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu H, et al. Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor cell death. Clin. Cancer Res. 2018;24:6066–6077. doi: 10.1158/1078-0432.CCR-18-1478. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 113.Sarhan J, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA. 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung CH, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/S1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 115.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol. 2017;24:507–14.e4. doi: 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Willson J. A matter of life and death for caspase 8. Nat. Rev. Mol. Cell Biol. 2020;21:63. doi: 10.1038/s41580-019-0201-8. [DOI] [PubMed] [Google Scholar]
- 117.Sun Q, et al. Caspase 1 activation is protective against hepatocyte cell death by up-regulating beclin 1 protein and mitochondrial autophagy in the setting of redox stress. J. Biol. Chem. 2013;288:15947–15958. doi: 10.1074/jbc.M112.426791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi C-S, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yuk J-M, Silwal P, Jo E-K. Inflammasome and mitophagy connection in health and disease. Int. J. Mol. Sci. 2020;21:4714. doi: 10.3390/ijms21134714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen L, et al. Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell Mol. Life Sci. 2018;75:2045–2058. doi: 10.1007/s00018-017-2720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang H, et al. NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer. 2018;18:500. doi: 10.1186/s12885-018-4403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bae JY, et al. P2X7 receptor and NLRP3 inflammasome activation in head and neck cancer. Oncotarget. 2017;8:48972–48982. doi: 10.18632/oncotarget.16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang CF, et al. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2017;36:116. doi: 10.1186/s13046-017-0589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feng X, et al. The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2017;36:81. doi: 10.1186/s13046-017-0553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stanam A, Gibson-Corley KN, Love-Homan L, Ihejirika N, Simons AL. Interleukin-1 blockade overcomes erlotinib resistance in head and neck squamous cell carcinoma. Oncotarget. 2016;7:76087–76100. doi: 10.18632/oncotarget.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang F, et al. Alcohol accumulation promotes esophagitis via pyroptosis activation. Int. J. Biol. Sci. 2018;14:1245–1255. doi: 10.7150/ijbs.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ainouze M, et al. Human papillomavirus type 16 antagonizes IRF6 regulation of IL-1β. PLoS Pathog. 2018;14:e1007158. doi: 10.1371/journal.ppat.1007158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.So D, et al. Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense. Oncogene. 2018;37:5191–5204. doi: 10.1038/s41388-018-0339-4. [DOI] [PubMed] [Google Scholar]
- 129.Noguchi A, et al. SIRT1 expression is associated with good prognosis for head and neck squamous cell carcinoma patients. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013;115:385–392. doi: 10.1016/j.oooo.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 130.Di Vincenzo S, et al. Cigarette smoke impairs Sirt1 activity and promotes pro-inflammatory responses in bronchial epithelial cells. Eur. Respir. J. 2015;46:PA5105. [Google Scholar]
- 131.Song Y, et al. HPV E7 inhibits cell pyroptosis by promoting TRIM21-mediated degradation and ubiquitination of the IFI16 inflammasome. Int. J. Biol. Sci. 2020;16:2924–2937. doi: 10.7150/ijbs.50074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rogers C, et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Segovia M. et al. Targeting TMEM176B enhances antitumor immunity and augments the efficacy of immune checkpoint blockers by unleashing inflammasome activation. Cancer Cell. 35, 767–781.e6 (2019). [DOI] [PMC free article] [PubMed]
- 134.Zhang Z, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rogers C, et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 2019;10:1689. doi: 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang W. et al. DFNA5 attenuated in head and neck squamous cell carcinoma acquired with radio-resistance and associated with PD-L2 (2020).
- 137.Shan L, et al. Visualizing head and neck tumors in vivo using near-infrared fluorescent transferrin conjugate. Mol. Imaging. 2008;7:42–49. doi: 10.2310/7290.2008.0006. [DOI] [PubMed] [Google Scholar]
- 138.Lenarduzzi M, et al. Hemochromatosis enhances tumor progression via upregulation of intracellular iron in head and neck cancer. PloS ONE. 2013;8:e74075. doi: 10.1371/journal.pone.0074075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Järvinen AK, et al. Identification of target genes in laryngeal squamous cell carcinoma by high-resolution copy number and gene expression microarray analyses. Oncogene. 2006;25:6997–7008. doi: 10.1038/sj.onc.1209690. [DOI] [PubMed] [Google Scholar]
- 140.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang L, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Viswanathan VS, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dohadwala M, et al. The role of ZEB1 in the inflammation-induced promotion of EMT in HNSCC. Otolaryngol. Head Neck Surg. 2010;142:753–759. doi: 10.1016/j.otohns.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 144.Wu J, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2–YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jung YS, Kato I, Kim HR. A novel function of HPV16-E6/E7 in epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2013;435:339–344. doi: 10.1016/j.bbrc.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 146.Yagoda N, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu Z, et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl Acad. Sci. USA. 2019;116:2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao M, et al. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Belleudi F, Nanni M, Raffa S, Torrisi MR. HPV16 E5 deregulates the autophagic process in human keratinocytes. Oncotarget. 2015;6:9370–9386. doi: 10.18632/oncotarget.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mattoscio D., Medda A. & Chiocca S. Human papilloma virus and autophagy. Int. J. Mol. Sci. 19, 1775 (2018). [DOI] [PMC free article] [PubMed]
- 151.Tingting C, et al. Human papillomavirus 16E6/E7 activates autophagy via Atg9B and LAMP1 in cervical cancer cells. Cancer Med. 2019;8:4404–4416. doi: 10.1002/cam4.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sewell A, et al. Reverse-phase protein array profiling of oropharyngeal cancer and significance of PIK3CA mutations in HPV-associated head and neck cancer. Clin. Cancer Res. 2014;20:2300–2311. doi: 10.1158/1078-0432.CCR-13-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pietrocola F, et al. Aspirin recapitulates features of caloric restriction. Cell Rep. 2018;22:2395–2407. doi: 10.1016/j.celrep.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Castoldi F, Pietrocola F, Maiuri MC, Kroemer G. Aspirin induces autophagy via inhibition of the acetyltransferase EP300. Oncotarget. 2018;9:24574–24575. doi: 10.18632/oncotarget.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hedberg ML, et al. Use of nonsteroidal anti-inflammatory drugs predicts improved patient survival for PIK3CA-altered head and neck cancer. J. Exp. Med. 2019;216:419–427. doi: 10.1084/jem.20181936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu SY, et al. Ionizing radiation induces autophagy in human oral squamous cell carcinoma. J. BUON. 2014;19:137–144. [PubMed] [Google Scholar]
- 157.Kamarajan P, et al. Head and neck squamous cell carcinoma metabolism draws on glutaminolysis, and stemness is specifically regulated by glutaminolysis via aldehyde dehydrogenase. J. Proteome Res. 2017;16:1315–1326. doi: 10.1021/acs.jproteome.6b00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao M, Jiang X. To eat or not to eat—the metabolic flavor of ferroptosis. Curr. Opin. Cell Biol. 2018;51:58–64. doi: 10.1016/j.ceb.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang J, et al. Targeting cellular metabolism to reduce head and neck cancer growth. Sci. Rep. 2019;9:4995. doi: 10.1038/s41598-019-41523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Okazaki S, et al. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019;110:3453–3463. doi: 10.1111/cas.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang Z, et al. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br. J. Cancer. 2020;122:82–93. doi: 10.1038/s41416-019-0637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zimmermann P, Bette M, Giel G, Stuck BA, Mandic R. Influence of the xc-cystine/glutamate antiporter inhibitor sulfasalazine on the growth of head and neck squamous cell carcinoma cell lines. Laryngo Rhino Otol. 2018;97:10050. [Google Scholar]
- 163.Okazaki S, et al. Synthetic lethality of the ALDH3A1 inhibitor dyclonine and xCT inhibitors in glutathione deficiency-resistant cancer cells. Oncotarget. 2018;9:33832–33843. doi: 10.18632/oncotarget.26112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tao X, et al. AP1G1 is involved in cetuximab-mediated downregulation of ASCT2-EGFR complex and sensitization of human head and neck squamous cell carcinoma cells to ROS-induced apoptosis. Cancer Lett. 2017;408:33–42. doi: 10.1016/j.canlet.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoshikawa M, et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855–1866. doi: 10.1158/0008-5472.CAN-12-3609-T. [DOI] [PubMed] [Google Scholar]
- 166.Zhang P, et al. xCT expression modulates cisplatin resistance in Tca8113 tongue carcinoma cells. Oncol. Lett. 2016;12:307–314. doi: 10.3892/ol.2016.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 169.Yang L, et al. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab. 2016;24:685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ferber MJ, et al. Preferential integration of human papillomavirus type 18 near the c-myc locus in cervical carcinoma. Oncogene. 2003;22:7233–7242. doi: 10.1038/sj.onc.1207006. [DOI] [PubMed] [Google Scholar]
- 171.Liu L, et al. Identification of reliable biomarkers of human papillomavirus 16 methylation in cervical lesions based on integration status using high-resolution melting analysis. Clin. Epigenetics. 2018;10:10. doi: 10.1186/s13148-018-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Palmieri EM, et al. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumor metastasis. Cell Rep. 2017;20:1654–1666. doi: 10.1016/j.celrep.2017.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fazzari J, Linher-Melville K, Singh G. Tumour-derived glutamate: linking aberrant cancer cell metabolism to peripheral sensory pain pathways. Curr. Neuropharmacol. 2017;15:620–636. doi: 10.2174/1570159X14666160509123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bertero T, et al. Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 2019;29:124–40.e10. doi: 10.1016/j.cmet.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Y, et al. Fibroblasts in head and neck squamous cell carcinoma associated with perineural invasion have high-level nuclear yes-associated protein (YAP) expression. Acad. Pathol. 2015;2:2374289515616972. doi: 10.1177/2374289515616972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ge L, et al. Yes-associated protein expression in head and neck squamous cell carcinoma nodal metastasis. PloS ONE. 2011;6:e27529. doi: 10.1371/journal.pone.0027529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.García-Escudero R, et al. Overexpression of PIK3CA in head and neck squamous cell carcinoma is associated with poor outcome and activation of the YAP pathway. Oral. Oncol. 2018;79:55–63. doi: 10.1016/j.oraloncology.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 178.LeBlanc L. et al. Yap1 safeguards mouse embryonic stem cells from excessive apoptosis during differentiation. Elife. 7, e40167 (2018) [DOI] [PMC free article] [PubMed]
- 179.Wang W, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yamazaki T, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21:69–78. doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019;510:278–283. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 182.Wild CA, et al. HMGB1 is overexpressed in tumor cells and promotes activity of regulatory T cells in patients with head and neck cancer. Oral. Oncol. 2012;48:409–416. doi: 10.1016/j.oraloncology.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 183.Yang WS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim EH, Shin D, Lee J, Jung AR, Roh J-L. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 2018;432:180–190. doi: 10.1016/j.canlet.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 185.Roh JL, Kim EH, Jang HJ, Park JY, Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381:96–103. doi: 10.1016/j.canlet.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 186.Lang X, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Disco. 2019;9:1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lin R, et al. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 2016;381:165–175. doi: 10.1016/j.canlet.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 188.Shaw AT, et al. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl Acad. Sci. USA. 2011;108:8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen JJ, Galluzzi L. Fighting resilient cancers with iron. Trends Cell Biol. 2018;28:77–78. doi: 10.1016/j.tcb.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 190.Pisanti S, Picardi P, Ciaglia E, D’Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharm. Res. 2014;88:84–98. doi: 10.1016/j.phrs.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 191.Cruz SA, Qin Z, Stewart AFR, Chen HH. Dabrafenib, an inhibitor of RIP3 kinase-dependent necroptosis, reduces ischemic brain injury. Neural Regen. Res. 2018;13:252–256. doi: 10.4103/1673-5374.226394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fulda S. Repurposing anticancer drugs for targeting necroptosis. Cell Cycle. 2018;17:829–832. doi: 10.1080/15384101.2018.1442626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang H, et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology. 2016;5:e1149673–e1149673. doi: 10.1080/2162402X.2016.1149673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oliver Metzig M, et al. Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-α-dependent necroptosis driven by RIP1 kinase and NF-κB. Oncogene. 2016;35:3399–3409. doi: 10.1038/onc.2015.398. [DOI] [PubMed] [Google Scholar]
- 195.Huang X, et al. Bypassing drug resistance by triggering necroptosis: recent advances in mechanisms and its therapeutic exploitation in leukemia. J. Exp. Clin. Cancer Res. 2018;37:310. doi: 10.1186/s13046-018-0976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Valter K, Zhivotovsky B, Gogvadze V. Cell death-based treatment of neuroblastoma. Cell Death Dis. 2018;9:113. doi: 10.1038/s41419-017-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang CC, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24:312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 198.Johnson DC, et al. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat. Med. 2018;24:1151–1156. doi: 10.1038/s41591-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Woo JH, et al. Elucidating compound mechanism of action by network perturbation analysis. Cell. 2015;162:441–451. doi: 10.1016/j.cell.2015.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lachaier E, et al. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res. 2014;34:6417–6422. [PubMed] [Google Scholar]
- 201.Möckelmann N, et al. Effect of sorafenib on cisplatin-based chemoradiation in head and neck cancer cells. Oncotarget. 2016;7:23542–23551. doi: 10.18632/oncotarget.8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guo J, et al. Ferroptosis: a novel anti-tumor action for Cisplatin. Cancer Res. Treat. 2018;50:445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu T., Zhang J., Li K., Deng L. & Wang H. Combination of an autophagy inducer and an autophagy inhibitor: a smarter strategy emerging in cancer therapy. Front. Pharmacol. 11, 408 (2020). [DOI] [PMC free article] [PubMed]
- 204.Chude C. I. & Amaravadi R. K. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int. J. Mol. Sci. 18, 1279 (2017). [DOI] [PMC free article] [PubMed]
- 205.Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015;70:1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Maiuri MC, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 207.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol. Biomark. Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 208.Descamps G, Wattiez R, Saussez S. Proteomic study of HPV-positive head and neck cancers: preliminary results. BioMed. Res. Int. 2014;2014:430906. doi: 10.1155/2014/430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Canning M, et al. Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front. Cell Dev. Biol. 2019;7:52. doi: 10.3389/fcell.2019.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kobayashi K, et al. A review of HPV-related head and neck cancer. J. Clin. Med. 2018;7:241. doi: 10.3390/jcm7090241. [DOI] [PMC free article] [PubMed] [Google Scholar]