Abstract
Background
Increased exercise and physical activity levels are recommended throughout cancer therapy and survivorship. Nonetheless, the COVID-19 pandemic and consequent social distancing are likely to cause a decline in physical activity.
Objective
to evaluate the level of unsupervised physical activity of breast cancer survivors during the COVID-19 pandemic, and the factors associated with difficulties in engaging and maintaining recommended physical activity levels.
Methods
This is a cross-sectional epidemiological study with a sample of 37 breast cancer survivors. They participated in a canoeing training program (project Remama) at the University of São Paulo before the COVID-19 pandemic. Socioeconomic aspects, engagement in physical activity, motivation, and potential exposure to COVID-19 were investigated through an online survey, administered in September of 2020.
Results
During the pandemic, participants increased their body weight (5 ± 3.4 kg); 90% reported decreasing physical activity levels associated with increased sedentary time. Twenty-one (58%) participants exhibited some COVID-19-related symptoms, most used public transportation (59%), or returned to work during the period of a high incidence of COVID-19. The only factor associated with perceived difficulty in engaging in physical activities was having had more than three cancer treatments (RR: 2.14; 95% CI: 1.07–4.27).
Conclusion
The COVID-19 pandemic led to a group of previously active breast cancer survivors to decrease their physical activity, gain weight, and have sedentary behavior. Specific tailored-care interventions are needed to prevent these occurrences, as overweight and physical inactivity may impose an additional risk for breast cancer recurrence and a severe course of COVID-19 in cancer patients.
Keywords: physical activity, COVID-19, breast neoplasms, survivorship, pandemic (COVID-19)
Introduction
A series of pneumonia cases of unknown etiology was reported in the city of Wuhan, China, in late 2019 (Xu et al., 2020). By sequencing a patient’s lower respiratory tract, a new virus was identified called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its disease, coronavirus disease 2019 (COVID-19). It rapidly spread across countries and a new pandemic was declared by the World Health Organization (WHO) in March 2020. COVID-19 reached a mark of 20,162,474 cases and 737417 deaths globally in August of 2020 (World Health Organization, 2020). In Brazil, the Ministry of Health notified the first confirmed case on February 26, 2020. From that date until August 8, 2020, 3,012,412 cases were confirmed, and 100,477 deaths occurred as a result of COVID-19 in the country (Ministério da Saúde, 2020).
Social distancing recommendations significantly reduced levels of physical activity of the overall population and more profoundly in the population at increased risk, such as the elderly and those living with chronic non-communicable diseases (Damiot et al., 2020). Periods of confinement can be identified as a barrier to regular physical activity. A recent study showed that middle-aged individuals who adopt sedentary behavior have an increased risk of cancer mortality (Gilchrist et al., 2020). Physical inactivity may exacerbate comorbidities amongst older adults, including cardiovascular disease, cancer, and dysfunctional inflammatory responses (Flynn et al., 2019). In this scenario, older adults and individuals living with underlying conditions are at a greater risk for complications during COVID-19 disease (Damiot et al., 2020).
Worldwide, the incidence of cancer and mortality remain high. Over 18 million new cases were registered in 2018, alongside 9.6 million deaths (Bray et al., 2018). Among women, the most diagnosed neoplasm is breast cancer (excluding non-melanoma skin cancer), which also represents the major oncological cause of death in this population, with 2.1 million diagnosed cases in 2018, accounting for almost one in four cancer cases (Bray et al., 2018). Despite the escalating cancer incidence, advances in early diagnosis and breast cancer therapy improved five-year overall survival rates, now exceeding 90% when diagnosed in the early stages (Simon et al., 2019; Siegel et al., 2020).
In this context, particular attention should be dedicated to metabolic aspects including weight control and management of physical inactivity, through lifestyle interventions. In fact, increased physical activity levels, exercise, and body weight control have been considered strong allies to cancer patients and survivors, as they positively impact physical capacities, fatigue, depressive symptoms, anxiety, and quality of life (Campbell et al., 2019; Mctiernan et al., 2019; Nardin et al., 2020). Since the literature of the past two decades has provided evidence that supports the practice of physical activity during and after cancer treatment (Schmitz et al., 2019); and that physical activity helps to prevent neoplastic recurrence (Patel et al., 2019), it is of utmost importance to develop public health strategies to encourage this practice.
The present study aimed to evaluate the level of unsupervised physical activity of breast cancer survivors during the COVID-19 pandemic, and the factors associated with difficulties in engaging and maintaining recommended physical activity levels.
Materials and Methods
This is a cross-sectional study designed to address physical activity levels in a convenience sample of breast cancer survivors. All subjects of this study participate in a group canoeing training project called Remama at the University of São Paulo, in which they are longitudinally followed-up.
Remama is a collaborative project between the Cancer Institute of the State of Sao Paulo, the School of Physical Education of the University of São Paulo (USP), and the Sports Center of USP. The inclusion criteria were participants who have finished their prescribed breast cancer treatment with curative intent, including surgery, systemic cytotoxic chemotherapy, and radiotherapy; who are aged between 35 and 75 years old; and have concluded their treatment within a time span of at least 6 months up to 3 years. The exclusion criteria were patients who had metastatic disease, severe lymphedema, organic dysfunction, or uncontrolled risk factors (hypercholesterolemia, diabetes, hypertension).
Previously to the pandemic, Remama participants received a physical activity recommendation booklet (based on WHO guidelines). The booklet suggests different exercise modalities such as aerobic, strength and flexibility, and an increase in overall physical activity level as part of a behavioral change. It also recommends precautions participants should adopt while exercising. Due to the COVID-19 pandemic, access to public and private spaces is restricted. The participants were instructed to increase physical activity levels at home because of the discontinuation of face-to-face training sessions. We sent online questionnaires to 41 Remama participants in September 2020. The participants were instructed through videoconference.
Instruments
The level of physical activity, sport, and leisure was assessed using an instrument built especially for this study and adapted from the Minnesota Leisure Time Physical Activity Questionnaire (Taylor et al., 1978), that is a widely used tool to address physical activity levels in different populations (Elosua et al., 2000; Lozano-Lozano et al., 2016) and that has been validated for Brazilian population (Lustosa et al., 2011). It quantified: (a) the time that participants spent on accumulated physical activities in daily life (lifestyle), called “not programmed movement”; (b) the time spent on physical activities to meet the goal of 150 min per week of physical exercise; and (c) the total time they spent sitting, whether in leisure activities (such as watching television, cell phones, etc.) or in professional work-related activities.
To assess current patterns of physical activity upon the COVID-19 pandemic, a survey adapted from a questionnaire used by Lesser and Nienhuis (2020) was applied. This was based on the Nature Relatedness scale (Nguyen and Brymer, 2018) and on the Godin Leisure Questionnaire, which has been validated for the Brazilian population (São-João et al., 2013). This included questions on the (a) the importance of carrying out outdoor activities (Nisbet et al., 2009); (b) sedentary behavior, questions based on a study by Ekelund et al. (2016); and, (c) the Behavioral Regulations in Exercise questionnaire (BREQ-3), also validated to adult Brazilian population (Guedes and Sofiati, 2015) to assess the participants’ motivation for physical activity at home (Rutten et al., 2014). Participants were asked to answer questions related to motivation and training opportunities, indicating answers on eight statements using a 5-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Finally, we also investigated the potential exposure to SARS-CoV-2. A validated questionnaire adapted from the Mount Sinai Hospital (2020) survey was administered. The participants were asked to answer the questions considering the period starting on March 1, 2020.
Dependent Variable
Participants were asked about adherence to physical activity in the context of measuring an individuals’ motivation, support, and opportunity to engage in physical activity. Each answer was scored on the Likert scale from one (strongly disagree), two (partially disagree), three (neutral), four (partially agree) to five (strongly agree). Next, we generated dichotomous variables from the Likert scale and used them for measuring the participants’ difficulty in engage with physical activity. The scores 1 and 2 were accounted as “no,” while the scores from three to five were considered “yes.”
Independent Variables
Demographic data were evaluated, including age, education, occupation, return to work, and professional working routine. Clinical data were retrieved on body mass index, menopausal status, presence of underlying conditions, and cancer treatment adopted (chemotherapy, radiotherapy, surgery, immunotherapy, and hormone-based therapy). Behavioral factors included opportunity and motivation for physical activity. Lastly, patients were asked whether they had experienced any symptoms of COVID-19 during social distancing.
Statistical Analyses
Two groups were compared: participants who did experience difficulty in engaging in physical activity against those who did not. Results are presented as relative risk (RR) and 95% confidence intervals (CI). A 95% CI that did not include 1.00 was considered statistically significant. Statistical analysis was performed using EpiInfoTM version 7.2.4.0.
Ethical Considerations
This study was approved by the School of Physical Education Research Ethical Board (CAAE: 12242919.9.0000.5391) in accordance with the 1964 Declaration of Helsinki amendment in 2013. All participants signed a consent form. The identity and individual information of the subjects are confidential, and the data were analyzed in an aggregate form. The raw data supporting the conclusions of this article will be made available by the authors upon request.
Results
The survey was sent to 41 members of the project Remama of which 37 responded (90%). The demographic and clinical data and, the characteristics of their breast cancer can be seen in Table 1.
TABLE 1.
Demographic and clinical characteristics, and responses of 37 breast cancer survivors, enrolled in a program for physical activity, who answered a survey on their level of activity and perceptions on the subject, on possible COVID-19 symptoms, and on potential exposure to the disease during the pandemic.
| Age, years, mean ± SD | 57 ± 7.4 |
| Ethnicity n (%) | |
| White | 19(51%) |
| Black/brown | 13(35%) |
| Others | 5(14%) |
| Education n (%) | |
| Up to high school | 15(41%) |
| University | 12(32%) |
| Post-graduation | 10(27%) |
| Menopausal n (%) | 22(59%) |
| Obesity status n (%) | |
| Overweight | 22(59%) |
| Weight unchanged | 7(19%) |
| Weight loss | 6(16%) |
| Unknown | 2(5%) |
| Weight gain (Kg), median (range) | 3.75 (1–15) |
| Type of treatment n (%) | |
| Surgery | 32(86%) |
| Chemotherapy | 32(86%) |
| Radiotherapy | 31(84%) |
| Endocrine | 12(32%) |
| Immunotherapy | 2(5%) |
| Type of malignancy n (%) | |
| Invasive ductal carcinoma | 15(41%) |
| In situ ductal carcinoma | 5(14%) |
| Invasive lobular carcinoma | 3(8%) |
| Unknown | 10(27%) |
| Other | 4(11%) |
| Time since treatment completion, months, median (range) | 46 (1–95) |
| Use of tamoxifen n (%) | 15(41%) |
| Under cancer treatment n (%) | 4(11%) |
| Current treatments n (%) Systemic arterial hypertension | 11(30%) |
| Diabetes mellitus | 5(14%) |
| Symptoms suggestive of COVID-19 n (%) | |
| No symptoms | 16(43%) |
| Headache | 13(35%) |
| Myalgia | 7(19%) |
| Cough | 5(14%) |
| Coryza | 5(14%) |
| Sore throat | 4(11%) |
| Other | 5(14%) |
| Duration of symptoms (days), median (range) | 4 (4–20) |
| Admitted to hospital n (%) | 3(8%) |
| Contact with suspected or confirmed case of COVID-19 at home n (%) | 5(14%) |
| Duration of exposure (days), median (range) | 3 (3–10) |
| Contact with suspected or confirmed case of COVID-19 outside the home n (%) | 8(22%) |
| Worked outside the home n (%) | 13(35%) |
| Number of times per week worked outside the home, median (range) | 3.5 (1–6) |
| Used public transportation n (%) | 22(59%) |
| Number of times per week used public transportation, median (range) | 2 (0–6) |
| Levels and characteristics of physical activity during the COVID-19 pandemic | |
| Prefers outdoor training n (%) | 22(59%) |
| Considers outdoor activities very important n (%) | 25(68%) |
| Reduced physical activity levels during the pandemic n (%) | 33(89%) |
SD, standard deviation.
The mean age of the volunteers was 57 years old, with the majority aged 55 years or older. Twenty-two (59%) participants reported increase in bodyweight (5 kg, ranging from 1 to 15 kgs). The five most frequently reported symptoms were headache, myalgia, cough, coryza, and sore throat. These symptoms occurred mainly in July and lasted a few days. Although three participants were hospitalized, no subject from the population developed severe COVID-19 or post-COVID19 complications.
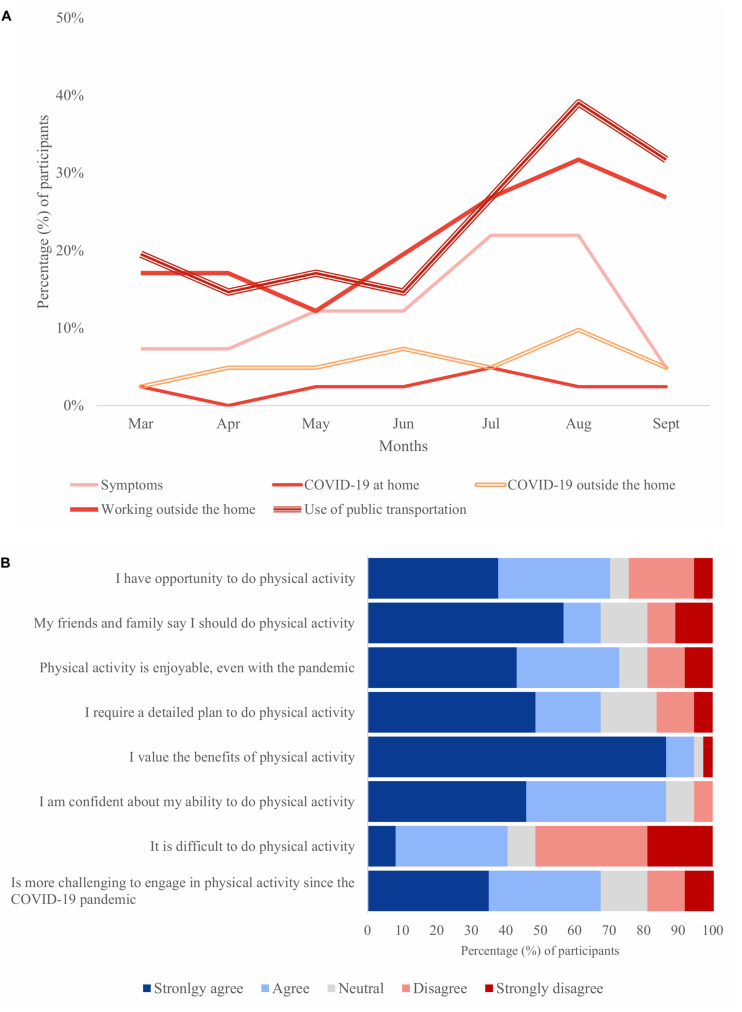
Participants worked outside the home mainly in July, August, and September, roughly between three and four times a week. This coincides with the use of public transportation in July, August, and September. They used public transportation between two and three times a week. Figure 1A summarizes participants’ potential exposure to SARS-CoV-2 reported from March to September 2020.
FIGURE 1.
Participants’ behavior outside and inside the home during the COVID-19 pandemic. (A) Proportion of participants who reported exposure, from March to September 2020, to suspected or confirmed cases of COVID-19 at home, outside the home, and who used public transportation (n = 37). (B) Barriers and facilitators for physical activity perceived in September 2020 (n = 37). Results are presented in percentage.
Barriers and facilitators of physical activity perceived by the participants can be seen in Figure 1B. The vast majority reported that it is challenging to engage in physical activity since the pandemic, although most had the opportunity and valued the effect of exercise and being physically active.
The levels of programmed and not programmed movement are described in Table 2. Most volunteers reported having adopted alternative movement and used stairs. Besides, the majority stated they performed housework activities. Roughly half of them achieved 150 min/week of physical activity in September 2020. As to sedentary behavior, 20 (54%) participants reported remaining seated for over two consecutive hours during the week, and 19 (51%), during the weekend.
TABLE 2.
Evaluation of programmed and non-programmed physical activities of breast cancer survivors enrolled in a program for physical activity, who answered a survey on their level of activity, (n = 37).
| Non-programmed movement/daily activities | |
| Adopted alternative movement n (%) | 28(76%) |
| Used stairs n (%) | 26(70%) |
| Walked n (%) | 24(65%) |
| Worked in the last 2 weeks n (%) | 25(68%) |
| Movement in professional activities n (%) | 16(43%) |
| Movement in leisure activities n (%) | 15(41%) |
| Performed housework n (%) | 31(84%) |
| Felt discomfort during housework activities n (%) | 11(30%) |
| Programmed activities | |
| Achieved 150 min/week physical activity n (%) | 18(49%) |
| Sedentary behavior during week/weekend | |
| Remained seated for more than two consecutive hours during week n (%) | 20(54%) |
| Remained seated for more than two consecutive hours during weekend n (%) | 19(51%) |
The potential association between the patients having reported having difficulty engaging in physical activities can be seen in Table 3. Women who underwent at least three anticancer treatments found difficulty in doing physical activity.
TABLE 3.
Evaluation of factors potentially associated with the perception of the patients that it is difficult to engage in physical activities (Survey taken during the COVID-19 pandemic with breast cancer survivors previously enrolled in a canoeing program, n = 37).
| Find it difficult to engage in physical activity | |||
| Yes (%) n = 15 | No (%) N = 22 | RR (CI 95%) | |
| Age > 55 years old | 9 (60%) | 12 (55%) | 1.14 (0.51–2.55) |
| Obesity | 11 (73%) | 14 (64%) | 1.03 (0.53–3.29) |
| Studied up to high school | 9 (40%) | 13 (59%) | 1.02 (0.46–2.27) |
| Menopause | 6 (40%) | 16 (73%) | 0.75 (0.32–1.78) |
| Has hypertension | 5 (33%) | 6 (27%) | 1.18 (0.52–2.65) |
| Had > 3 cancer treatments | 5 (33%) | 2 (9%) | 2.14 (1.07–4.27) * |
| Prefers outdoor activities | 8 (53%) | 14 (64%) | 0.72 (0.33–1.55) |
| Has opportunity to do physical activity | 9 (60%) | 18 (82%) | 0.55 (0.26–1.15) |
| Works outside the home | 7 (47%) | 6 (27%) | 1.60 (0.75–3.44) |
| Presented COVID-19 symptoms | 9 (60%) | 12 (55%) | 1.14 (0.51–2.55) |
*Statistically significant.
Discussion
Our survey with 37 breast cancer survivors revealed that most of the participants reduced their physical activity level and gained weight upon temporary suspension of the canoeing training project Remama at COVID-19 pandemic onset. Although no factors were associated with reducing activity, our study discloses that having been submitted to more than three cancer treatments was associated with their perception of difficulty to do physical activity. Taken together, these aspects may impose an additional risk of a severe course of COVID-19 with a worse prognosis. No other modifiable factor could be associated with this outcome. This finding was crucial to support our research group in developing a new strategy to engage participants in a physical activity program that is active via online classes currently.
Exercise, preferably following an exercise program (Newton et al., 2020), can improve outcomes in people who have or have had cancer, such as well-being, body weight control, and reduce the cancer recurrence risk. Bodyweight gain and physical inactivity are known to increase the cancer recurrence risk (Meyerhardt et al., 2006; Renehan et al., 2008), cardiovascular disease, and metabolic disorders (Ford and Caspersen, 2012). Physical activity enhances quality of life and improves the effectiveness of therapies, mitigating potential adverse effects inherent to antineoplastic therapy and drug toxicity. Furthermore, it may minimize or reverse the progression of other chronic diseases. Besides that, cancer patients may be at an increased risk of developing severe COVID-19 due to the presence of underlying conditions, anticancer therapy, and old age, among others (Galluzzi et al., 2015; Dai et al., 2020; Damiot et al., 2020).
In our study, most women reported they preferred outdoor activities, and the majority considered outdoor spaces especially important for physical activity. However, due to the pandemic, the health authorities restricted these outdoor activities. This may have been one of the contributing factors to our results. A larger number of cancer treatments was significantly associated with perceived difficulty in being physically active. This might be due to the long-term side effects associated with antineoplastic therapies. This includes fatigue, insomnia, persistent pain, lymphedema, among other unwanted effects that might impose barriers to physical activity engagement (Campbell et al., 2019). In fact, the probability of developing long-term side effects increases as the number of antineoplastic treatment increases (Cheville, 2001; Stout and Sabo Wagner, 2019).
A large proportion of the participants reported having had symptoms, mostly during July and August, the months with the highest incidence of COVID-19 in São Paulo. This was also the period in which the participants reported more frequent use of public transportation and return to work. Many of our participants were self-employed. They may have found themselves pushed back to work to provide for their families. This behavior possibly increased the chance of exposure to symptomatic or asymptomatic people outside the home. Brazilian women who are workers in the informal economy were disproportionally affected by the pandemic (International Labour Organization, 2020a), as they do not have access to social protection mechanisms. Thus, we believe that our study reflects a particular socioeconomic scenario that exacerbates social inequality. This scenario differs from that of other countries that provide social protection, allowing the population to stay at home and follow social distancing orders, minimizing the spread of SARS-CoV-2. We believe that adequate responses to the pandemic should observe the particularities of each country, and policies ensuring measures for social health protection and extending financial protection should prioritize vulnerable workers (International Labour Organization, 2020b).
Our study has limitations. Self-reported physical activity levels may impose imprecision on the data. Furthermore, this survey does not fully report all aspects of BREQ-3 or Minnesota questionnaire, including psychological aspects. The interpretation of our findings might be limited by the sample size. Nevertheless, understanding the practice of physical activity in the context of the life cycle and macro determinants of behavior is of vital importance (Hallal, 2014). Although using a small sample, this study provides fresh insights into a complex problem and may provide a direction for interventions. In this sense, alternative ways of delivering supervised and structured physical activity (exercise) based on telehealth should be studied as an alternative for increasing the adherence to exercise aiding to maintain an active lifestyle in the pandemic and even afterward (Newton et al., 2020).
In conclusion, previously active breast cancer survivors found themselves inactive or with reduced physical activity levels and gained weight during the pandemic. Those who underwent multiple antineoplastic treatments found it difficult to engage in physical activity. Therefore, our study calls for tailored-care interventions and alternative ways of delivering supervised exercise to cancer survivors during the COVID-19 pandemic and beyond.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of School of Physical Education and Sports of University of São Paulo. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
PB, AL, and AG planned the design of the study and data collection. PM-N carried out data collection through an online survey. AG and PM-N worked on data organization and treatment. AL provided guidance on statistical analysis. PB and AL contributed to the interpretation of the results. AG drafted the manuscript and designed the figures. JF is the coordinator of the Cancer institute rehabilitation program supporting Remama group health care. AG and PB drafted the manuscript with critical revision from AL and CB. All authors provided critical feedback on the present manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.624169/full#supplementary-material
References
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Campbell K. L., Winters-Stone K. M., Wiskemann J., May A. M., Schwartz A. L., Courneya K. S., et al. (2019). Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51 2375–2390. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheville A. (2001). Rehabilitation of patients with advanced cancer. Cancer 92 1039–1048. [DOI] [PubMed] [Google Scholar]
- Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. (2020). Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 10 783–791. 10.1158/2159-8290.CD-20-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiot A., Pinto A. J., Turner J. E., Gualano B. (2020). Immunological implications of physical inactivity among older adults during the COVID-19 pandemic. Gerontology 66 431–438. 10.1159/000509216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U., Steene-Johannessen J., Brown W. J., Fagerland M. W., Owen N., Powell K. E., et al. (2016). Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 388:10051. 10.1016/S0140-6736(16)30370-1 [DOI] [PubMed] [Google Scholar]
- Elosua R., Garcia M., Aguilar A., Molina L., Covas M.-I., Marrugat J., et al. (2000). Validation of the minnesota leisure time Spanish women. Med. Sci. Sports Exerc. 32 1431–1437. 10.1097/00005768-200008000-00011 [DOI] [PubMed] [Google Scholar]
- Flynn M. G., Markofski M. M., Carrillo A. E. (2019). Elevated inflammatory status and increased risk of chronic disease in chronological aging: inflamm-aging or inflamm-inactivity? Aging Dis. 10 147–156. 10.14336/AD.2018.0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E. S., Caspersen C. J. (2012). Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int. J. Epidemiol. 41 1338–1353. 10.1093/ije/dys078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. (2015). Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28 690–714. 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Gilchrist S. C., Howard V. J., Akinyemiju T., Judd S. E., Cushman M., Hooker S. P., et al. (2020). Association of sedentary behavior with cancer mortality in middle- aged and older US adults. JAMA Oncol. 6 1210–1217. 10.1001/jamaoncol.2020.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes D. P., Sofiati S. L. (2015). Translation and psicometric validation of the behavioral regulation in exercise questionnaire for use in Brazilian adults. Rev. Bras. Ativ. Fís. Saúde 20 397–412. 10.12820/rbafs.v.20n4p397 [DOI] [Google Scholar]
- Hallal P. C. (2014). Atividade física e saúde no Brasil: Pesquisa, vigilância e políticas. Cad. Saude Publica 30 2487–2489. 10.1590/0102-311XPE011214 [DOI] [PubMed] [Google Scholar]
- International Labour Organization (2020a). COVID-19 and the World of Work: Impact and Policy Responses. Available online at: https://www.ilo.org/wcmsp5/groups/public/---dgreports/---dcomm/documents/briefingnote/wcms_738753.pdf (Acessed September, 2020). [Google Scholar]
- International Labour Organization (2020b). Extending Social Protection to Informal Workers in the COVID-19 Crisis: Country Responses and Policy Considerations. Available online at: https://www.ilo.org/wcmsp5/groups/public/—ed_protect/—soc_sec/documents/publication/wcms_754731.pdf (acessed September, 2020). [Google Scholar]
- Lesser I. A., Nienhuis C. P. (2020). The impact of COVID-19 on physical activity behavior and well-being of canadians. Int. J. Environ. Res. Public Health 17:3899. 10.3390/ijerph17113899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Lozano M., Martín-Martín L., Galiano-Castillo N., Álvarez-Salvago F., Cantarero-Villanueva I., Fernández-Lao C., et al. (2016). Integral strategy to supportive care in breast cancer survivors through occupational therapy and a m-health system: design of a randomized clinical trial. BMC Med. Inform. Decis. Mak. 16:150. 10.1186/s12911-016-0394-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustosa L. P., Pereira D. S., Dias R. C., Britto R. R., Parentoni A. N., Pereira L. S. M., et al. (2011). Tradução e adaptação transcultural do minnesota leisure time activities questionnaire em idosos. Geriatr. Gerontol. Aging 5 57–65. [Google Scholar]
- Mctiernan A., Friedenreich C. M., Katzmarzyk P. T., Powell K. E., Macko R., Buchner D., et al. (2019). Physical activity in cancer prevention and survival: a systematic review. Med. Sci. Sports Exerc. 51 1252–1261. 10.1249/MSS.0000000000001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt J. A., Heseltine D., Niedzwiecki D., Hollis D., Saltz L. B., Mayer R. J., et al. (2006). Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J. Clin. Oncol. 24 3535–3541. 10.1200/JCO.2006.06.0863 [DOI] [PubMed] [Google Scholar]
- Ministério da Saúde (2020). Boletim Epidemiológico Especial. Doença pelo Coronavírus COVID-19. Available online at: http://antigo. saude.gov.br/images/pdf/2020/August/12/Boletim-epidemiologico-COVID-26.pdf (accessed August 23, 2020). [Google Scholar]
- Mount Sinai Hospital (2020). COVID-19 Symptoms. Available online at: https://www.mountsinai.org/health-library/symptoms/covid-19-symptoms (Accessed August, 2015). [Google Scholar]
- Newton R. U., Hart N. H., Clay T. (2020). Keeping patients with cancer exercising in the age of COVID-19. JCO Oncol. Pract. 16 656–664. 10.1200/OP.20.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J., Brymer E. (2018). Nature-based guided imagery as an intervention for state anxiety. Front. Psychol. 9:1858. 10.3389/fpsyg.2018.01858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardin S., Mora E., Varughese F. M., D’Avanzo F., Vachanaram A. R., Rossi V., et al. (2020). Breast cancer survivorship, quality of life, and late toxicities. Front. Oncol. 10:864. 10.3389/fonc.2020.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet E. K., Zelenski J. M., Murphy S. A. (2009). The nature relatedness scale: linking individuals’ connection with nature to environmental concern and behavior. Environ. Behav. 41 715–740. 10.1177/0013916508318748 [DOI] [Google Scholar]
- Patel A. V., Friedenreich C. M., Moore S. C., Hayes S. C., Silver J. K., Campbell K. L., et al. (2019). American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med. Sci. Sports Exerc. 51 2391–2402. 10.1249/MSS.0000000000002117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan A. G., Roberts D. L., Dive C. (2008). Obesity and cancer: pathophysiological and biological mechanisms. Arch. Physiol. Biochem. 114 71–83. 10.1080/13813450801954303 [DOI] [PubMed] [Google Scholar]
- Rutten G. M., Meis J. J. M., Hendriks M. R. C., Hamers F. J. M., Veenhof C., Kremers S. P. J. (2014). The contribution of lifestyle coaching of overweight patients in primary care to more autonomous motivation for physical activity and healthy dietary behaviour: results of a longitudinal study. Int. J. Behav. Nutr. Phys. Act. 11:86. 10.1186/s12966-014-0086-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- São-João T. M., Rodrigues R. C. M., Gallani M. C. B. J., Miura C. T. D. P., Domingues G. D. B. L., Godin G. (2013). Cultural adaptation of the Brazilian version of the godin-shephard leisure-time physical activity questionnaire. Rev. Saude Publica 47 3. 10.1590/S0034-8910.2013047003947 [DOI] [PubMed] [Google Scholar]
- Schmitz K. H., Campbell A. M., Stuiver M. M., Pinto B. M., Schwartz A. L., Morris G. S., et al. (2019). Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 69 468–484. 10.3322/caac.21579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2020). Cancer statistics, 2020. CA Cancer J. Clin. 70 7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Simon S. D., Bines J., Werutsky G., Nunes J. S., Pacheco F. C., Segalla J. G., et al. (2019). Characteristics and 238 prognosis of stage I-III breast cancer subtypes in Brazil: the AMAZONA retrospective cohort 239 study. Breast 44 113–119. 10.1016/j.breast.2019.01.008 [DOI] [PubMed] [Google Scholar]
- Stout N. L., Sabo Wagner S. (2019). Antineoplastic therapy side effects and polypharmacy in older adults with cancer. Top Geriatr. Rehabil. 35 15–30. 10.1097/TGR.0000000000000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. L., Jacobs D. R., Schucker B., Knudsen J., Leon A. S., Debacker G. (1978). A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 31 741–755. 10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020). Coronavirus Disease 2019. Situation Report – 205. Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200812-covid-19-sitrep-205.pdf?sfvrsn=627c9aa8_2 (accessed August 23, 2020). [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., et al. (2020). Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 63 457–460. 10.1007/s11427-020-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.