Abstract
Enormous studies have corroborated that long non-coding RNAs (lncRNAs) extensively participate in crucial physiological processes such as metabolism and immunity, and are closely related to the occurrence and development of tumors, cardiovascular diseases, nervous system disorders, nephropathy, and other diseases. The application of lncRNAs as biomarkers or intervention targets can provide new insights into the diagnosis and treatment of diseases. This paper has focused on the emerging research into lncRNAs as pharmacological targets and has reviewed the transition of lncRNAs from the role of disease coding to acting as drug candidates, including the current status and progress in preclinical research. Cutting-edge strategies for lncRNA modulation have been summarized, including the sources of lncRNA-related drugs, such as genetic technology and small-molecule compounds, and related delivery methods. The current progress of clinical trials of lncRNA-targeting drugs is also discussed. This information will form a latest updated reference for research and development of lncRNA-based drugs.
KEY WORDS: LncRNAs, Targeted drug, Gene therapy, Small molecules, Delivery, ASncmtRNA, Translational medicine, Clinical trials
Abbreviations: AD, Alzheimer's disease; ANRIL, antisense noncoding RNA gene at the INK4 locus; ASncmtRNA, antisense noncoding mitochondrial RNA; ASO, antisense oligonucleotide; BCAR4, breast cancer anti-estrogen resistance 4; BDNF-AS, brain-derived neurotrophic factor antisense; CASC9, cancer susceptibility candidate 9; CDK, cyclin dependent kinase 1; CHRF, cardiac hypertrophy related factor; CRISPR, clustered regularly interspaced short palindromic repeats; DACH1, dachshund homolog 1; DANCR, differentiation antagonizing non-protein coding RNA; DKD, diabetic kidney disease; DPF, diphenyl furan; EBF3-AS, early B cell factor 3-antisense; ENE, element for nuclear expression; Erbb4-IR, Erb-B2 receptor tyrosine kinase 4-immunoreactivity; FDA, U.S. Food and Drug Administration; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GAS5, growth arrest specific 5; HISLA, HIF-1α-stabilizing long noncoding RNA; HOTAIR, HOX transcript antisense intergenic RNA; HULC, highly upregulated in liver cancer; lincRNA-p21, long intergenic noncoding RNA p21; LIPCAR, long intergenic noncoding RNA predicting cardiac remodeling; LNAs, locked nucleic acids; lncRNAs, long non-coding RNAs; MALAT1, metastasis associated lung adenocarcinoma transcript 1; MEG3, maternally expressed gene 3; MHRT, myosin heavy chain associated RNA transcripts; MM, multiple myeloma; mtlncRNA, mitochondrial long noncoding RNA; NEAT1, nuclear enriched abundant transcript 1; NKILA, NF-kappaB interacting lncRNA; Norad, non-coding RNA activated by DNA damage; NPs, nanoparticles; OIP5-AS1, opa-interacting protein 5 antisense transcript 1; PD, Parkinson's disease; PEG, polyethylene glycol; PNAs, peptide nucleic acids; pHLIP, pH-low insertion peptide; PTO, phosphorothioate; PVT1, plasmacytoma variant translocation 1; RGD, arginine-glycine-aspartic acid peptide; RISC, RNA-induced silencing complex; SALRNA1, senescence associated long non-coding RNA 1; sgRNA, single guide RNA; siRNAs, small interfering RNAs; SncmtRNA, sense noncoding mitochondrial RNA; SNHG1, small nucleolar RNA host gene 1; THRIL, TNF and HNRNPL related immunoregulatory; TncRNA, trophoblast-derived noncoding RNA; TTTY15, testis-specific transcript, Y-linked 15; TUG1, taurine-upregulated gene 1; TWIST1, twist family BHLH transcription factor 1; UCA1, urothelial carcinoma-associated 1; UTF1, undifferentiated transcription factor 1; XIST, X-inactive specific transcript
Graphical abstract
This review summarizes the current knowledge on pre- and clinical transformation of lncRNAs-based drugs, covering latest strategies to target pathogenic lncRNAs, indispensable delivery systems, arising clinical trials, future directions and challenges.
1. Introduction
Long non-coding RNAs (lncRNAs) are defined as RNAs that have a transcript length exceeding 200 nucleotides and will not be translated into proteins1. Although lncRNAs were once thought to be by-products of RNA polymerase II transcription without biological functions, a large repertoire of lncRNAs have been certified to regulate cellular processes, such as chromosome and genome modification, transcription activation and interference, and nuclear transport, thus driving more researchers to explore how lncRNAs influence human biology. LncRNAs can be categorized in terms of length, function, location, and targeting mechanism and currently there is no unified standard for their classification. According to their position in the genome relative to protein-coding genes, they can be classified as sense, antisense, bidirectional, intronic, intergenic, and enhancer lncRNAs, with their functions dependent on their position2. Simultaneously, lncRNAs are commonly sorted into bait, scaffold, signal, and guide lncRNAs based on their function mechanisms3. In recent years, several studies have reported that lncRNAs can encode small peptides to fine-tune general biological processes in a tissue-specific manner, further unveiling the value of lncRNAs as well as their complexity4, 5, 6, 7.
The mechanisms by which lncRNAs regulate gene expression are rather complicated and have not yet been fully elucidated. They can function through heterogeneous modes of operation, usually being sorted into the following categories: (1) binding to DNA directly or transcription factors so as to achieve gene expression regulation at the transcriptional level; (2) targeting mRNAs, miRNAs, or proteins and modulating their activities and stability to act post-transcriptionally; and (3) interfering with chromatin complexes to repress or activate gene expression in an epigenetic fashion8, 9, 10.
The mechanisms of lncRNAs allow their involvement in almost all physiological activities in living cells, and they are associated with a broad range of diseases. LncRNAs have emerged as potential novel molecules shaping disease diagnosis, treatment, and prognosis. Owing to their key roles in disease, scientists are currently developing technologies and tools to target lncRNAs and create lncRNA-based drugs. This represents a major opportunity and new frontier for drug development; however, there are still notable challenges, including the absence of successful reference cases and the lack of clinical data on the safety and efficiency of lncRNA-targeted drugs. In the current review, we have summarized the rapid advances that have occurred in this field in recent years, and examined the remaining challenges.
2. Overview of lncRNAs in diseases
The relationship between lncRNAs and diseases, especially chronic diseases, is explicit. However, cancer is the most intensively studied disease related to lncRNAs.
H19 is one of the first lncRNAs to be associated with various types of cancer, including bladder cancer, colorectal cancer, and hepatocellular carcinoma. The mutually inhibiting interplay between H19 and p53, a master tumor suppressor gene, endows in part the role of H19 in tumorigenesis11. Mainly via the modulation of miRNAs, H19 can promote angiogenesis, the major element in tumorigenesis12. In metastasis, ectopic H19 expression may directly involve in the epithelial–mesenchymal transition through the downregulation of cell-to-cell adhesion molecules, such as E-cadherin13. PVT1, another highly expressed lncRNA in cancer tissues, appears to be an effective diagnostic and prognostic biomarker14,15. The positive feedback between PVT1 and c-Myc is well-known; these two molecules act synergistically to promote proliferation, metastasis, and tumor escape16. Moreover, emerging studies have revealed that PVT1 is tightly correlated with chemotherapy resistance17. NEAT1, a well-known lncRNA, is also closely related to chemotherapy resistance in various types of cancers, such as triple-negative breast cancer18. These discoveries support the idea that tumor resistance can be overcome by utilizing lncRNAs. Tumor immunology and tumor metabolism are hotspots in basic medical research and the clinical treatment of cancer, and there is a crosstalk between these two fields and lncRNAs. For example, NKILA mediates the apoptotic sensitivity of different subsets of T cells to orchestrate the balance of immune activation and immunosuppressive T cell subsets in the tumor microenvironment, which results in tumor immune escape19. HISLA maintains the continuous activation of HIF-1a signaling in tumor cells under aerobic conditions to promote lactic acid secretion, which in turn upregulates macrophage HISLA expression and forms a positive feedback loop between lncRNA and tumor metabolism20. At present, there exists a lncRNA cascade, including but not limited to MALAT1, HOTAIR, UCA1, XIST, HULC, and BCAR4, which gives rise to comprehensive mechanism networks for carcinogenesis. More details of this topic are discussed elsewhere21,22. Therefore, lncRNAs represent a treasure to excavate carcinogenesis and corresponding drug development.
More recently, amounting evidence has begun to emphasize lncRNAs modulation in other diverse physiological and pathological processes. Among these, neurological and cardiovascular diseases are two areas of particular interest. LncRNAs are engaged in the pathogenesis of various neurological diseases, such as Parkinson's (PD), Alzheimer's (AD), Huntington's diseases, and lateral spinal cord sclerosis, and the associated mechanisms have gradually been elucidated23. Among these, multiple lncRNAs (e.g., H19, MALAT1, TncRNA, lincRNA-p21, and SNHG1) related to synapse formation and neural cell survival are differentially expressed in PD, which is indicative of their potential as drug targets and biomarkers23. In addition, in AD, there is a cluster of lncRNAs that regulate neuronal apoptosis, such as MALAT124, H1925, BDNF-AS26 and EBF3-AS27, suggesting that the regulation of lncRNA networks exerts unneglectable influence on the pathology of AD and that lncRNAs may shed new light on the unclear etiology of AD and the current unsatisfactory drug therapy.
LncRNAs are reported to participate in cardiovascular system development and biology, as well as in cardiovascular diseases28, so the functional characterization of lncRNAs may aid in the prevention, monitoring, and treatment of diseases, including hypertension, heart failure (HF), cardiac hypertrophy, coronary artery disease, and myocardial infarction. For example, DACH1, a newly discovered lncRNA that regulates cardiac function, was upregulated in patients with HF, whereas lncDACH1 knockout/knockdown in HF mice impeded the development of HF29. LncRNA-Safe (AK137033) promoted myocardial fibrosis during myocardial infarction, and the inhibition of Safe in fibroblasts improved cardiac function; thus, Safe may be a new target for antifibrotic therapy30. To date, MALTA1, MHRT, CHRF, and other lncRNAs have been studied in cardiovascular fields2. However, whether the detection or modulation of these lncRNAs is helpful for the diagnosis, prevention, treatment, and prognosis calls for more data. Undoubtedly, the functions of lncRNAs in cardiac biology and disease are largely unclear and should be explored to identify novel cardiovascular biomarkers and therapeutic targets.
In other chronic diseases, such as diabetes, lncRNAs are involved in pancreatic β-cell disorder (e.g., TUG131), insulin resistance (e.g., MEG332, THRIL, and SALRNA132), and its complications. One of the major diabetic complications, diabetic kidney disease (DKD) has become the leading cause of chronic kidney disease and end-stage renal disease33, thus innovative biomarkers and therapies are urgently needed. Inflammation and fibrosis are fundamental steps in the onset and progression of DKD. In recent years, lncRNAs such as MALAT134, PVT135, ANRIL36 and Erbb4-IR37 have been found to regulate ECM proteins and the expression of pro-fibrosis factors in DKD. MALAT1 is also able to govern inflammation in DKD through the regulation of inflammatory genes and cytokines. More studies are being conducted to clarify the role of lncRNAs in DKD.
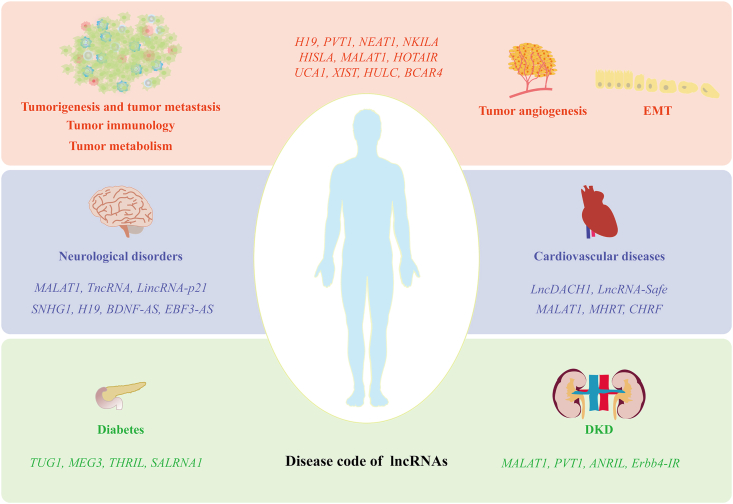
The well-studied lncRNAs involved in different diseases mentioned in this review are summarized in Fig. 1. Information on lncRNAs and their functions in diseases is continuously being decoded, which should provide a theoretical basis to utilize lncRNAs to treat diseases, provide abundant potential drug target candidates for subsequent drug design, and finally pave the way for a new drug arena of lncRNAs.
Figure 1.
Representative well-studied lncRNAs involved in the different diseases mentioned in this review. LncRNAs participate in multi-faceted process in tumorigenesis and tumor metastasis such as tumor angiogenesis and EMT, involving tumor immunology and metabolism. In addition, lncRNAs have been reported to be associated with many other diseases, especially chronic diseases, including but not limited to neurological disorders, cardiovascular diseases, diabetes, and DKD, which provides a basis for drug research and development targeting lncRNAs. LncRNAs, long non-coding RNAs; EMT, epithelial mesenchymal transition; DKD, diabetic kidney disease; H19, imprinted maternally expressed transcript; PVT1, plasmacytoma variant translocation 1; NEAT1, nuclear enriched abundant transcript 1; NKILA, NF-kappaB interacting lncRNA; HISLA, HIF-1α-stabilizing long noncoding RNA; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; HOTAIR, HOX antisense intergenic RNA; UCA1, urothelial carcinoma-associated 1; XIST, X-inactive specific transcript; HULC, human universal load carrier; BCAR4, breast cancer anti-estrogen resistance 4; TncRNA, trophoblast-derived noncoding RNA; LincRNA-p21, long intergenic noncoding RNA p21; SNHG1, small nucleolar RNA host gene 1; BDNF-AS, brain-derived neurotrophic factor antisense RNA; EBF3-AS, early B cell factor 3 antisense RNA; LncDACH1, dachshund homolog 1; MHRT, myosin heavy chain associated RNA transcripts; CHRF, cardiac hypertrophy related factor; TUG1, taurine upregulated gene 1; MEG3, maternally expressed 3; THRIL, TNF and HNRNPL related immunoregulatory; SALRNA1, senescence associated long non-coding RNA 1; ANRIL, antisense non-coding RNA in the INK4 locus; Erbb4-IR, Erb-B2 receptor tyrosine kinase 4-immunoreactivity.
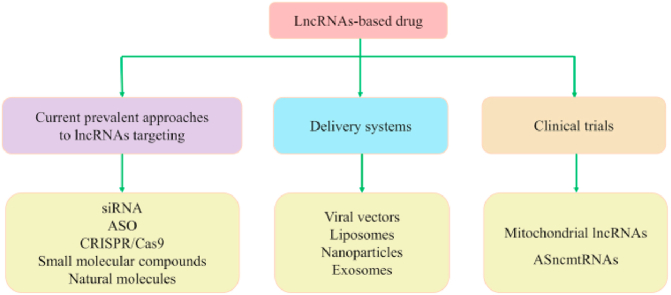
3. Current prevalent approaches to lncRNA targeting
3.1. Small interfering RNAs
Small interfering RNAs (siRNAs) complementary to the target lncRNAs can be applied to target lncRNAs. SiRNAs recruit the RNA-induced silencing complex (RISC) containing arginine to induce lncRNA degradation. In lncRNA research, siRNAs have been used successfully in several preclinical models to study the therapeutic significance of targeting lncRNAs in various diseases38. For example, in the in vivo and in vitro functional analysis of lncRNA CASC9, siRNA was used to target different sites within CASC9, and two sites, CASC9-2 and CASC9-3, were determined to have the highest knockout efficiency. In subsequent experiments, CASC9-2 siRNA cloned into a lentiviral vector significantly reduced the invasion and migration of esophageal squamous cell carcinoma39. Moreover, tumor progression was conspicuously impeded when 1 mg/kg DANCR siRNA nanoparticles (NPs) was systematically administered in a triple-negative breast cancer mouse model40. SiRNA (1 mg/kg) targeting Linc00311 and lncRNA AK141205 could effectively relieve neuropathic pain in rats by inhibiting the activation of the STAT3 signaling pathway41. SiRNA-mediated LINC01296 knockdown in a non-small cell lung cancer model reduced the number of cancer cells in vitro and shrunk the tumor mass in vivo42, demonstrating the ability of siRNA to systematically target specific lncRNAs.
Definitely, there is still one major challenge for siRNA now: off-target. Although this will be mitigated by lower doses and modifications of siRNA or delivery systems (overviewed later), the off-target effects have not been completely addressed43, 44, 45. With the world's first siRNA drug Onpattro (patisiran) being used to treat patients with polyneuropathy caused by hereditary parathyroid amyloidosis and another siRNA drug, Givlaari (givosiran) being approved for the market last year for acute hepatic porphyria in adults, the investigation of lncRNA-based siRNA drugs may enter a new era.
3.2. Antisense oligonucleotides
Antisense oligonucleotides (ASOs) are a type of chemically synthesized short single-stranded oligonucleotides that have a 15- to 25-nucleotide DNA sequence. They can bind to complementary RNA and recruit RNase H, provoking RNA degradation and altering the expression of downstream proteins. Moreover, they can also inhibit transcription without the assistance of RNase H cleavage, such as by the spatial blocking of ribosomes46. The chemical modification of ASOs has allowed the disadvantages of low cell permeability and poor stability to be overcome, and remarkable progress has been made in the improvement of their pharmacological properties. ASOs can be divided into three generations according to the chemical modification. The first generation is represented by phosphorothioate (PTO) oligonucleotides. PTO is nuclease-resistant and highly soluble; however, its specificity and affinity are insufficient and sometimes incur off-target effects, such as non-specific protein downregulation and cell apoptosis47,48. The second-generation 2′-O-substituted ASOs have stronger binding ability49, greater stability50, lower toxicity, and lower immunostimulatory effects51 but a lower ability to induce cleavage by RNase H152. The third generation ASOs, peptide nucleic acids (PNAs), phosphorodiamidate morpholino oligomers, and locked nucleic acids (LNAs), were designed for decent affinity and stability, but still have disadvantages. For example, RNase H1 recognition and cleavage specificity are not qualified enough, thus inducing hepatotoxicity along with the off-target effects. In addition, there are still difficulties with the delivery of third-generation ASOs, as they cannot be orally administered, mostly by injection53, 54, 55. Thus, each generation has its own problems to some degree.
In the past few years, ASO-based therapies have led to clinical breakthroughs. The U.S. Food and Drug Administration (FDA) has approved three ASOs drugs for the treatment of neurodegenerative or muscular dystrophy. Nusinersen was approved for treating multiple forms of spinal muscular atrophy, eteplirsen for treating Duchenne muscular dystrophy, and inotersen for treating familial amyloid polyneuropathy, suggesting that ASOs may form the next generation of cutting-edge drugs for the treatment of neurological diseases54. The application of ASOs is mainly focused on the treatment of PD, AD, amyotrophic lateral sclerosis, Huntington's disease, and cancer. Furthermore, a large number of ASO-based drugs are undergoing clinical trials.
With the use of ASO technology, research into lncRNAs targeted by ASOs has burgeoned, indicating the clinical prospects of lncRNA-targeted therapy. The subcutaneous delivery of MALAT1 phosphorothioate-modified ASO successfully inhibited primary tumor differentiation and reduced lung metastasis in a mouse model of breast cancer, which offers preliminary evidence that MALAT1 ASO is a therapeutic drug able to inhibit breast cancer progression56.
In addition, the most recent third generation ASOs are often used to target lncRNAs. PNAs with modified nucleotide furanose rings are artificially synthesized DNA mimics resistant to nuclease degradation that can be further modified for systematic applications57, and researchers have tried to use them for targeting various non-coding RNAs, including lncRNAs58. For example, cross-linking pH-low insertion peptide (pHLIP) with PNAs can resist the acidic tumor microenvironment, effectively inhibiting HOTAIR activity both in vitro and in vivo, hence the proposal of pHLIP-PNA targeting lncRNAs for solid tumors treatment systematically59. The combination of PNAs with cholesterol groups can increase hydrophobicity, thus promoting cell absorption and enhancing tissue targeting. The attachment of lysine groups can reinforce the release of endoplasmic bodies after the target cells taking up PNA58.
LNA gapmeR, an ASO designed specifically for lncRNA and mRNA60,61, has become one of the most widely used ASO-targeting lncRNAs in preclinical research. In the case of NEAT1, this technology was used to develop a new lncRNA function suppressor, g#N1_E LNA gapmeR, to trigger the RNase H-dependent degradation of lncRNAs. The main mechanism was that the LNA-gapmeRs sequence was complementary to the 5ʹ region of NEAT1, which led to the NEAT1 degradation. After in vitro delivery was optimized, g#N1_E LNA gapmeR resulted in 80%–90% of NEAT1 downregulation. CD138+ cells from patients with multiple myeloma (MM) showed downregulation of NEAT1 after 6 days of exposure to g#N1_E, and finally, a large number of cells died. Later, in vivo experiments in mice revealed that #N1#E LNA gapmeR could induce striking antitumor activity, simultaneously showing the best tumor absorption without systemic toxicity, consistent with previously documented data61. It was found that MALAT1 has a tumor-promoting effect in MM through the inhibition of proteasome activity. LNA gapmeR ASO, which was used to degrade MALAT1, displayed superior antitumor activity in a humanized mouse MM model and could induce cytotoxic effects through the destruction of the MALAT1/RF1 interrelation cycle and in combination with bortezomib, which provided preclinical evidence for the use of this new effective ASO-targeting lncRNA for the treatment of MM62. In addition to cancer, ASO-targeting lncRNAs have been applied in other diseases such as bone disease. It was discovered that Lnc-ob1 (its human homolog is LNC-OB1) was rich in osteoblasts and significantly reduced during the loss of mouse and human bones. In lnc-ob1 knocked-in mice, bone density and bone formation rate were upregulated. An osteoblast-targeted drug delivery system63 was used to package and deliver lnc-ob1 ASO intravenously in lnc-ob1 knockdown mice, which drastically inhibited the osteogenic process64. Osteoblast-targeted Lnc-ob1 can ameliorate ovariectomy-induced bone loss in mice, demonstrating the potential of targeted delivery of specific lncRNAs to treat osteoporosis in humans.
Existing randomized clinical trial results disclose the stable pharmacokinetics and safety of apolipoprotein-targeting ASOs65. However, ASO, as a special nucleic acid drug, may also have potent off-target effects and difficulties associated with highly specific delivery as previously mentioned; thus, despite the numerous preclinical applications for targeting lncRNAs, further technological progress is required.
3.3. Clustered regularly interspaced short palindromic repeats
Clustered regularly interspaced short palindromic repeats (CRISPR) are found in many bacteria, and its related proteins (CRISPR-associated proteins, the Cas family) are involved in protection against mobile genetic elements. Essentially, the CRISPR/Cas system is an immune defense system for prokaryotes. One type of CRISPR system, the type II system, relies on a Cas protein that anchors to a defined DNA sequence, making it the basis for a genome-editing tool. The type II Cas protein in Streptococcus pyogenes has been shown to conduct RNA-guided DNA cutting in mammalian cells, opening a new stage in which CRISPR/Cas9 has become a widely used gene-editing tool.
The CRISPR/Cas9 system includes a single guide RNA (sgRNA) and a Cas9 enzyme; sgRNA guides the Cas9 nuclease to specific sites in the genome via complementary base pairing, and Cas9 cleaves the DNA sequence followed by the protospacer-adjacent motif. Owing to its outstanding accuracy, efficiency, permanence, and ease of programming66,67, CRISPR/Cas9 has been successfully used to target lncRNAs and has stimulated research into lncRNAs. Norad is a non-coding RNA activated by DNA damage. To study the function and mechanism of lncRNA Norad at the whole animal level, a mouse model of lncRNA Norad deletion was created using the CRISPR/Cas9 system. Norad-deficient mice showed clear similarities in the multisystem degenerative phenotype of premature aging, causing severe genomic instability and mitochondrial dysfunction68. Hence, CRISPR/Cas9 has been employed to explore lncRNA function. In addition, CRISPR/Cas9 is often used for the screening and annotation of lncRNAs69.
Furthermore, the therapeutic effect of CRISPR/Cas9-targeting lncRNAs is still limited to basic research owing to the unpredictable clinical risk. However, according to in vitro and in vivo preclinical data, it is still a potential therapeutic tool. TTTY15 is a lncRNA highly expressed on the Y chromosome. In a knockdown experiment aimed to confirm that TTTY15 promoted the proliferation and migration of prostate cancer cells, a double-sgRNA guided CRISPR/Cas9 system was used to achieve complete TTTY15 knockout and simultaneous CRISPR/Cas9-mediated transcription terminator knock-in to stop TTTY15 transcription. These different CRISPR/Cas9 strategies successfully knocked out TTTY15 in vitro, resulting in the substantially reduced growth of TTTY15 knockout clones and weakened tumor phenotype in a xenograft mouse model70. Another example is the use of a dual sgRNA CRISPR/Cas9 system to knockout NEAT1, which led to glioblastoma cells apoptosis and metastatic phenotype suppression71. These results indicate that CRISPR/Cas9 represents a possible brand-new technology to translate lncRNAs into clinical therapy.
In a genome-wide analysis, however, CRISPR/Cas9 was found to safely manipulate only 38% of 15,929 lncRNA loci without deregulating the expression of their divergent neighboring genes, which occurred mainly due to their intragenic or bidirectional promoters72. While this issue didn't happen when using siRNA or ASOs, hence adding the deficiency and limitation of CRIPR/Cas9. Globally, off-target effects, caused by inaccurate recognition and cleavage of similar sequences, are the first major hurdles for CRISPR/Cas9 bench-to-bedside transition, as off-target effects will induce undesired genetic modifications. The current antidotes are off-target prediction, detection, and prevention, and there is no complete solution so far. Different algorithms and continuously improving technologies have been developed to predict and detect off-target respectively, such as the kinetic biophysical model of sequential target recognition established by Klein et al.73 for off-target determinants prediction as well as off-target rate calculation, and the recently proposed prominent genome-wide off-target analysis to detect off-target mutations via whole genome sequencing without individual single-nucleotide variant interference74. Modifications of Cas9 or sgRNA75, as well as sgRNA truncation, are viable to reduce off-target effects76,77, and the design of sgRNA with high-specificity for lncRNAs is not always easy but is a key component. Considerable software and online tools have been developed to assist in the design of sgRNAs78.
Similar to siRNA and ASO, in vivo delivery for CRISPR/Cas9 is different from traditional medicine. Compared with in vitro/ex vivo systems, the in vivo delivery of CRISPR/Cas is immature. Thus, the clinical applications, especially to treat human disease, will be unachievable, unless we prevent off-target effects and solve systematic delivery and other potential thorny technical problems.
3.4. Selected applications of siRNA, ASOs, and CRISPR/Cas9
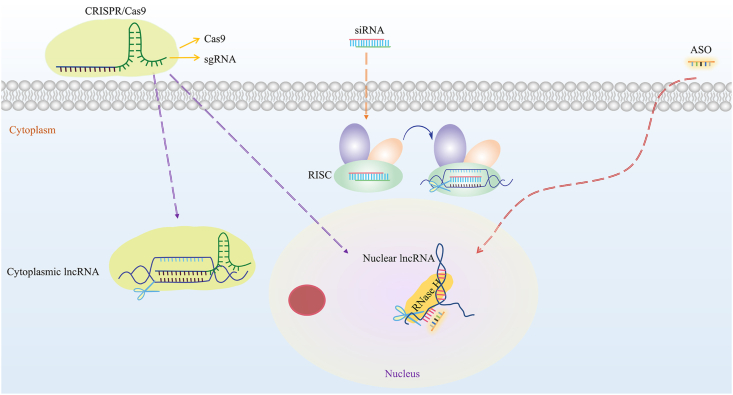
The cellular location of lncRNAs should be considered when choosing siRNA, ASOs or CRISPR/Cas9 for lncRNA targeting in preclinical models. ASOs can more effectively inhibit nuclear lncRNAs such as MALAT1 and NEAT1, and siRNA can more effectively inhibit cytoplasmic lncRNAs such as DANCR and OIP5-AS1 (for which RISC is present mainly in the cytoplasm). CRISPR/Cas9 can be applied for lncRNA knockout regardless of cellular location, but this technology has a relatively narrow lncRNA spectrum, as mentioned above. Therefore, for lncRNAs with unclear or dual cellular localization, using these methods alone makes it difficult to achieve effective interference. At this time, using both ASOs and siRNA concurrently can inhibit dual-localized lncRNAs. The recently improved Smart Silencer, a hybrid approach combining ASOs and siRNA reagents, is a mixture containing three siRNAs and three ASOs, and has been successfully applied in countless experiments involving lncRNA knockdown in vivo and in vitro79, 80, 81, 82. For example, given the uncertain localization of lncRNA RP11-544D21.2 and XLOC_014288, some researchers believed the expected knockout effect was difficult to achieve with traditional siRNAs, so they used Smart Silencer at a concentration of 50 nmol/L, which resulted in massive downregulation of the target lncRNAs79. The optimal application of siRNA, ASOs, and CRISPR/Cas9, based on lncRNA intracellular localization, is shown in Fig. 2.
Figure 2.
Respective optimal applications of siRNA, CRISPR/Cas9, and ASOs based on lncRNA intracellular localization. siRNAs are appropriate for cytoplasmic lncRNAs, ASOs for nuclear lncRNAs, and CRISPR/Cas9 for dual-localized lncRNAs or those with unknown cellular localization. CRISPR, clustered regularly interspaced short palindromic repeats; Cas9, CRISPR associated protein 9; sgRNA, single guide RNA; ASO, antisense oligonucleotide; RISC, RNA-induced silencing complex.
4. Small-molecule compounds
Similar to proteins, lncRNAs are equipped with multidimensional architecture; these secondary and tertiary structures are complicated but attractive targets awaiting understanding and exploitation. It is envisioned that specific lncRNA structures can be bound or blocked by small molecules. Therefore, in addition to nucleic acid technology, small-molecule compounds identified by library screening have been proven to be effective entities. They disrupt lncRNA spatial structure or lncRNA–protein interaction and will overcome the extra obstacles to the delivery of ASO/siRNA. These are based on the decipherment of lncRNAs functions in a definite disease, the appropriate structure within the exact lncRNA, and favorable “pockets” that can be stably bound by ligand molecules. Earlier, Pedram Fatemi et al.83 developed ALPHA screening technology to quantify lncRNA–protein interactions and identify a small molecule, ellipticine, that could inhibit the interaction of BDNF-AS–EZH2 and HOTAIR–EZH2, which demonstrated the use of high-throughput screening to identify lncRNA–protein interactions and target lncRNAs with small molecules83. Here, we first used MALAT1, a lncRNA with notable drug potential84,85, as an example of the roadmap to the discovery of small-molecule modulators based on their chemical structure, to illustrate the tremendous potential of small molecules as lncRNA-targeted drugs.
There is a highly conserved element for nuclear expression (ENE) at the 3ʹ end of MALAT1. The triple helix structure of ENE, which ensures that MALAT1 is not degraded, is necessary for the physiological function of MALAT186, 87, 88. Recently, Donlic et al.89 confirmed that the triple helix structure at the 3ʹ end of MALAT1 could be selectively targeted by small molecules. In their study, a series of diphenyl furan (DPF)-derivates based on RNA-binding scaffolds were synthesized, and molecules with different structural shapes, which are essential for lncRNA recognition, were screened to assess the binding strength with the MALAT1 triple helix and stem loop via fluorescence-based screening. Rod-like DPFs were found to be the most promising ligands from principal moments of inertia calculations. This work encouraged the exploration of other small molecules for lncRNAs89. Abulwerdi et al.90 used small-molecule microarray and molecular docking technology to select two specific compounds that could specifically bind the MALAT1 3ʹ triple helix structure with different mechanisms and binding modes, as measured with docking poses analysis using AutoDock 4, and compound 5 presented better regulation of MALAT1 downstream gene Csn2 than ASO90. This further displayed the development prospects of small lncRNA-targeting molecules.
The second example is lncRNA GAS5, whose decrease is closely related to insulin resistance and diabetes91, 92, 93, and it has been tested as a drug target through compound library screening. Briefly, Shi et al.94 synthesized a γ-AApeptide combinatorial library and employed a one-bead-two-compound screening strategy with the help of fluorescein-tagged oligonucleotide, which identified hit small molecules that bound and stabilized lncRNA GAS5 through disruption of the interaction between GAS5 and UTF1, a transcriptional factor downregulating GAS5. Finally, they tested the ability of NP-C86 to competitively bind to GAS5 transcript with high affinity and specificity, thus restoring the GAS5 level to that of normal physiological levels without causing GAS5 overexpression, which was confirmed at the tissue level94. This is an elegant example of translating the idea of using small-molecular compounds targeting lncRNAs into practice. Among the existing small-molecular drugs, there are also some molecules acting prominently on lncRNAs to exert pharmacological effects. For example, in Ewing sarcoma cells, HULC acted as a sponge for miR-186 to promote the expression of the oncogene TWIST1, whereas the small-molecule YK-4-279 can inhibit this axis by releasing miR-186 from HULC; however, the exact mechanism remains to be determined95. These achievements, as stated above, will support the use of lncRNA-targeting small molecular compounds.
In this respect, it is necessary to acquire information on three principal stages: elucidating the action mechanisms of lncRNAs, analyzing the functional structure pocket of lncRNAs, and finding molecules that can form specific binding sites with the pocket. Among them, the development of techniques for lncRNAs structural analysis and the recognition of interactions between lncRNAs and other molecules is essential96. Then, establishing screening methods and libraries is helpful for the rapid identification of drug-like molecules and highly complex lncRNA motifs to improve binding specificity and affinity88,97. On the other hand, the compounds obtained by this approach require further in vitro and in vivo model tests to confirm their structure–function relationships and actual pharmacological effects.
Based on currently published literature, research into small-molecule inhibitors of lncRNAs is still relatively limited, so it is uncertain whether small molecules will outperform nucleic acid technology for the development of lncRNA-based drugs. However, compared with the nucleic acid drugs described above, small-molecule inhibitors have the advantages of lower cost and more convenient administration modes, so it is reasonable to develop them as lncRNA-targeted drugs, either alone or in combination with other techniques. More critically, small molecules target lncRNAs in a structure-specific manner rather than in a sequence-complementary manner and may orchestrate lncRNA functions without changing their expression, which cannot be achieved via siRNAs, ASOs, and CRISPR, because small molecules can only disrupt the binding/interaction between lncRNAs and other biomolecules. Naturally, to determine the qualified fine-tuning of small molecules, extensive research into lncRNAs is required.
5. LncRNA regulators derived from natural plants
A large number of studies have shown that in models of diseases such as cancer, non-alcoholic fatty liver disease, osteoporosis, and PD98, 99, 100, 101, plant-derived natural compounds have a credible regulatory effect on lncRNAs, including MALAT1, PVT1, HOTAIR, H19, and NEAT1. For example, resveratrol ameliorates PD through the regulation of MALAT1 and its downstream signaling pathways99. Besides, resveratrol inhibits the proliferation and migration of MM cells via NEAT1/Wnt/β-catenin, showing the lncRNA-related mechanism of resveratrol in cancer treatment102. However, this should be confirmed in vivo. Curcumin, another natural molecule with anticancer effects, can downregulate H19 and promote the expression of p53 in a time- and concentration-dependent manner103. When combining curcumin with si-MALAT1, downregulation of MALAT1 was more effective in vitro104, suggesting the potential of phytochemicals to regulate lncRNA. In vivo, berberine altered the expression of 538 lncRNAs in a rat model of non-alcoholic fatty liver disease105, indicating a new possible mechanism for the effect of berberine98.
By searching website “ClinicalTrial.gov”, several natural compounds mentioned above have been tested in clinical trials, such as curcumin against colorectal cancer, and resveratrol and berberine have moved into phase II or phase III clinical trials for some metabolic related diseases (e.g., NCT02439385, NCT03597568, NCT02114892, and NCT03251716). However, the lncRNAs are not claimed as major targets of these natural compounds at beginning, but are novel possible intracellular targets that can be referred to explain their pharmacological activities to some extent.
Optimistically, phytochemicals come from a wide range of herbal sources and are relatively safe with a long history in traditional Chinese medicine. The lack of accurate targets and mechanisms is a shortcoming of phytochemicals. Traditionally, proteins in the human body are the most possible targets for phytochemicals. Howbeit, targeting lncRNAs currently provides an innovative perspective to explain their bioactivities and to possibly extend their usage in clinical trials. Approaches targeting lncRNAs and their examples are summarized in Table 1.
Table 1.
Examples of applications of targeting strategies in lncRNAs-based intervention as mentioned in this account.
| Approach | Mechanism/origin | Targeted lncRNA | Condition | Ref. |
|---|---|---|---|---|
| siRNA | Being complementary to the target lncRNAs and recruiting RISC to induce lncRNA degradation | CASC9 | Esophageal squamous cell carcinoma | 39 |
| DANCR | Triple-negative breast cancer | 40 | ||
| LncRNAAK141205 | Neuropathic pain | 41 | ||
| LINC01296 | Non-small cell lung cancer | 42 | ||
| ASO | Binding to complementary RNA and recruiting RNase H to degrade lncRNAs | MALAT1 | Breast cancer | 56 |
| MALAT1 | Multiple myeloma | 62 | ||
| HOTAIR | Breast cancer | 59 | ||
| NEAT1 | Multiple myeloma | 61 | ||
| Lnc-ob1 | Osteoporosis | 64 | ||
| CRISPR/Ca9 | sgRNA recognizing targeted lncRNA sequence Cas9 nuclease conducting lncRNA degradation |
Norad | Normal physiology and aging | 68 |
| TTTY15 | Prostate cancer | 70 | ||
| NEAT1 | Glioblastoma | 71 | ||
| Small molecule | ALPHA screening quantifying lncRNA–protein interactions and identifying small molecules ellipticine | BDNF-AS&HOTAIR | – | 83 |
| Synthesizing a small molecule library based on an RNA-binding scaffold and then screening | MALAT1 | – | 89 | |
| Small molecule microarray and molecular docking technology selecting two specific compounds | MALAT1 | – | 90 | |
| One-bead-two-compound screening strategy identifying NP-C86 | GAS5 | Diabetes | 94 | |
| Existing small molecule YK-4-279 inducing lncRNA downregulation | HULC | Ewing sarcoma cells | 95 | |
| Natural molecule | Resveratrol |
MALAT1 NEAT1 |
Parkinson's disease Multiple myeloma |
99 102 |
| Curcumin |
H19 MALAT1 |
Gastric cancer cells Colon cancer cells |
103 104 |
|
| Berberine | 538 lncRNAs | Non-alcoholic fatty liver disease | 105 |
CASC9, cancer susceptibility candidate 9; DANCR, differentiation antagonizing non-protein coding RNA; Norad, non-coding RNA activated by DNA damage; TTTY15, testis-specific transcript, Y-linked 15; GAS5, growth arrest specific 5.
–Not applicable.
6. Delivery systems
The delivery system is the key to the envisioned drug role for lncRNAs. It must have a satisfactory specificity, stability, cell permeability, and low immunogenicity. Classical carriers are mainly divided into viral vectors and non-viral vectors.
6.1. Exogenous vectors
6.1.1. Viral vectors
Lentiviral vectors are the most widely used lncRNAs-carrying systems. They have a high transfection efficiency and long expression time. As a typical tool, lentiviral vectors are used in most studies to interfere with lncRNAs, such as by loading specific lncRNAs or siRNAs, to achieve targeted lncRNA cellular overexpression and knockdown106,107.
Adenoviral vectors are also commonly used for lncRNA overexpression or interference29,108. An adenovirus is an unencapsulated linear double-stranded DNA virus, with an approximate diameter of 80–120 nm, which can rapidly infect dividing and non-dividing cells. The knockdown models of the LncLGR gene can be effectively constructed by the injection of an adenovirus carrying LncLGR siRNA into mice108. By modifying the wild-type adenovirus, preserving the packaging signal sequence, and removing the viral protein-coding sequence, the loading capacity for foreign genes increases while cytotoxicity and immune response simultaneously reduce in the body; overall, this results in a safer system for the introduction of foreign nucleic acids.
In light of the controversy over the safety of viral vectors, they have been gradually replaced by other vectors in systemic drug administration, which we discuss as below.
6.1.2. Non-viral vectors
Liposomes are ideal materials for the targeted delivery of lncRNAs, especially given the development of nanomaterials. Nanoliposomes have become increasingly popular in preclinical trials in which the function and therapeutic potential of lncRNAs are studied. Liposomes have many desirable qualities109. In addition to reduced toxicity and immunogenicity, liposome encapsulation has also improved drug stability and targeting to liver tissues, which can ensure that the encapsulated material, such as siRNA, is not filtered and removed by the kidney, and is gradually absorbed by target cells in the liver during blood circulation. Several liposome-encapsulated DNA (NCT01502358) or mRNA (NCT03382405) in vivo delivery systems have entered into clinical trials, and they are also frequently used to deliver lncRNAs in preclinical experiments in vitro and in vivo110, 111, 112. Liposomes can be cationic or cationic-anionic exchangeable up to certain pH values, allowing flexibility in the increase of drug-carrying efficiency, as well as the control of drug delivery and release113, 114, 115, 116.
Lipid NPs are similar to liposomes; however, they have greater diversity and are more suitable for encapsulating a variety of nucleic acid drugs, and have become the most popular non-viral delivery system used in gene therapy117,118. In lncRNA delivery, RGD-PEG-ECO/siRNA DNCR-targeted NPs have been proven to effectively inhibit tumor growth in preclinical animal trials40, with no obvious side effects, showing the high efficiency and safety of lncRNA nanoparticle administration. Intranuclear lncRNAs are more difficult to target because of the nuclear envelope protection. Nevertheless, the recent application of ASO-gold-TAT NPs targeting the nuclear lncRNA MALAT1 in a mouse model of xenotransplanted lung cancer noticeably suppressed tumor metastasis, and the survival time of mice was prolonged by 80%. This was a result of the highly specific nuclear localization ability of ASOs conducted by NPs119. Therefore, gold NPs bounded with transmembrane peptide (or TAT), which have good biocompatibility, chemical inertia, multivalent effect, and easy functionalization, can promote nuclear-lncRNA-targeted ASO applications. Moreover, rod-like supramolecular nanoassemblies, combining the unique characteristics of CNC, PGEA, and PAsp, when co-transfected with MEG3 and miR101, can be condensed into nanocomposites with a particle size less than 200 nm to achieve cooperative delivery of non-coding RNAs with different sizes and enhance drug targeting and therapeutic efficacy120.
In many cases, liposomes and lipid NPs are coupled with additional molecules, including nucleic acid aptamers, antibodies, peptides, protein ligands, polymers, or small molecules, to form an active or passive targeted drug delivery system (Fig. 3). Similarly, sometimes non-coding RNA drugs themselves are modified; for example, in the paper of Das et al.121, the S1 aptamer is fused with lncRNAs to increase the amount of lncRNA LOC284454 in target cells. Moreover, through the use of diverse organic polymers, especially plant-derived polymers, polyplex/polymer micelles122, 123, 124 and polymer NPs125,126, can also contribute to better drug delivery. For example, it has been proposed that polyurethane nanocapsules act differently from traditional lipid NPs as they encapsulate small nucleic acids via electrostatic interactions. Preclinical results have validated their good biocompatibility, low toxicity, and good mechanical elasticity63, some of which are also suitable for lncRNAs127.
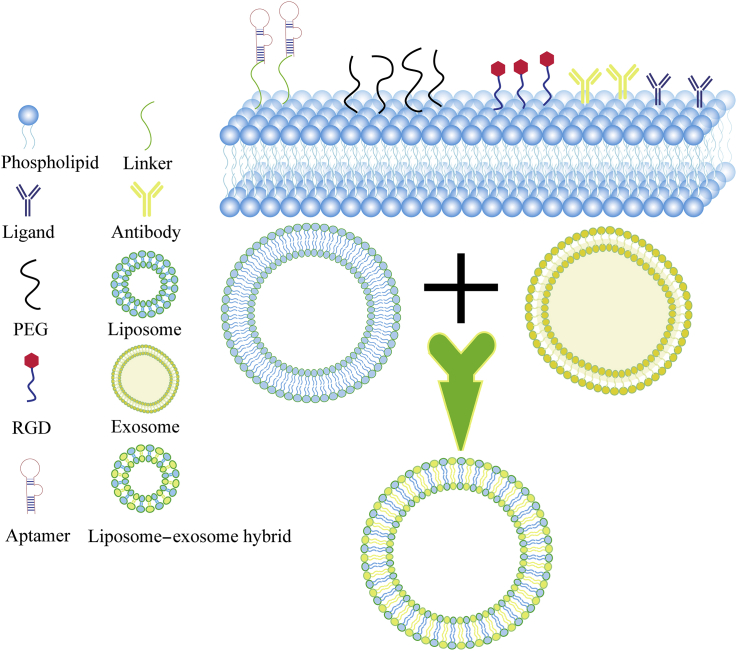
Figure 3.
Mainstream surface modifications for liposomes/lipid nanoparticles, as well as liposome–exosome hybrids in lncRNA drug delivery. Liposomes/lipid nanoparticles can be moderated by ligands, PEG, RGD, aptamers, linkers, or antibodies to improve delivery performance. In addition, liposome–exosome hybrids are new drug delivery agents that can be successfully applied in preclinical experiments. PEG, polyethylene glycol; RGD, arginine-glycine-aspartic acid peptide.
6.2. Endogenous vector: Exosomes
Exosomes are 30–200 nm membrane-bound vesicles naturally secreted by cells and contain lipids, proteins, DNA, mRNAs, miRNAs, and lncRNAs128. Evidence is accumulating to confirm that exosomes have instrumental value in disease diagnosis and targeted therapy. As endogenous nano-vesicles, exosomes are of paramount priority for the delivery of protein and nucleic acid drugs, and even exceed liposomes in loading efficiency129.
Going back to two decades ago, Alvarez-Erviti et al.130 injected self-exosomes secreted from dendritic cells and engineeringly loaded with GAPDH siRNA into mice, demonstrating that siRNA encapsulated in targeting ligand-expressing exosomes can pass through the blood–brain barrier to specifically target brain tissue, and knockdown specific genes. This was the first proposed concept of exosomes as nucleic acid drug delivery vehicles. Recently, Haney et al.131 delivered exosomes via nasal injection into a PD mouse model and identified specific neuroprotective effects. Kamerkar et al.129 designed exosomes from normal fibroblast-like mesenchymal cells as a vector carrying specific siRNAs or shRNAs, and observed a drastic reduction in tumor volume and improved survival rate in mice with pancreatic cancer.
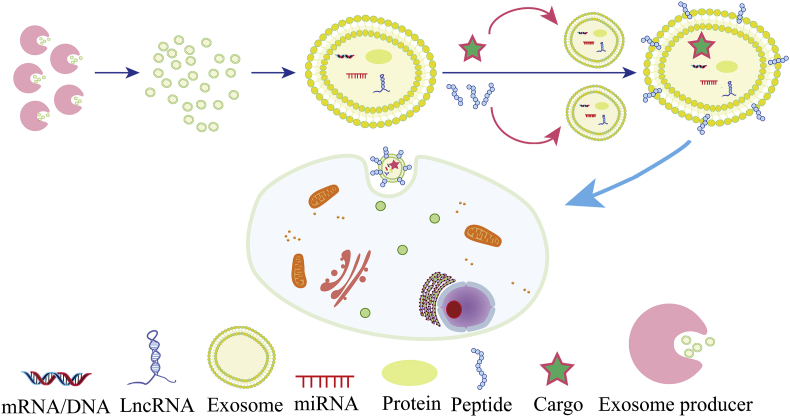
Therefore, extracellular vesicles, represented by exosomes, are very promising delivery vectors and can be engineered to restore or knockdown pathogenic lncRNAs expression in lncRNA-dominating diseases. Compared with viral and non-viral nanocarriers, exosomes have lower immunogenicity and higher stability in vivo; specially, patient-derived tissues are suitable as a source of individualized and biocompatible drug-packaged exosomes. Due to the mediocre efficiency of exosomes in packaging large nucleic acids, exosomes can now be integrated with liposomes (Fig. 3), organic NPs, or polymer NPs to improve the specificity and controllability of drug delivery systems. For example, exosome–liposome hybrids have been used successfully for in vivo and in vitro delivery in CRISPR-Cas9 systems, symbolizing a new direction for precision medicine132,133. Parallel to exogenous NPs, surface modification is also a practical tactic for exosome delivery optimization; these can be cataloged as genetic engineering or chemical modifications134. Most essentially, the exosome membrane is always incorporated specific peptides which bind their receptors within the target tissue130,135,136, as shown in Fig. 4.
Figure 4.
Schematic illustration of peptide-decorated exosomes for targeted lncRNA delivery. Exosome producers can be living cells, in particular, cells from patients themselves. The exosome membrane is always modified with specific peptides binding to their receptors within the target tissue, thus enhancing the specificity of exosome delivery.
The described delivery systems have their own fine features and defects, which suggests that it is a preferable choice to allow their comprehensive and conjunctive use134,136. In contrast, the purification and stability of exosomes are paradoxical. To this end, exosome drug delivery, especially for RNAs with special structure, is still in its infancy and requires further improvement.
7. Clinical trials: Mitochondrial lncRNAs
Although most of the studies on lncRNA targeting are still in the preclinical stage, research into mitochondrial long non-coding RNA (mtlncRNA) has progressed rapidly.
When the two strands of mitochondrial DNA are fully transcribed, the light chain carrying only seven tRNA genes and the gene encoding ND6 protein generates and releases large non-coding sequences, including lncRNA, during transcription processing137. Mitochondrial energy metabolism and functions related to the induction of apoptosis, inflammation, and metastasis are mediated, to a certain extent, by mtlncRNA. The lncRNAs produced by mtlncRNA mainly include sense mtlncRNA (sense non-coding mitochondrial RNAs, SncmtRNA) and antisense non-coding mitochondrial RNA (ASncmtRNA)138. Undoubtedly, the regulatory property of mtlncRNA in diseases and its clinical application are of great value. For example, mtlncRNA LIPCAR is a new biomarker for cardiac remodeling that can predict the survival of patients with heart failure139. In recent years, two types of ASncmtRNAs have been identified: ASncmtRNA-1 and ASncmtRNA-2 (hereinafter collectively referred to as ASncmtRNAs), which are downregulated in various tumor tissues140. It was then reported that ASOs targeting ASncmtRNAs could induce death in a large area of cancer cells without affecting normal cells. Subsequent in vivo experiments in immunoreactive mice showed that targeted therapy of ASncmtRNAs could prevent lung metastasis after melanoma resection141. The lentiviral construct targeting ASncmtRNAs obviously blocked B16F10 primary tumor proliferation142. These results supported the clinical application of ASncmtRNAs-targeting ASOs for the treatment of melanoma. Similarly, in vivo studies in isogenic mouse renal cancer models manifested that ASncmtRNAs-targeting ASOs could completely reverse tumor growth143. Based on these animal experiments, ASOs targeting specific mtlncRNAs may be a safe and effective tumor treatment strategy, providing a reliable basis for subsequent clinical trials.
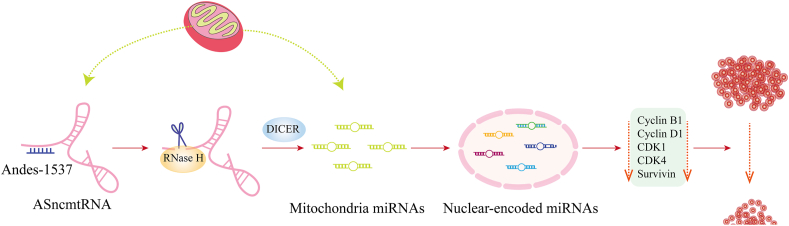
To this end, FDA has approved a clinical trial of Andes-1537, a short single-stranded phosphorothioate ASO, which binds to ASncmtRNAs through complementary base pairs, for the targeted treatment of solid tumors, including a phase I clinical trial recruiting patients with advanced metastatic cancer (NCT02508441). The phase I clinical results showed that Andes-1537 was well-tolerated144. Although it has been discontinued (the second part was terminated to redefine a new regimen), the first part of the study was completed and the maximum tolerated dose was determined. Another clinical trial of Andes-1537 for multiple solid tumors (NCT03985072) was started last year. Furthermore, to a deeper degree, the related research group carried out mechanistic experiments to explain how Andes-1537 realize its anti-tumor effects145; for details see Fig. 5, presented according to the results of Fitzpatrick et al.145
Figure 5.
Schematic illustration of the mechanism of Andes-1537. The binding between Andes-1537 and ASncmtRNA activates RNase H degradation in this binding region. DICER then releases mitochondrial miRNAs, mainly hsa-miR-4485, inducing an increase in some specific nuclear-encoded miRNAs. These miRNAs (such as hsa-miR-5096 and hsa-miR-3609) downregulate cell cycle progression factors including cyclin B1, cyclin D1, CDK1, CDK4, and survivin, and consequently reduce cell cycle and tumor cells. Further details can be found in the previous study145 [Adapted with modification from Ref. 145 © The Author(s) 2019]. ASncmtRNA, antisense non-coding mitochondrial RNA; CDK, cyclin dependent kinase.
However, the roles and mechanisms of mtlncRNAs in tumors, especially tumor metabolism and other functions, have not been unraveled completely; as such, further research is indisputably needed, which will lay the foundation for the reasonable clinical application of mtlncRNA.
8. Summary and outlook
Although the future is bright, some concerns remain about the possible adverse effects of gene therapy targeting lncRNAs as it is still an emerging concept and strategy compared with the traditional drug targets and proteins. The greatest risk is that basic research on the function of druggable lncRNAs and the potential downstream effects is insufficient currently; therefore, unexpected risks and inappropriate pathological effects may occur when lncRNA-targeted drugs are used clinically. Specifically, off-target effects may lead to adverse effects. Highly specific targeting approaches and delivery systems require improvement to guarantee that only the selected lncRNA is affected. Owing to the lack of sufficient data on clinical trials, the efficacy and safety of lncRNA drugs in humans remain inconclusive.
Target selection is a key element of drug development; therefore, identifying the most potential lncRNAs is the first step and the most important process. All the possible interactions between druggable lncRNAs and the intracellular miRNAs or proteins they regulated should be elucidated to clarify their role in the physiological activity in vitro and in vivo. Further advances in lncRNA-targeted drugs are clearly dependent on the in-depth basic research into the function and mechanisms of lncRNAs. Of course, accurate approaches to the lncRNAs targeting and efficient delivery systems can significantly speed up this progress.
LncRNA-targeted drug design provides a plethora of opportunities and challenges for the drug industry. Current gene therapy aims to treat disease by artificially controlling gene expression and is considered to be the “third generation” of therapeutic drugs, following chemical small-molecule drugs and biological macromolecular drugs. This type of therapy is promising for modulating diseases at the genetic level, and able to overcome the limitations of incompatible proteins. From the perspective of the clinical breakthrough in targeted mRNA drugs and the cumulative recruitment for clinical trials of miRNAs, it is conceivable that targeting lncRNAs will likely play a pivotal role in gene therapy in the near future, enabling new options for precision medicine.
Acknowledgments
This work was supported by the Drug Innovation Major Project of China (Grant No. 2018ZX09711001-002-010); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (Grant No. 2016-I2M-3–011, China); and Beijing Natural Science Foundation (Grant Nos. 7202138 and 7181007, China).
Author contributions
Yuanyuan Chen collected literature and wrote this review under the guidance of Sen Zhang. Zhaojun Li and Xiaoguang Chen helped to revise the manuscript.
Conflicts of interest
The authors declare no conflicts of competitive interest.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Xiaoguang Chen, Email: chxg@imm.ac.cn.
Sen Zhang, Email: zhangs@imm.ac.cn.
References
- 1.Dahariya S., Paddibhatla I., Kumar S., Raghuwanshi S., Pallepati A., Gutti R.K. Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol. 2019;112:82–92. doi: 10.1016/j.molimm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzen J.M., Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol. 2016;12:360–373. doi: 10.1038/nrneph.2016.51. [DOI] [PubMed] [Google Scholar]
- 3.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson B.R., Makarewich C.A., Anderson D.M., Winders B.R., Troupes C.D., Wu F. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–275. doi: 10.1126/science.aad4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J.Z., Chen M., Chen D., Gao X.C., Zhu S., Huang H. A peptide encoded by a putative lncRNA HOXB-AS3 suppresses colon cancer growth. Mol Cell. 2017;68:171–184.e6. doi: 10.1016/j.molcel.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Choi S.W., Kim H.W., Nam J.W. The small peptide world in long noncoding RNAs. Briefings Bioinf. 2019;20:1853–1864. doi: 10.1093/bib/bby055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto A., Pasut A., Matsumoto M., Yamashita R., Fung J., Monteleone E. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017;541:228–232. doi: 10.1038/nature21034. [DOI] [PubMed] [Google Scholar]
- 8.Marchese F.P., Raimondi I., Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salviano-Silva A., Lobo-Alves S.C., Almeida R.C., Malheiros D., Petzl-Erler M.L. Besides pathology: long non-coding RNA in cell and tissue homeostasis. Noncoding RNA. 2018;4:3. doi: 10.3390/ncrna4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes J.C.R., Acuna S.M., Aoki J.I., Floeter-Winter L.M., Muxel S.M. Long non-coding RNAs in the regulation of gene expression: physiology and disease. Noncoding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matouk I.J., Mezan S., Mizrahi A., Ohana P., Abu-Lail R., Fellig Y. The oncofetal H19 RNA connection: hypoxia, p53 and cancer. Biochim Biophys Acta. 2010;1803:443–451. doi: 10.1016/j.bbamcr.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Jia P., Cai H., Liu X., Chen J., Ma J., Wang P. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Canc Lett. 2016;381:359–369. doi: 10.1016/j.canlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Luo M., Li Z., Wang W., Zeng Y., Liu Z., Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Canc Lett. 2013;333:213–221. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Chen X., Gao G., Liu S., Yu L., Yan D., Yao X. Long noncoding RNA PVT1 as a novel diagnostic biomarker and therapeutic target for melanoma. BioMed Res Int. 2017;2017:7038579. doi: 10.1155/2017/7038579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu S., Shuai P., Yang C., Zhang Y., Zhong S., Liu X. Prognostic value of long non-coding RNA PVT1 as a novel biomarker in various cancers: a meta-analysis. Oncotarget. 2017;8:113174. doi: 10.18632/oncotarget.22830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin K., Wang S., Zhang Y., Xia M., Mo Y., Li X. Long non-coding RNA PVT1 interacts with MYC and its downstream molecules to synergistically promote tumorigenesis. Cell Mol Life Sci. 2019;76:4275–4289. doi: 10.1007/s00018-019-03222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ping G., Xiong W., Zhang L., Li Y., Zhang Y., Zhao Y. Silencing long noncoding RNA PVT1 inhibits tumorigenesis and cisplatin resistance of colorectal cancer. Am J Transl Res. 2018;10:138. [PMC free article] [PubMed] [Google Scholar]
- 18.Shin V.Y., Chen J., Cheuk I.W., Siu M.T., Ho C.W., Wang X. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019;10:270. doi: 10.1038/s41419-019-1513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang D., Chen J., Yang L., Ouyang Q., Li J., Lao L. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat Immunol. 2018;19:1112. doi: 10.1038/s41590-018-0207-y. [DOI] [PubMed] [Google Scholar]
- 20.Chen F., Chen J., Yang L., Liu J., Zhang X., Zhang Y. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21:498. doi: 10.1038/s41556-019-0299-0. [DOI] [PubMed] [Google Scholar]
- 21.Arun G., Diermeier S.D., Spector D.L. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24:257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Fang Z., Hong M., Yang D., Xie W. Long-noncoding RNAs (lncRNAs) in drug metabolism and disposition, implications in cancer chemo-resistance. Acta Pharm Sin B. 2020;10:105–112. doi: 10.1016/j.apsb.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L., Zhuang Y., Zhao X., Li X. Long non-coding RNA in neuronal development and neurological disorders. Front Genet. 2018;9:744. doi: 10.3389/fgene.2018.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma P., Li Y., Zhang W., Fang F., Sun J., Liu M. Long non-coding RNA MALAT1 inhibits neuron apoptosis and neuroinflammation while stimulates neurite outgrowth and its correlation with miR-125b mediates PTGS2, CDK5 and FOXQ1 in Alzheimer's disease. Curr Alzheimer Res. 2019;16:596–612. doi: 10.2174/1567205016666190725130134. [DOI] [PubMed] [Google Scholar]
- 25.Wan P., Su W., Zhang Y., Li Z., Deng C., Li J. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ. 2020;27:176–191. doi: 10.1038/s41418-019-0351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo C.C., Jiao C.H., Gao Z.M. Silencing of LncRNA BDNF-AS attenuates Aβ25-35-induced neurotoxicity in PC12 cells by suppressing cell apoptosis and oxidative stress. Neurol Res. 2018;40:795–804. doi: 10.1080/01616412.2018.1480921. [DOI] [PubMed] [Google Scholar]
- 27.Gu C., Chen C., Wu R., Dong T., Hu X., Yao Y. Long noncoding RNA EBF3-AS promotes neuron apoptosis in Alzheimer's disease. DNA Cell Biol. 2018;37:220–226. doi: 10.1089/dna.2017.4012. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Du W., Yang B. Long non-coding RNAs as new regulators of cardiac electrophysiology and arrhythmias: molecular mechanisms, therapeutic implications and challenges. Pharmacol Ther. 2019;203:107389. doi: 10.1016/j.pharmthera.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Cai B., Zhang Y., Zhao Y., Wang J., Li T., Zhang Y. Long noncoding RNA-DACH1 (dachshund homolog 1) regulates cardiac function by inhibiting SERCA2a (sarcoplasmic reticulum calcium ATPase 2a) Hypertension. 2019;74:833–842. doi: 10.1161/HYPERTENSIONAHA.119.12998. [DOI] [PubMed] [Google Scholar]
- 30.Liu S., Zhao M., Zhou Y., Wang C., Yuan Y., Li L. Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: a potential risk to individuals with impaired kidney function. Phytomedicine. 2019;57:223–235. doi: 10.1016/j.phymed.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Yin D., Zhang E., You L., Wang N., Wang L., Jin F. Downregulation of lncRNA TUG1 affects apoptosis and insulin secretion in mouse pancreatic β cells. Cell Physiol Biochem. 2015;35:1892–1904. doi: 10.1159/000373999. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X., Wu Y.B., Zhou J., Kang D.M. Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochem Biophys Res Commun. 2016;469:319–325. doi: 10.1016/j.bbrc.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 33.Koye D.N., Magliano D.J., Nelson R.G., Pavkov M.E. The global epidemiology of diabetes and kidney disease. Adv Chron Kidney Dis. 2018;25:121–132. doi: 10.1053/j.ackd.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu B., Qiang L., Wang G., Duan Q., Liu J. LncRNA MALAT1 facilities high glucose induced endothelial to mesenchymal transition and fibrosis via targeting miR-145/ZEB2 axis. Eur Rev Med Pharmacol Sci. 2019;23:3478–3486. doi: 10.26355/eurrev_201904_17713. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez M.L., DiStefano J.K. Functional characterization of the plasmacytoma variant translocation 1 gene (PVT1) in diabetic nephropathy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas A.A., Feng B., Chakrabarti S. ANRIL regulates production of extracellular matrix proteins and vasoactive factors in diabetic complications. Am J Physiol Endocrinol Metab. 2018;314:E191–E200. doi: 10.1152/ajpendo.00268.2017. [DOI] [PubMed] [Google Scholar]
- 37.Sun S.F., Tang P.M.K., Feng M., Xiao J., Huang X.R., Li P. Novel lncRNA Erbb4-IR promotes diabetic kidney injury in db/db mice by targeting miR-29b. Diabetes. 2018;67:731–744. doi: 10.2337/db17-0816. [DOI] [PubMed] [Google Scholar]
- 38.Khorkova O., Wahlestedt C. Oligonucleotide therapies for disorders of the nervous system. Nat Biotechnol. 2017;35:249–263. doi: 10.1038/nbt.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang Y., Chen X., Wu Y., Li J., Zhang S., Wang K. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ. 2018;25:1980–1995. doi: 10.1038/s41418-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaidya A.M., Sun Z., Ayat N., Schilb A., Liu X., Jiang H. Systemic delivery of tumor-targeting siRNA nanoparticles against an oncogenic lncRNA facilitates effective triple-negative breast cancer therapy. Bioconjugate Chem. 2019;30:907–919. doi: 10.1021/acs.bioconjchem.9b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang H., Ren Y., Li H., Chen C., Zheng X. LncRNAs linc00311 and AK141205 are identified as new regulators in STAT3-mediated neuropathic pain in bCCI rats. Eur J Pharmacol. 2020;868:172880. doi: 10.1016/j.ejphar.2019.172880. [DOI] [PubMed] [Google Scholar]
- 42.Xu L., Wei B., Hui H., Sun Y., Liu Y., Yu X. Positive feedback loop of lncRNA LINC01296/miR-598/Twist1 promotes non-small cell lung cancer tumorigenesis. J Cell Physiol. 2019;234:4563–4571. doi: 10.1002/jcp.27235. [DOI] [PubMed] [Google Scholar]
- 43.Iribe H., Miyamoto K., Takahashi T., Kobayashi Y., Leo J., Aida M. Chemical modification of the siRNA seed region suppresses off-target effects by steric hindrance to base-pairing with targets. ACS Omega. 2017;2:2055–2064. doi: 10.1021/acsomega.7b00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson A.L., Linsley P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 45.Caffrey D.R., Zhao J., Song Z., Schaffer M.E., Haney S.A., Subramanian R.R. siRNA off-target effects can be reduced at concentrations that match their individual potency. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crooke S.T. Molecular mechanisms of antisense oligonucleotides. Nucleic Acid Therapeut. 2017;27:70–77. doi: 10.1089/nat.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stessl M., Marchetti-Deschmann M., Winkler J., Lachmann B., Allmaier G., Noe C.R. A proteomic study reveals unspecific apoptosis induction and reduction of glycolytic enzymes by the phosphorothioate antisense oligonucleotide oblimersen in human melanoma cells. J Proteomics. 2009;72:1019–1030. doi: 10.1016/j.jprot.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Winkler J., Stessl M., Amartey J., Noe C.R. Off-target effects related to the phosphorothioate modification of nucleic acids. ChemMedChem. 2010;5:1344–1352. doi: 10.1002/cmdc.201000156. [DOI] [PubMed] [Google Scholar]
- 49.McKay R.A., Miraglia L.J., Cummins L.L., Owens S.R., Sasmor H., Dean N.M. Characterization of a potent and specific class of antisense oligonucleotide inhibitor of human protein kinase C-α expression. J Biol Chem. 1999;274:1715–1722. doi: 10.1074/jbc.274.3.1715. [DOI] [PubMed] [Google Scholar]
- 50.Lubini P., Zürcher W., Egli M. Stabilizing effects of the RNA 2′-substituent: crystal structure of an oligodeoxynucleotide duplex containing 2′-O-methylated adenosines. Chem Biol. 1994;1:39–45. doi: 10.1016/1074-5521(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 51.Henry S., Stecker K., Brooks D., Monteith D., Conklin B., Bennett C.F. Chemically modified oligonucleotides exhibit decreased immune stimulation in mice. J Pharmacol Exp Therapeut. 2000;292:468–479. [PubMed] [Google Scholar]
- 52.Crooke S.T. Progress in antisense technology. Annu Rev Med. 2004;55:61–95. doi: 10.1146/annurev.med.55.091902.104408. [DOI] [PubMed] [Google Scholar]
- 53.Khvorova A., Watts J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol. 2017;35:238–248. doi: 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rinaldi C., Wood M.J.A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14:9–21. doi: 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 55.Burel S.A., Hart C.E., Cauntay P., Hsiao J., Machemer T., Katz M. Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts. Nucleic Acids Res. 2016;44:2093–2109. doi: 10.1093/nar/gkv1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arun G., Diermeier S., Akerman M., Chang K.C., Wilkinson J.E., Hearn S. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ray A., Norden B. Peptide nucleic acid (PNA): its medical and biotechnical applications and promise for the future. FASEB J. 2000;14:1041–1060. doi: 10.1096/fasebj.14.9.1041. [DOI] [PubMed] [Google Scholar]
- 58.Adams B.D., Parsons C., Walker L., Zhang W.C., Slack F.J. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127:761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozes A.R., Wang Y., Zong X., Fang F., Pilrose J., Nephew K.P. Therapeutic targeting using tumor specific peptides inhibits long non-coding RNA HOTAIR activity in ovarian and breast cancer. Sci Rep. 2017;7:894. doi: 10.1038/s41598-017-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai J., Ozen A., Mouritzen P., Tolstrup N., Frandsen N.M. Potent knock down of lncRNAs in vitro and in vivo with antisense LNA™ GapmeRs. Cancer Res. 2016;76(6 Suppl) In: Proceedings of the AACR special conference on noncoding RNAs and cancer: mechanisms to medicines; 2015 Dec 4–7; Boston, MA, USA. Philadelphia (PA): AACR. Abstract nr PR14. [Google Scholar]
- 61.Taiana E., Favasuli V., Ronchetti D., Todoerti K., Pelizzoni F., Manzoni M. Long non-coding RNA NEAT1 targeting impairs the DNA repair machinery and triggers anti-tumor activity in multiple myeloma. Leukemia. 2020;34:234–244. doi: 10.1038/s41375-019-0542-5. [DOI] [PubMed] [Google Scholar]
- 62.Amodio N., Stamato M.A., Juli G., Morelli E., Fulciniti M., Manzoni M. Drugging the lncRNA MALAT1 via LNA gapmeR ASO inhibits gene expression of proteasome subunits and triggers anti-multiple myeloma activity. Leukemia. 2018;32:1948–1957. doi: 10.1038/s41375-018-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y., Ye X., Cai M., Liu X., Xiao J., Zhang C. Osteoblast-targeting-peptide modified nanoparticle for siRNA/microRNA delivery. ACS Nano. 2016;10:5759–5768. doi: 10.1021/acsnano.5b07828. [DOI] [PubMed] [Google Scholar]
- 64.Sun Y., Cai M., Zhong J., Yang L., Xiao J., Jin F. The long noncoding RNA lnc-ob1 facilitates bone formation by upregulating Osterix in osteoblasts. Nat Metab. 2019;1:485–496. doi: 10.1038/s42255-019-0053-8. [DOI] [PubMed] [Google Scholar]
- 65.Viney N.J., van Capelleveen J.C., Geary R.S., Xia S., Tami J.A., Rosie Z.Y. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 66.Smith C., Gore A., Yan W., Abalde-Atristain L., Li Z., He C. Whole-genome sequencing analysis reveals high specificity of CRISPR/Cas9 and TALEN-based genome editing in human iPSCs. Cell Stem Cell. 2014;15:12–13. doi: 10.1016/j.stem.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X., Homma A., Sayadi J., Yang S., Ohashi J., Takumi T. Sequence features associated with the cleavage efficiency of CRISPR/Cas9 system. Sci Rep. 2016;6:19675. doi: 10.1038/srep19675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kopp F., Elguindy M.M., Yalvac M.E., Zhang H., Chen B., Gillett F.A. PUMILIO hyperactivity drives premature aging of Norad-deficient mice. Elife. 2019;8 doi: 10.7554/eLife.42650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esposito R., Bosch N., Lanzós A., Polidori T., Pulido-Quetglas C., Johnson R. Hacking the cancer genome: profiling therapeutically actionable long non-coding RNAs using CRISPR-Cas9 screening. Canc Cell. 2019;35:545–557. doi: 10.1016/j.ccell.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 70.Yao J., Kong D., Ye C., Chen R., Li L., Zeng T. The long noncoding RNA TTTY15, which is located on the Y chromosome, promotes prostate cancer progression by sponging let-7. Eur Urol. 2019;76:315–326. doi: 10.1016/j.eururo.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Chen Q., Cai J., Wang Q., Wang Y., Liu M., Yang J. Long noncoding RNA NEAT1, regulated by the EGFR pathway, contributes to glioblastoma progression through the WNT/beta-catenin pathway by scaffolding EZH2. Clin Canc Res. 2018;24:684–695. doi: 10.1158/1078-0432.CCR-17-0605. [DOI] [PubMed] [Google Scholar]
- 72.Goyal A., Myacheva K., Gross M., Klingenberg M., Duran Arque B., Diederichs S. Challenges of CRISPR/Cas9 applications for long non-coding RNA genes. Nucleic Acids Res. 2017;45:e12. doi: 10.1093/nar/gkw883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klein M., Eslami-Mossallam B., Arroyo D.G., Depken M. Hybridization kinetics explains CRISPR-Cas off-targeting rules. Cell Rep. 2018;22:1413–1423. doi: 10.1016/j.celrep.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 74.Zuo E., Sun Y., Wei W., Yuan T., Ying W., Sun H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science. 2019;364:289–292. doi: 10.1126/science.aav9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slaymaker I.M., Gao L., Zetsche B., Scott D.A., Yan W.X., Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kocak D.D., Josephs E.A., Bhandarkar V., Adkar S.S., Kwon J.B., Gersbach C.A. Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat Biotechnol. 2019;37:657–666. doi: 10.1038/s41587-019-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu Y., Sander J.D., Reyon D., Cascio V.M., Joung J.K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32:279. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhan T., Rindtorff N., Betge J., Ebert M.P., Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Canc Biol. 2019;55:106–119. doi: 10.1016/j.semcancer.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Li H., Chen C., Fan J., Yin Z., Ni L., Cianflone K. Identification of cardiac long non-coding RNA profile in human dilated cardiomyopathy. Cardiovasc Res. 2018;114:747–758. doi: 10.1093/cvr/cvy012. [DOI] [PubMed] [Google Scholar]
- 80.Li W., Dong X., He C., Tan G., Li Z., Zhai B. LncRNA SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by miR-21 in hepatocellular carcinoma cells. J Exp Clin Canc Res. 2019;38:183. doi: 10.1186/s13046-019-1177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tao S.C., Rui B.Y., Wang Q.Y., Zhou D., Zhang Y., Guo S.C. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25:241–255. doi: 10.1080/10717544.2018.1425774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J., Zhang X., Chen K., Cheng Y., Liu S., Xia M. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity. 2019;50:600–615. doi: 10.1016/j.immuni.2019.01.021. e15. [DOI] [PubMed] [Google Scholar]
- 83.Pedram Fatemi R., Salah-Uddin S., Modarresi F., Khoury N., Wahlestedt C., Faghihi M.A. Screening for small-molecule modulators of long noncoding RNA–protein interactions using AlphaScreen. J Biomol Screen. 2015;20:1132–1141. doi: 10.1177/1087057115594187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amodio N., Raimondi L., Juli G., Stamato M.A., Caracciolo D., Tagliaferri P. MALAT1: a druggable long non-coding RNA for targeted anti-cancer approaches. J Hematol Oncol. 2018;11:63. doi: 10.1186/s13045-018-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen T.M., Kabotyanski E.B., Reineke L.C., Shao J., Xiong F., Lee J.H. The SINEB1 element in the long non-coding RNA Malat1 is necessary for TDP-43 proteostasis. Nucleic Acids Res. 2019;48:2621–2642. doi: 10.1093/nar/gkz1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ageeli A.A., McGovern-Gooch K.R., Kaminska M.M., Baird N.J. Finely tuned conformational dynamics regulate the protective function of the lncRNA MALAT1 triple helix. Nucleic Acids Res. 2019;47:1468–1481. doi: 10.1093/nar/gky1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCown P.J., Wang M.C., Jaeger L., Brown J.A. Secondary structural model of human MALAT1 reveals multiple structure-function relationships. Int J Mol Sci. 2019;20:5610. doi: 10.3390/ijms20225610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yonkunas M.J., Baird N.J. A highly ordered, nonprotective MALAT1 ENE structure is adopted prior to triplex formation. RNA. 2019;25:975–984. doi: 10.1261/rna.069906.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donlic A., Morgan B.S., Xu J.L., Liu A., Roble C., Jr., Hargrove A.E. Discovery of small molecule ligands for MALAT1 by tuning an RNA-binding scaffold. Angew Chem Int Ed Engl. 2018;57:13242–13247. doi: 10.1002/anie.201808823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abulwerdi F.A., Xu W., Ageeli A.A., Yonkunas M.J., Arun G., Nam H. Selective small-molecule targeting of a triple helix encoded by the long noncoding RNA, MALAT1. ACS Chem Biol. 2019;14:223–235. doi: 10.1021/acschembio.8b00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie C., Wu W., Tang A., Luo N., Tan Y. LncRNA GAS5/miR-452-5p reduces oxidative stress and pyroptosis of high-glucose-stimulated renal tubular cells. Diabetes Metab Syndr Obes. 2019;12:2609–2617. doi: 10.2147/DMSO.S228654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y., Liu Y., Liu H., Tang W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2020;34:2703–2714. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang L., Zhao S.Q., Zhu Y.F. Long noncoding RNA growth arrest-specific transcript 5 alleviates renal fibrosis in diabetic nephropathy by downregulating matrix metalloproteinase 9 through recruitment of enhancer of zeste homolog 2. FASEB J. 2020;34:2703–2714. doi: 10.1096/fj.201901380RR. [DOI] [PubMed] [Google Scholar]
- 94.Shi Y., Parag S., Patel R., Lui A., Murr M., Cai J. Stabilization of lncRNA GAS5 by a small molecule and its implications in diabetic adipocytes. Cell Chem Biol. 2019;26:319–330.e6. doi: 10.1016/j.chembiol.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mercatelli N., Fortini D., Palombo R., Paronetto M.P. Small molecule inhibition of Ewing sarcoma cell growth via targeting the long non coding RNA HULC. Canc Lett. 2020;469:111–123. doi: 10.1016/j.canlet.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 96.Engreitz J.M., Sirokman K., McDonel P., Shishkin A.A., Surka C., Russell P. RNA–RNA interactions enable specific targeting of noncoding RNAs to nascent pre-mRNAs and chromatin sites. Cell. 2014;159:188–199. doi: 10.1016/j.cell.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warner K.D., Hajdin C.E., Weeks K.M. Principles for targeting RNA with drug-like small molecules. Nat Rev Drug Discov. 2018;17:547–558. doi: 10.1038/nrd.2018.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mishra S., Verma S.S., Rai V., Awasthee N., Chava S., Hui K.M. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol Life Sci. 2019;76:1947–1966. doi: 10.1007/s00018-019-03053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xia D., Sui R., Zhang Z. Administration of resveratrol improved Parkinson's disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J Cell Biochem. 2019;120:4942–4951. doi: 10.1002/jcb.27769. [DOI] [PubMed] [Google Scholar]
- 100.Saghafi T., Taheri R.A., Parkkila S., Emameh R.Z. Phytochemicals as modulators of long non-coding RNAs and inhibitors of cancer-related carbonic anhydrases. Int J Mol Sci. 2019;20:2939. doi: 10.3390/ijms20122939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang S., Xin H., Li Y., Zhang D., Shi J., Yang J. Skimmin, a coumarin from hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti-inflammatory effects and inhibiting immune complex deposition. Evid Based Complement Alternat Med. 2013;2013:819296. doi: 10.1155/2013/819296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geng W., Guo X., Zhang L., Ma Y., Wang L., Liu Z. Resveratrol inhibits proliferation, migration and invasion of multiple myeloma cells via NEAT1-mediated Wnt/beta-catenin signaling pathway. Biomed Pharmacother. 2018;107:484–494. doi: 10.1016/j.biopha.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 103.Liu G., Xiang T., Wu Q.F., Wang W.X. Curcumin suppresses the proliferation of gastric cancer cells by downregulating H19. Oncol Lett. 2016;12:5156–5162. doi: 10.3892/ol.2016.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dai W., Li S.Y., Xiao D.J., Liu C., He J.H., Lin Y. Curcumin combining with si-MALAT1 inhibits the invasion and migration of colon cancer SW480 cells. Brazilian J Pharm Sci. 2019;55 [Google Scholar]
- 105.Yuan X., Wang J., Tang X., Li Y., Xia P., Gao X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med. 2015;13:24. doi: 10.1186/s12967-015-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang L., Li Y., Gong R., Gao M., Feng C., Liu T. The long non-coding RNA-ORLNC1 regulates bone mass by directing mesenchymal stem cell fate. Mol Ther. 2019;27:394–410. doi: 10.1016/j.ymthe.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li S., Zhao H., Li J., Zhang A., Wang H. Downregulation of long non-coding RNA LET predicts poor prognosis and increases Notch signaling in non-small cell lung cancer. Oncotarget. 2018;9:1156. doi: 10.18632/oncotarget.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruan X., Li P., Cangelosi A., Yang L., Cao H. A long non-coding RNA, lncLGR, regulates hepatic glucokinase expression and glycogen storage during fasting. Cell Rep. 2016;14:1867–1875. doi: 10.1016/j.celrep.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Hammadi M.M., Arias J.L. An update on liposomes in drug delivery: a patent review (2014–2018) Expert Opin Ther Pat. 2019;29:891–907. doi: 10.1080/13543776.2019.1679767. [DOI] [PubMed] [Google Scholar]
- 110.Yu Q., Qiu Y., Wang X., Tang J., Liu Y., Mei L. Efficient siRNA transfer to knockdown a placenta specific lncRNA using RGD-modified nano-liposome: a new preeclampsia-like mouse model. Int J Pharm. 2018;546:115–124. doi: 10.1016/j.ijpharm.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 111.Chang L., Wang G., Jia T., Zhang L., Li Y., Han Y. Armored long non-coding RNA MEG3 targeting EGFR based on recombinant MS2 bacteriophage virus-like particles against hepatocellular carcinoma. Oncotarget. 2016;7:23988–24004. doi: 10.18632/oncotarget.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Z.C., Tang C., Dong Y., Zhang J., Yuan T., Li X.L. Targeting LncRNA-MALAT1 suppresses the progression of osteosarcoma by altering the expression and localization of beta-catenin. J Canc. 2018;9:71–80. doi: 10.7150/jca.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhen S., Li X. Application of CRISPR-Cas9 for long noncoding RNA genes in cancer research. Hum Gene Ther. 2019;30:3–9. doi: 10.1089/hum.2018.063. [DOI] [PubMed] [Google Scholar]
- 114.Yu Q., Zhang B., Zhou Y., Ge Q., Chang J., Chen Y. Co-delivery of gambogenic acid and VEGF-siRNA with anionic liposome and polyethylenimine complexes to HepG2 cells. J Liposome Res. 2019;29:322–331. doi: 10.1080/08982104.2018.1473423. [DOI] [PubMed] [Google Scholar]
- 115.Sato Y., Hashiba K., Sasaki K., Maeki M., Tokeshi M., Harashima H. Understanding structure–activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo. J Control Release. 2019;295:140–152. doi: 10.1016/j.jconrel.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 116.Sato Y., Okabe N., Note Y., Hashiba K., Maeki M., Tokeshi M. Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta Biomater. 2020;102:341–350. doi: 10.1016/j.actbio.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 117.Yang J. Patisiran for the treatment of hereditary transthyretin-mediated amyloidosis. Expet Rev Clin Pharmacol. 2019;12:95–99. doi: 10.1080/17512433.2019.1567326. [DOI] [PubMed] [Google Scholar]
- 118.Guo Q., Zheng X., Yang P., Pang X., Qian K., Wang P. Small interfering RNA delivery to the neurons near the amyloid plaques for improved treatment of Alzheimer's disease. Acta Pharm Sin B. 2019;9:590–603. doi: 10.1016/j.apsb.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gong N., Teng X., Li J., Liang X.J. Antisense oligonucleotide-conjugated nanostructure-targeting lncRNA MALAT1 inhibits cancer metastasis. ACS Appl Mater Interfaces. 2019;11:37–42. doi: 10.1021/acsami.8b18288. [DOI] [PubMed] [Google Scholar]
- 120.Song H.Q., Pan W., Li R.Q., Yu B., Liu W., Yang M. Rodlike supramolecular nanoassemblies of degradable poly(aspartic acid) derivatives and hydroxyl-rich polycations for effective delivery of versatile tumor-suppressive ncRNAs. Small. 2018;14:1703152. doi: 10.1002/smll.201703152. [DOI] [PubMed] [Google Scholar]
- 121.Das M., Renganathan A., Dighe S.N., Bhaduri U., Shettar A., Mukherjee G. DDX5/p68 associated lncRNA LOC284454 is differentially expressed in human cancers and modulates gene expression. RNA Biol. 2018;15:214–230. doi: 10.1080/15476286.2017.1397261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Uchida S., Kataoka K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J Biomed Mater Res. 2019;107:978–990. doi: 10.1002/jbm.a.36614. [DOI] [PubMed] [Google Scholar]
- 123.Dirisala A., Uchida S., Tockary T.A., Yoshinaga N., Li J., Osawa S. Precise tuning of disulphide crosslinking in mRNA polyplex micelles for optimising extracellular and intracellular nuclease tolerability. J Drug Target. 2019;27:670–680. doi: 10.1080/1061186X.2018.1550646. [DOI] [PubMed] [Google Scholar]
- 124.Musacchio T., Torchilin V.P. Polymeric biomaterials. CRC Press; Boca Raton: 2019. Advances in polymeric and lipid-core micelles as drug delivery systems; pp. 84–103. [Google Scholar]
- 125.Dormenval C., Lokras A., Cano-Garcia G., Wadhwa A., Thanki K., Rose F. Identification of factors of importance for spray drying of small interfering RNA-loaded lipidoid-polymer hybrid nanoparticles for inhalation. Pharm Res (N Y) 2019;36:142. doi: 10.1007/s11095-019-2663-y. [DOI] [PubMed] [Google Scholar]
- 126.Halman J.R., Kim K.T., Gwak S.J., Pace R., Johnson M.B., Chandler M.R. A cationic amphiphilic co-polymer as a carrier of nucleic acid nanoparticles (Nanps) for controlled gene silencing, immunostimulation, and biodistribution. Nanomedicine. 2020;23:102094. doi: 10.1016/j.nano.2019.102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ren Y., Li R.Q., Cai Y.R., Xia T., Yang M., Xu F.J. Effective codelivery of lncRNA and pDNA by pullulan-based nanovectors for promising therapy of hepatocellular carcinoma. Adv Funct Mater. 2016;26:7314–7325. [Google Scholar]
- 128.Pegtel D.M., Gould S.J. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 129.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 131.Haney M.J., Klyachko N.L., Zhao Y.L., Gupta R., Plotnikova E.G., He Z.J. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tao S.C., Guo S.C., Zhang C.Q. Modularized extracellular vesicles: the dawn of prospective personalized and precision medicine. Adv Sci (Weinh) 2018;5:1700449. doi: 10.1002/advs.201700449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L. Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh) 2018;5:1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Richardson J.J., Ejima H. Surface engineering of extracellular vesicles through chemical and biological strategies. Chem Mater. 2019;31:2191–2201. [Google Scholar]
- 135.Kim G., Kim M., Lee Y., Byun J.W., Hwang D.W., Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Control Release. 2020;317:273–281. doi: 10.1016/j.jconrel.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 136.Sancho-Albero M., Encabo-Berzosa M.D., Beltrán-Visiedo M., Fernández-Messina L., Sebastián V., Sánchez-Madrid F. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11:18825–18836. doi: 10.1039/c9nr06183e. [DOI] [PubMed] [Google Scholar]
- 137.Lung B., Zemann A., Madej M.J., Schuelke M., Techritz S., Ruf S. Identification of small non-coding RNAs from mitochondria and chloroplasts. Nucleic Acids Res. 2006;34:3842–3852. doi: 10.1093/nar/gkl448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olavarria J.V., Burzio V.A., Borgna V., Lobos-Gonzalez L., Araya M., Guevara F. Mitochondrial DNA—new insights. IntechOpen; London: 2018. Long noncoding mitochondrial RNAs (LncmtRNAs) as targets for cancer therapy; pp. 179–188. [Google Scholar]
- 139.Kumarswamy R., Bauters C., Volkmann I., Maury F., Fetisch J., Holzmann A. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res. 2014;114:1569–1575. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 140.Burzio V.A., Villota C., Villegas J., Landerer E., Boccardo E., Villa L.L. Expression of a family of noncoding mitochondrial RNAs distinguishes normal from cancer cells. Proc Natl Acad Sci U S A. 2009;106:9430–9434. doi: 10.1073/pnas.0903086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lobos-González L., Silva V., Araya M., Restovic F., Echenique J., Oliveira-Cruz L. Targeting antisense mitochondrial ncRNAs inhibits murine melanoma tumor growth and metastasis through reduction in survival and invasion factors. Oncotarget. 2016;7:58331–58350. doi: 10.18632/oncotarget.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Varas-Godoy M., Lladser A., Farfan N., Villota C., Villegas J., Tapia J.C. In vivo knockdown of antisense non-coding mitochondrial RNAs by a lentiviral-encoded shRNA inhibits melanoma tumor growth and lung colonization. Pigment Cell Melanoma Res. 2018;31:64–72. doi: 10.1111/pcmr.12615. [DOI] [PubMed] [Google Scholar]
- 143.Borgna V., Villegas J., Burzio V.A., Belmar S., Araya M., Jeldes E. Mitochondrial ASncmtRNA-1 and ASncmtRNA-2 as potent targets to inhibit tumor growth and metastasis in the RenCa murine renal adenocarcinoma model. Oncotarget. 2017;8:43692–43708. doi: 10.18632/oncotarget.18460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dhawan M.S., Aggarwal R.R., Boyd E., Comerford K., Zhang J., Méndez B. Phase 1 study of ANDES-1537: a novel antisense oligonucleotide against non-coding mitochondrial DNA in advanced solid tumors. J Clin Oncol. 2018;36(suppl 15):2557. [Google Scholar]
- 145.Fitzpatrick C., Bendek M.F., Briones M., Farfan N., Silva V.A., Nardocci G. Mitochondrial ncRNA targeting induces cell cycle arrest and tumor growth inhibition of MDA-MB-231 breast cancer cells through reduction of key cell cycle progression factors. Cell Death Dis. 2019;10:423. doi: 10.1038/s41419-019-1649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]