Abstract
Clear cell renal cell carcinoma (ccRCC) is a common kidney malignancy characterized by a poor prognosis. Suppressor of variegation 3–9 homolog 1 (SUV39H1), which encodes a histone H3 lysine 9 methyltransferase, has been reported to act as an oncogene in many cancers. However, it is unclear whether SUV39H1 is involved in ccRCC. Here, we report that SUV39H1 expression is frequently upregulated in ccRCC tumors and is significantly correlated with ccRCC progression. SUV39H1 expression level is an independent risk factor for cancer prognosis, and integration with several known prognostic factors predicted ccRCC patient prognosis with improved accuracy than the conventional SSIGN (stage, size, grade and necrosis) prognostic model. Mechanistically, we discovered that siRNA knockdown or pharmacological inhibition of SUV39H1 induced iron accumulation and lipid peroxidation, leading to ferroptosis that disrupted ccRCC cell growth in vitro and in vivo. We also show that SUV39H1 deficiency modulated the H3K9me3 status of the DPP4 (dipeptidyl-peptidase-4) gene promoter, resulting in upregulation of its expression that contributes to ferroptosis. Taken together, our findings provide the mechanistic insight into SUV39H1-dependent epigenetic control of ccRCC tumor growth and indicate that SUV39H1 may serve as a potential therapeutic target for ccRCC treatment.
KEY WORDS: Clear cell renal cell carcinoma, SUV39H1, Progression, Prognostic model, Ferroptosis, DPP4, Epigenetics, Therapeutic target
Graphical abstract
SUV39H1 is an epigenetic repressor to suppress DPP4 expression. Function loss of SUV39H1 in ccRCC tumors contributes the hypomethylation of the DPP4 promoter to upregulate DPP4 expression and induces DPP4-mediated ferroptosis to suppress cell proliferation.
1. Introduction
Renal cell carcinoma (RCC) is the most common adult renal cancer originating from renal tubular epithelial cells1. Clear cell renal cell carcinoma (ccRCC) is the major histopathological RCC subtype, and accounts for approximately 75% of all RCC cases2. Localized ccRCC is generally cured by surgery, whereas patients with advanced ccRCC still face limited treatment options due to its intrinsic resistance to conventional chemotherapy or radiotherapy3. Recently, tremendous progress in our understanding of the underlying biology of this tumor type and the discovery of effective drugs have resulted in the development of several therapeutic methods, including immune-checkpoint inhibitors, vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitors and mammalian target of rapamycin (mTOR) inhibitors, which have exhibited promising clinical results4. However, each of these management strategies has specific limitations, such as frequently occurring severe side effects and intrinsic or acquired drug resistance5. Clinicians therefore face a significant decision-making challenge when choosing the most appropriate therapeutic regimen for patients with advanced ccRCC. Thus, an individualized therapeutic regimen is desperately needed to provide hope for patients with advanced ccRCC.
Epigenetic alterations, such as histone modification, DNA methylation and the expression of non-coding RNAs, are closely involved in ccRCC initiation and progression, indicating that targeting the epigenome could be a promising approach to treating ccRCC6,7. Suppressor of variegation 3–9 homolog 1 (SUV39H1), a SET domain-containing histone methyltransferase (HMTase), is responsible for tri-methylation of histone 3 lysine 9 (H3K9me3), which is associated with heterochromatin formation and transcriptional repression of targeted genes8. SUV39H1 is essential for mouse germ cell development and cell cycle regulation9,10. In addition, SUV39H1 has been reported to participate in tumorigenesis in various types of cancer. SUV39H1 is generally regarded as a tumor suppressor, due to its roles in suppressing genes required for proliferation and in promoting senescence11. However, increasing evidence indicates that SUV39H1 may also serve as an oncogene in some human cancers. SUV39H1 expression is upregulated in many cancers, including human colon carcinoma, bladder cancer and hepatocellular carcinoma, compared with nontumor tissues12, 13, 14. SUV39H1 has been reported to function as an oncogene in melanoma via inhibiting the expression of retinoblastoma (RB)15. To date, the role of SUV39H1 in ccRCC progression is still largely unknown.
Ferroptosis, an iron-dependent form of non-apoptotic regulated cell death, was originally identified in cancer cells with oncogenic RAS mutations16. Two central biochemical events, iron accumulation and lipid peroxidation, result in reactive oxygen species (ROS) production and subsequent cell death17. More importantly, ferroptosis has been implicated in the progression of neurodegenerative and neuropsychiatric diseases, ischemia–reperfusion injury and kidney degeneration18. Recent work has also indicated that dysfunction of ferroptosis is closely related to tumor progression. Inducing ferroptosis has emerged as an attractive strategy for managing various types of cancer19. Therefore, factors that regulate ferroptosis are potential therapeutic targets for cancer treatment. Dipeptidyl-peptidase-4 (DPP4, also known as CD26) is a glycoprotein mainly located at the plasma membrane that plays a critical role in regulating ferroptosis. In particular, DPP4 binds to NADPH oxidase 1 (NOX1) to form the DPP4–NOX1 complex, which facilitates intracellular lipid peroxidation and ultimately results in ferroptosis20. Of note, the expression levels of a variety of iron-related genes are significantly associated with ccRCC patient prognosis, suggesting that ferroptosis plays a vital role in ccRCC progression, and that targeting ferroptosis could be an effective option for ccRCC treatment21.
In this study, we examined SUV39H1 expression in ccRCC tumor tissues and analyzed its association with ccRCC progression and prognosis. Further, SUV39H1 expression and SUV39H1 activity were inhibited by genetic knockdown and pharmacological inhibition, respectively, to determine the functional role of SUV39H1 in ccRCC cell growth both in vitro and in vivo. Finally, we investigated the exact mechanism underlying the effects of SUV39H1 on ccRCC tumor growth. Our study provides proof-of-concept that targeting an epigenetic factor could be a promising strategy for ccRCC treatment.
2. Materials and methods
2.1. Patients and tissue samples
Two ccRCC patient cohorts for which clinical information was available were used for the analysis. For the first cohort, ClinicalMatrix and RNA sequencing data (HiSeqV2) for 534 ccRCC patients were obtained from the Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/) data portal. Patients for whom clinical information was missing were excluded when analyzing the relationship between SUV39H1 expression level and clinical characteristics. The median mRNA expression value was considered as the cutoff value for low/high expression of SUV39H1 or DPP4 in ccRCC samples. For the second cohort, SUV39H1 expression in ccRCC tumor and normal tissue samples from 358 patients (for whom follow-up data was available) was analyzed by immunohistochemistry (IHC). Overall survival (OS) was calculated from the time of surgery to the latest follow-up or death for any reason, while recurrence-free survival (RFS) was calculated from the date of nephrectomy to the date of recurrence. Tissues samples collected from ccRCC patients who had undergone nephrectomy were used for mRNA or protein extraction and IHC staining. Written consent was obtained from all the patients for sample collection. This study was approved by the Ethics and Research Committees of Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
2.2. RNA isolation and RT-qPCR
Total RNA was isolated using Trizol and reverse transcribed into cDNA following the manufacturer's instructions. Gene expression levels were measured by reverse transcription quantitative polymerase chain reaction (RT-qPCR) using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) and an ABI ViiA™ 7 System (Thermo Fisher, Waltham, MA, USA). Primers used in our study are listed in Supporting Information Table S1.
2.3. Western blot
Western blot analysis was performed as previously described22. Briefly, after collecting protein lysates in 2% SDS, we measured the protein concentration of each sample and separated the samples on a 7.5%–12.5% SDS-PAGE gel (Bio-Rad Laboratories Inc., Hercules, CA, USA). Next, the proteins were transferred to a membrane, the membrane was blocked and then treated with various antibodies (with washing between steps), and target protein bands were detected by an enhanced chemiluminescence system (GE Healthcare Life Sciences, Chalfont, UK). All antibodies used in this study are listed in Supporting Information Table S2.
2.4. Immunohistochemistry
Tissue microarrays (TMAs) were constructed and stained as previously described23. A primary anti-SUV39H1 antibody was used for IHC staining. Tissue staining intensity and percentage were scored, and a comprehensive score (staining percentage × intensity) was used to evaluate the expression level of SUV39H1. A comprehensive score of 6 was set as the cutoff value to define low/high SUV39H1 expression in ccRCC tissues24.
2.5. Cell culture and chemical reagents
RCC cell lines 786-O, Caki-1, A498, 769-P and ACHN and the normal cell line HK-2 were obtained from the American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in the recommended medium supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco, Australia) at 37 °C with 5% CO2. Chemical reagents, including ferrostatin-1 (CSN12654), z-VAD-FMK (CSN19230), necrostatin 1 (CSN11637), bafilomycin A1 (CSN10374), VX-765 (CSN15837), UNC0638 (CSN16350) and chaetocin (CSN19229) were provided by CSNpharm (Chicago, IL, USA).
2.6. Small interfering RNA (siRNA) transfection
Cells were seeded into plates at an appropriate density. After 12 h, small interfering RNAs (siRNAs, 50–100 nmol/L) and lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) were added, according to the manufacturer's instructions. Western blotting was performed to detect the efficiency of gene knockdown. The sequence-specific siRNAs used to knock down SUV39H1 and DPP4 expression are listed in Supporting Information Table S3.
2.7. Cell proliferation assay
The effects of siRNA transfection and treatment with various compounds on cellular proliferation were determined by SulforhodamineB (SRB) assay according to the manufacturer's instructions. In brief, ccRCC cells were seeded into 96-well plates (1 × 103 cells/well) and incubated overnight at 37 °C. After treatment, cells were fixed, stained, and Soft Max pro plate reader was used to assess the cell viability at 560 nm.
2.8. Colony growth assay
Cells were seeded in 6- or 12- or 24-well plates at an appropriate density and incubated overnight at 37 °C. After managements, colony cell growth was measured at Day 3 or 7, and the colonies were fixed, stained, photographed and counted.
2.9. Cell cycle assay
Cell cycle analysis after siRNAs and drug treatments were performed by flow cytometry (Becton–Dickinson, Franklin Lakes, NJ, USA) as previous reported22. Briefly, cells were collected, fixed, stained with propidium iodide (PI) and analyzed by flow cytometry. The results were analyzed using ModFit software.
2.10. Lentiviral vector construction and transfection
Lentiviral particles were constructed as described25. In brief, a short hairpin RNA (shRNA) homologous to SUV39H1 or a control shRNA was integrated into the pGMLV-SC5 backbone. The shRNA sequences are listed in Supporting Information Table S4. Cells were infected and subjected to puromycin selection before the experiments were performed.
2.11. Animal xenograft models and treatment
Female SCID mice (4–6 weeks old) were purchased and used for the xenograft models. Approximately 5 × 106 ccRCC cells were injected subcutaneously into the flank, or patient-derived tumor xenograft (PDX) was established using human ccRCC tissue (PDX#1002523691) from patients from Renji Hospital, with written patient consent. Tumor volume was estimated as Eq. (1):
| Tumor volume = Length × Width2/2 | (1) |
The mice were treated with vehicle (control) or chaetocin (0.5 mg/kg/day) by daily intraperitoneal injection. Mice body weights and tumor volumes were measured twice a week. After treatment, the tumors were harvested, weighed, photographed and fixed with 4% formaldehyde. Animal studies were performed in accordance with the guidelines of the Experimental Animal Ethics Committee of Shanghai Jiao Tong University (Shanghai, China).
2.12. Lipid ROS assay
Lipid ROS in cells was detected with C11-BODIPY (Cat. #D3861, Thermo Fisher) following the manufacturer's protocols. Briefly, after 2 days of treatment, cells were incubated with 10 μmol/L of C11-BODIPY and incubated in the dark at 37 °C, 5% CO2 for 30 min. After washing three times with PBS, the C11-BODIPY green fluorescence (484 nm/510 nm) was measured with a flow cytometer.
2.13. Mitochondrial superoxide measurement
Mitochondrial superoxide production in ccRCC cells was detected using MitoSOX™ Red Mitochondrial Superoxide Indicator for live-cell imaging (Invitrogen) according to the manufacturer's protocols. After 2 days of treatment, the cells were stained with 5 μmol/L MitoSOX reagent in PBS for 10 min at 37 °C, 5% CO2 in the dark, and then washed three times to remove excess MitoSOX. Cellular fluorescence was examined at an excitation/emission value of 510 nm/580 nm.
2.14. Iron assay
An Iron Assay Kit (Sigma–Aldrich, St. Louis, MO, USA) was used to measure the Fe2+ and total iron content of cells, according to the manufacturer's protocols. After 2 days of treatment, cells (5 × 106) were homogenized in 300 μL of iron assay buffer and centrifuged at 16,000×g for 10 min at 4 °C. Next, 50 μL of the samples were transferred to a 96-well plate, and the volume was brought to 100 μL per well with assay buffer. To measure Fe2+, 5 μL of iron assay buffer was added to each sample. To measure total iron, 5 μL of iron reducer was added to samples. Then, the 96-well plate was incubated for 30 min at room temperature. Next, 100 μL of iron probe was added to each sample, and the samples were then incubated in the dark for 60 min at room temperature. Finally, the absorbance was measured at 593 nm (A593).
2.15. RNA-sequencing analysis
RNA-sequencing (RNA-seq) analysis was performed as described previously26. Briefly, cells were collected, and total RNA was isolated. RNA-seq was performed on the Illumina HiSeq2000, the data were aligned to the UCSC human hg19 genome using STAR 2.5, and hits were quantified using feature count software. The R package tools “DESeq2” and “ClusterProfiler” were used to analyze the RNA-seq data, using a fold change of 2 and an adjusted P value of 0.01 as the cut-off values, respectively.
2.16. Chromatin immunoprecipitation analysis (ChIP)
ChIP analysis was performed using a ChIP Kit from Cell Signal Technology (Cat. No. #9005, Danvers, MA, USA) according to the manufacturer's instructions. The percentage of DPP4 gene promoter copies bound to H3K9me3 was quantified by qPCR using DPP4 promoter-specific primers (Supporting Information Table S5).
2.17. Statistical analysis
Statistical analysis was performed using SPSS 22 software, R software or Graphpad Prism 6.0 (La Jolla, CA, USA). The chi-square function of SPSS 22 was used to investigate the correlations between SUV39H1 expression and clinical features, and the Kaplan–Meier method and log-rank test were used to analyze survival curves. The “rms” tool of R was used to analyze the nomograms, calibration plots, Harrell concordance index (C-index) and Akaike information criteria (AIC). Data was presented as mean ± standard deviation (SD), and a student's t-test was performed to calculate the statistical significance of differences between groups using GraphPad Prism. ∗P < 0.05, **P < 0.01 and ***P < 0.001 were considered significantly different.
3. Results
3.1. SUV39H1 expression is upregulated in ccRCC tumors and correlates with the progression and prognosis
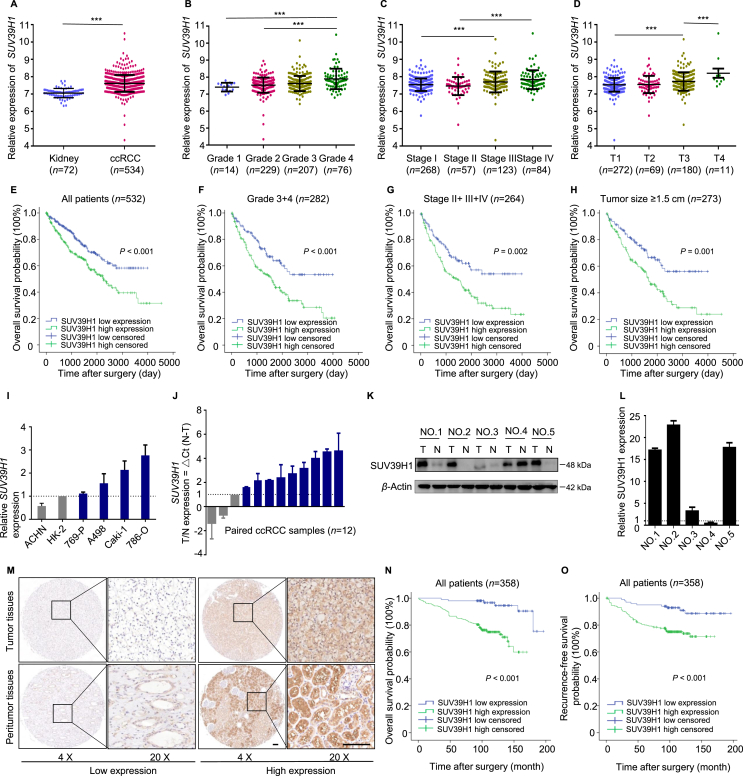
Studies have indicated that SUV39H1 plays a vital role in the progression of multiple cancers12,14. To assess the clinical role of SUV39H1 in ccRCC progression, we first investigated the expression pattern of SUV39H1 in ccRCC by analyzing publicly available data from TCGA. As shown in Fig. 1A, SUV39H1 expression was significantly higher in ccRCC tumor tissues than that in normal tissues. SUV39H1 expression was also correlated with various features of tumor progression, including histological grade, clinical TNM stage, invasion depth (T stage) and tumor size (Fig. 1B–D, Supporting Information Fig. S1A–S1C and Table S6).
Figure 1.
SUV39H1 expression is upregulated in tumors and correlates with the progression and prognosis of ccRCC patients. (A) The mRNA levels of SUV39H1 were analyzed in the ccRCC tissues in TCGA dataset compared to the normal controls. (B)–(D) The mRNA levels of SUV39H1 in different histological grades, clinical TNM stages and T stages of ccRCC tissues. Patients with clinical information missing were excluded. (E) Kaplan–Meier analysis of OS for all ccRCC patients with low or high SUV39H1 expression level in TCGA dataset. (F) Kaplan–Meier analysis of OS for patients with high histological grade (Grades 3+4). (G) Kaplan–Meier analysis of OS for patients with intermediate and advanced tumor stage (Stages II+III+IV). (H) Kaplan–Meier analysis of OS for patients with large tumor size (tumor size ≥ 1.5 cm). (I) RT-qPCR experiment was performed to analyze the SUV39H1 mRNA level in ccRCC cell lines (786-O, Caki-1, A498, 769-P, and ACHN) and the normal kidney cell line HK-2. The expression level of β-actin was used as the normalized control. The experiment was repeated three times and data was presented as mean ± SD. (J) The mRNA level of SUV39H1 was detected in 12 pairs of human ccRCC tumor tissues and adjacent normal tissues. The expression level of β-actin was used as normalized control. The experiment was repeated three times and data was presented as mean ± SD. (K) and (L) Western blot experiment was performed to evaluate the protein level of SUV39H1 in 5 paired ccRCC tissue samples, and β-Actin was used as control. The statistical analysis comes from technical replicates and the experiment was repeated three times. (“T” means tumor tissue and “N” means paired normal tissue). Data was presented as mean ± SD. (M) Representative images of SUV39H1 staining with high or low expression level in ccRCC tumor tissues and peritumor tissues. nTumor tissues = 358, nPeritumor tissues = 233. Scale bar: 100 μm. (N) Kaplan–Meier analysis of ccRCC OS based on SUV39H1 expression level in ccRCC TMAs. n = 358. (O) Kaplan–Meier analysis of ccRCC RFS based on SUV39H1 expression level in ccRCC TMAs. n = 358. ∗∗∗P < 0.001.
A survival analysis was performed to investigate the prognostic value of SUV39H1 expression level in ccRCC, and the results show that patients exhibiting high SUV39H1 expression had significantly shorter OS than those with low SUV39H1 expression (Fig. 1E). Moreover, SUV39H1 expression level had significant prognostic value for ccRCC with a high histological grade (Grades 3+4), intermediate and advanced tumor stages (Stages II+III+IV), large tumors (tumor size ≥ 1.5 cm), deep invasion (T2+T3+T4), and negative for lymph node metastasis (N0 stage), while there was no significant prognostic value for patients with distant metastasis (Fig. 1F–H and Fig. S1D–S1L).
To further investigate SUV39H1 expression in ccRCC, RT-qPCR and Western blot experiments were performed with ccRCC cell lines and paired normal and ccRCC tissue samples from patients. The results show that most ccRCC cell lines (786-O, Caki-1, A498, and 769-P) have a higher SUV39H1 expression levels than the normal kidney cell line HK-2 (Fig. 1I). Consistent with this, ccRCC tumor tissues exhibited increased levels of SUV39H1 mRNA and protein compared with normal tissues (Fig. 1J–L).
Next, we evaluated the SUV39H1 expression in ccRCC by performing an IHC staining analysis of a TMA consisting of 358 ccRCC tissue samples. As shown in Fig. 1M, variable SUV39H1 staining intensity was detected both in tumor tissues and paired peritumor tissues. Upregulated SUV39H1 expression was also observed in ccRCC tissues compared with peritumor tissues (Fig. S1M and S1N). The association between patients’ clinicopathological features and SUV39H1 levels are summarized in Table 1. Of note, SUV39H1 expression was positively associated with clinical stage (P = 0.020), invasion depth (T stage, P = 0.040) and Fuhrman grade (P = 0.002). Furthermore, survival analysis indicated that a high level of SUV39H1 expression was significantly correlated with shorter OS and RFS in ccRCC patients (Fig. 1N and O). Taken together, these findings suggest that SUV39H1 expression is increased in ccRCC tumors and correlates with the progression and prognosis.
Table 1.
Association of SUV39H1 expression with clinicopathological characteristics of 358 ccRCC patients in TMAs.
| Characteristic | Patient |
SUV39H1 expression |
P | |||
|---|---|---|---|---|---|---|
| n | % | Low | High | |||
| All patients | 358 | 100 | 99 | 259 | ||
| Gender | 0.950 | |||||
| Male | 254 | 70.9 | 70 | 184 | ||
| Female | 104 | 29.1 | 29 | 75 | ||
| Age (years) | 0.259 | |||||
| ≤55 | 178 | 49.7 | 54 | 124 | ||
| >55 | 180 | 50.3 | 45 | 135 | ||
| Stage | 0.020∗ | |||||
| I+II | 341 | 95.3 | 99 | 242 | ||
| III+IV | 17 | 4.7 | 0 | 17 | ||
| T stage | 0.040∗ | |||||
| T1+T2 | 344 | 96.1 | 99 | 245 | ||
| T3+T4 | 14 | 3.9 | 0 | 14 | ||
| N stage | 0.133 | |||||
| N0 | 349 | 97.5 | 99 | 250 | ||
| N1 | 9 | 2.5 | 0 | 9 | ||
| M stage | 0.286 | |||||
| M0 | 352 | 98.3 | 99 | 253 | ||
| M1 | 6 | 1.7 | 0 | 6 | ||
| Fuhrman grade | 0.002∗ | |||||
| G1+G2 | 297 | 83.0 | 92 | 205 | ||
| G3+G4 | 61 | 17.0 | 7 | 54 | ||
| Tumor size (cm) | 0.280 | |||||
| ≤4 | 186 | 52.0 | 56 | 130 | ||
| >4 | 172 | 48.0 | 43 | 129 | ||
∗P < 0.05 indicates a significant association among the variables.
3.2. Construction of nomogram models for predicting ccRCC patient prognosis
We next investigated whether SUV39H1 expression level is an independent prognostic factor for ccRCC patient outcomes. Significant prognostic factors (P < 0.05) identified by univariate analysis were further assessed by multivariate analysis. The results indicate that SUV39H1 expression level, as well as TNM stage, Fuhrman grade and tumor size, was an independent prognostic predictor for OS and RFS of ccRCC patients (Table 2).
Table 2.
Multivariate Cox regression analysis for overall survival and recurrence-free survival in 358 ccRCC patients.
| Characteristic | Overall survival |
Recurrence-free survival |
||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | |
| SUV39H1 in cancer tissues | ||||||
| Low | 1 | 1 | ||||
| High | 4.678 | 1.851–11.821 | 0.001∗ | 2.420 | 1.183–4.949 | 0.016∗ |
| TNM stage | ||||||
| I+II | 1 | 1 | ||||
| III+IV | 4.373 | 2.373–8.058 | <0.001∗ | 3.336 | 1.754–6.346 | <0.001∗ |
| Fuhrman grade | ||||||
| I+II | 1 | 1 | ||||
| III+IV | 1.981 | 1.216–3.225 | 0.006∗ | 1.924 | 1.174–3.154 | 0.009∗ |
| Tumor size (cm) | ||||||
| ≤4 | 1 | 1 | ||||
| >4 | 3.891 | 2.081–7.274 | <0.001∗ | 5.064 | 2.676–9.582 | <0.001∗ |
HR, hazard ratio; 95% CI, 95% confidence interval. ∗P < 0.05 was considered statistically significant.
To evaluate the predictive value of SUV39H1 expression levels, C-index and AIC analyses were performed27. As shown in Table 3, the C-index values were increased and the AIC values were decreased for OS or RFS when SUV39H1 expression level was considered together with conventional prognostic factors, suggesting the SUV39H1 expression level has good predictive ability for ccRCC prognosis. Moreover, compared with the SSIGN outcome algorithm, the nomogram model integrating all factors (SUV39H1 expression, TNM stage, Fuhrman grade and tumor size) has a higher C-index and a lower AIC, which means that the nomogram model performed better than the conventional SSIGN outcome algorithm in predicting ccRCC prognosis.
Table 3.
Comparison of the predictive accuracies of prognostic factors.
| Model | Overall survival (n = 358) |
Recurrence free survival (n = 358) |
||
|---|---|---|---|---|
| C-index | AIC | C-index | AIC | |
| SUV39H1 | 0.628 | 780.1949 | 0.600 | 828.7987 |
| TNM stage | 0.589 | 771.5985 | 0.576 | 816.8801 |
| TNM stage+SUV39H1 | 0.688 | 752.7648 | 0.652 | 808.8266 |
| Fuhrman grade | 0.614 | 788.2059 | 0.609 | 825.5831 |
| Fuhrman grade+SUV39H1 | 0.696 | 767.8118 | 0.665 | 817.0112 |
| Tumor size | 0.683 | 766.2329 | 0.703 | 793.0312 |
| Tumor size+SUV39H1 | 0.748 | 742.5755 | 0.749 | 781.6016 |
| Nomogram | 0.791 | 722.4722 | 0.782 | 768.5916 |
| SSIGN | 0.678 | 768.2519 | 0.702 | 789.5096 |
AIC, Akaike information criterion; C-index: Harrell's concordance index.
To further assess the utility of SUV39H1 expression as a prognostic factor, we constructed two nomogram models to predict ccRCC patient prognosis by integrating all of the independent prognostic predictors identified by the multivariate analysis28. Patients’ OS or RFS probabilities could be predicted from the total score, which was calculated by adding up the points for each parameter (Supporting Information Fig. S2A and S2B). The calibration plots for the probability of OS or RFS at 3, 5, 7 and 10 years after surgery show good consistency between observed survival and that predicted by the nomogram models (Fig. S2C–S2J). Moreover, analysis of ccRCC data from TCGA largely supported our findings (Supporting Information Tables S7 and S8, and Fig. S2K–S2O). Our results suggest that these two nomogram models may be used to reliably predict the probable prognosis of ccRCC patients.
3.3. SUV39H1 knockdown inhibits ccRCC tumor growth and induces ferroptosis
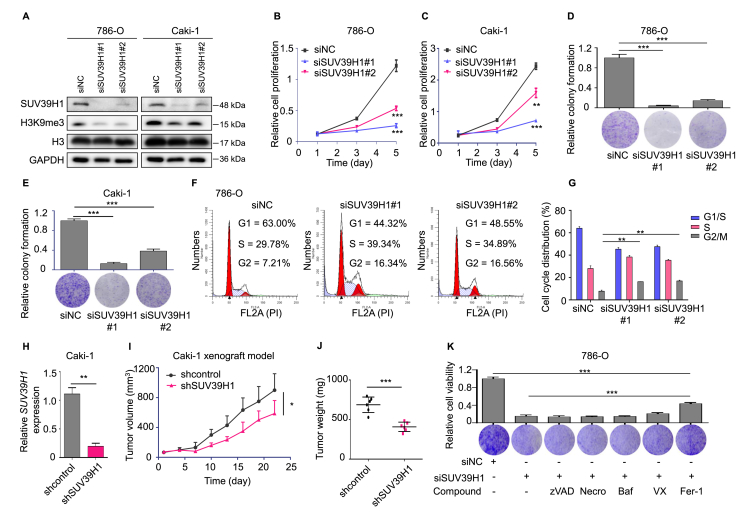
Based on our finding that SUV39H1 expression is correlated with ccRCC prognosis, we next investigated the functional role of SUV39H1 in ccRCC cells. We initially evaluated the correlation between SUV39H1 expression level and expression of KI67, a marker that is closely associated with tumor malignancy. As expected, SUV39H1 expression was significantly correlated with KI67 expression (R = 0.4099, P < 0.001) in ccRCC tumors from TCGA (Supporting Information Fig. S3A). To further investigate the effect of SUV39H1 on ccRCC growth, SUV39H1 was depleted by siRNAs in ccRCC cells, and the knockdown efficiency was assessed by Western blot (Fig. 2A). Compared with the negative control, cells in which SUV39H1 expression was knocked down showed reduced growth and colony formation (Fig. 2B–E) and the cells were arrested in G2/M phase (Fig. 2F and G and Fig. S3B–S3C). We also employed a ccRCC cell-derived xenograft mouse model to investigate whether SUV39H1 expression is essential for tumor growth in vivo. Caki-1 cells with stable depletion of SUV39H1 significantly impaired tumor growth, as indicated by tumor volume, weight and size (Fig. 2H–J and Fig. S3D). The average body weight of mice did not change significantly in either group (Fig. S3E). These findings suggest that SUV39H1 plays a crucial role in promoting ccRCC growth.
Figure 2.
Knockdown of SUV39H1 attenuates cell growth and induces ferroptotic cell death. (A) Knockdown of SUV39H1 by specific siRNAs in ccRCC cells, after 48 h, and the expression of SUV39H1, H3K9me3, H3 and GAPDH were detected by Western blot. (B) and (C) Knockdown of SUV39H1 suppressed cell growth ability in ccRCC cells (786-O and Caki-1). SUV39H1 was knocked down with SUV39H1 siRNAs and OD values were used to compare cell growth ability between different groups. Data was presented as mean ± SD, n = 3. (D) and (E) Knockdown of SUV39H1 suppressed 786-O or Caki-1 cell colony formation ability. Three view fields were selected to count the number of cells and data was presented as mean ± SD. (F) and (G) Knockdown of SUV39H1 significantly induced G2/M cell cycle arrest in 786-O cells. The experiment was repeated three times and data was presented as mean ± SD. (H) RT-qPCR analysis was performed to detect the level of SUV39H1 mRNA in Caki-1 cells transfected with the SUV39H1-specific shRNA and control (shcontrol). Data was presented as mean ± SD, n = 3. (I) and (J) Tumor growth curves in SUV39H1 stable depleted Caki-1 xenograft models, and tumor weights were expressed as mean ± SD, n = 6 for each group. (K) Treatment with RCD specific inhibitors, including z-VAD-FMK (an apoptosis inhibitor, 10 μmol/L), necrostatin 1 (a necroptosis inhibitor, 20 μmol/L), bafilomycin A1 (an autophagy inhibitor, 100 nmol/L), VX-765 (a pyroptosis inhibitor, 5 μmol/L), and ferrostatin-1 (a ferroptosis inhibitor, 5 μmol/L), in SUV39H1 specific siSUV39H1#1 transfected 786-O cells, and cell proliferation was evaluated after 3 days. Three view fields were selected to count the number of cells and data was presented as mean ± SD. (L) and (M) 786-O and Caki-1 cells were transfected with SUV39H1 specific siRNA (siSUV39H1#1) or negative siRNA (siNC) in the presence and absence of 5 μmol/L Fer-1 (ferrostatin-1), and cell proliferation was evaluated after 3 days. Three view fields were selected to count the number of cells and data was presented as mean ± SD. (N) and (O) Levels of lipid ROS were analyzed in SUV39H1-depleted Caki-1 cells. Data was presented as mean ± SD, n = 3. (P) and (Q) Levels of mitochondrial superoxide were analyzed in SUV39H1-depleted Caki-1 cells. Data was presented as mean ± SD, n = 3. (R) and (S) Levels of Fe2+ and total iron were analyzed in Caki-1 cells transfected with the SUV39H1-specific shRNA and control (shcontrol). Data was presented as mean ± SD, n = 3. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Several forms of regulated cell death (RCD), including apoptosis, necroptosis, pyroptosis, ferroptosis and autophagy-dependent cell death, have been well-characterized29. We next attempted to determine whether SUV39H1 depletion inhibits ccRCC tumor growth by inducing any of these forms of RCD. To test this, five different RCD-specific small-molecule compounds were used to inhibit individual cell death pathways. The death of SUV39H1-depleted cells was significantly reduced by treatment with ferrostatin-1, an inhibitor of ferroptosis, suggesting that SUV39H1 knockdown may attenuate cell growth partly by inducing ferroptosis (Fig. 2K–M and Fig. S3F). To confirm this finding, we assessed intracellular iron, lipid ROS levels and the morphological changes in ccRCC cells. As expected, we found that SUV39H1 knockdown increased intracellular lipid ROS, mitochondrial superoxide levels, intracellular iron and Fe2+ levels, and induced ferroptosis associated morphological changes in ccRCC cells (Fig. 2N–S and Fig. S3G–S3K). Together, our results suggest that SUV39H1 knockdown attenuates tumor growth partly by inducing ferroptotic cell death in ccRCC.
3.4. Inhibition of SUV39H1 enzymatic activity induces ferroptosis and suppresses cell proliferation in ccRCC cells
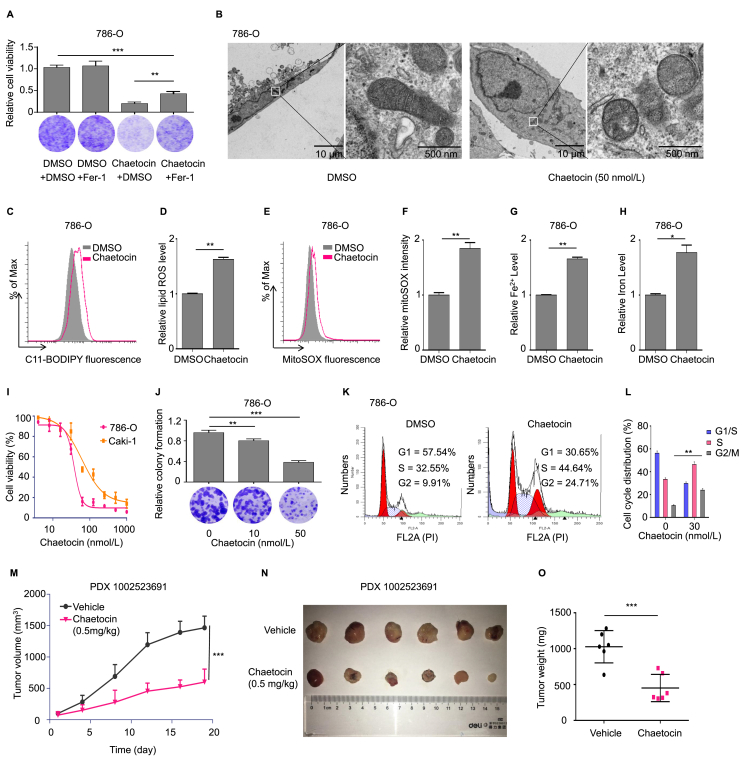
To determine whether SUV39H1 enzymatic activity participates into ferroptosis, we examined the effect of the SUV39H1 inhibitor chaetocin on ccRCC cell ferroptosis30. Western blot analysis was performed to evaluate the effect of chaetocin on H3K9me3 levels in ccRCC cells. As shown in Supporting Information Fig. S4A, chaetocin treatment reduced the level of H3K9me3 in ccRCC cells. Importantly, we found ferrostatin-1 significantly reduced the cell death induced by chaetocin (Fig. 3A and Fig. S4B). Importantly, electron microscopy analysis indicated that chaetocin treatment significantly induces ferroptosis specific changes of mitochondria such as the disappearance of the mitochondrial crest and increased dense of mitochondrial membrane (Fig. 3B). In addition, chaetocin treatment also increased intracellular levels of lipid ROS, mitochondrial superoxide, iron and Fe2+ levels, and induced ferroptosis associated morphological changes in ccRCC cells (Fig. 3C–H and Fig. S4C–S4I). Since chaetocin is a non-selective histone lysine methyltransferase inhibitor (targeting for Suv39H1, G9a and DIM5), we next to test whether the inhibition of G9a or DIM5 impacts the occurrence of ferroptosis in ccRCC. We firstly examined the impacts of G9a inhibition on intracellular iron level, lipid ROS level and the morphology of ccRCC cells with a G9a-selective inhibitor (UNC0638), and found that G9a has no significant impact on ferroptosis-specific phenotypes in ccRCC (Supporting Information Fig. S5). Besides, we found DIM5 gene is not detected in Homo sapiens31. These results together largely exclude that the impacts of chaetocin was via other targets like G9a and DIM5. Together, we reveal that the inhibition of SUV39H1 enzymatic activity could induce ccRCC cell ferroptosis.
Figure 3.
Inhibition of SUV39H1 enzymatic activity induces ferroptosis and suppresses cell proliferation. (A) 786-O cells were treated with chaetocin (80 nmol/L) or DMSO in the presence and absence of 5 μmol/L Fer-1 (ferrostatin-1), and cell proliferation was evaluated after 3 days. Three view fields were selected to count the number of cells and data was presented as mean ± SD. (B) Transmission electron microscopy analysis of 786-O cells treated with DMSO (24 h) and chaetocin (50 nmol/L, 24 h). (C) and (D) Levels of lipid ROS were analyzed in chaetocin (100 nmol/L) treated 786-O cells. Data was presented as mean ± SD, n = 3. (E) and (F) Levels of mitochondrial superoxide were analyzed in chaetocin (100 nmol/L) treated 786-O cells. Data was presented as mean ± SD, n = 3. (G) and (H) Levels of Fe2+ and total iron were analyzed in chaetocin (100 nmol/L) treated 786-O cells. Data was presented as mean ± SD, n = 3. (I) Cell proliferation assay was performed to determine the proliferation ability of ccRCC cells (786-O and Caki-1) treated with chaetocin for 5 days in a dose-dependent manner. The experiment was repeated three times and data was presented as mean ± SD. (J) Treatment with chaetocin suppressed 786-O cell colony formation ability. Three view fields were selected to count the number of cells and data was presented as mean ± SD. (K) and (L) Treatment with chaetocin (30 nmol/L) significantly induced G2/M cell cycle arrest in 786-O cells. Data was presented as mean ± SD, n = 3. (M) Tumor growth curves in chaetocin (0.5 mg/kg/day) treated ccRCC PDX animal model (PDX 1002523691). n = 6 for each group. (N) and (O) Representative photographs of ccRCC PDX animal model tumor tissues treated with chaetocin, and tumor weights were expressed as mean ± SD, n = 6 for each group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
To evaluate whether SUV39H1 could serve as a potential therapeutic target for ccRCC treatment, we examined the effect of chaetocin on ccRCC cell growth. In vitro experiments showed that chaetocin inhibited ccRCC cell proliferation and colony formation, and increased cell death in a dose-dependent manner (Fig. 3I and J and Supporting Information Fig. S6A). Consistent with this, chaetocin treatment significantly increased the proportion of ccRCC cells that arrested in the G2/M phase of the cell cycle (Fig. 3K–L, Fig. S6B and S6C). To assess the anti-tumor effects of chaetocin in vivo, we treated a patient-derived xenograft (PDX) ccRCC model with chaetocin and found chaetocin treatment resulted in substantially inhibited tumor growth, as indicated by tumor volume, size and weight (Fig. 3M–O). In terms of tolerance and toxicity, no significant weight loss was observed in either group (Fig. S6D). In summary, our results show that inhibiting SUV39H1 enzymatic activity induces ferroptosis and suppresses ccRCC tumor growth, indicating that SUV39H1 may be an effective therapeutic target for treating ccRCC.
Given that SUV39H1 depletion also induces G2/M cell cycle arrest, we next asked whether cell cycle arrest is mediated by ferroptosis. Result showed that the inhibition of ferroptosis barely impacts the occurrence of G2/M cell cycle arrest, indicating the regulation of cell cycle is independent of ferroptosis in SUV39H1 suppressed ccRCC cells (Supporting Information Fig. S7). Considering apoptosis is a key pattern of inducing tumor cell death, we further asked whether cell apoptosis is involved in the SUV39H1-induced cell death. Our data indicate that knockdown or inhibition of SUV39H1 has no significant impact on apoptosis phenotype of ccRCC cells (Supporting Information Fig. S8). Besides, we want to know whether SUV39H1 knockdown or inhibition have cell cytotoxicity in the normal kidney cell line HK-2. Results showed that SUV39H1 knockdown or inhibition of SUV39H1 enzymatic activity by chaetocin have some extent effect of cell cytotoxicity in HK-2 cells, which is relatively marginal compared with that in ccRCC cells (Supporting Information Fig. S9). Taken together, our data showed that targeting SUV39H1 has a potential value in treating ccRCC.
3.5. SUV39H1 inhibition upregulates DPP4 in ccRCC
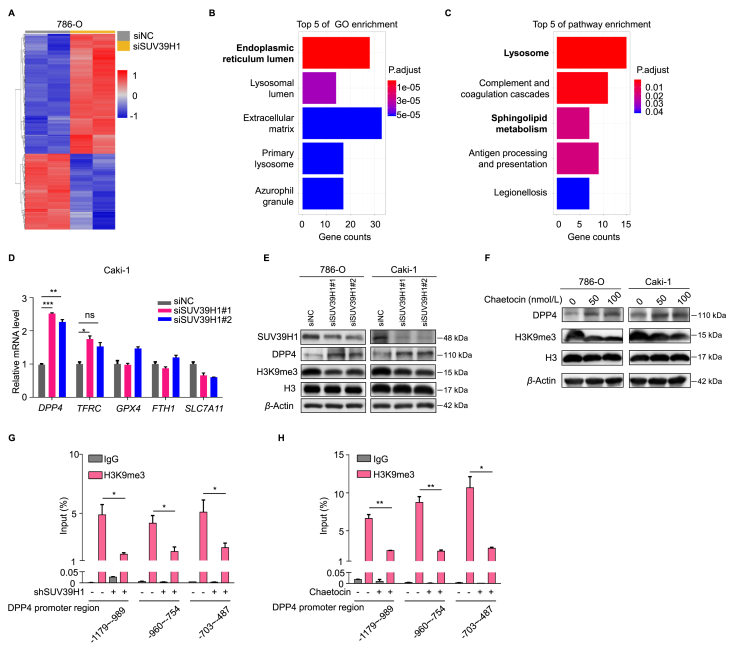
To identify the molecular signaling pathway involved SUV39H1-mediated regulation of ferroptotic cell death, we performed an RNA-seq analysis of 786-O cells after SUV39H1 knockdown. Heat-map analysis of differentially expressed genes (DEGs) showed the overall changes (Fig. 4A). Given the functional role of SUV39H1 in transcriptional repression of target genes, DEGs that were upregulated following SUV39H1 knockdown were selected for further analysis. GO enrichment and KEGG pathway analyses were performed to evaluate the functional role of SUV39H1 in ccRCC. The GO analysis results showed that the upregulated DEGs were enriched with proteins involved in the endoplasmic reticulum lumen, and the KEGG analysis indicated that SUV39H1 may participate in lysosome function and sphingolipid metabolism in ccRCC (Fig. 4B and C), which have been previously reported play a critical role in ferroptosis19,32,33.
Figure 4.
SUV39H1 deficiency upregulates DPP4 expression in ccRCC. (A) RNA-seq analysis was performed to analyze gene expression changes in 786-O cells transfected with the SUV39H1 specific siSUV39H1#1 or negative siRNA (siNC), and upregulated genes presented in red while downregulated genes in blue. n = 2 for each group. (B) GO functional annotation for upregulated genes in DEGs following SUV39H1 knockdown. Top 5 of GO enrichments were showed. (C) KEGG functional annotation for upregulated genes in DEGs following SUV39H1 knockdown. Top 5 of KEGG enrichments were showed. (D) Ferroptosis associated key regulators, including TFRC, DPP4, GPX4, FTH1 and SLC7A11, were verified by RT-qPCR experiment in SUV39H1 deleted Caki-1 cells. Data was presented as mean ± SD, n = 3. (E) DPP4 expression levels were detected by Western blot in SUV39H1 knockdown ccRCC cells. (F) DPP4 expression levels were detected by Western blot in ccRCC cells treated with different concentrations of chaetocin. (G) H3K9me3 binding at the three sections of DPP4 promoter in SUV39H1 knockdown 786-O cells. Data was presented as mean ± SD. (H) H3K9me3 binding at the three sections of DPP4 promoter in chaetocin (100 nmol/L) treated 786-O cells after 3 days. Data was presented as mean ± SD, n = 3. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
We next sought to identify the potential targeted genes that may contribute to ferroptosis induction in ccRCC. An ever-expanding number of regulators, such as GPX4, FTH1, SLC7A11, TFRC and DPP4, are consecutively reported to involve in modulating ferroptosis19,29. We found two ferroptosis associated genes (TFRC and DPP4) expression levels were significantly changed among the Top200 upregulated DEGs (ranked by Padj) in response to SUV39H1 knockdown (Supporting Information Table S9). Next, RT-qPCR was performed to verify the expression level of TFRC and DPP4, as well as GPX4, FTH1 and SLC7A11, and we found that DPP4 was the most significantly upregulated gene after SUV39H1 knockdown (Fig. 4D). To confirm this finding, we performed Western blot analyses to detect DPP4 protein expression levels in SUV39H1 deficiency cells. Consistently, DPP4 was significantly upregulated in SUV39H1-depleted or inhibition cells compared with the negative control cells (Fig. 4E and F). These data indicate that DPP4 was transcriptionally upregulated when SUV39H1 expression was suppressed.
As SUV39H1 primarily catalyzes H3K9me3 status, we next asked whether SUV39H1 modulates the H3K9me3 status of the DPP4 promoter to regulate its expression. To test this, we performed ChIP-qPCR analysis in ccRCC cells upon SUV39H1 depletion or inhibition. The results show that H3K9me3 modification in the DPP4 promoter region was noticeably decreased following SUV39H1 knockdown or inhibition (Fig. 4G and H). Taken together, these data suggest that SUV39H1 suppression decreased H3K9me3 localization to the DPP4 promoter region, which in turn led to the transcriptional upregulation of DPP4 in ccRCC cells.
3.6. SUV39H1 deficiency induces ferroptosis and inhibits cell proliferation by targeting DPP4
Previously report showed that DPP4 plays differential regulation roles of ferroptosis dependent on its subcellular localization in cells and P53 status20. We first detected the subcellular localization of the upregulated DPP4 protein in ccRCC cells, and result showed that SUV39H1 deficiency increased the expression of DPP4 in both membrane and nuclear of ccRCC cells (Supporting Information Fig. S10A). We next want to figure out whether the P53 status impacts the DPP4-induced ferroptosis in ccRCC cells. Two ccRCC cell lines 786-O (mutant P53) and Caki-1 (wild-type P53) were used in our study and both cell lines showed ferroptosis after SUV39H1 depleted, which indicated that the regulation role of DPP4 is independent of P53 status in ccRCC cells34. Together, our data indicate that SUV39H1 deficiency upregulate the expression of membrane and nucleus DPP4, which is P53-independent in ccRCC.
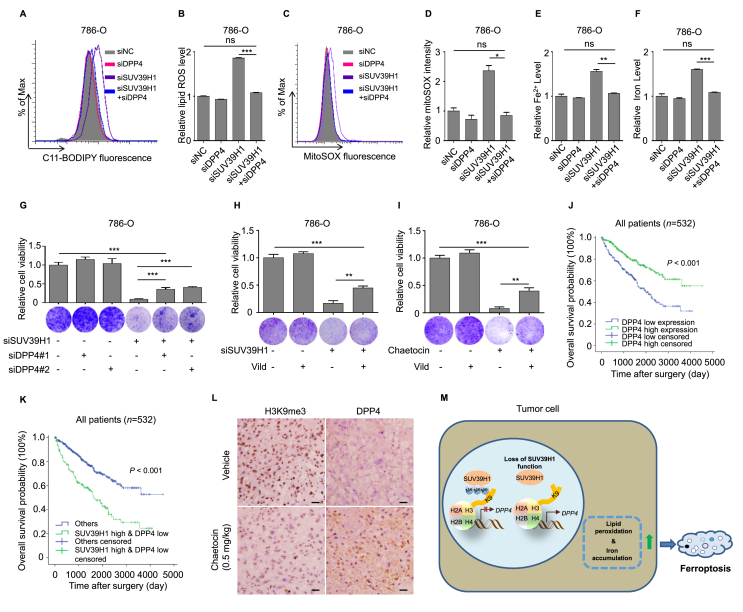
Given that membrane DPP4 contributes the occurrence of ferroptosis via cooperating with NOX1, we next to investigate the association between upregulated DPP4 and NOX1 activity in SUV39H1 deficient ccRCC cells. We observed that NOX activity was remarkably upregulated in 786-O cells following SUV39H1 depleted (Supporting Information Fig. S10B and S10C), indicating DPP4 may interact with NOX1 to induce ferroptosis in SUV39H1 deficient ccRCC cells. To determine whether inhibiting DPP4 expression or activity could reverse SUV39H1-mediated ferroptosis and its associated phenotypes, we performed DPP4 knockdown or enzyme activity inhibition experiments in SUV39H1-deficient ccRCC cells. Vildagliptin was used to specifically inhibit DPP4 enzyme activity. Knockdown of DPP4 expression restored the intracellular lipid ROS, mitochondrial superoxide, iron and Fe2+ levels in ccRCC cells (Fig. 5A–F). Furthermore, DPP4 knockdown or enzyme inhibition partially restored cell growth in SUV39H1-deficient ccRCC cells (Fig. 5G–I and Supporting Information Fig. S11A–S11E). These findings suggest that DPP4 is the functional target gene in SUV39H1-mediated ferroptosis and its associated phenotypes in ccRCC.
Figure 5.
SUV39H1 deficiency induces ferroptosis and inhibits cell proliferation by targeting DPP4. (A) and (B) Levels of lipid ROS were analyzed in 786-O cells transfected with the SUV39H1-specific siSUV39H1#1 and/or DPP4-specific siDPP4#1. Data was presented as mean ± SD, n = 3. (C) and (D) Levels of mitochondrial superoxide were analyzed in 786-O cells transfected with the SUV39H1-specific siSUV39H1#1 and/or DPP4-specific siDPP4#1. Data was presented as mean ± SD, n = 3. (E) and (F) Levels of Fe2+ and total iron were analyzed in 786-O cells transfected with the SUV39H1-specific siSUV39H1#1 and/or DPP4-specific siDPP4#1. Data was presented as mean ± SD, n = 3. (G) Cell proliferation was evaluated in 786-O cells transfected with SUV39H1 specific siSUV39H1#1 and/or DPP4-specific siRNAs after 3 days. Three view fields were selected to count the number of cells and data was presented as mean ± SD. (H) 786-O cells were transfected with SUV39H1 specific siSUV39H1#1 or negative siRNA (siNC) in the presence and absence of 10 μmol/L Vild (vildagliptin), and cell proliferation was evaluated after 3 days. Three view fields were selected to count the number of cells and data was presented as mean ± SD. (I) 786-O cells treated with chaetocin (80 nmol/L) or DMSO in the presence and absence of 10 μmol/L Vild (vildagliptin), and cell proliferation was evaluated after 3 days. Three view fields were selected to count the number of cells and data was presented as mean ± SD. (J) Kaplan–Meier analysis of OS for all ccRCC patients with low or high DPP4 expression level in TCGA dataset. n = 532. (K) SUV39H1 and DPP4 prognostic interactions. Kaplan–Meier analysis was performed to show the association between OS and SUV39H1 high & DPP4 low expression levels in ccRCC patients in TCGA. n = 532. (L) IHC analysis of tissues harvested from the mouse tumor model stained for H3K9me3 and DPP4 (scale bar: 10 μm). (M) Schematic diagram depicting the regulation of DPP4 in ccRCC cells. SUV39H1 is an epigenetic repressor to suppress DPP4 expression. Loss function of SUV39H1 in ccRCC tumors contributes the hypomethylation of the DPP4 promoter to upregulate DPP4 expression and induces DPP4-mediated ferroptosis to suppress cell proliferation. Each experiment was performed three times, and data was presented as mean ± SD. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Based on the above findings, we next asked whether DPP4 expression is associated with ccRCC prognosis. The role of DPP4 in ccRCC is poorly understood. We found that ccRCC patients in the TCGA dataset with low DPP4 transcript levels had a poor prognosis, with a median OS of 38.5 months compared with 42.3 months in the high DPP4 expression group (Fig. 5J). Finally, patients with a high level of SUV39H1 expression and a low level of DPP4 expression had an even worse prognosis (Fig. 5K and Fig. S11F). Additionally, we also evaluated H3K9me3 and DPP4 expression level in the chaetocin-treated group and control group tumor tissues by IHC and verified that chaetocin treatment was associated with obviously increased DPP4 expression (Fig. 5L). Taken together, our findings outline a functional role for the SUV39H1–DPP4 axis in ccRCC progression (Fig. 5M). Loss of SUV39H1 function in ccRCC tumors leads to hypomethylation of the DPP4 promoter, upregulating DPP4 expression, which then induces ferroptosis and suppresses cell proliferation. Our data indicate that SUV39H1 may serve as a therapeutic target for ccRCC treatment.
4. Discussion
Aberrant histone modification is one of the most common events observed in carcinogenesis35,36. As a H3K9 methyltransferase, SUV39H1 has been reported to function as an oncogene in many tumors. However, the exact role of SUV39H1 in ccRCC progression remains poorly understood. In the present study, we show that SUV39H1 plays a critical role in ccRCC progression and prognosis, and impacts tumor growth by regulating ferroptotic cell death. By analyzing data from the public TCGA database and our own ccRCC cohorts, we found that SUV39H1 is overexpressed in ccRCC tumors, and that SUV39H1 expression levels are significantly correlated with shortened OS and RFS in ccRCC patients. We further showed that integrating assessment of SUV39H1 expression level with several prognostic factors could predict clinical outcomes in ccRCC patients with greater accuracy than the conventional SSIGN prognostic model. In addition, we demonstrated that knocking down SUV39H1 expression or pharmacologically inhibiting SUV39H1 activity suppresses ccRCC cell growth in vitro and in vivo. Furthermore, DPP4, a well-known ferroptosis regulator, was identified as the direct functional target of SUV39H1 in mediating ccRCC cell proliferation. Thus, our findings provide an insight into the functional role of SUV39H1 in ccRCC progression and prognosis, and suggest that SUV39H1 could be used as a prognostic marker for ccRCC progression and a therapeutic target for ccRCC treatment.
Our data show that SUV39H1 is mostly overexpressed in ccRCC tissues while a small number of patients may have low expression of SUV39H1. Typically, the reason for this phenomenon may due to the heterogeneity of ccRCC. As is a highly heterogeneous and complex disease, ccRCC patients are always facing limited effective treatment options. Therefore, there is an urgent need for improved outcome prediction models to guild clinical decisions following surgery. To this end, numerous nomogram models have been proposed to predict ccRCC clinical outcomes37, 38, 39. Unfortunately, many of these models have innate limitations, such as insufficient validation and poor accuracy40. Here, we found that SUV39H1 expression level is an independent prognostic factor for ccRCC patient outcome. SUV39H1 expression levels were especially accurate in predicting outcomes in ccRCC patients with a high histological grade, intermediate and advanced tumor stages, large tumors, deep invasion, and no lymph node metastasis, suggesting that analysis of SUV39H1 expression may be most appropriate for predicting prognosis in this subset of ccRCC patients. Importantly, when combined with several clinical features, analyzing SUV39H1 expression levels could predict ccRCC patient outcomes with greater accuracy than the conventional SSIGN prognostic model, which is a powerful predictor of prognosis in ccRCC patients who have undergone radical nephrectomy37. Ideally, with more and more external validations of our findings, we believe that SUV39H1 could be considered as a powerful predictor of prognosis in ccRCC patients.
Recently, ferroptosis has been identified as a nonapoptotic form of cell death that is involved in the progression of many diseases, especially cancer18,41. Inducing ferroptosis using small molecules is a focus of intense investigation in the cancer therapy field42. Although many molecular factors are involved in ferroptosis, several key regulators, such as RAS and TP53, play critical roles in tumor development19. However, the correlation between epigenetic alterations and ferroptosis remains poorly understood. Here we show that SUV39H1 deficiency increases intracellular lipid ROS, mitochondrial superoxide and iron levels, indicating that the HMTase SUV39H1 may regulate ferroptosis in ccRCC cells. More importantly, sorafenib, a first-line clinical drug for advanced ccRCC treatment, is also a ferroptosis inducer, which suggests that the SUV39H1 inhibitor chaetocin could be effective in treating sorafenib-resistant ccRCC patients. Our findings provide the insight into the role of epigenetic alteration in regulating ferroptosis, as well as the biological processes underlying ccRCC progression.
Loss function of GPX4 is a canonical way to induce ferroptosis. GPX4 is a member of the GSH peroxidases that protects biomembranes against peroxidation damage through repressing phospholipid hydroperoxide43. However, our RNA-seq data show that there is no significant expression change for GPX4 after SUV39H1 knockdown. Additionally, DPP4, an important ferroptosis-associated regulator that binds to NOX1 to form the DPP4–NOX1 complex, which facilitates lipid peroxidation in ferroptosis, plays a key role in the clinical progression of ccRCC20. DPP4 is primarily located at the plasma membrane, where it functions as a serine exopeptidase that cleaves X-proline dipeptides from the N-terminus of polypeptides44. In addition to its role in diabetes45, the biological role of DPP4 in various types of cancers, including ccRCC, has also been investigated. DPP4 activity is negatively correlated with tumor grade and positively correlated with survival in ccRCC patients46,47. In our study, we demonstrated that SUV39H1 deficiency upregulated DPP4 expression. Knocking down DPP4 expression or inhibiting DPP4 enzyme activity restored intracellular lipid ROS and iron levels, and partially restored cell growth in SUV39H1-deficient ccRCC cells, suggesting that DPP4 is a functional target of SUV39H1 in mediating ccRCC ferroptosis and cell proliferation. Furthermore, we found that SUV39H1 deficiency upregulates DPP4 expression by decreasing H3K9me3 levels at the DPP4 promoter. Taken together, these results demonstrate that SUV39H1 negatively regulates DPP4 expression by modulating H3K9me3 levels at the DPP4 promoter during ccRCC cell growth. Thus, our study provides a mechanistic insight into the impact of SUV39H1 on ccRCC tumor growth, which may provide a strategy for ccRCC treatment.
Although our study presents meaningful findings regarding the role of SUV39H1 in ccRCC progression, it does have several limitations. Specifically, the role of SUV39H1 expression level in the clinical behavior of ccRCC was investigated by retrospectively analyzing data from TCGA and our own ccRCC cohort. However, the clinical features of our cohort were somewhat different from those of the patients in the TCGA database, so we were unable to analyze enough patients with advanced disease to confirm the role of SUV39H1 expression levels in advanced ccRCC. Thus, data from multicentric cohorts with comprehensive clinical information are needed to confirm our findings. Besides, we used chaetocin as the inhibitor to inhibit SUV39H1 enzymatic activity. However, chaetocin is a non-selective histone lysine methyltransferase inhibitor which targeted SUV39H1, G9a and DIM5 at the same time. Although we excluded the potential interferences in our study, a SUV39H1-specific inhibitor is still needed to confirm our finding. Additionally, our data show that suppressing ferroptosis only partially restored cell growth in SUV39H1-deficient ccRCC cells, suggesting the SUV39H1 may also regulate ccRCC cell proliferation through other ferroptosis-independent mechanisms. As shown in our data, SUV39H deficiency could also induce G2/M cell cycle arrest, which is independent of ferroptosis. Thus, the potential involvement of other molecular mechanisms involved in SUV39H1 function in ccRCC needs to be explored further.
In conclusion, our study provides the mechanistic insight into SUV39H1-dependent epigenetic regulation of ccRCC tumor growth, indicating that SUV39H1 may serve as a prognostic biomarker for ccRCC progression and a therapeutic target for ccRCC treatment.
Acknowledgments
This study was supported by the advanced technology promotion project of Shanghai Municipal Commission of Health and Family Planning (No. 27 HYR 2013, China) and Doctoral Innovation Fund Projects from Shanghai Jiao Tong University School of Medicine (BXJ201919, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.09.015.
Contributor Information
Wei Xue, Email: uroxuewei@sjtu.edu.cn.
Jin Zhang, Email: med-zhangjin@vip.sina.com.
Yiran Huang, Email: huangyiran@renji.com.
Author contributions
Yiran Huang, Jin Zhang and Wei Xue conceived and designed the experiments; Jianfeng Wang, Xiaomao Yin and Wei He performed the experiments, analyzed the data, wrote and validated the paper. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hsieh J.J., Purdue M.P., Signoretti S., Swanton C., Albiges L., Schmidinger M. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rini B.I., Campbell S.C., Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 3.Capitanio U., Montorsi F. Renal cancer. Lancet. 2016;387:894–906. doi: 10.1016/S0140-6736(15)00046-X. [DOI] [PubMed] [Google Scholar]
- 4.Kotecha R.R., Motzer R.J. Towards individualized therapy for metastatic renal cell carcinoma. Nat Rev Clin Oncol. 2019;16:621–633. doi: 10.1038/s41571-019-0209-1. [DOI] [PubMed] [Google Scholar]
- 5.Posadas E.M., Limvorasak S., Figlin R.A. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13:496–511. doi: 10.1038/nrneph.2017.82. [DOI] [PubMed] [Google Scholar]
- 6.Joosten S.C., Smits K.M., Aarts M.J., Melotte V., Koch A. Epigenetics in renal cell cancer: mechanisms and clinical applications. Nat Rev Urol. 2018;15:430–451. doi: 10.1038/s41585-018-0023-z. [DOI] [PubMed] [Google Scholar]
- 7.Chen L., Chen L., Qin Z., Lei J., Ye S., Zeng K. Upregulation of miR-489-3p and miR-630 inhibits oxaliplatin uptake in renal cell carcinoma by targeting OCT2. Acta Pharm Sin B. 2019;9:1008–1020. doi: 10.1016/j.apsb.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B., Wang Z., Zhang L., Ghosh S., Zheng H., Zhou Z. Depleting the methyltransferase Suv39h1 improves DNA repair and extends lifespan in a progeria mouse model. Nat Commun. 2013;4:1868. doi: 10.1038/ncomms2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Carroll D., Scherthan H., Peters A.H., Opravil S., Haynes A.R., Laible G. Isolation and characterization of Suv39h2, a second histone H3 methyltransferase gene that displays testis-specific expression. Mol Cell Biol. 2000;20:9423–9433. doi: 10.1128/mcb.20.24.9423-9433.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandel L., Nicolas E., Vaute O., Ferreira R., Ait-Si-Ali S., Trouche D. Transcriptional repression by the retinoblastoma protein through the recruitment of a histone methyltransferase. Mol Cell Biol. 2001;21:6484–6494. doi: 10.1128/MCB.21.19.6484-6494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao V.K., Pal A., Taneja R. A drive in SUVs: from development to disease. Epigenetics. 2017;12:177–186. doi: 10.1080/15592294.2017.1281502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba T., Saito T., Yuki K., Zen Y., Koide S., Kanogawa N. Histone lysine methyltransferase SUV39H1 is a potent target for epigenetic therapy of hepatocellular carcinoma. Int J Canc. 2015;136:289–298. doi: 10.1002/ijc.28985. [DOI] [PubMed] [Google Scholar]
- 13.Lu C., Yang D., Klement J.D., Oh I.K., Savage N.M., Waller J.L. SUV39H1 represses the expression of cytotoxic T-lymphocyte effector genes to promote colon tumor immune evasion. Canc Immunol Res. 2019;7:414–427. doi: 10.1158/2326-6066.CIR-18-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z., He L., Lin K., Zhang Y., Deng A., Liang Y. The KMT1A-GATA3-STAT3 circuit is a novel self-renewal signaling of human bladder cancer stem cells. Clin Canc Res. 2017;23:6673–6685. doi: 10.1158/1078-0432.CCR-17-0882. [DOI] [PubMed] [Google Scholar]
- 15.Kim G., Kim J.Y., Lim S.C., Lee K.Y., Kim O., Choi H.S. SUV39H1/DNMT3A-dependent methylation of the RB1 promoter stimulates PIN1 expression and melanoma development. FASEB J. 2018;32:5647–5660. doi: 10.1096/fj.201700645RRRRR. [DOI] [PubMed] [Google Scholar]
- 16.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschhorn T., Stockwell B.R. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130–143. doi: 10.1016/j.freeradbiomed.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B., Liu J., Kang R., Klionsky D.J., Kroemer G., Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Canc Biol. 2019;S1044–579X:30006–30009. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y., Zhu S., Song X., Sun X., Fan Y., Liu J. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20:1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S., Chang W., Wu H., Wang Y.H., Gong Y.W., Zhao Y.L. Pan-cancer analysis of iron metabolic landscape across the Cancer Genome Atlas. J Cell Physiol. 2020;235:1013–1024. doi: 10.1002/jcp.29017. [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Xu Y., Zhu L., Zou Y., Kong W., Dong B. Cannabinoid receptor 2 as a novel target for promotion of renal cell carcinoma prognosis and progression. J Canc Res Clin Oncol. 2018;144:39–52. doi: 10.1007/s00432-017-2527-y. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Xu Y., Zhu L., Zou Y., Kong W., Dong B. High expression of stearoyl-CoA desaturase 1 predicts poor prognosis in patients with clear-cell renal cell carcinoma. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hang J., Hu H., Huang J., Han T., Zhuo M., Zhou Y. Sp1 and COX2 expression is positively correlated with a poor prognosis in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:28207–28217. doi: 10.18632/oncotarget.8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C., Jiang H., Jin J., Xie Y., Chen Z., Zhang H. Development of potent type i protein arginine methyltransferase (PRMT) inhibitors of leukemia cell proliferation. J Med Chem. 2017;60:8888–8905. doi: 10.1021/acs.jmedchem.7b01134. [DOI] [PubMed] [Google Scholar]
- 26.Zhu L., Wang J., Kong W., Huang J., Dong B., Huang Y. LSD1 inhibition suppresses the growth of clear cell renal cell carcinoma via upregulating P21 signaling. Acta Pharm Sin B. 2019;9:324–334. doi: 10.1016/j.apsb.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss A., Chavez-MacGregor M., Lichtensztajn D.Y., Yi M., Tadros A., Hortobagyi G.N. Validation study of the American Joint Committee on Cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4:203–209. doi: 10.1001/jamaoncol.2017.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y., Liu L., Xia Y., Wang J., Xi W., Bai Q. Low CCL17 expression associates with unfavorable postoperative prognosis of patients with clear cell renal cell carcinoma. BMC Canc. 2017;17:117. doi: 10.1186/s12885-017-3106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang D., Kang R., Berghe T.V., Vandenabeele P. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai Y.S., Chen J.Y., Tsai H.J., Chen T.Y., Hung W.C. The SUV39H1 inhibitor chaetocin induces differentiation and shows synergistic cytotoxicity with other epigenetic drugs in acute myeloid leukemia cells. Blood Canc J. 2015;5:e313. doi: 10.1038/bcj.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X., Tamaru H., Khan S.I., Horton J.R., Keefe L.J., Selker E.U. Structure of the neurospora SET domain protein DIM-5, a histone H3 lysine methyltransferase. Cell. 2002;111:117–127. doi: 10.1016/s0092-8674(02)00999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y.S., Lee D.H., Choudry H.A., Bartlett D.L., Lee Y.J. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol Canc Res. 2018;16:1073–1076. doi: 10.1158/1541-7786.MCR-18-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novgorodov S.A., Voltin J.R., Gooz M.A., Li L., Lemasters J.J., Gudz T.I. Acid sphingomyelinase promotes mitochondrial dysfunction due to glutamate-induced regulated necrosis. J Lipid Res. 2018;59:312–329. doi: 10.1194/jlr.M080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berglind H., Pawitan Y., Kato S., Ishioka C., Soussi T. Analysis of p53 mutation status in human cancer cell lines: a paradigm for cell line cross-contamination. Canc Biol Ther. 2008;7:699–708. doi: 10.4161/cbt.7.5.5712. [DOI] [PubMed] [Google Scholar]
- 35.Dawson M.A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Chen X., Pan X., Zhang W., Guo H., Cheng S., He Q. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. 2020;10:723–733. doi: 10.1016/j.apsb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank I., Blute M.L., Cheville J.C., Lohse C.M., Weaver A.L., Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 38.Karakiewicz P.I., Briganti A., Chun F.K., Trinh Q.D., Perrotte P., Ficarra V. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–1322. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 39.Leibovich B.C., Blute M.L., Cheville J.C., Lohse C.M., Frank I., Kwon E.D. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–1671. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 40.Leibovich B.C., Lohse C.M., Cheville J.C., Zaid H.B., Boorjian S.A., Frank I. Predicting oncologic outcomes in renal cell carcinoma after surgery. Eur Urol. 2018;73:772–780. doi: 10.1016/j.eururo.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Lu B., Chen X.B., Ying M.D., He Q.J., Cao J., Yang B. The role of ferroptosis in cancer development and treatment response. Front Pharmacol. 2017;8:992. doi: 10.3389/fphar.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang C., Zhang X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31 doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 43.Seibt T.M., Proneth B., Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Matteucci E., Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943–2951. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
- 45.Liu J., Huan Y., Li C., Liu M., Shen Z. Establishment of a selective evaluation method for DPP4 inhibitors based on recombinant human DPP8 and DPP9 proteins. Acta Pharm Sin B. 2014;4:135–140. doi: 10.1016/j.apsb.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larrinaga G., Blanco L., Sanz B., Perez I., Gil J., Unda M. The impact of peptidase activity on clear cell renal cell carcinoma survival. Am J Physiol Ren Physiol. 2012;303:F1584–F1591. doi: 10.1152/ajprenal.00477.2012. [DOI] [PubMed] [Google Scholar]
- 47.Varona A., Blanco L., Perez I., Gil J., Irazusta J., Lopez J.I. Expression and activity profiles of DPP IV/CD26 and NEP/CD10 glycoproteins in the human renal cancer are tumor-type dependent. BMC Canc. 2010;10:193. doi: 10.1186/1471-2407-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.