Abstract
Immune effector responses against Plasmodium falciparum include antibody-mediated activation of innate immune cells, which can induce Fc effector functions, including antibody-dependent cellular cytotoxicity, and the secretion of cytokines and chemokines. These effector functions are regulated by the composition of immunoglobulin G (IgG) Fc N-linked glycans. However, a role for antibody-mediated natural killer (NK) cells activation or Fc N-linked glycans in pregnant women with malaria has not yet been established. Herein, we studied the capacity of IgG antibodies from pregnant women, with placental malaria or non-placental malaria, to induce NK cell activation in response to placental malaria-associated antigens DBL2 and DBL3. Antibody-mediated NK cell activation was observed in pregnant women with malaria, but no differences were associated with susceptibility to placental malaria. Elevated anti-inflammatory glycosylation patterns of IgG antibodies were observed in pregnant women with or without malaria infection, which were not seen in healthy non-pregnant controls. This suggests that pregnancy-associated anti-inflammatory Fc N-linked glycans may dampen the antibody-mediated activation of NK cells in pregnant women with malaria infection. Overall, although anti-inflammatory glycans and antibody-dependent NK cell activation were detected in pregnant women with malaria, a definitive role for these antibody features in protecting against placental malaria remains to be proven.
Subject terms: Immunology, Diseases
Introduction
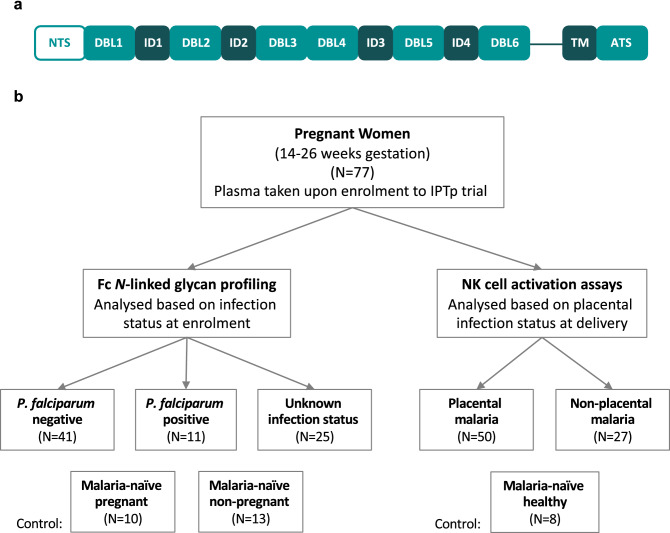
Plasmodium falciparum, the main causative agent of malaria, poses a serious threat to the health of pregnant women and to their unborn babies. Malaria in pregnant women can not only cause maternal death and life-threatening symptoms, such as anemia, pulmonary edema, hypoglycemia, puerperal sepsis, but also miscarriages, stillbirths, prematurity and fetal growth restriction1. Globally, malaria contributes to more than 20% of all maternal deaths in malaria endemic areas1. Pregnant women are more susceptible to malaria than their non-pregnant counterparts2, not only due to immunological changes during pregnancy, but also to the unique characteristics of P. falciparum parasites that can accumulate and sequester in the maternal blood spaces of the placenta3,4. In placental malaria, a single member of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family5,6, called VAR2CSA, is expressed on P. falciparum-infected erythrocytes (IEs), and mediates adhesion to the glycosaminoglycan chondroitin sulphate A (CSA), on the syncytiotrophoblast cell surface lining of the maternal blood spaces7–10. This adhesion avoids splenic clearance of IEs from the blood circulation, which leads to inflammation and localized endothelial dysfunction of the placenta11. The variant surface antigen VAR2CSA is a large ~ 350 kDa transmembrane protein consisting of a cytoplasmic acidic terminal segment, six extracellular Duffy binding-like (DBL) domains, four inter-domain (ID) regions, and a N-terminal segment12,13 (Fig. 1a).
Figure 1.
Schematic representation of VAR2CSA and overview of cohort groups. (a) The extracellular region of VAR2CSA contains a N-terminal sequence (NTS) followed by Duffy binding-like (DBL) domains and interdomain (ID) regions. It is anchored in the membrane by a transmembrane (TM) domain connected to an acidic terminal segment (ATS). (b) Plasma samples were obtained from pregnant women in Papua New Guinea between November 2009 and August 2012 upon enrolment into an Intermittent Preventive Treatment in Pregnancy (IPTp) randomized controlled trial at 14–26 gestation weeks. Samples were grouped based on infection status at enrolment for Fc N-linked glycan profiling and grouped based on infection status at delivery for functional NK cell activation assays.
Whilst antibody (Ab) responses to several VAR2CSA domains are positively associated with the presence of placental and peripheral infections, there is little evidence that Ab levels to recombinant proteins protect from placental malaria14. However, studies have shown that immunoglobulin G (IgG) Abs recognize VAR2CSA in a sex-specific and parity-dependent manner10. High anti-VAR2CSA IgG levels can be found in multigravid pregnant women in P. falciparum-endemic regions15, and women with high plasma levels of anti-VAR2CSA IgG have a decreased risk of delivering low-birthweight babies10. The predominant IgG subclasses produced in response to P. falciparum in pregnancy are IgG1 and IgG315–17. Both IgG subclasses have been linked to protective immunity against P. falciparum infections18,19, possibly due to opsonization of IEs and the Ab-mediated activation of Fc gamma receptor (FcγR) expressing innate immune cells including phagocytes and natural killer (NK) cells20,21.
NK cells can mediate Ab-dependent cellular cytotoxicity (ADCC) upon recognition of target cells via FcγRIIIa22, which is hypothesized to play a possible role in direct cytotoxic killing of IEs, and therefore is suggested to be beneficial against P. falciparum infections23. Ab-mediated activation of NK cells can also induce the secretion of a range of cytokines, including interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα)24–26. These cytokines may be beneficial during the early phase of Plasmodium infection by reducing parasitemia22,23. However, overproduction of pro-inflammatory cytokines can also result in immunopathology and adverse clinical outcomes, especially in pregnancy27–29.
Antigen-specific Ab engagement with FcγRIIIa on NK cells was recently identified as a key vaccine-induced functional immune responses linked to protection by RTS,S/AS01, the only licensed P. falciparum vaccine30. In addition, in vitro assays demonstrated the ability of NK cells to kill IEs via ADCC, and IgG Abs to PfEMP1 were sufficient to promote NK-dependent growth inhibition of P. falciparum in IEs31. This study also showed that naturally acquired IgG of multigravid women specific for VAR2CSA promotes NK-dependent lysis of IEs31. The ability of IgG Abs against the DBL2 and its flanking ID regions of VAR2CSA to induce ADCC is still unexplored32, but is of special interest, since the two leading placental malaria vaccine candidates PRIMVAC (Institut National de la Santé et de la Recherche Médicale, France) and PAMVAC (University Hospital Tuebingen, Germany) both include DBL2 domains33,34.
Fc effector functions such as ADCC are regulated through multiple structural and genetic components of the Ab, FcγR, and effector cell35, including post-translational modifications of glycans on the Fc domain of Abs, specifically at asparagine 297 on IgG36. Multiple factors can influence glycosylation patterns of IgG Abs including age, sex37, epigenetics38, disease state39,40, infection41–43, or vaccination44. Glycosylation patterns of IgG Abs can also undergo temporary changes during pregnancy, when galactosylation and sialylation of IgG Abs increase45,46. This has been associated with a less inflammatory profile47, which may contribute to acceptance of the placenta by the maternal immune system during pregnancy48,49. Changes in the composition of the asparagine 297 glycan can also influence the binding affinity of IgG Abs to FcγRs, and thereby change the magnitude of effector functions initiated, including ADCC and Ab-dependent cellular phagocytosis50. Human NK cells primarily express one Fc gamma receptor (FcγRIIIa), and responses through FcγRIIIa are highly regulated by IgG N-linked glycosylation, more so than any other human FcγR51–53. Some studies suggest that the presence/absence of key glycoforms can modulate FcγR affinity and ADCC activity by up to 20-fold36,51,54,55.
Here, we investigate the ability of IgG Abs of pregnant women from a malaria-endemic area specific to DBL2 and DBL3 (both VAR2CSA domains) to activate human primary NK cells from malaria-naïve donors to secrete IFNγ and TNFα cytokines, and upregulate CD107a expression, which is a surrogate for granzyme B degranulation and ADCC activity56. In addition, we evaluated pregnancy-associated glycosylation patterns of IgG Abs and their effect on NK-mediated effector functions in the context of P. falciparum infection during pregnancy.
Results
Primary human NK cells are activated by DBL2 or DBL3-specific IgG Abs from pregnant women with malaria
NK cells are major innate immune mediators of cytotoxicity. To evaluate the capacity of DBL2 and DBL3-specific IgG Abs to induce NK-mediated effector functions, we used purified IgG from two groups of pregnant women at mid pregnancy with peripheral P. falciparum parasitemia at delivery, and who were either positive (N = 50) or negative for P. falciparum IEs in the placenta (N = 27) (Fig. 1b).
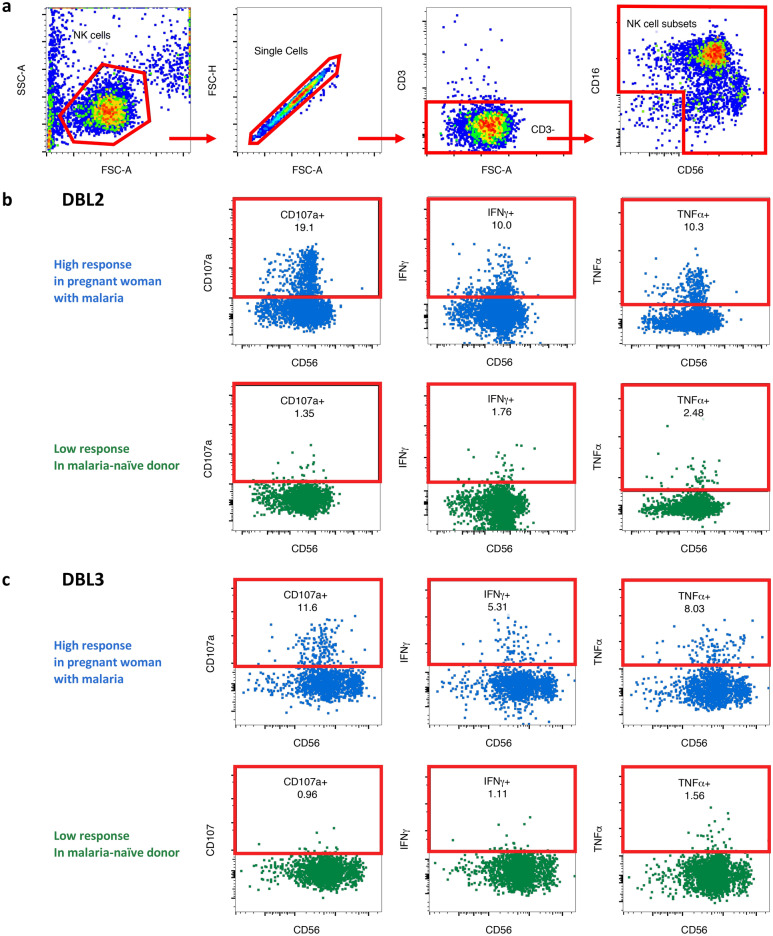
We modified previously described Ab-dependent NK cell activation assays that have been utilized to assess responses to influenza, human immunodeficiency virus (HIV) and Mycobacterium tuberculosis proteins24–26,57 for the use with VAR2CSA domain antigens (Fig. 1a). DBL2 was chosen because of its relevance in the development of placental malaria vaccines33,34. DBL3 is another domain of the VAR2CSA protein, which can be recognized by IgG Abs generated by pregnant women with malaria58. We characterized the ability of Abs against these domains to activate primary human NK cells, isolated from the blood of three malaria-naïve healthy donors. NK cells were identified via flow cytometry (Fig. 2a) and the levels of Ab-mediated NK cell activation in response to DBL2 and DBL3 were measured as indicated by intracellular cytokine production of IFNγ and TNFα, and the upregulation of cell surface degranulation marker CD107a (Fig. 2b-c). In order to optimize the Ab-dependent NK cell activation assay for malaria antigens, DBL2 (50–300 ng/well), DBL3 (50–300 ng/well) and IgG Ab (0.0625–0.25 mg/ml) concentrations were first titrated using four individual control Ab samples from pregnant women with malaria and a malaria-naïve individual (Fig. S1a–d).
Figure 2.
Gating strategy to identify NK cell activation markers. Purified NK cells were incubated with IgG test samples in presence of DBL2 or DBL3 for 5 h and then analyzed via flow cytometry. Representative flow cytometry plots of one sample to visualize gating strategy. (a) NK cells were identified by sequentially gating on lymphocytes, single cells, CD3- cells, and NK cell subsets. NK cells subsets were gated as one and assessed for surface CD107a expression and intracellular IFNγ and TNFα production in presence of DBL2 (b) and DBL3 (c) (High response = blue; malaria-naïve response = green).
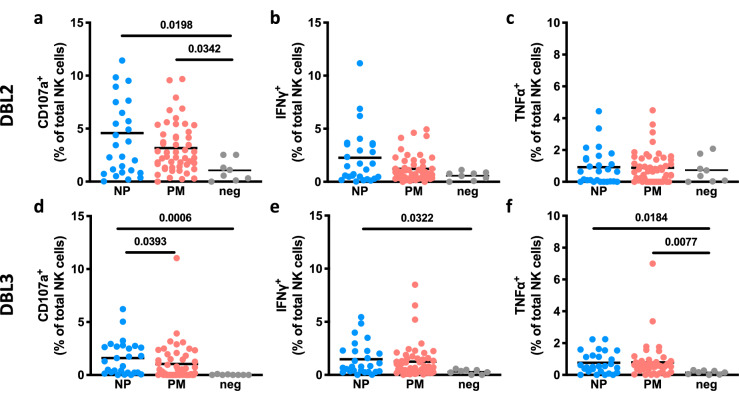
We evaluated purified IgG from pregnant women at mid pregnancy with peripheral P. falciparum parasitemia at delivery, and who were either positive or negative for P. falciparum IEs in the placenta. Their purified IgG was assessed in the presence of DBL2 or DBL3 for induction of Ab-mediated NK cell activation (Fig. 3). For both antigens, we observed upregulation of NK cell degranulation (CD107a; Fig. 3a,d, DBL2: p-value = 0.0198, DBL3: p-value = 0.0006) mid pregnancy in pregnant women who have non-placental malaria at delivery compared to non-pregnant malaria-naïve healthy individuals. In addition, DBL3-specific IgG mid pregnancy from pregnant women with non-placental malaria at delivery induced significantly higher IFNγ and TNFα production (Fig. 3e,f, IFNγ: p-value = 0.0322, TNFα: p-value = 0.0184) compared to IgG from non-pregnant malaria-naïve healthy individuals. Relative to IgG from non-pregnant malaria-naïve healthy individuals, IgG from pregnant women with placental malaria were associated with significantly higher NK cell degranulation (CD107a upregulation; Fig. 3a, p-value = 0.0342) in response to DBL2 and TNFα production (Fig. 3f, p-value = 0.0077) in response to DBL3 antigen. Differences in NK cell degranulation or cytokine production between pregnant women with non-placental malaria and women with placental malaria were only observed in CD107a expression (Fig. 3d, p-value = 0.0393) in response to DBL3.
Figure 3.
Human NK cells lack activation in presence of DBL2- or DBL3-specific Abs from pregnant women with malaria. NK cells were assessed for surface CD107a expression and intracellular IFNγ and TNFα production in the presence of VAR2CSA subdomains DBL2 (a–c) or DBL3 (d–f). Percentage of activation markers expressed by NK cells (mean of three separate donors) are shown. NK cells were stimulated with purified IgG Abs from pregnant women mid pregnancy with placental malaria (PM; N = 50; red) or from pregnant women with non-placental malaria (NP; N = 27; blue) at delivery in the presence of VAR2CSA subdomains DBL2 or DBL3. IgG Abs from malaria-naïve donors were used as negative control (N = 8; grey). Activation marker expression of NK cells incubated without Abs and median of SIV gp120-specific responses were subtracted as background. Statistical comparison between groups was performed using a Kruskal–Wallis test corrected for multiple comparisons using Dunn’s multiple comparison method (p-values are shown on graphs).
Ab-dependent NK cell activation assays were validated with the use of two control antigens. In the presence of IgG Abs from malaria-naïve healthy individuals, influenza H3 (positive control) induced NK cell activation26, whereas the negative control SIV gp120 did not (Fig. S2a–c). Using human primary NK cells from malaria-naïve healthy donors, H3-specific Abs induced significant CD107a upregulation and cytokine expression (median expression [interquartile range (IQR)]: CD107a: 11.1% [10.2–13.1%], IFNγ: 7.0% [5.1–10.8%], TNFα: 6.2% [3.9–6.7%]), whereas SIV gp120 did not (median expression [IQR]: CD107a: 1.3% [0.7–1.6%], IFNγ: 0.3% [0.1–0.7%], TNFα: 0.0% [0.0–0.3%]). Overall, weaker NK cell degranulation or expression of intracellular cytokines IFNγ and TNFα were observed in the presence of DBL2 and DBL3-specific Abs compared to H3-specific Abs (Figs. 3 and S2). The mean of activation markers expressed by NK cells in the presence of IgG-coated DBL2 or DBL3 never reached the expression observed in the presence of influenza H3-specific IgG.
In addition, DBL2- and DBL3-binding capacities of IgG1-4 subclasses from pregnant women with placental (N = 50) and non-placental malaria (N = 27) were investigated via multiplex assays and correlated to the expression of CD107a, IFNγ and TNFα by Ab-activated NK cells (Figs. S3 and S4). The majority of non-placental malaria Ab-mediated NK cell activation was driven by IgG1; however, these correlations, if present, were weak to moderate (max Spearman ρ = 0.553, p-value = 0.0051). No significant correlations between IgG subclasses and Ab-mediated NK cell activation were observed for the placental malaria cohort, suggesting that other Ab features in addition to IgG subclasses may contribute to the modulation of Ab-mediated NK cell activation.
These results show that IgG to DBL2 and DBL3 from malaria-exposed pregnant women can activate NK cells, but that DBL2- or DBL3-specific Ab-mediated NK cell activation does not appear to predict subsequent placental malaria.
Polyfunctional NK cell activation profiles in pregnant women with malaria
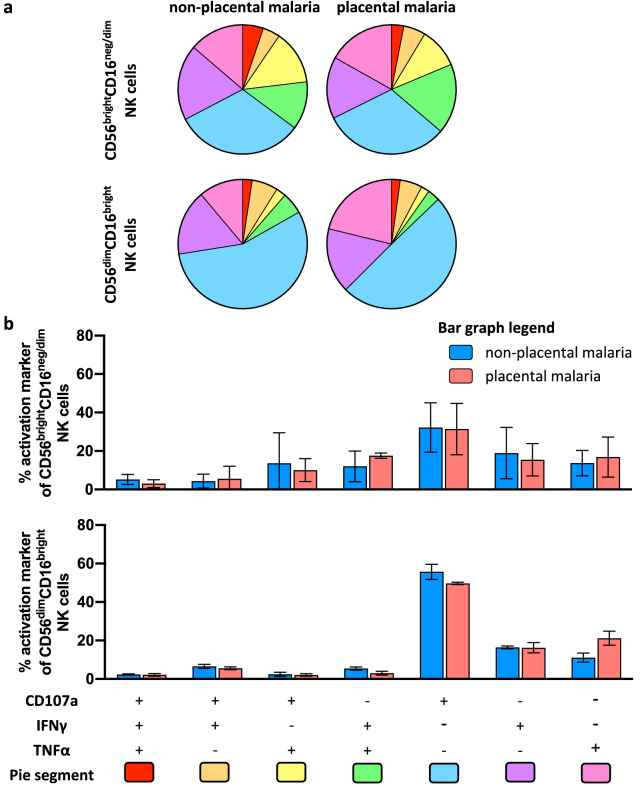
Although levels of individual DBL2- or DBL3-specific NK cell activation markers did not differ the groups of pregnant women with and without placental malaria, it is possible that the proportion of activated NK cells expressing multiple activation markers (“polyfunctional NK cell activation”) differed between the two groups of pregnant women. We therefore assessed the polyfunctional ability of NK cells to secrete TNFα, IFNγ and/or to express CD107a in different combinations. Activated NK cells were selected based on their CD56 and CD16 expression (CD56dimCD16bright and CD56brightCD16neg/dim) (Fig. S5). The levels of CD56 expression have been associated with NK effector function59. CD56bright NK cell subsets have been combined here due to low cell numbers, but are mainly characterized by their poor cytotoxic capacity and their high capacity to secrete several types of post-activation cytokines60. The CD56dimCD16bright NK cell population represents around 90% of peripheral blood NK cells and exhibit potent cytotoxic activity60,61.
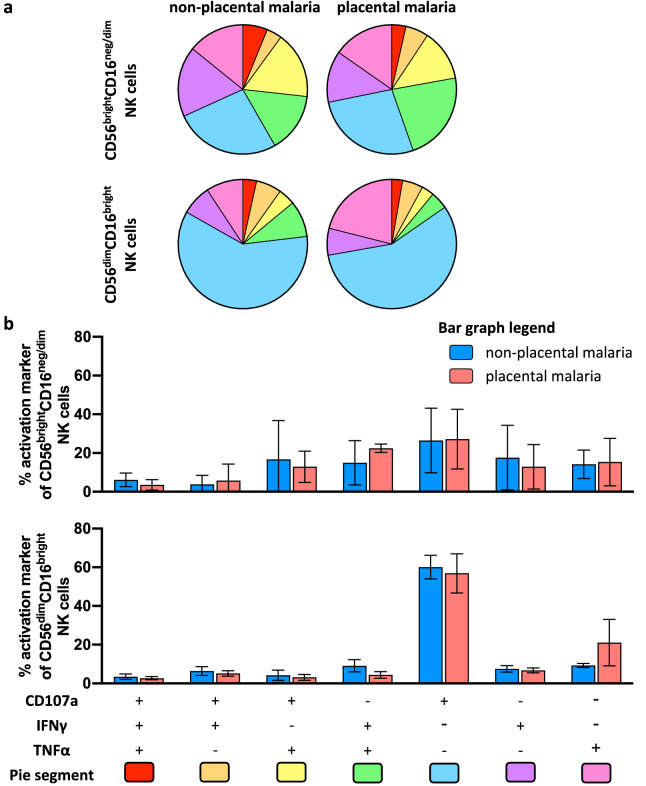
We observed only a small proportion of CD56dim NK cells which were polyfunctional as indicated by expression of two or more activation markers (DBL2: non-placental malaria: 23.1% of CD56dimCD16bright NK cells, placental malaria: 15.4%; DBL3: non-placental malaria: 16.8%, placental malaria: 12.8%). The majority of CD56dimCD16bright NK cells were associated with a single function, with a great portion of NK cells exclusively expressing CD107a (Figs. 4 and 5). The smaller subset of CD56brightCD16neg/dim NK cells (~ 1–5%) was more polyfunctional, more so for DBL2-specific responses than DBL3 (DBL2: non-placental malaria: 47.7% of CD56bright NK cells, placental malaria: 44.6%; DBL3: non-placental malaria: 35.1%, placental malaria: 36.2%) (Figs. 4 and 5).
Figure 4.
Polyfunctional responses of NK cells induced by DBL2-specific Abs from pregnant women with malaria. NK cells from three separate donors were stimulated with IgG from pregnant women mid pregnancy with non-placental malaria (N = 27), women with placental malaria (N = 50) at delivery in presence of DBL2 and assessed for expression of CD107a, IFNγ and TNFα. (a) NK cells were selected based on their CD56 expression (CD56dim and CD56bright). Pie and Bar charts show the proportion (b) and relative frequency (c) of each activation marker combination of only activated NK cells. (b) The pie segments correspond to NK cells expressing different combinations of activation markers and are color coded (pie segment legend: pink-red) to indicate increasing polyfunctional NK cell activation. (c) The bar graph shows relative frequencies of combinations of activation markers by NK cells stimulated with IgG from non-placental malaria-infected women (blue) or women with placental malaria (red) in presence of DBL2. Mean of three NK cell donors with standard deviation is shown. Statistical analysis between groups was performed using multiple t tests corrected for multiple comparisons using the Holm-Šídák method.
Figure 5.
Polyfunctional responses of NK cells induced by DBL3-specific Abs from pregnant women with malaria. NK cells from three separate donors were stimulated with IgG from pregnant women mid pregnancy with non-placental malaria (N = 27), women with placental malaria (N = 50) at delivery in presence of DBL2 and assessed for expression of CD107a, IFNγ and TNFα. NK cells were selected based on their CD56 expression (CD56dim and CD56bright). Pie and Bar charts show the proportion (a) and relative frequency (b) of each activation marker combination of only activated NK cells. (a) The pie segments correspond to NK cells expressing different combinations of activation markers and are color coded (pie segment legend: pink-red) to indicate increasing polyfunctional NK cell activation. (b) The bar graph shows relative frequencies of combinations of activation markers by NK cells stimulated with IgG from non-placental malaria-infected women (blue) or women with placental malaria (red) in presence of DBL3 (bottom bar graph). Mean of three NK cell donors with standard deviation is shown. Statistical analysis between groups was performed using multiple t tests corrected for multiple comparisons using the Holm-Šídák method.
The polyfunctional ability of IgG Abs from pregnant women with non-placental malaria or placental malaria to activate NK cells was not significantly different (Figs. 4b and 5b). These findings indicate that IgG Abs against DBL2 and DBL3 from malaria-exposed pregnant women can induce NK cells, with the main response being an upregulation of CD107a expression, but also with a small subset inducing polyfunctional NK cell responses.
Glycan profiles of IgG Abs in pregnant women with malaria are potentially masked by pregnancy
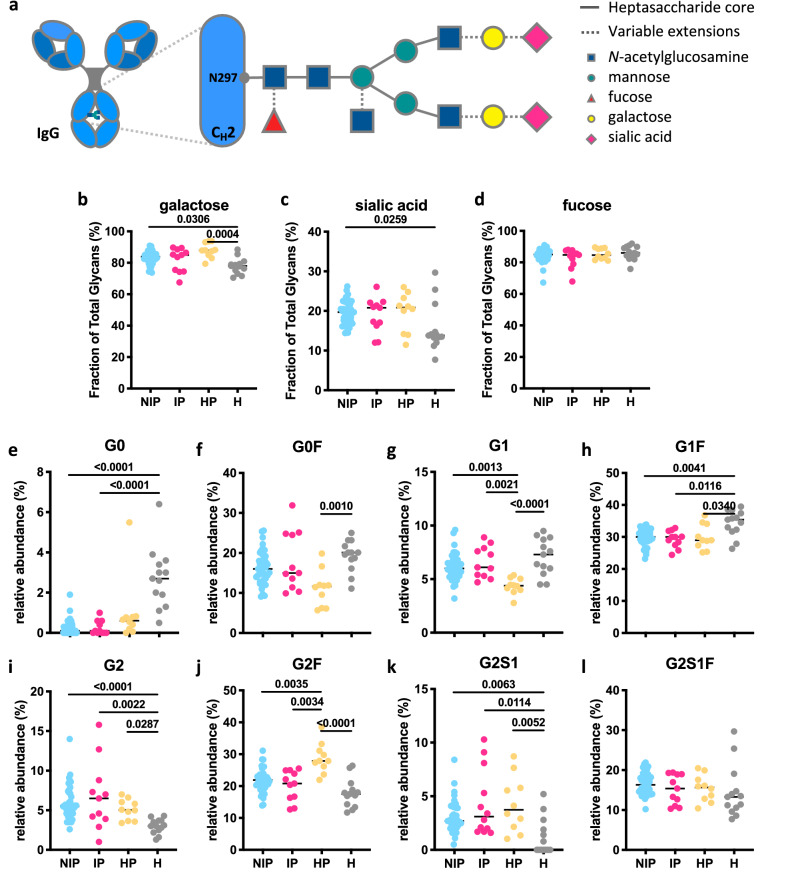
Another mechanism by which the immune system regulates Ab-mediated activation of innate immune cells is the modulation of glycosylation patterns of IgG Abs at a single asparagine residue at position 297 in the CH2 domain36. N-linked glycans are composed of a core complex biantennary structure of mannose and N-acetylglucosamine (GlcNAc) with variable additions of sugars such as fucose, galactose, sialic acid and bisecting GlcNAc (Fig. 6a)36. These post-translational modifications tune the affinity of IgG Abs for FcγRs, such as FcγRIIIa on NK cells, and regulate effector function25,62,63. We evaluated N-linked glycosylation patterns of IgG Abs in pregnant women using plasma collected at 14–26 weeks’ gestation. Samples from pregnant women with P. falciparum infection (N = 11) and uninfected pregnant women (N = 41) at enrolment were analyzed, together with malaria-naïve healthy pregnant women (N = 10) and non-pregnant women controls (N = 13). N-linked glycosylation profiles were analyzed via microchip capillary electrophoresis-laser-induced fluorescence. No statistically significant differences were observed between the two groups of pregnant women (Fig. 6b–l), suggesting that malaria infection in second trimester of pregnancy does not change the total IgG N-linked glycan profile. However, comparing N-linked glycosylation profiles of IgG from pregnant women, regardless of infection or malaria exposure status, with the profiles of uninfected non-pregnant women, the total IgG of pregnant women exhibited a higher degree of total galactosylation (Fig. 6b, median [IQR]: pregnant non-infected: 83.9% [80.9–86.3%], pregnant infected: 85.0% [75.0–87.4%], pregnant malaria-naïve healthy: 87.90% [86.1–90.6], non-pregnant malaria-naïve healthy: 78.0% [75.2–79.3]) and total sialylation (Fig. 6c, median [IQR]: pregnant non-infected: 19.7% [17.5–22.0%], pregnant infected: 20.8% [16.7–21.8%], pregnant malaria-naïve healthy: 20.8% [17.1–22.3], non-pregnant malaria-naïve healthy: 13.8% [13.3–14.7%]). No differences in total fucosylation were observed. Examining the distribution of specific glycan structures, significantly decreased proportions of G0 and G1F glycan structures were observed in pregnant women compared to non-pregnant women (Fig. 6e,h), whereas elevated proportions of G2 and G2S1, structures were observed in pregnant women compared to non-infected non-pregnant women (Fig. 6i–l). However, differences between malaria-naïve healthy pregnant women and malaria-exposed pregnant women were observed for G1 and G2F glycan structures (Fig. 6g,j), suggesting that malaria-naïve healthy pregnant women have slightly higher anti-inflammatory glycan structures (more galactose and fucose) in comparison to malaria-exposed pregnant women. Placental malaria infections may induce slightly more inflammatory glycan structures within pregnant women. However, no significant differences were observed for total galactose or fucose glycan structures were compared healthy pregnant control (Fig. 6b,d).
Figure 6.
Glycan profiles of IgG Abs in pregnant women with malaria. (a) Schematic representation of N-linked glycan composition of human IgG Abs. The glycans are attached to asparagine (N) at position 297 in the CH2 domain of IgG and have a biantennary heptasaccharide core (solid line) and variable extensions (dash line), such as fucose, galactose and/or sialic acid. Relative abundance of specific types of N-linked glycan structures of purified IgG Abs from non-infected pregnant women (NIP; N = 41; blue), pregnant women with malaria infection (IP; N = 11; pink), malaria-naïve healthy pregnant women (HP; N = 10; yellow) and uninfected healthy non-pregnant women (H; N = 13; grey) were profiled. % of Fc glycans with the presence of (b) galactose (monogalactosylated or digalactosylated), (c) sialic acid and (d) fucose. (e–l) The relative prevalence of several major glycan structures (G0 agalactosylated, G1 monogalactosylated, G2 digalactosylated, F fucosylated, S1 sialylated). Statistical comparison between groups was performed using a Kruskal–Wallis test corrected for multiple comparisons using Dunn’s multiple comparison method (p-values are shown on graphs).
N-linked glycosylation profiles of purified total IgG from pregnant women at 14–26 weeks of gestation were also assessed to determine if they could predict future clinical outcome (placental malaria status), with no significant differences observed (Fig. S6a–k). We did note that similar differences in N-linked glycosylation profiles were maintained between infected pregnant and non-infected non-pregnant women. Furthermore, DBL2- and DBL3-specific Ab-mediated expression of activation markers by NK cells and IgG N-linked glycosylation patterns of malaria-exposed pregnant women did not correlate (Fig. S6), except for DBL2-mediated IFNγ production and total fucose (ρ = − 0.4255, p-value = 0.0108) along with a trend for DBL2-mediated CD107a expression and total fucose (ρ = − 0.3744, p-value = 0.0543), and DBL2-mediated TNFα production and total galactose (ρ = 0.3625, p-value = 0.0323). This suggests that the N-linked glycosylation profiles of total IgG in pregnant women may be more influenced by pregnancy than by malaria infection.
Discussion
Naturally acquired immunity to malaria is complex, and likely requires a combination of cell-mediated and humoral immune responses, including the secretion of cytokines, cellular cytotoxicity, and production of functional Abs in order to efficiently clear parasites18,64. It has previously been shown that antigen-specific Ab-mediated phagocytosis and engagement with FcγRIIIa on NK cells are linked to protection by the sporozoite-based malaria vaccine RTS,S/AS0130. In addition, Ab-dependent NK cell cytotoxicity towards IEs in malaria-exposed individuals can inhibit P. falciparum growth31. Furthermore, adaptive NK cells, a sub-population of differentiated specialized NK cells, were associated with lower parasitemia and protection against malaria infection through enhanced ADCC response to IEs in the presence of naturally acquired Abs from malaria-resistant individuals32,65. The potential of NK cell-mediated ADCC to protect individuals against placental malaria is still to be determined. A limited number of studies have investigated NK cells in the placenta and in the blood at various timepoints during malaria infections66–69, however none have considered the implications of ADCC. Here, we demonstrated that Abs generated by pregnant women with malaria from a malaria endemic area in their second trimester against the VAR2CSA subdomains DBL2 or DBL3 were able to induce NK cell activation, but no significant differences in responses were associated with susceptibility to subsequent placental malaria. We observed that the majority of Ab-mediated NK cell activation in women with placental malaria was driven by IgG1, even though a recent study identified malaria-specific IgG1 and IgG3, and engagement with FcγRIIIa (linked to Ab-mediated NK cell activity), as key prediction parameters for protection in malaria RTS,S/AS01 vaccinees30. However, even though IgG3 shows high affinity to FcγRs and especially to FcγRIIIa, within this study, it did not correlate with NK cell activation in pregnant women. This suggests that IgG subclass distribution may not be the only factor that modulates NK cell activation during pregnancy, and that other IgG features, such as N-linked glycans may impact Ab-mediated NK cell activation. Currently, the importance of VAR2CSA-specific Ab-mediated responses in protection from placental malaria is unclear, as Ab responses to recombinant VAR2CSA antigens at delivery are associated with the presence of placental infection, and may represent markers of infection, rather than correlates of protection14. In addition, there is no malaria-specific antigen, which can be used as a universal antigenic control to assess blood stage parasite Ab-mediated NK cell responses.
Within our study, Ab-mediated activation of NK cells was largely associated with the upregulation of CD107a expression, a surrogate marker of ADCC activity56,70, with smaller fractions of activated NK cells producing only IFNγ and TNFα or in combination with CD107a, suggesting that the majority of Ab-activated NK cells were potentially cytotoxic in the absence of inflammation. We speculate that this balance of Ab-mediated activation may be beneficial, as excessive secretion of pro-inflammatory cytokines, such as IFNγ and TNFα, in the placenta of malaria-infected women, especially in primigravidae, has been associated with placental pathology and adverse clinical outcomes27,71. Placental malaria is associated with activation of pro-inflammatory host cells, such as monocytes and macrophages causing inflammation of the placenta8,72. Our findings suggest that Ab-mediated activation of NK cells, potentially does not contribute to the overproduction of pro-inflammatory cytokines and resulting pathologies. Several studies suggest that progesterone, estrogen and cortisol dampen NK cell cytotoxic activities during pregnancy73–75. However, the increased concentration of cortisol during pregnancy could also inhibit NK cell activity against P. falciparum IEs76. Here, NK cells purified from whole blood of malaria-naïve healthy donors were used instead of pregnant women with malaria, and effects of pregnancy-associated hormones were not represented by non-pregnant malaria-naïve healthy donor blood NK cells.
We would like to acknowledge limitations of our study. NK cells from healthy malaria-naïve donors were used instead of pregnant women with malaria. This could have skewed for specific NK cell subsets, which may be underrepresented during pregnancy. Our work studied peripheral NK cells which may have substantially different responses to uterine NK cells and adaptive NK cells. Uterine NK cells are functionally different, do not circulate outside the uterus, and are more difficult to access for functional studies77. The analysis of peripheral NK cells is however relevant in that they can access the site of infection, the syncytiotrophoblast cell surface lining of the maternal peripheral blood8. Nevertheless, in future studies, parasite loads in the placenta at birth, or other clinical markers of disease severity, could also be considered, but this information was not collected for the majority of individuals in this cohort.
One limitation regarding the plate-bound Ab-mediated NK cell assay is that it does not mimic the interaction between NK cell and IEs as shown before31,78. However, our study is complementary to current placental malaria vaccine studies, which also only use DBL2 antigens33,34, and in vitro NK cell ADCC assays could be used for high-throughput screens of serum samples32. Furthermore, additional activation markers of NK cell subpopulations, such as CD57, CD25, CD69 or the inhibitory receptor programmed death-1 (PD-1) could be considered for future studies23,78–80.
IgG N-linked glycosylation profiles can influence the engagement of IgG Abs with FcγRIIIa on NK cells36. Surprisingly, we did not observe any differences in Fc N-linked glycan profiles between pregnant women infected with P. falciparum and their non-infected counterparts. Consistent with previous studies45,46, we observed a higher degree of galactosylation and sialylation of IgG Abs from pregnant women, regardless of malaria infection, compared to non-pregnant women. Overall, these results suggest that anti-inflammatory Fc N-linked glycans are elevated in both healthy and malaria-exposed pregnant women, which may dampen the Ab-mediated activation of NK cells in pregnant women with malaria infection. These changes have been associated with a less inflammatory profile during pregnancy. Fc N-linked glycan patterns of IgG Abs can be globally modulated during the course of inflammation, autoimmune disease or pregnancy46,81. For example, in patients with lupus erythematosus or rheumatoid arthritis (RA), reduced galactosylation and sialylation of IgG Abs correlates with pro-inflammatory immune responses and disease severity82. Intriguingly, the majority of pregnant women with RA undergo pregnancy-induced remission, which occurs simultaneously with the upregulation of IgG galactosylation and sialylation, such that inflammatory RA-associated glycosylation patterns are masked by pregnancy83–85. We observe a similar increase in galactosylation and sialylation of the IgG Abs in pregnant women, regardless of malaria infection status46,81. We acknowledge that the malaria-naïve healthy pregnant women in our study are more progressed in their pregnancy, which could affect the glycosylation profiles. However, these Fc N-linked glycans may explain why secretion of pro-inflammatory cytokines was suppressed from NK cells within our assays.
In healthy pregnancy, highly galactosylated Abs may be more effectively transferred across the placenta and may be able to mediate CD107a degranulation of both maternal and cord NK cells86. The transfer of maternal Abs across the placenta is mediated by binding to the neonatal Fc receptor (FcRn), which is a key process for neonatal immunity, as neonates cannot sufficiently generate IgG Abs87. However, contradictory roles for IgG glycosylation on FcRn binding have been reported86,88,89, including studies which show significant Ab galactosylation-driven changes in FcRn affinity and NK cell-activating Abs are selectively transferred across the placenta86,88, while another study showed that placental IgG transport is not Fc glycosylation selective89. In addition, Jennewein et al. considered transfer of maternal Abs across the placenta via binding to FcRn and FcγRIIIa, while other more recent studies suggest that FcγRIIIa do not play a role in maternal–fetal Ab exchange87. Defining the mechanisms of placental transfer, including the role of Fc glycosylation, may offer novel insights for the rational development of maternal vaccines to enhance transfer of protective Abs to fetuses and reduce their vulnerability86,90, and should be considered in the further development of vaccine candidates.
A limitation of capillary electrophoresis-laser-induced fluorescence is that not all Fc N-linked glycan profiles are clearly detectable. However, these additional patterns make up only a small fraction of human IgG Fc N-linked glycans91, and more sensitive techniques such as liquid chromatography mass spectrometry require extensive protein clean up and in-solution digestion, in-depth proteome and glycoform analysis92. Furthermore, the evaluation of DBL2- and DBL3-specific IgG glycosylation profiles would allow us to more accurately assess the contribution of N-linked glycans to Ab-mediated NK cell activation, unfortunately these assays require large volumes of plasma samples25,93, which were not available for this cohort. Previous studies examining antigen-specific N-linked glycosylation of IgG from HIV-infected pregnant women have observed significantly different profiles between HIV, tetanus and pertussis toxin specific-IgG94, thus future studies where adequate sample is available should assess for malaria-specific IgG glycan patterns. Vaccine studies assessing healthy non-pregnant volunteers have demonstrated that antigen-specific IgG glycosylation profiles can be modulated by vaccination44. It is still unclear if antigen-specific IgG glycosylation profiles can be modulated in pregnant women, or if pregnancy-associated global glycan changes will mask any antigen-specific glycosylation effects as observed in RA83–85. Determining if antigen-specific Ab glycosylation patterns are associated with clinically relevant outcomes of placental malaria could inform the design of the next generation of maternal vaccines95. Overall, our study highlights the necessity to better understand Ab effector functions, such as Ab-mediated NK cell activation, and the potential effect of N-linked glycans modulation during pregnancy upon protection from, or susceptibility to, malaria and other infectious diseases.
Methods
Study participants
During a randomized controlled trial of Intermittent Preventive Treatment in Pregnancy (IPTp), plasma samples were collected from pregnant women in Madang Province, Papua New Guinea (PNG) between November 2009 and August 201296. Plasma samples were obtained upon enrolment into the prospective study at 14–26 gestation weeks, when pregnant women presented at the hospital for their first medical examination and were stored at − 80 °C. Infection status of pregnant women was determined at collection time point by light microscopy of Giemsa-stained peripheral blood smears (with malaria, N = 11; without malaria, N = 41, 25 pregnant women had unknown malaria infection status at sample collection).
In this cross-sectional study, parasitemia status was determined at delivery by light microscopy of Giemsa-stained peripheral blood smears, as well as by polymerase chain reaction of peripheral blood at delivery97. Samples were categorized based on the presence of P. falciparum parasites in peripheral blood at delivery. Groups included women who were positive for P. falciparum IEs in the placenta (placental malaria, N = 50) or women who were positive for P. falciparum IEs in peripheral blood but did not show any sequestering of IEs in the placenta (non-placental malaria, N = 27) (Fig. 1). The groups were frequency matched for primigravidae, age, bed net use, rural residency and type of malaria preventive treatment received (Table 1). Ethical approval was obtained from the PNG Institute of Medical Research Institutional Review Board, the PNG Medical Research Advisory Council, and the Melbourne Health Human Research Ethics Committee.
Table 1.
Characteristics of study participants.
| Characteristic | Placental malaria N = 50 |
Non-placental malaria N = 27 |
|---|---|---|
| Age (years) | 24.6 [5.24] | 23.7 [5.05] |
| Gravidity | ||
| Primigravidae | 29 (58) | 14 (51.9) |
| Secundigravidae | 7 (14) | 8 (29.6) |
| Multigravidae | 14 (28) | 5 (18.5) |
| Mean gestational age (days) | 147.5 [31.3] | 152.2 [19.8] |
| Mean maternal weight (kg) | 53.5 [8.2] | 54.1 [7.4] |
| Mean maternal height (cm) | 154.6 [6.9] | 148.4 [32] |
| IPTp regime | ||
| SPAZ | 20 (40.0) | 9 (33.3) |
| SPCQ | 30 (60.0) | 18 (66.7) |
| Residence | ||
| Urban | 6 (12) | 2 (7.4) |
| Periurban | 4 (8) | 7 (25.9) |
| Rural | 38 (76) | 18 (66.7) |
| Migrant | 2 (4) | 0 (0) |
| Bed net use | 40 (80) | 21 (77.8) |
Data shown as mean [standard deviation], or number (%).
IPTp intermittent preventive treatment in pregnancy, SPAZ sulphadoxine-pyrimethamine and azithromycin, SPCQ sulphadoxine-pyrimethamine + chloroquine.
Plasma samples from malaria-naïve healthy Melbourne donors (N = 8) were chosen as negative controls, because many matched women from PNG would have been exposed to malaria and skewed negative responses. For the Fc N-linked IgG glycan profiling, samples of malaria-naïve healthy non-pregnant women (N = 13) were used (age: 34.3 ± 7.7 years). Plasma samples from individual healthy Melbourne donors were obtained in accordance with the University of Melbourne Human ethics approval (#1443420) and the Australian National Health and Medical Research Council Statement on Ethical Conduct in Human Research. Samples from malaria-naïve healthy pregnant women at the end of their second/beginning of their third trimester (N = 10) were obtained to compare pregnancy-specific Fc N-linked IgG glycan profiles (age: 31.3 ± 2.8 years; mean gestational age: 196 ± 4 days). Ethical approval was granted by the Mercy Health Board Human Research Ethics Committee (R10/16). All participants provided written informed consent.
IgG antibody purification
IgG Abs were purified from plasma of pregnant women at enrolment according to manufacturer’s protocol via Melon Gel chromatography (Melon Gel IgG Purification Kit, Thermo Fisher Scientific, USA)98. IgG Ab samples were centrifugated through 100 kDa Amicon Ultra filters (Merck & Co, USA) at 14,000 × g for 10 min to remove excess albumin proteins and buffer exchanged into phosphate buffered saline (PBS). The IgG concentration and purity were quantitated using a human IgG ELISA development kit (Mabtech AB, Sweden). The IgG Ab samples were diluted in PBS to adjust Ab concentration to 0.25 mg/ml for Ab-dependent NK cell activation assays and 2 mg/ml for N-linked glycan profiling. The samples were stored at − 20 °C until further use.
Natural killer cell isolation
NK cells were isolated from heparinized whole blood from malaria-naïve healthy donors with RosetteSep (Stemcell Technologies, Canada) and density gradient separation via Ficoll (Bio-Strategy Lab, Australia) centrifugation according to manufacturer’s protocols.
Antibody-dependent natural killer cell activation
Ab-dependent plate-bound NK cell activation assays were modified for use with DBL antigens24–26,99. In order to compare NK cell activation induced by DBL domains and controls, various IgG Ab (0.0625–0.25 mg/ml) and protein (50–300 ng/well) concentrations were tested. Purified IgG was used to control for IgG concentration for each individual sample and to ensure no other components in plasma contributed to activation of NK cells. Concentrations of 200 ng of protein/well and 0.25 mg/ml of IgG Abs were chosen to test individual samples.
High protein-binding plates (NUNC MaxiSorp flat bottom; Thermo Fisher Scientific) were coated with either DBL233 or DBL3100 (200 ng/well) at 4 °C for 12 h. Bovine serum albumin (BSA; Sigma-Aldrich, USA) was used to control for unspecific binding. Simian immunodeficiency virus (SIV) envelope protein gp120 (Sino Biological Inc., China) and influenza hemagglutinin (H)3 (A/Switzerland/9715293/2013; Immune Technology Corp., USA) were used as negative and positive antigen controls, respectively. H3 was selected as a universal technical control, as all individuals have been previously exposed to influenza, and Abs to H3 are highly cross reactive and strong inducers of NK cell activation24.
After washing with PBS, the plate was blocked with 1% PBS-BSA for 1 h. Purified IgG (0.25 mg/ml) was added to each well and incubated at 37 °C for 2 h. NK cells (0.25 × 106 cells/ml) were then incubated with anti-CD107a-APC-H7 (BD, USA), brefeldin A (10 mg/ml; Sigma-Aldrich) and GolgiStop (BD) for 5 h at 37 °C. NK cells were stained for surface markers using anti-FcγRIII–BV605 (BD), anti-CD56-BUV737 (BD), anti-FcγRII-APC (BioLegend, USA) and anti-CD3-PerCP (BD), and intracellularly with anti-IFNγ-PE (BD) and anti-TNFα-BV785 (BD) via fixation with 10% paraformaldehyde and Perm B solutions (Thermo Fisher Scientific). NK cells were analyzed via flow cytometry and combination gates in FlowJo (BD) were used to include all NK cells expressing activation marker CD107a (degranulation marker), IFNγ and TNFα (cytokines). Mean fluorescence intensity (MFI) of NK cells incubated without Abs was subtracted as background to determine Ab-mediated activation and median of SIV gp120-specific responses was subtracted as non-specific NK cell activation.
Multiplex assays of antibodies binding to Duffy binding-like domains
DBL2 and DBL3 domains were coupled to Bio-Plex magnetic carboxylated microspheres (Bio-Rad, Hercules, USA) as per manufacturer’s instructions. The antigen-coupled microspheres were resuspended in storage buffer (PBS, 0.05% sodium azide), and stored in the dark at 4 °C for immediate use. Their concentration was determined using a hemocytometer.
The DBL-coupled microspheres were mixed, resuspended in 1% PBS-BSA and added to wells of a 96-well round bottom plate (Greiner Bio-One, Kremsmünster, Austria) containing plasma in a 1:100 dilution in PBS. The sealed plates were incubated on a plate shaker overnight at 4 °C. After incubation, the plates were centrifuged and washed with PBS-0.1% Tween using a magnetic plate-washer (Bio-Plex Pro wash station, Bio-Rad). The anti-human Ab (total IgG, IgG1, IgG2, IgG3, IgG4) detectors conjugated with phycoerythrin (PE; all SouthernBiotech, Birmingham, USA) were added and the mixture was incubated for 2 h on a plate shaker. After washing with PBS and resuspending in xMAP drive fluid (Life Technologies, Carlsbad, USA), the plates were read on a Bio-Plex MAGPIX multiplex reader (Bio-Rad), and analysed using Bio-Plex Manager software (Bio-Rad). The median fluorescence intensity is directly proportional to the amount of Ab bound to the antigens101.
IgG N-linked glycan profiling
N-linked glycan profiles of purified IgG Abs (2 mg/ml) were measured on the LabChip GXII Touch instrument (PerkinElmer, USA) according to the ProfilerPro glycan profiling LabChip GXII Touch protocol. Microchip capillary electrophoresis-laser-induced fluorescence analysis of digested and labelled N-linked glycans was performed. The relative prevalences of several glycan profiles of IgG Abs were analyzed using the LabChip GX Reviewer (PerkinElmer) software. Peaks were assigned based on migration of known standards and glycan digests91. Peak area and therefore the relative prevalence of each glycan pattern was calculated.
Statistical methods
Statistical analyses were performed in Prism version 8 (GraphPad, USA). Statistical comparison of NK cell activation markers between groups was performed using Kruskal–Wallis test with Dunn’s multiple comparison method. Statistical comparison between groups for the analysis of activated NK cells polyfunctionality was performed using multiple t tests corrected for multiple comparisons using the Holm-Šídák method. Spearman's rank correlation coefficients of antigen binding and NK cell activation were calculated. Kruskal–Wallis tests with Dunn’s multiple comparison method were conducted to determine the significance of differences observed in glycan prevalence between pregnant women and their non-pregnant counterparts. Statistical significance was considered when p-values were less than 0.05.
Supplementary Information
Acknowledgements
We thank the study participants who took part in this study, the staff of the PNG Institute of Medical Research, particularly Dr Sarah Hanieh, Dr Regina Wangnapi, Dr Maria Ome-Kaius and the nurses, midwives, health extension officers and other staff of the IPTp study team. We also thank Dr Patrick Duffy and Dr David Narum (Laboratory of Malaria Immunology and Vaccinology at National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, Maryland, United States) for providing DBL3 protein.
Author contributions
T.D. designed the study, performed experiments, analyzed data and wrote the original manuscript draft; E.H.A. designed the study, W.H. performed antibody purifications; M.K. and E.L. assisted with experimental procedures for glycan profiling; H.W.U., A.S., E.M., K.K. and M.L. collected plasma samples; A.S. provided DBL2 protein, S.J.K. designed the study; S.J.R. and A.W.C. conceptualized and designed the study; all author assisted with manuscript editing.
Funding
This work was supported by a Project Grant from the National Health and Medical Research Council of Australia (NHMRC) to SJR, EHA and AWC (APP1143946) and a Program Grant (APP1092789) also from the NHMRC. AWC is supported by an NHMRC Career Development Fellowship (APP1140509). Sample collection was primarily supported by the Malaria in Pregnancy Consortium, through a Grant from the Bill & Melinda Gates Foundation (46099). AS, EM and ML were funded by the Medical Research Foundation for Women and Babies and the Mercy Research Foundation. KK is supported by an NHMRC Investigator Fellowship (1173871). KK and AWC are supported by the University of Melbourne Dame Kate Campbell Fellowship.
Data availability
Derived data supporting the findings of this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83093-4.
References
- 1.Sharma L, Shukla G. Placental malaria: A new insight into the pathophysiology. Front. Med. 2017;4:117. doi: 10.3389/fmed.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai M, et al. Epidemiology and burden of malaria in pregnancy. Lancet. Infect. Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 3.Ataíde R, Mayor A, Rogerson SJ. Malaria, primigravidae, and antibodies: Knowledge gained and future perspectives. Trends. Parasitol. 2014;30:85–94. doi: 10.1016/j.pt.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Wahlgren M, Goel S, Akhouri RR. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat. Rev. Microbiol. 2017;15:479–491. doi: 10.1038/nrmicro.2017.47. [DOI] [PubMed] [Google Scholar]
- 5.Smith JD. The role of PfEMP1 adhesion domain classification in Plasmodium falciparum pathogenesis research. Mol. Biochem. Parasitol. 2014;195:82–87. doi: 10.1016/j.molbiopara.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeson JG, Reeder JC, Rogerson SJ, Brown GV. Parasite adhesion and immune evasion in placental malaria. Trends. Parasitol. 2001;17:331–337. doi: 10.1016/S1471-4922(01)01917-1. [DOI] [PubMed] [Google Scholar]
- 8.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: Pathogenesis and immunity. Lancet. Infect. Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 9.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 10.Salanti A, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JD, Rowe JA, Higgins MK, Lavstsen T. Malaria's deadly grip: Cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2013;15:1976–1983. doi: 10.1111/cmi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salanti A, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 13.Bockhorst J, et al. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol. Biochem. Parasitol. 2007;155:103–112. doi: 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Cutts JC, et al. Pregnancy-specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: A systematic review. BMC Med. 2020;18:14. doi: 10.1186/s12916-019-1467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Megnekou R, Staalsoe T, Taylor DW, Leke R, Hviid L. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infect. Immun. 2005;73:4112–4118. doi: 10.1128/IAI.73.7.4112-4118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keen J, et al. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Med. 2007;4:e181. doi: 10.1371/journal.pmed.0040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott SR, et al. Placental malaria induces variant-specific antibodies of the cytophilic subtypes immunoglobulin G1 (IgG1) and IgG3 that correlate with adhesion inhibitory activity. Infect. Immun. 2005;73:5903–5907. doi: 10.1128/IAI.73.9.5903-5907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards JS, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin. Infect. Dis. 2010;51:50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 19.Damelang T, Rogerson SJ, Kent SJ, Chung AW. Role of IgG3 in infectious diseases. Trends. Immunol. 2019;40:23–37. doi: 10.1016/j.it.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Kana IH, et al. Cytophilic antibodies against key Plasmodium falciparum blood stage antigens contribute to protection against clinical malaria in a high transmission region of Eastern India. J. Infect. Dis. 2018;218:956–965. doi: 10.1093/infdis/jiy258. [DOI] [PubMed] [Google Scholar]
- 21.Osier, F. H. A. et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: Mechanism in human immunity and a correlate of protection against malaria. BMC Med.12. 10.1186/1741-7015-12-108 (2014). [DOI] [PMC free article] [PubMed]
- 22.Burrack KS, Hart GT, Hamilton SE. Contributions of natural killer cells to the immune response against Plasmodium. Malar. J. 2019;18:1–9. doi: 10.1186/s12936-019-2953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf, A.-S., Sherratt, S. & Riley, E. M. NK cells: Uncertain allies against malaria. Front. Immunol.8. 10.3389/fimmu.2017.00212 (2017). [DOI] [PMC free article] [PubMed]
- 24.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J. Virol. 2013;87:5512–5522. doi: 10.1128/JVI.03030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu LL, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167:433–443. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristensen AB, et al. Antibody responses with Fc-mediated functions after vaccination of HIV-infected subjects with trivalent influenza vaccine. J. Virol. 2016;90:5724–5734. doi: 10.1128/JVI.00285-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King T, Lamb T. Interferon-γ: The Jekyll and Hyde of malaria. PLoS Pathog. 2015;11:e1005118. doi: 10.1371/journal.ppat.1005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogerson SJ, et al. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 2003;68:115–119. doi: 10.4269/ajtmh.2003.68.1.0680115. [DOI] [PubMed] [Google Scholar]
- 29.Boeuf P, et al. Plasmodium falciparum malaria elicits inflammatory responses that dysregulate placental amino acid transport. PLoS Pathog. 2013;9:e1003153–e1003153. doi: 10.1371/journal.ppat.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suscovich TJ, et al. Mapping functional humoral correlates of protection against malaria challenge following RTS, S/AS01 vaccination. Sci. Transl. Med. 2020;12:eabb4757. doi: 10.1126/scitranslmed.abb4757. [DOI] [PubMed] [Google Scholar]
- 31.Arora G, et al. NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. eLife. 2018;7:e36806. doi: 10.7554/eLife.36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart GT, et al. Adaptive NK cells in people exposed to Plasmodium falciparum correlate with protection from malaria. J. Exp. Med. 2019;216:1280–1290. doi: 10.1084/jem.20181681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mordmüller B, et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin. Infect. Dis. 2019;69:1509–1516. doi: 10.1093/cid/ciy1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sirima SB, et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: A first-in-human, randomised, double-blind, placebo-controlled study. Lancet. Infect. Dis. 2020;20:585–597. doi: 10.1016/S1473-3099(19)30739-X. [DOI] [PubMed] [Google Scholar]
- 35.Arnold KB, Chung AW. Prospects from systems serology research. Immunology. 2018;153:279–289. doi: 10.1111/imm.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidarsson, G., Dekkers, G. & Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol.5, 10.3389/fimmu.2014.00520 (2014). [DOI] [PMC free article] [PubMed]
- 37.Baković MPI, et al. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 2013;12:821–831. doi: 10.1021/pr300887z. [DOI] [PubMed] [Google Scholar]
- 38.Menni C, et al. Glycosylation of immunoglobulin g: Role of genetic and epigenetic influences. PLoS ONE. 2013;8:e82558. doi: 10.1371/journal.pone.0082558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akmačić IT, et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis. 2015;21:1237–1247. doi: 10.1097/MIB.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wuhrer M, et al. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflamm. 2015;12:235. doi: 10.1186/s12974-015-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardinassi LG, et al. Clinical severity of visceral leishmaniasis is associated with changes in immunoglobulin G Fc N-glycosylation. mBio. 2014;5:e01844–e11814. doi: 10.1128/mBio.01844-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ackerman ME, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLean, M. R., Lu, L. L., Kent, S. J. & Chung, A. W. An inflammatory story: Antibodies in tuberculosis comorbidities. Front. Immunol.10. 10.3389/fimmu.2019.02846 (2019). [DOI] [PMC free article] [PubMed]
- 44.Mahan AE, et al. Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog. 2016;12:e1005456. doi: 10.1371/journal.ppat.1005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dekkers G, Rispens T, Vidarsson G. Novel concepts of altered immunoglobulin G galactosylation in autoimmune diseases. Front. Immunol. 2018;9:553–553. doi: 10.3389/fimmu.2018.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen BC, et al. MALDI-TOF-MS reveals differential N-linked plasma-and IgG-glycosylation profiles between mothers and their newborns. Sci. Rep. 2016;6:34001. doi: 10.1038/srep34001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowden TA, et al. Chemical and structural analysis of an antibody folding intermediate trapped during glycan biosynthesis. J. Am. Chem. Soc. 2012;134:17554–17563. doi: 10.1021/ja306068g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zenclussen AC, Gentile T, Margni R, Kortebani G, Mazzolli A. Asymmetric antibodies and pregnancy. Am. J. Reprod. Immunol. 2001;45:289–294. doi: 10.1111/j.8755-8920.2001.450504.x. [DOI] [PubMed] [Google Scholar]
- 49.Bondt A, et al. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol. Cell. Proteom. 2014;13:3029. doi: 10.1074/mcp.M114.039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temming AR, et al. Functional attributes of antibodies, effector Cells, and target cells affecting NK cell-mediated antibody-dependent cellular cytotoxicity. J. Immunol. 2019;203:3126–3135. doi: 10.4049/jimmunol.1900985. [DOI] [PubMed] [Google Scholar]
- 51.Dekkers G, et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front. Immunol. 2017;8:877. doi: 10.3389/fimmu.2017.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niwa R, et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Methods. 2005;306:151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Kapur R, Einarsdottir HK, Vidarsson G. IgG-effector functions: "The Good, The Bad and The Ugly". Immunol. Lett. 2014;160:139–144. doi: 10.1016/j.imlet.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 55.Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 56.Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J. Immunol. 2009;182:1202–1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 57.Chung AW, et al. Viral control in chronic HIV-1 subtype C infection is associated with enrichment of p24 IgG1 with Fc effector activity. AIDS. 2018;32:1207. doi: 10.1097/QAD.0000000000001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambert LH, et al. Antigen reversal identifies targets of opsonizing IgGs against pregnancy-associated malaria. Infect. Immun. 2014;82:4842–4853. doi: 10.1128/IAI.02097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the immune system: More than a marker for cytotoxicity? Front. Immunol. 2017;8:892–892. doi: 10.3389/fimmu.2017.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper MA, et al. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.V97.10.3146. [DOI] [PubMed] [Google Scholar]
- 61.Ferlazzo G, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J. Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 62.Herter S, et al. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J. Immunol. 2014;192:2252–2260. doi: 10.4049/jimmunol.1301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung AW, et al. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS. 2014;28:2523. doi: 10.1097/QAD.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gowda, D. & Wu, X. Parasite recognition and signaling mechanisms in innate immune responses to malaria. Front. Immunol.9, 10.3389/fimmu.2018.03006 (2018). [DOI] [PMC free article] [PubMed]
- 65.Tukwasibwe S, et al. Variations in killer-cell immunoglobulin-like receptor and human leukocyte antigen genes and immunity to malaria. Cell Mol. Immunol. 2020;17:799–806. doi: 10.1038/s41423-020-0482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sartelet H, et al. Less HLA-G expression in Plasmodium falciparum-infected third trimester placentas is associated with more natural killer cells. Placenta. 2005;26:505–511. doi: 10.1016/j.placenta.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Ordi J, et al. Placental malaria is associated with cell-mediated inflammatory responses with selective absence of natural killer cells. J. Infect. Dis. 2001;183:1100–1107. doi: 10.1086/319295. [DOI] [PubMed] [Google Scholar]
- 68.Othoro C, et al. Elevated gamma interferon-producing NK cells, CD45RO memory-like T cells, and CD4 T cells are associated with protection against malaria infection in pregnancy. Infect. Immun. 2008;76:1678–1685. doi: 10.1128/IAI.01420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Omosun YO, et al. Differential association of gene content polymorphisms of killer cell immunoglobulin-like receptors with placental malaria in HIV− and HIV+ mothers. PLoS ONE. 2012;7:e38617. doi: 10.1371/journal.pone.0038617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Maestre A, Carmona-Fonseca J. Immune responses during gestational malaria: A review of the current knowledge and future trend of research. J. Infect. Dev. Countr. 2014;8:391–402. doi: 10.3855/jidc.3777. [DOI] [PubMed] [Google Scholar]
- 72.Ortega-Pajares, A. & Rogerson, S. J. The rough guide to monocytes in malaria infection. Front. Immunol.9. 10.3389/fimmu.2018.02888 (2018). [DOI] [PMC free article] [PubMed]
- 73.Arruvito L, et al. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J. Immunol. 2008;180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- 74.Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30) Clin. Exp. Immunol. 2005;139:287–296. doi: 10.1111/j.1365-2249.2004.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bouyou-Akotet MK, et al. Depressed Natural Killer cell cytotoxicity against Plasmodium falciparum-infected erythrocytes during first pregnancies. Clin. Infect. Dis. 2004;38:342–347. doi: 10.1086/380646. [DOI] [PubMed] [Google Scholar]
- 76.Le Gars, M. et al. Pregnancy-induced alterations in NK cell phenotype and function. Front. Immunol.10. 10.3389/fimmu.2019.02469 (2019). [DOI] [PMC free article] [PubMed]
- 77.Sojka DK, Yang L, Yokoyama WM. Uterine natural killer cells. Front. Immunol. 2019;10:960–960. doi: 10.3389/fimmu.2019.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moebius J, et al. PD-1 expression on NK cells in malaria-exposed individuals is associated with diminished natural cytotoxicity and enhanced antibody-dependent cellular cytotoxicity. Infect. Immun. 2020;88:e00711–00719. doi: 10.1128/IAI.00711-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez-Vergès S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walk, J. & Sauerwein, R. W. Activatory receptor NKp30 predicts NK cell activation during controlled human malaria infection. Front. Immunol.10. 10.3389/fimmu.2019.02864 (2019). [DOI] [PMC free article] [PubMed]
- 81.Bondt A, et al. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J. Proteome. Res. 2013;12:4522–4531. doi: 10.1021/pr400589m. [DOI] [PubMed] [Google Scholar]
- 82.Biermann M, et al. Sweet but dangerous—The role of immunoglobulin G glycosylation in autoimmunity and inflammation. Lupus. 2016;25:934–942. doi: 10.1177/0961203316640368. [DOI] [PubMed] [Google Scholar]
- 83.Rook G, et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J. Autoimmun. 1991;4:779–794. doi: 10.1016/0896-8411(91)90173-A. [DOI] [PubMed] [Google Scholar]
- 84.van de Geijn FE, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis. Res. Ther. 2009;11:R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hazes JMW, et al. Rheumatoid arthritis and pregnancy: Evolution of disease activity and pathophysiological considerations for drug use. Rheumatology. 2011;50:1955–1968. doi: 10.1093/rheumatology/ker302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jennewein MF, et al. Fc glycan-mediated regulation of placental antibody transfer. Cell. 2019;178:202–215.e214. doi: 10.1016/j.cell.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borghi S, et al. FcRn, but not FcgRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc. Natl. Acad. Sci. USA. 2020;117:12943–12951. doi: 10.1073/pnas.2004325117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dashivets T, et al. Multi-angle effector function analysis of human monoclonal IgG glycovariants. PLoS ONE. 2015;10:e0143520. doi: 10.1371/journal.pone.0143520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Einarsdottir HK, et al. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj. J. 2013;30:147–157. doi: 10.1007/s10719-012-9381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rice TF, Holder B, Kampmann B. Antibody glycosylation in pregnancy and in newborns: Biological roles and implications. Curr. Opin. Infect. Dis. 2020;33:225–230. doi: 10.1097/QCO.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 91.Mahan AE, et al. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. J. Immunol. Methods. 2015;417:34–44. doi: 10.1016/j.jim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hughes CS, et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019;14:68–85. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 93.Brown EP, et al. Microscale purification of antigen-specific antibodies. J. Immunol. Methods. 2015;425:27–36. doi: 10.1016/j.jim.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez DR, et al. Fc characteristics mediate selective placental transfer of IgG in HIV-infected women. Cell. 2019;178:190–201.e111. doi: 10.1016/j.cell.2019.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saso, A. & Kampmann, B. Maternal immunization: Nature meets nurture. Front. Microbiol.11. 10.3389/fmicb.2020.01499 (2020). [DOI] [PMC free article] [PubMed]
- 96.Unger HW, et al. Sulphadoxine-pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: A randomised controlled trial. BMC Med. 2015;13:9. doi: 10.1186/s12916-014-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lufele E, et al. Risk factors and pregnancy outcomes associated with placental malaria in a prospective cohort of Papua New Guinean women. Malar. J. 2017;16:427. doi: 10.1186/s12936-017-2077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopez, E. et al. Low pH exposure during immunoglobulin G purification methods results in aggregates that avidly bind Fcγ receptors: Implications for measuring Fc dependent antibody functions. Front. Immunol.10. 10.3389/fimmu.2019.02415 (2019). [DOI] [PMC free article] [PubMed]
- 99.Vanderven HA, et al. Antibody-dependent cellular cytotoxicity (ADCC) responses to seasonal influenza vaccination in older adults. J. Infect. Dis. 2017;217:12–23. doi: 10.1093/infdis/jix554. [DOI] [PubMed] [Google Scholar]
- 100.Avril M, Cartwright MM, Hathaway MJ, Smith JD. Induction of strain-transcendent antibodies to placental-type isolates with VAR2CSA DBL3 or DBL5 recombinant proteins. Malar. J. 2011;10:36. doi: 10.1186/1475-2875-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taylor DW, et al. Measuring antibody avidity to Plasmodium falciparum merozoite antigens using a multiplex immunoassay approach. Malar. J. 2020;19:171–171. doi: 10.1186/s12936-020-03243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author upon request.