Abstract
Statin intolerance, primarily myalgia, is not uncommon in patients treated for elevated low-density lipoprotein cholesterol. Nonstatin drugs, such as ezetimibe, can spare patients from statin exposure, while still reducing low-density lipoprotein cholesterol. Ezetimibe is generally very well tolerated, although gastrointestinal and musculoskeletal symptoms have been occasionally reported. We describe an extremely rare case of an ezetimibe-associated liver injury who required protracted treatment with prednisone and azathioprine. Ezetimibe-associated liver injury should be suspected with development of hepatic abnormalities concurrent with the timing of ezetimibe treatment and in the absence of other possible precipitating factors.
Résumé
L’intolérance aux statines, se manifestant principalement par une myalgie, n’est pas rare chez les patients traités pour des taux élevés de cholestérol à lipoprotéines à basse densité (LDL). Des médicaments n’appartenant pas à la classe des statines, comme l’ézétimibe, permettent d’éviter l’exposition aux statines chez les patients, tout en réduisant le taux le cholestérol LDL. L’ézétimibe est généralement bien toléré, bien que des symptômes gastro-intestinaux et musculosquelettiques aient été signalés à l’occasion. Nous décrivons un cas extrêmement rare de lésion hépatique associée à l’ézétimibe ayant nécessité un traitement prolongé par la prednisone et l’azathioprine. Il faut soupçonner une lésion hépatique liée à l’ézétimibe lors de la survenue d’anomalies hépatiques pendant le traitement par l’ézétimibe en l’absence d’autres facteurs déclencheurs.
Statins are the cornerstone therapy to reduce low-density lipoprotein (LDL) cholesterol levels and its associated risk of atherosclerotic cardiovascular disease.1 However, statin intolerance, primarily statin-associated muscle symptoms affect 2.5%-5% of patients.2 Nonstatin drugs are thus essential to spare affected patients from statin exposure, while still reducing LDL cholesterol.1 Treatment guidelines endorse the use of both ezetimibe and inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9) as safe and effective second-line LDL-lowering treatments to consider in response to statin intolerance.1 Ezetimibe lowers LDL cholesterol by blocking sterol uptake via the Niemann-Pick C1-like 1 protein (NPC1L1) in the upper small intestine.3 It has been available in Canada since 2003. Ezetimibe is generally very well tolerated, although gastrointestinal and musculoskeletal symptoms have been reported in approximately 0.1%-2% of patients.4, 5, 6, 7 Although no cases of ezetimibe-induced liver injury were observed in randomized clinical trials, there are a few case reports of this complication.4, 5, 6, 7 We describe an extremely rare case of an ezetimibe-associated liver injury.
Case Report
A 59-year-old asymptomatic man of European descent was referred to the lipid clinic with high LDL cholesterol levels. His medical history was positive only for appendectomy and he was not taking any medication including neither natural health products nor supplements. He had no history of the use of either alcohol or recreational drugs. His family history was positive for dyslipidemia in 2 brothers, one of whom died of a myocardial infarction at age 67. The patient’s father and mother each also died from sudden cardiac events at ages 62 and 80, respectively. On physical examination, his height was 180 cm, weight was 81.5 kg, body mass index (BMI) was 25.2 kg/m2, supine blood pressure was 104/60 mm Hg, and radial pulse was 75 beats/min. No physical stigmata of familial hypercholesterolemia were detected, that is, no xanthomas, xanthelasmas, or corneal arcus. There was no hepatosplenomegaly, and cardiovascular examination was normal.
His lipid profile showed total cholesterol, triglyceride, high-density lipoprotein cholesterol, and LDL cholesterol of 5.62, 1.12, 1.02, and 4.09 mmol/L, respectively. His apolipoprotein B level was 1.41 g/L (target < 0.8 g/L) and lipoprotein (a) was normal at < 10 mg/dL.1 His 2008 Framingham 10-year risk of cardiovascular disease was 11.2%.1 Baseline liver enzymes included alanine transaminase (ALT) 54 U/L (normal < 46 U/L), aspartate transaminase (AST) 33 U/L (normal < 37 U/L), bilirubin 10.2 μmol/L (normal 3.4-17.1 μmol/L), and alkaline phosphatase (ALP) 68 U/L (normal 40-129 U/L). Creatine kinase was 94 U/L (normal < 190 U/L). His mean carotid intima medial thickness was at the 50th percentile for age and sex. Targeted DNA sequencing found no pathogenic familial hypercholesterolemia mutation but showed a high polygenic score for LDL cholesterol (unweighted 15/20, or the 92nd percentile). His clinical and biochemical pictures were thus consistent with polygenic hypercholesterolemia, with a positive family history of early atherosclerotic cardiovascular disease and intermediate Framingham cardiovascular risk.
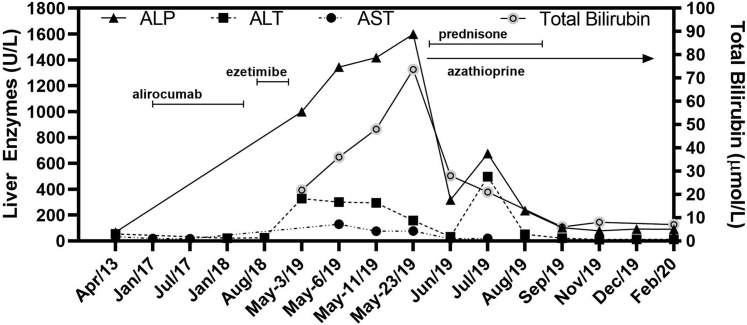
The patient was prescribed atorvastatin 20 mg daily but discontinued this treatment after 8 weeks because of severe myalgia, with normal creatine kinase at 103 U/L. After discussion, his preference was to avoid another statin and instead to try the PCSK9 inhibitor alirocumab 75 mg subcutaneously biweekly. As monotherapy, this treatment effectively reduced his plasma LDL cholesterol level to 1.41 mmol/L (55 mg/dL) within 8 weeks with no adverse effects (Fig. 1). Unfortunately, after 18 months, the patient's medical insurer declined to continue coverage of alirocumab therapy until a 3-month trial of ezetimibe 10 mg daily had been completed.
Figure 1.
Timeline showing liver function tests. Periods of medication (doses described in text) are shown with the horizontal lines. Azathioprine was still taken at the time of last assessment. ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase.
Within a month of starting ezetimibe therapy, the patient began to experience progressive and eventually debilitating frontal headaches, which led him to discontinue the medication within 8 weeks. After stopping ezetimibe, his headaches abated, but his health continued to deteriorate. One month after ezetimibe therapy had been stopped, he presented to the emergency department with anorexia, right upper quadrant abdominal pain radiating to the back, profound pruritus with no obvious rash, diarrhoea, debilitating sleeping difficulties, and new-onset arthralgia. His BMI had decreased to 23.4 kg/m2. His liver was palpable 4 cm below the right costal margin. ALT, AST, and ALP were 300, 129, and 1344 U/L, respectively. Bilirubin and international normalized ratio were normal at 14 μmol/L and 1.0, respectively. Viral serology was negative for hepatitis A, B, and C, and HIV. The antinuclear antibody test was positive. There were no other contributing factors that may have precipitated acute liver injury after ezetimibe discontinuation.
A trial of ursodiol and cholestyramine resulted in no significant improvement in pruritus. He was prescribed hydromorphone for pain. A month later, his BMI was further reduced to 22.8 kg/m2. ALT, AST, and ALP were 300, 158, and 1600 U/L, respectively. Fibroscan of the liver was 12.9 kPa, which was consistent with fibrosis and/or severe inflammation. A liver biopsy showed active parenchymal inflammation and bile duct injury, but without fibrosis. A causal relationship was assessed using the Roussel Uclaf Causality Assessment Method causality scale,8 which is a well-established and commonly used tool to determine causality in cases of suspected drug-induced liver injury, by quantitatively assessing factors such as age, time of onset, liver function, drug use, and other comorbidities. In this case, ezetimibe-induced liver injury was determined as being probable for this patient with a score of 8. He was prescribed prednisone 40 mg daily and azathioprine 100 mg daily. Within 1 month, his ALT, AST, ALP, and bilirubin each decreased by > 50%. Prednisone was gradually tapered and stopped after a total of 7 months, but azathioprine was maintained (Fig. 1). After stopping prednisone, his BMI was 24.7 kg/m2 and his liver function tests had returned to the normal range. He restarted alirocumab 75 mg daily subcutaneously biweekly. Six months after stopping prednisone, on azathioprine 100 mg daily and alirocumab, his total cholesterol, triglyceride, high-density lipoprotein and LDL cholesterol were 3.63, 1.51, 0.86 and 2.08, respectively. His ALT, AST, and ALP were 12, 10, and 91 U/L, respectively, whereas total bilirubin was 7.0 μmol/L. He felt improved overall, although he still reported fatigue with exertion.
Discussion
We report a patient with severe ezetimibe-induced liver injury lasting several months after discontinuation of the medication. Suggestive features include the development of hepatic abnormalities in relation to the timing of ezetimibe treatment, and the absence of other possible precipitating agents and causes of acute liver injury. He remained symptomatic for more than 7 weeks after discontinuation of ezetimibe and in the acute stage of illness was at high risk for liver failure. Despite the severe ALP elevation, liver biopsy showed active parenchymal inflammation. Ursodiol was initially used because of the early predominance of the ALP elevation, but inflammation observed on liver biopsy led to switch to prednisone plus azathioprine. He required prednisone for a total of 7 months together with ongoing azathioprine before biochemical and clinical resolution, and has now returned close to his baseline state of health.
Ezetimibe is a useful second-line therapy for hypercholesterolemia that produces a modest reduction in LDL cholesterol compared with statin therapy.3 Severe side effects are very rare, particularly when the drug is used as monotherapy in patients with statin intolerance.2,3 Most previous published cases of ezetimibe-induced liver injury have been reported in combination with a statin.4,5 Indeed, the drug label warns of possible liver enzyme elevation when ezetimibe is prescribed in combination with a statin, but with no mention of such effects when used as monotherapy. Only 2 case reports describe liver injury when ezetimibe was used as monotherapy, but both subjects had other medical conditions such as treated hypertension.6,7 In contrast, our subject was on ezetimibe monotherapy solely, without any other medication or underlying condition except for hypercholesterolemia.
It remains unclear how ezetimibe induced the liver injury observed here. Ezetimibe does not interact with cytochrome P450 system or liver enzymes.3 However, ezetimibe circulates enterohepatically and likely blocks sterol absorption from hepatocytes via the inhibition of hepatic NPC1L1.3 Thus, there is some plausible connection to the liver as a site of action. This unique patient's history illustrates an extremely rare but potentially life-threatening hazard of ezetimibe monotherapy.
Novel Teaching Points.
-
•
Although very rare, ezetimibe-associated liver injury is a potentially serious complication.
-
•
With hepatic inflammation due to ezetimibe, discontinue treatment and consider systemic steroids and anti-inflammatory agents, with weekly monitoring of liver function tests (ALT, AST, ALP, total bilirubin).
-
•
Inhibitors of PCSK9 may be safe and effective alternatives to consider for LDL lowering in certain patients intolerant to statins and ezetimibe.
Acknowledgements
We gratefully acknowledge the written consent, support, and cooperation of the patient in preparation of this report.
Funding Sources
J.L. is supported by the Canadian Institutes of Health Research (Doctoral Research Award) and the Schulich School of Medicine and Dentistry (Cobban Student Award in Heart and Stroke Research). RAH is supported by the Jacob J. Wolfe Distinguished Medical Research Chair, the Edith Schulich Vinet Research Chair in Human Genetics, and the Martha G. Blackburn Chair in Cardiovascular Research. R.A.H. has also received operating grants from the Canadian Institutes of Health Research (Foundation award), the Heart and Stroke Foundation of Ontario (G-18-0022147).
Disclosures
R.A.H. reports consulting fees from Acasti, Aegerion, Akcea/Ionis, Amgen, HLS Therapeutics Novartis, Regeneron, and Sanofi. The rest of the authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported has adhered to ethical guidelines (Western University protocol 0379).
See page 197 for disclosure information.
References
- 1.Anderson T.J., Grégoire J., Pearson G.J. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–1282. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 2.Mancini G.B., Baker S., Bergeron J. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016) Can J Cardiol. 2016;32(Suppl):S35–65. doi: 10.1016/j.cjca.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Phan B.A., Dayspring T.D., Toth P.P. Ezetimibe therapy: mechanism of action and clinical update. Vasc Health Risk Manag. 2012;8:415–427. doi: 10.2147/VHRM.S33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stolk M.F., Becx M.C., Kuypers K.C., Seldenrijk C.A. Severe hepatic side effects of ezetimibe. Clin Gastroenterol Hepatol. 2006;4:908–911. doi: 10.1016/j.cgh.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Ellingsen S.B., Nordmo E., Lappegard K.T. Recurrence and severe worsening of hepatotoxicity after reintroduction of atorvastatin in combination with ezetimibe. Clin Med Insights Case Rep. 2017;10 doi: 10.1177/1179547617731375. 1179547617731375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q., Tobias H., Petrovic L.M. Drug-induced liver injury associated with ezetimibe therapy. Dig Dis Sci. 2007;52:602–605. doi: 10.1007/s10620-006-9497-2. [DOI] [PubMed] [Google Scholar]
- 7.Castellote J., Ariza J., Rota R., Girbau A., Xiol X. Serious drug-induced liver disease secondary to ezetimibe. World J Gastroenterol. 2008;14:5098–5099. doi: 10.3748/wjg.14.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17:14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]