Abstract
Background
Although cardiac rehabilitation (CR) has proven to have short- and mid-term benefit in treatment of coronary artery disease, its long-term benefit in patients who have undergone coronary artery bypass grafting (CABG) is less certain. Our objective was to examine the late outcomes of patients who attended CR within the first year after CABG.
Methods
Adult CABG patients referred to Toronto Rehabilitation Institute (CR group: were referred and attended at least 1 session; No-CR group: were referred but did not attend) between January 1996 and September 2008 were identified through linkages with clinical and provincial administrative databases for comorbidities and outcome ascertainment. The primary outcome was a composite of all-cause mortality, acute myocardial infarction, stroke or repeat revascularization (major adverse cardiac and cerebrovascular events [MACCE]). The secondary outcome was all-cause mortality. Multivariable Cox proportional hazard models were used to assess the CR treatment effect, adjusting for baseline characteristics.
Results
The study cohort consisted of 5,000 patients—3,685 (73.7%) in the CR group and 1,315 (26.3%) in the No-CR group. Median referral time was 32.5 days, and follow-up was 13.1 years. The CR group patients, compared with the No-CR group, were younger (age 62.6 ± 9.6 vs 64.0 ± 10.5 years), were more likely to be male (85.0% vs 79.5%), and had fewer cardiac comorbidities. In adjusted analyses, the CR group was associated with decreased MACCE (hazard ratio 0.83, 95% confidence interval 0.75-0.91, P < 0.0001) and a higher adjusted survival at 15 years (66.3% vs 60.1%, hazard ratio 0.76, 95% confidence interval 0.68-0.84, P < 0.0001), as compared with the No-CR group.
Conclusions
There was a reduction in MACCE and late mortality associated with CR attendance, highlighting the importance of patient referral and participation in CR after CABG.
Résumé
Contexte
La réadaptation cardiaque (RC) s’est révélée bénéfique à court et à moyen terme dans le traitement des coronaropathies, mais on en sait moins sur ses bienfaits à long terme chez les patients ayant subi un pontage aortocoronarien (PAC). Nous avons donc examiné les issues à long terme chez des patients ayant participé à un programme de RC dans l’année suivant un PAC.
Méthodologie
À partir des données couplées des bases de données des cliniques et de l’administration provinciale, nous avons relevé tous les patients adultes ayant subi un PAC qui ont été orientés vers l’Institut de réadaptation de Toronto (groupe RC : patients orientés vers le programme et ayant participé à au moins 1 séance; groupe sans RC : patients orientés vers le programme, mais n’ayant participé à aucune séance) entre janvier 1996 et septembre 2008, afin d’établir les affections concomitantes et les résultats obtenus. Le critère d’évaluation principal composé comprenait la mortalité toutes causes confondues, l’infarctus du myocarde aigu, l’accident vasculaire cérébral (AVC) ou une nouvelle revascularisation en raison d’un événement cardiaque ou cérébrovasculaire majeur (ECCVM). Le critère d’évaluation secondaire était la mortalité toutes causes confondues. Nous avons utilisé des modèles à risques proportionnels de Cox multivariés pour évaluer l’effet thérapeutique de la RC, en apportant les corrections nécessaires pour tenir compte des caractéristiques initiales des patients.
Résultats
La cohorte de l’étude réunissait 5 000 patients – 3 685 (73,7 %) dans le groupe RC et 1 315 (26,3 %) dans le groupe sans RC. Les valeurs médianes du temps écoulé avant l’orientation vers un programme de RC et de la période du suivi étaient de 32,5 jours et de 13,1 ans, respectivement. Comparativement aux patients du groupe sans RC, les patients du groupe RC étaient plus jeunes (62,6 ± 9,6 ans vs 64,0 ± 10,5 ans), étaient dans une plus forte proportion des hommes (85,0 % vs 79,5 %) et présentaient un moins grand nombre d’affections cardiaques concomitantes. À l’issue des analyses après corrections, on a observé dans le groupe RC une réduction du taux d’ECCVM (rapport des risques instantanés de 0,83; intervalle de confiance [IC] à 95 %, de 0,75 à 0,91; p < 0,0001) et une augmentation du taux de survie à 15 ans corrigé (66,3 % vs 60,1 %; rapport des risques instantanés de 0,76; IC à 95 %, de 0,68 à 0,84; p < 0,0001), comparativement au groupe sans RC.
Conclusions
La participation à un programme de RC a été associée à une diminution du risque d’ECCVM et de mortalité tardive, ce qui fait ressortir l’importance d’orienter les patients ayant subi un PAC vers de tels programmes et de les encourager à y participer.
Cardiac rehabilitation (CR) is an important component in the management of chronic cardiovascular disease, supporting provision of secondary prevention and lifestyle changes, which successfully reduces the risk of recurrence and mortality.1 The 2011 American College for Cardiology Foundation/American Heart Association (ACCF/AHA) guideline recommends CR for all eligible patients post–coronary artery bypass graft surgery (CABG) beginning 4-8 weeks after surgery, 3 times per week for 3 months (Class I, Level of Evidence: A).2 The 2016 European guidelines on cardiovascular disease prevention in clinical practice recommend participation in CR for all patients hospitalized for revascularization (Class I, Level of Evidence: A).3 A recent Cochrane systematic review and meta-analysis of 63 randomized controlled trials (RCTs) of more than 14,486 participants with coronary artery disease (CAD) showed that CR led to a 26% relative risk reduction in cardiovascular mortality and lowered the risk of hospital readmissions at a median follow-up of 12 months.4
All the existing RCTs and observational studies related to CR attendance have reported only all-cause mortality or cardiovascular mortality as the primary outcome.4, 5, 6, 7, 8, 9, 10, 11 The CABG subgroup has been studied in a meta-analysis of primarily observational studies at a mean follow-up of 40 months in 14,583 patients, and a significant reduction in mortality in patients participating in CR was reported.11 There is little evidence regarding long-term mortality or other relevant outcomes after CR in CABG patients.
Therefore, the primary objective of this study was to examine the long-term MACCE (major adverse cardiac and cerebrovascular events, here defined as the composite of all-cause mortality, myocardial infarction, stroke or repeat revascularization) in postoperative CABG patients who attended CR vs those who did not attend CR. Our secondary objective was to examine all-cause mortality. Our hypotheses are that patients who attend postoperative CR care have better long-term freedom from MACCE, and survival, as compared to those who are referred to but do not attend postoperative CR.
Methods
Study design
A retrospective comparison of late outcomes in CABG patients attending CR at the Toronto Rehabilitation Institute (TRI) was performed through linkages of multiple clinical and administrative databases housed at the ICES (formerly known as the Institute for Clinical Evaluative Sciences) in Toronto, Ontario, Canada. TRI is one of the oldest rehabilitation institutes in Canada and across the world; it receives >2400 referrals per year and provides comprehensive CR care in accordance with the Canadian Association for Cardiac Rehabilitation Guidelines.12 ICES is Canada’s largest health services research institute and holds multiple population-based health databases of the Ontario population. ICES is a prescribed entity under Ontario’s Personal Health Information Protection Act, which allows for researchers to link together encoded population-based administrative databases and clinical registries for conducting approved research studies under strict privacy and security policies, procedures, and practices (see link to Data and Privacy at www.ices.on.ca). The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board. The need for individual patient consent is waived. These datasets were linked using unique encoded identifiers and analyzed at ICES.
Study population
All adult CABG patients identified from the CorHealth Ontario Cardiac Registry (a repository of patients undergoing any cardiac procedure in Ontario) with a surgery date between January 1, 1996 and September 30, 2008 who were referred to TRI were linked at ICES to create our patient population. All patients were followed to March 31, 2017; this end date was selected based on the linkages available at ICES when the dataset creation plan was executed. The index date used for this study is the “referral date,” defined as the date when the referral was processed at TRI and contact with the patient was initiated. Patients who died before the referral date were excluded, as were those who had a CABG to referral time of >365 days. The latter group is unique, and studies have shown that delayed referral into the CR program is associated with poorer compliance and outcomes in CABG patients.13 The study cohort was divided into 2 groups (CR and No-CR), based on attendance. Patients in the CR group were referred and attended at least one session; the No-CR group patients were referred but did not attend any CR sessions.
Baseline demographics such as age and sex were derived from the Registered Persons Database. The ethnic origin was extracted from the Canadian Immigration Database and the ICES ethnic database. Variables including body mass index, hypertension, diabetes, Canadian Cardiovascular Society Class, left ventricular ejection fraction, previous myocardial infarction, prior percutaneous coronary intervention, peripheral vascular disease, congestive heart failure, chronic obstructive pulmonary disease, and cerebrovascular disease were identified in the CorHealth Ontario Cardiac Registry. The ICES hypertension and diabetes databases were used to identify missing hypertension and diabetes variables. The income quintile for this study was computed by cross-referencing patient postal codes with the average household income by area from census data, with 1 being the lowest. This was ascertained from completed referral forms and administrative databases. All the databases had a look-back window of 15 years. The number of arterial and overall grafts was based on the physician’s billing through the Ontario Health Insurance Plan (OHIP).14 Perioperative and postoperative outcomes were identified by linkage to the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) and the International Classification of Diseases, ninth and tenth revisions (ICD 9 and ICD10) (Supplemental Table 1). The Ontario Registered Deaths Database was used to identify all-cause mortality. Patients with an invalid identification number, with age data missing, or who died on or before the cohort entry date were excluded.
Toronto Rehabilitation Institute—CR program
The exposure was defined as referral for CR at TRI and attendance of at least one CR session. The control group included CABG patients who were referred for CR at TRI but did not attend any session. The date of treatment exposure was the referral date for both groups. A CR referral is routinely made at the time of discharge following CABG, although it also can be made afterward by a family physician, cardiologist, surgeon, or other healthcare professional. The TRI-CR program, led by an interprofessional team, consisted of an active phase of a total of 24 to 36 prescheduled once-per-week supervised aerobic and resistance training exercise sessions over a 6-12–month period, dietary counseling, stress management, health education, and smoking cessation. Patients also performed additional home-based physical activities for a total of 5 exercise sessions per week.15
Outcome definition
The primary outcome was MACCE, a composite endpoint of all-cause mortality, acute myocardial infarction (AMI), stroke or repeat revascularization (percutaneous coronary intervention [PCI] or redo CABG). The secondary outcome was all-cause mortality. The tertiary outcomes were AMI, stroke, and repeat revascularization. As referral date was used as the index date for analysis, only outcomes that occurred after the referral date contributed to the primary outcome, MACCE. However, we also examined in-hospital perioperative outcomes, including AMI, stroke, and dialysis, as well as all-cause readmissions that occurred after discharge from the CABG hospitalization and before the referral date. In-hospital outcomes (AMI, stroke, and dialysis) and all-cause readmissions after discharge were treated as covariates in the adjusted multivariable models, as these nonfatal events may have occurred differentially between the 2 groups and could potentially affect the outcomes. However, patients who were readmitted for AMI, stroke, or repeat revascularization prior to the referral date (n = 56) were left censored for the MACCE outcome, as the dataset included only the first date of any of the post-CABG nonfatal cardiovascular events. These patients remained in the analysis of mortality, and for the other individual nonfatal cardiovascular events.
Statistical analyses
Continuous variables were reported as the mean ± standard deviation or median (interquartile range [IQR]), whereas categorical variables are reported as frequencies and percentages. Baseline demographics were compared between the CR and No-CR groups using t-tests (continuous data) or χ2 tests (categorical data). A 2-tailed P <0.05 was considered statistically significant. Standardized mean differences were determined, in order to compare the baseline balance in variables between the 2 groups (CR vs No-CR), and a standardized mean difference of >10% was considered to be a substantial imbalance.16 Kaplan-Meier curves were used to compare the event-free survival rates for each group. Effect estimates for CR for the primary and secondary endpoints are presented as hazard ratios (HRs) and their associated 95% confidence intervals (CIs). A multivariable Cox proportional hazards model was used to derive a measure of the CR treatment effect in the unadjusted and adjusted analyses (adjusting for baseline characteristics, in-hospital outcomes, and all-cause readmissions before the referral date). Prior to modelling, we tested for multicollinearity by calculating the tolerance for all predictor variables. A tolerance value of <0.2 was considered significant, and in cases with such values, only one member of a correlated set of variables was retained for the models. The assumptions of proportional hazards was assessed for MACCE and all-cause mortality by examining the cumulative hazards graph for proportional parallel lines (log-negative log survival graph for “parallel lines”).17 The cumulative incidence functions for AMI, stroke, and repeat revascularization were determined and Fine-Gray sub-distribution hazard models were generated, accounting for death as a competing risk.18 To validate our findings, we compared the results to a model in which we imputed missing data using the Markov chain Monte Carlo method.19
Secondary analyses
An exploratory secondary analysis was performed based on the percentage of attendance after multivariable adjustment as described above, for both MACCE and all-cause mortality, using a Cox proportional hazards model. The study cohort was divided into 4 groups—high attendance: >67% (25-36 visits); mid attendance: 33%-67% (17-24 visits); low attendance: <33% (1-16 visits); and no attendance (0% of CR sessions, ie, the No-CR group). We sought to determine whether a dose–response relationship existed between CR attendance and both the primary outcome (MACCE) and secondary outcome (all-cause mortality).
Per ICES policy, all cells with fewer than 5 events were presented as a range to avoid patient identification. All analyses were conducted with SAS version 14.3 (SAS Institute, Cary, NC).
Results
Study cohort
Of the 98,681 CABG patients who underwent CABG in the CorHealth Ontario Registry during the study period, 90,654 were linkable to all the necessary databases to ascertain outcome. During the same time frame, 25,470 patients were referred to TRI, of which 5,000 had undergone CABG, who were linked to the ICES datasets (Supplemental Figure S1). The final cohort of 5,000 patients included 3,685 (73.7%) in the CR group and 1,315 (26.3%) in the No-CR group. The median discharge-to-referral time was 32.5 days (IQR: 15-56 days). The median follow-up was 13 years (IQR: 10.0-16.6 years), with a maximum of 20 years.
Overall, patients in the CR group were younger (62.6 ± 9.6 vs 64.0 ± 10.5 years, P < 0.001), were more likely to be male (85.0% vs 79.5%, P < 0.001), had lower body mass index (<30: 73.8% vs 59.0%, P < 0.001), were more likely to be from a higher income quintile (31.1% vs 19.3%, P < 0.001), and had fewer cardiac comorbidities compared to the No-CR patients (Table 1).
Table 1.
Baseline characteristics of the CR group and the No-CR group
| Variable | CR group |
No-CR group |
Standardized difference | P |
|---|---|---|---|---|
| n = 3,685 | n = 1,315 | |||
| Age, y | –62.6 ± 9.6 | 64.0 ± 10.5 | 0.14 | < 0.001 |
| Sex | ||||
| Female | 552 (15.0) | 270 (20.5) | 0.15 | < 0.001 |
| Male | 3133 (85.0) | 1045 (79.5) | 0.15 | |
| BMI, kg/m2 | ||||
| <25 | 925 (25.1) | 313 (23.8) | 0.03 | < 0.001 |
| 25-30 | 1793 (48.7) | 463 (35.2) | 0.28 | |
| 31-35 | 702 (19.1) | 264 (20.1) | 0.03 | |
| > 35 | 215 (5.8) | 88 (6.7) | 0.04 | |
| Missing | 50 (1.4) | 187 (14.2) | 0.49 | |
| Income quintile | ||||
| 1 | 594 (16.1) | 286 (21.7) | 0.14 | < 0.001 |
| 2 | 616 (16.7) | 282 (21.4) | 0.12 | |
| 3 | 640 (17.4) | 242 (18.4) | 0.03 | |
| 4 | 689 (18.7) | 251 (19.1) | 0.01 | |
| 5 | 1146 (31.1) | 254 (19.3) | 0.27 | |
| Surname-based ethnic group | ||||
| Chinese | 92 (2.5) | 39 (3.0) | 0.03 | 0.12 |
| General population | 3428 (93.0) | 1201 (91.3) | 0.06 | |
| South Asian | 165 (4.5) | 75 (5.7) | 0.06 | |
| Charlson Index | 0.9 ± 0.7 | 1.0 ± 0.7 | 0.16 | < 0.001 |
| Creatinine, μmol | ||||
| <120 | 3312 (89.9) | 1140 (86.7) | 0.10 | 0.003 |
| 120-180 | 222 (6.0) | 91 (6.9) | 0.04 | |
| > 180 | 33 (0.9) | 23 (1.7) | 0.07 | |
| Missing | 118 (3.2) | 61 (4.6) | 0.07 | |
| CCS class | ||||
| 0 | 16-20 | 6-10 | 0.03 | |
| 1 | 228 (6.2) | 82 (6.2) | 0.00 | |
| 2 | 579 (15.7) | 179 (13.6) | 0.06 | |
| 3 | 1045 (28.4) | 345 (26.2) | 0.05 | |
| 4 | 1809 (49.1) | 698 (53.1) | 0.08 | |
| Missing | 4-8 | 1-5 | 0.01 | 0.08 |
| LVEF grade | ||||
| 1 | 1770 (48.0) | 563 (42.8) | 0.10 | < 0.001 |
| 2 | 1269 (34.4) | 474 (36.0) | 0.03 | |
| 3 | 542 (14.7) | 224 (17.0) | 0.06 | |
| 4 | 77 (2.1) | 41 (3.1) | 0.06 | |
| Missing | 27 (0.7) | 13 (1.0) | 0.03 | |
| HTN | 2502 (67.9) | 986 (75.0) | 0.16 | < 0.001 |
| Diabetes | 1159 (31.5) | 521 (39.6) | 0.17 | < 0.001 |
| Hyperlipidemia | 2460 (66.8) | 882 (67.1) | 0.01 | 0.83 |
| Smoking | 2136 (58.0) | 798 (60.7) | 0.06 | 0.08 |
| PVD | 362 (9.8) | 179 (13.6) | 0.12 | < 0.001 |
| COPD | 192 (5.2) | 86 (6.5) | 0.06 | 0.07 |
| CVD | 271 (7.4) | 122 (9.3) | 0.07 | 0.02 |
| Redo CABG | 96 (2.6) | 47 (3.6) | 0.06 | 0.07 |
| Previous PCI | 243 (6.6) | 104 (7.9) | 0.05 | 0.10 |
| Previous MI | 1299 (35.3) | 534 (40.6) | 0.11 | < 0.001 |
| Previous MI within 30 d | 719 (19.5) | 225 (17.1) | 0.06 | 0.05 |
| Number of grafts based on OHIP billing | 3.2 ± 0.9 | 3.1 ± 0.9 | 0.12 | < 0.001 |
| Missing | 90 (2.4) | 33 (2.5) | 0.00 | < 0.001 |
| 1 | 83 (2.3) | 38 (2.9) | 0.04 | |
| 2 | 503 (13.6) | 216 (16.4) | 0.08 | |
| 3 | 1472 (39.9) | 551 (41.9) | 0.04 | |
| 4 | 1191 (32.3) | 391 (29.7) | 0.06 | |
| 5 | 323 (8.8) | 80 (6.1) | 0.10 | |
| 6 | 23 (0.6) | 6 (0.5) | 0.02 | |
| Episode length of stay in days, median (IQR) | 7.0 (6-10) | 8.0 (6-12) | 0.16 | < 0.001 |
| Arterial graft, number | ||||
| 0 | 240 (6.5) | 106 (8.1) | 0.06 | |
| 1 | 2456 (66.6) | 899 (68.4) | 0.04 | |
| 2 | 796 (21.6) | 252 (19.2) | 0.06 | |
| 3 | 193 (5.2) | 58 (4.4) | 0.04 | 0.007 |
| Left main disease | 823 (22.3) | 304 (23.1) | 0.02 | 0.35 |
| Proximal LAD + one or more Cx & RCA (value: 2) | 1918 (52.0) | 689 (52.4) | 0.01 | |
| TVD without proximal LAD (value: 3) | 126 (3.4) | 56 (4.3) | 0.04 | |
| SVD of proximal LAD (value: 4) | 296 (8.0) | 83 (6.3) | 0.07 | |
| 1- or 2-vessel disease or none of the above (value: 5) | 497 (13.5) | 171 (13.0) | 0.01 | |
| Missing disease location | 25 (0.7) | 12 (0.9) | 0.03 |
Values are n (%) or mean (± standard deviation), unless otherwise specified. The CR group consists of patients who were referred to and attended at least one CR session. The No-CR group consists of patients who were referred to but did not attend any CR sessions. Previous MI is defined as any MI within 15 years prior to index CABG. Previous MI within 30 days indicates any MI within 30 days prior to index CABG. Per ICES (formerly the Institute for Clinical Evaluative Sciences in Toronto, Ontario, Canada) policy, all cells with fewer than 5 events were presented as a range, to avoid patient identification. For standardized difference, < 0.1 is negligible.
CABG, coronary artery bypass graft; CR, cardiac rehabilitation; CCS, Canadian Cardiovascular Society; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; PVD, peripheral vascular disease; HTN, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OHIP, Ontario Health Insurance Plan; IQR, interquartile range; PCI, percutaneous coronary intervention; Cx, circumflex; LAD, left anterior descending artery; RCA, right coronary artery; SVD, single-vessel disease; TVD, triple-vessel disease.
Early outcomes
There were no significant differences between groups with respect to the unadjusted in-hospital outcomes (CR vs No-CR, for AMI: 2.1% vs 1.4%, P = 0.09; stroke: 0.8% vs 0.8%, P = 0.92; and dialysis: 2.4% vs 3.3%, P = 0.05; Supplemental Table S2). Patients in the CR group had fewer all-cause readmissions (excluding readmissions for cardiac events) prior to the referral date, compared with the No-CR group (6.2% vs 9.4%, P < 0.001; Supplemental Table S2). The number of readmissions for PCI prior to the referral date was also lower for CR, compared with No-CR patients (0.2% vs 0.6%, P = 0.03; Supplemental Table S2).
Late outcomes: MACCE and all-cause mortality
MACCE-free survival and survival were significantly higher in the CR patients after adjusting for baseline characteristics and outcomes prior to the referral date (see Supplemental Tables S3 and S4 for full models). Freedom from MACCE at 10, 15, and 20 years was 69.9%, 50.9%, and 27.1% in patients who attended CR, compared to 65.4%, 45.2%, and 21.9% in those who did not (HR 0.83, 95% CI 0.75-0.91, P < 0.001; Fig. 1). At 10, 15, and 20 years, survival was 84.0%, 66.3%, and 38.2% in the CR group, whereas survival was 80.1%, 60.1%, and 31.4% in the No-CR group (HR 0.76, 95% CI 0.68-0.84, P < 0.001; Fig. 2). Event rates and corresponding HRs at 5, 10, 15, and 20 years are shown in Table 2.
Figure 1.

Adjusted Kaplan-Meier curve for 20-year freedom from major adverse cardiac and cerebrovascular events (MACCE) for the cardiac rehabilitation (CR) vs No-CR groups. –The CR group consists of patients who were referred to and attended at least one CR session. The No-CR group consists of patients who were referred to but did not attend any CR sessions. MACCE is defined as a composite endpoint of all-cause mortality, acute myocardial infarction, stroke, or repeat revascularization. The shaded area represents the 95% confidence interval. MACCE was lower in patients who attended CR compared to those who did not attend.
Figure 2.
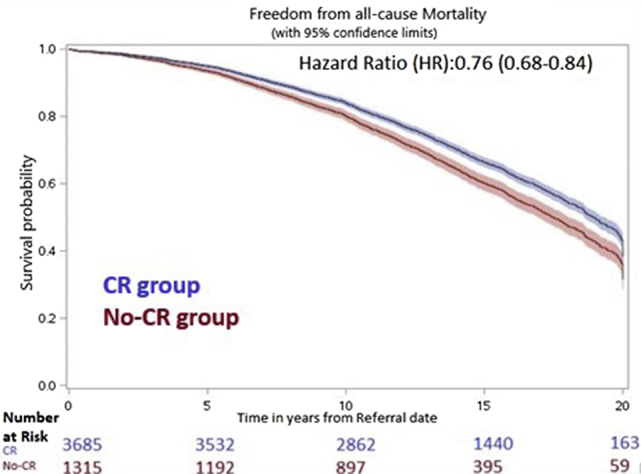
Adjusted Kaplan-Meier curve for 20-year freedom from all-cause mortality for the cardiac rehabilitation (CR) vs No-CR groups. –The CR group consists of patients who were referred to and attended at least one CR session. The No-CR group consists of patients who were referred to but did not attend any CR sessions. The shaded area represents the 95% confidence interval. Overall survival was higher in the CR group, compared to the No-CR group.
Table 2.
(Adjusted) time-to-event analysis for primary and secondary outcome
| Outcome | CR group, % (95% CI) |
No-CR group, % (95% CI) |
HR (95% CI) |
|---|---|---|---|
| Freedom from MACCE | |||
| 5-y | 86.1 (85.1-87.1) | 83.6 (82.3-85.0) | 0.71 (0.60-0.83) |
| 10-y | 69.9 (68.6-71.3) | 65.4 (63.4-67.6) | 0.80 (0.72-0.89) |
| 15-y | 50.9 (49.3-52.6) | 45.2 (42.7-47.9) | 0.80 (0.74-0.86) |
| 20-y | 27.1 (24.7-29.8) | 21.9 (19.2-25.1) | 0.83 (0.77-0.89) |
| Freedom from all-cause mortality | |||
| 5-y | 94.9 (94.4-95.5) | 93.5 (92.7-94.3) | 0.56 (0.44-0.73) |
| 10-y | 84.0 (83.0-85.0) | 80.1 (78.6-81.7) | 0.75 (0.66-0.85) |
| 15-y | 66.3 (64.9-67.8) | 60.1 (57.9-62.4) | 0.77 (0.71-0.83) |
| 20-y | 38.2 (35.8-40.8) | 31.4 (28.5-34.6) | 0.78 (0.72-0.83) |
The data in the table are the adjusted Kaplan-Meier estimates with the 95% CI. The CR group consists of patients who were referred to and attended at least one session. The No-CR group consists of patients who were referred to but did not attend any sessions.
CR, cardiac rehabilitation; HR, hazard ratio; CI, confidence interval; MACCE, all-cause mortality, myocardial infarction, stroke, or repeat revascularization.
The crude MACCE and survival Kaplan-Meier curves are depicted in Supplemental Figure S2a and b.
Late outcomes: Nonfatal cardiovascular events
There was a lower incidence of stroke at 20-year follow-up in the CR group compared to the No-CR group (adjusted sub-distribution HR 0.76, 95% CI 0.60-0.96, P = 0.02) after accounting for death as a competing risk. There was no statistically significant difference in AMI (adjusted sub-distribution HR 0.84, 95% CI 0.71-1.01, P = 0.06) or repeat revascularization (adjusted sub-distribution HR 0.90, 95% CI 0.75-1.07, P = 0.24) during the follow-up period (Table 3). Event rates and corresponding adjusted sub-distribution hazard ratios at 5, 10, 15, and 20 years are shown in Supplemental Table S5.
Table 3.
(Adjusted) sub-distribution HR for AMI, stroke, and repeat revascularization (PCI or CABG) with death as a competing risk at 20-year follow-up
|
Variable |
CR group (n = 3,685) | No-CR group (n = 1,315) | HR (95% CI) | P |
|---|---|---|---|---|
| AMI | 529 (14.3) | 243 (18.4) | 0.84 (0.71-1.01) | 0.06 |
| Stroke | 267 (7.2) | 140 (10.6) | 0.76 (0.60-0.96) | 0.02 |
| Repeat revascularization (PCI or CABG) | 579 (15.7) | 216 (16.4) | 0.90 (0.75-1.07) | 0.24 |
Values are number of events (%), unless otherwise indicated. –The CR group consists of patients who were referred to and attended at least one CR session. The No-CR group consists of patients who were referred to but did not attend any CR sessions.
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CI, confidence interval; CR, cardiac rehabilitation; HR, hazard ratio; PCI, percutaneous coronary intervention,
Additionally, in a post hoc fashion, the CR group had a lower risk of the composite outcome of AMI, stroke, or repeat revascularization, as compared to the No-CR group (adjusted sub-distribution HR 0.85, 95% CI 0.75-0.96, P = 0.013), after accounting for death as a competing risk.
Secondary analysis
We evaluated the association of low, medium, and high CR attendance on MACCE and mortality. Baseline characteristics (Supplemental Table S6) and early outcomes were compared among the 4 subgroups (Supplemental Table S7).
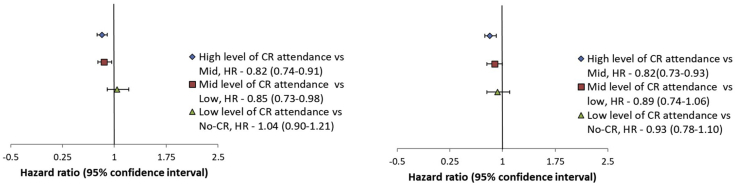
There was no benefit from low CR attendance compared to No-attendance for either MACCE or all-cause mortality. There was a graded response, with medium attendance as the minimum effective CR dose—the best results were observed in the high CR attendance group (Fig. 3; Supplemental Fig. S3a and b).
Figure 3.
Forest-plot showing stepwise comparison between levels of cardiac rehabilitation (CR) attendance—high vs mid, mid vs low, and low vs No-CR for the primary outcome—major adverse cardiac and cerebrovascular events (MACCE) and the secondary outcome—all-cause mortality. MACCE is defined as a composite endpoint of all-cause mortality, acute myocardial infarction, stroke, or repeat revascularization, all-cause mortality includes cardiovascular-related mortality and all other causes of mortality. (High attendance was defined as having attended > 67% of CR sessions, mid attendance as having attended 33%-67%, low attendance as having attended < 33%, and no attendance as having attended 0%). There was a stepwise graded response, for mid-level and high-level of attendance, but there was no CR effect with low attendance. HR, hazard ratio.
Sensitivity analysis
There was less than 4% missing data, and the findings were robust after multiple imputations for missing data. Five imputed datasets were created, and the summarized results across the 5 models were compared to the Cox model fit on the original data. In the adjusted analyses, CR continued to be associated with an improvement in MACCE (HR 0.86, 95% CI 0.7s8-0.93, P = 0.0008) and all-cause mortality (HR 0.81, 95% CI 0.73-0.89, P < 0.0001).
Discussion
To our knowledge, the current study is the first to evaluate the outcomes of freedom from MACCE and survival in CABG patients attending CR over 2 decades of follow-up. There were several important findings:
-
(i)
CABG patients who attended CR following hospital discharge had decreased MACCE and improved survival over the entire follow-up period.
-
(ii)
A high level of CR attendance, defined as > 67%, was associated with improved outcomes, as compared to a low level of attendance (defined as < 33%).
-
(iii)
A significant reduction in stroke was observed in patients who attended CR compared to those that did not attend CR.
Although some previous studies have investigated the potential long-term benefits of CR, these studies focused on the entire CAD population rather than only patients with advanced CAD that required CABG,4,11 and they did not assess longitudinal composite outcomes such as MACCE.6, 7, 8,11 Although it is from a single centre, our study is unique in that we had a moderately large sample size of 5,000 patients, a large number of events, and complete 20-year follow-up for both survival and freedom from a composite of nonfatal cardiac events.
In the Cochrane meta-analysis published in 2016, including 63 RCTs of patients diagnosed with CAD, exercise-based CR was associated with a significant reduction in cardiovascular mortality, (relative risk [RR] 0.74, 95% CI 0.64-0.86), whereas all-cause mortality was similar in the 2 groups (RR 0.96, 95% CI 0.88-1.04) at a median 1-year follow-up.4 However, over a longer follow-up period, a large registry-based study from Alberta, Canada of over 13,000 CAD patients reported improved survival with CR at a median of 6 years (HR 0.57, 95% CI 0.52-0.63, P < 0.001).7 Additionally, in the Cardiac Rehabilitation Outcome Study meta-analysis, 10 observational studies of patients with acute coronary syndrome reported a significant reduction in mortality after CR participation at a mean follow-up of 40 months (odds ratio 0.20, 95% CI 0.08-0.48).11 Overall, these findings suggest that there may be mid-term benefits from CR in patients with any CAD.
The evidence base around the benefit of CR in those with extensive CAD requiring CABG is smaller. The Cardiac Rehabilitation Outcome Study meta-analysis included only 5 observational studies that reported on the CABG subgroup (n = 14,583) and found reduced mortality at a mean follow-up of 40 months (HR 0.62, 95% CI 0.54-0.70) after participating in CR within 3 months of hospital discharge.11 Separately, Lee et al., in a separate Korean study, found a 20% reduction in all-cause mortality after phase I (at least one in-hospital CR session), including 1097 CR attendees , and a 40% reduction after phase II (outpatient CR) of 379 CR attendees within 3 months of discharge after CABG in a propensity-matched CABG registry cohort.9
In the same Cochrane meta-analysis, exercise-based CR was not associated with a lower incidence of AMI (RR 0.90, 95% CI 0.79-1.04) or need for repeat revascularization with either CABG (RR 0.96, 95% CI 0.80-1.16) or PCI (RR 0.85, 95% CI 0.70-1.04).4 Similar to the Cochrane findings, in our study, repeat revascularization was similar in the 2 groups (15.7% vs 16.4%, P = 0.24, adjusted sub-distribution HR 0.90, 95% CI 0.75-1.07). However, there was a trend toward a reduction in new myocardial infarction (14.3% vs 18.4%, P = 0.06, adjusted sub-distribution HR 0.84, 95% CI 0.71-1.01) throughout the follow-up period. We also observed a lower incidence of stroke at 20-year follow-up in the CR group, compared to the No-CR group (adjusted sub-distribution HR 0.76, 95% CI 0.60-0.96, P = 0.025), after accounting for death as a competing risk. To our knowledge, the reduction in stroke in this population is a novel finding. Furthermore, patients in the CR group had a lower incidence of the composite of AMI, stroke, and repeat revascularization in competing risk analysis, suggesting that a reduction in nonfatal outcomes may be a driver for reduced mortality.
Although we showed that any CR attendance was associated with better outcomes, a dose–response effect was also demonstrated in our analysis, which highlights the importance of a high level of participation in CR. Although the Cochrane meta-analysis compared subgroups of high and low CR attendance and did not find a significant difference, point estimates for both levels of intervention favored CR—this lack of difference may be related to sample size and length of follow-up.4 Consistent with our analysis, a single-centre observational Australian study found a dose–response survival benefits associated with CR in patients who underwent revascularization with CABG or PCI following AMI at late 14-year follow-up. Low CR attendance (<25%) was associated with a higher risk of all-cause mortality, although the CI was wide (odds ratio 2.57, 95% CI 1.04-6.38), likely reflecting the relatively small sample size of 544 total patients.8 A secondary interpretation of our dose–response finding is that the overall CR effect seen in our primary analysis is a true treatment effect rather than one related to unmeasured confounders.
Limitations
This observational study must be interpreted in the context of some significant limitations. As TRI is one of the oldest and largest rehabilitation centers in Ontario, findings from our single-centre study may not be easy to replicate at recently established rehabilitation centres in smaller communities, which may reduce external generalizability. The retrospective nature of this study raises concerns regarding measured and unmeasured confounders, which may not have been completely resolved despite extensive adjustments in multivariable models. In particular, although we used neighbourhood income quintile as a surrogate measure for socioeconomic status, we recognize that there are limitations to this approach and that true socioeconomic status measures include other factors such as education, available social supports, and mental health. Studies have shown that patients who undergo CR are more likely to be from higher socioeconomic status groups, and higher socioeconomic status is associated with improved outcomes.20 Furthermore, our study compared only those patients that were referred to CR; those that underwent CABG and were not referred to CR may differ. Although our exposure definition for the CR group was any attendance, we note that the majority of patients had moderate to high attendance (86.5% of the cohort). Furthermore, our dataset does not capture compliance with the program outside of in-person sessions. Also, we did not look at outcomes accounting for time to referral, which had an IQR of 15-56 days. Therefore, we cannot conclude from this study that these long-term outcome differences are attributable to early referral. We analyzed the time-to-first-event; the inclusion of recurrent nonfatal cardiac events may be informative. Although a dose–response effect was observed, this may reflect a change in patient behavior rather than being a function of program effectiveness. A systematic review of 29 studies across various countries showed that factors related to CR attendance and compliance followed a determined pattern, irrespective of the CR program.21 In addition, our study population is not a true contemporary cohort, as the final year was 2008; however, the key features of CR have remained the same over the past decade. Finally, we acknowledge that it is possible that the long-term outcome difference we observed may be attributable to the differences in lifestyle/exercise during the follow-up period rather than to CR referral itself.
Conclusions
Overall, this study highlights the importance of both patient referral to and enrollment in CR, with sustained participation, for improved outcomes after CABG.
Acknowledgements
The authors acknowledge that clinical registry data used in this publication are from CorHealth Ontario and its participating hospitals. CorHealth Ontario serves as an advisory body to the Ontario Ministry of Health and Long-Term Care (MOHLTC) and is dedicated to improving the quality, efficiency, access to, and equity of adult cardiovascular services in Ontario, Canada. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. We are thankful to Bing Yu and Jiming Fang at the Institute for Clinical Evaluative Sciences, Toronto, Canada, for their assistance in linking the datasets and creating the final cohort.
Funding Sources
This project was supported in part by the Bernard S. Goldman Chair in Cardiovascular Surgery.
H.C.W. is supported by a Phase 2 Clinician Scientist Award from the Heart and Stroke Foundation of Canada, Ontario Office. The other authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This project was completed as a part of an MSc thesis requirement at the Institute of Health Policy Management and Evaluation (IHPME) and was approved by the institutional review board of the University of Toronto. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board. The need for individual patient consent is waived.
The abstract was presented in part as a poster on November 18, 2019 at the American Heart Association Meeting scientific sessions.
See page 174 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.10.004.
Supplementary Material
References
- 1.WHO Expert Committee on Rehabilitation of Patients with Cardiovascular Diseases & World Health Organization. Technical Report Series G. Rehabilitation of patients with cardiovascular diseases: report of a WHO Expert Committee [meeting held in Geneva from 23 to 29 July 1963. Available at: https//apps. who.int/iris/handle/10665/40577. Accessed March 2019.
- 2.Hillis L.D., Smith P.K., Anderson J.L. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–e210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Piepoli M.F., Hoes A.W., Agewall S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson L., Oldridge N., Thompson D.R. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane Systematic Review and Meta-Analysis. J Am Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Heran B.S., Chen J.M., Ebrahim S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011:CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doimo S., Fabris E., Piepoli M. Impact of ambulatory cardiac rehabilitation on cardiovascular outcomes: a long-term follow-up study. Eur Heart J. 2019;40:678–685. doi: 10.1093/eurheartj/ehy417. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R., Norris C.M., Gyenes G., Senaratne M., Bainey K.R. Effect of cardiac rehabilitation on South Asian individuals with cardiovascular disease: results from the APPROACH Registry. Can J Cardiol. 2016;32(suppl 2):S397–S402. doi: 10.1016/j.cjca.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Beauchamp A., Worcester M., Ng A. Attendance at cardiac rehabilitation is associated with lower all-cause mortality after 14 years of follow-up. Heart. 2013;99:620–625. doi: 10.1136/heartjnl-2012-303022. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.-Y., Han S., Ahn J.-M. Impact of participation in Phase 1 and Phase 11 cardiac rehabilitation on long-term survival after coronary artery bypass graft surgery. Int J Cardiol. 2014;176:1429–1432. doi: 10.1016/j.ijcard.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Pack Q.R., Goel K., Lahr B.D. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation. 2013;128:590–597. doi: 10.1161/CIRCULATIONAHA.112.001365. [DOI] [PubMed] [Google Scholar]
- 11.Rauch B., Davos C.H., Doherty P. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies—The Cardiac Rehabilitation Outcome Study (CROS) Eur J Prev Cardiol. 2016;23:1914–1939. doi: 10.1177/2047487316671181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone J., Arthur H.M., Suskin N. 3rd edition. Canadian Association of Cardiac Rehabilitation; 2009. Canadian Guidelines for Cardiac Rehabiliation and Cardiovascular Disease Prevention: Translating Knowledge into Action. ISBN: 978-0-9685851-3-9. [Google Scholar]
- 13.Marzolini S.R., Blanchard C., Alter D.A., Grace S.L., Oh P.I. Delays in referral and enrolment are associated with mitigated benefits of cardiac rehabilitation after coronary artery bypass surgery. Circ Cardiovasc Qual Outcomes. 2015;8:608–620. doi: 10.1161/CIRCOUTCOMES.115.001751. [DOI] [PubMed] [Google Scholar]
- 14.Rocha R.V., Tam D.Y., Karkhanis R. Multiple arterial grafting is associated with better outcomes for coronary artery bypass grafting patients. Circulation. 2018;138:2081–2090. doi: 10.1161/CIRCULATIONAHA.118.034464. [DOI] [PubMed] [Google Scholar]
- 15.Grace S.L., Oh P.I., Marzolini S. Observing temporal trends in cardiac rehabilitation from 1996 to 2010 in Ontario: characteristics of referred patients, programme participation and mortality rates. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-009523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat - Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 17.Hess K.R. Graphical methods for assessing violations of the proportional hazards assumption in cox regression. Stat Med. 1995;14:1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 18.Austin P.C., Lee D.S., Fine J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan YC. Multiple imputation for Missing Data: Concepts and New Development (version 9.0), Published 2000; Computer Science.
- 20.Shanmugasegaram S., Oh P., Reid R.D., McCumber T., Grace S.L. Cardiac rehabilitation barriers by rurality and socioeconomic status: a cross-sectional study. Int J Equity Health. 2013;12:72. doi: 10.1186/1475-9276-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruano-Ravina A., Pena-Gil C., Abu-Assi E. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol. 2016;223:436–443. doi: 10.1016/j.ijcard.2016.08.120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.