Abstract
Background
It is unclear whether the coronary microvascular responses to multiple, mechanistically distinct hyperaemic agents exert similar dilatory responses or share common clinical predictors. This study therefore sought to characterize the index of microvascular resistance (IMR) response to multiple hyperaemic agents in the human coronary circulation.
Methods
Thermodilution-derived IMR was determined during intravenous adenosine, intracoronary acetylcholine, and intravenous dobutamine in patients with ischemic symptoms and nonobstructive coronary angiograms. A total of 128 patients were studied (44 with adenosine and acetylcholine, and 84 with all agents). Adenosine IMR >25, acetylcholine IMR >31, and dobutamine IMR >29 were used to define elevated responses.
Results
IMR responses demonstrated weak-to-moderate association (adenosine vs acetylcholine IMR: ρ = 0.33; adenosine vs dobutamine IMR: ρ = 0.51; acetylcholine vs dobutamine IMR: ρ = 0.28; all P < 0.01). Logistic regression analyses revealed that: (1) elevated adenosine IMR was associated with increasing age and left ventricle hypertrophy (odds ratio [OR] = 1.27 and 1.58; both P < 0.05, respectively), (2) elevated acetylcholine IMR was associated with increasing plasma uric acid (OR = 1.09; P < 0.05), and (3) elevated dobutamine IMR was associated with hypertension and left atrial volume index (OR = 3.99 and 1.07; both P < 0.05, respectively). Subset analyses to evaluate clinical utility of the acetylcholine and dobutamine IMR, independent of abnormal adenosine IMR, revealed that elevated acetylcholine and/or dobutamine IMR were associated with higher risk exercise stress tests, left atrial volumes, and burden of exertional chest pain.
Conclusions
Microvascular-specific IMR responses to different hyperaemic agents are only moderately associated, whereas the predictors for agent-specific IMR responses varied, suggesting that multiple pharmacologic agents interrogate different microvascular control mechanisms.
Résumé
Contexte
On ne sait pas vraiment si les réponses microvasculaires coronariennes à de multiples agents hyperémiques aux modes d’action distincts ont des effets vasodilatateurs similaires ou partagent des facteurs prédictifs cliniques communs. Cette étude visait donc à caractériser la réponse selon l’indice de résistance microvasculaire (IMR) aux multiples agents hyperémiques dans la circulation coronarienne chez l’humain.
Méhodologie
L’IMR obtenu par thermodilution a été déterminé pendant l’administration intraveineuse d’adénosine, intracoronarienne d’acétylcholine et intraveineuse de dobutamine chez des patients présentant des symptômes ischémiques et par angiogrammes coronariens non obstructifs. Un total de 128 patients (44 avec l’adénosine et l’acétylcholine, et 84 avec tous les agents) ont fait partie de l’étude. Des réponses élevées étaient définies par un IMR à l’adénosine > 25, un IMR à l’acétylcholine > 31 et un IMR à la dobutamine > 29.
Résultats
Les réponses selon l’IMR ont révélé une association faible à modérée (IMR à l’adénosine vs IMR à l’acétylcholine : ρ = 0,33; IMR à l’adénosine vs IMR à la dobutamine : ρ = 0,51; IMR à l’acétylcholine vs IMR à la dobutamine : ρ = 0,28; tous : p < 0,01). Des analyses de régression logistique ont révélé que : 1) un IMR à l’adénosine élevé était associé à l’avancement en âge et à une hypertrophie ventriculaire gauche (rapport des cotes [RC] = 1,27 et 1,58; p < 0,05 respectivement pour les deux), 2) un IMR à l’acétylcholine élevé était associé à l’augmentation de la concentration plasmatique d’acide urique (RC = 1,09; p < 0,05) et 3) un IMR à la dobutamine élevé était associé à l’hypertension et à l’indice de volume auriculaire gauche (RC = 3,99 et 1,07; p < 0,05 respectivement pour les deux). Des analyses par sous-groupes visant à évaluer l’utilité clinique de l’IMR à l’acétylcholine et à la dobutamine, indépendamment d’un IMR à l’adénosine anormal, ont révélé que des IMR à l’acétylcholine et/ou à la dobutamine élevés étaient associés à une augmentation du risque lors des épreuves à l’effort, à un volume auriculaire gauche plus élevé et à une augmentation du fardeau associé à la douleur thoracique à l’effort.
Conclusions
Les réponses microvasculaires selon l’IMR à différents agents hyperémiques sont seulement modérément associées, alors que les facteurs prédictifs des réponses selon l’IMR spécifique de l’agent varient, ce qui laisse croire que les multiples agents pharmacologiques font appel à différents mécanismes de contrôle microvasculaire.
Coronary microvascular dysfunction (CMD) is increasingly recognized as a significant contributor to cardiovascular morbidity and mortality.1 Classically, the diagnosis of CMD is considered in the setting of insufficient coronary microvascular vasodilation, usually provoked pharmacologically. The majority of studies assessing microvascular reactivity to pharmacologic hyperaemia have solely used intravenous adenosine and found that abnormal microvascular responses to adenosine predict adverse cardiac outcomes.1 However, it is unclear whether adenosine as the sole hyperaemic agent is sufficient or appropriate,2, 3, 4 because adenosine is not particularly important in the regulation of coronary blood flow in vivo.4 The control of the coronary microcirculation is complex and represents an interplay between neurohormonal, metabolic, myogenic, endothelial, and smooth muscle activity.5,6 This suggests that adenosine in isolation likely provides limited physiological insight into abnormalities related to other microvascular control mechanisms. Unfortunately, coronary microvascular reactivity to multiple pharmacologically distinct agents has been poorly explored. In particular, coronary flow responses to acetylcholine and dobutamine have demonstrated clinical utility in multiple cardiovascular conditions;7, 8, 9 however, microvascular responses to these mechanistically distinct pharmacologic agents are unclear.
The metric for assessing the coronary microcirculation has also not been standardized. Coronary flow reserve (CFR), quantified as the ratio of hyperaemic to basal coronary blood flow, has become the default clinical metric,10 because CFR can be measured using multiple techniques and has a proven prognostic value.11,12 Although clinically useful, recent work has demonstrated that CFR lacks physiological resolution of the coronary microcirculation relative to invasive thermodilution techniques that measure the index of microvascular resistance (IMR).13, 14, 15, 16 Unlike CFR, IMR is specific to the microcirculation and is generally unaffected by systemic hemodynamics.14 When compared with CFR, IMR is superior for the prediction of adverse clinical events.13,15,16 These data suggest that the IMR would provide microvascular-specific insight for examining the hyperaemic responses to mechanistically distinct agents.
Therefore, the primary aim of the current study was to characterize the IMR responses to adenosine, acetylcholine, and dobutamine among patients referred for possible CMD. Because adenosine, acetylcholine, and dobutamine elicit mechanistically distinct dilatory responses,2,4 we hypothesized that the IMR responses would demonstrate poor association. The secondary aim was to investigate clinical risk factors associated with agent-specific IMR responses. We hypothesized that clinical predictors would correlate with different pharmacologic agents.
Methods
Patient selection
This research was approved by the Research Ethics Board at Southlake Regional Health Centre and York University. Written informed consent was obtained by all patients. This study included men and women with ischemic chest pain and nonobstructive coronary arteries (defined by epicardial stenosis <50%), who underwent invasive coronary physiology testing for suspected CMD. Patients were excluded from this study if the invasive physiology study was performed without preprocedure assessment at a dedicated clinic with a comprehensive clinical assessment (described below). Patients were also excluded if technical or patient-specific issues were identified at the time of the procedure that resulted in termination of the procedure.
Study protocol
Clinical assessment
Clinical assessments included past medical history, cardiovascular risk factors, echocardiography, blood biomarkers, and graded exercise stress tests. A standard echocardiogram was performed, with key variables of interest: left ventricular (LV) thickness (measured as LV septal thickness) and left atrial (LA) volume (measured as LA volume index).17 Graded exercise stress tests were performed using the Bruce protocol with concurrent 12-lead electrocardiography, for subsequent calculation of the Duke Treadmill Score18—lower Duke Treadmill Scores indicate greater risk burden. The presence of limiting or nonlimiting angina during the graded exercise stress test was used to define the presence of exertional chest pain. Lastly, a positive stress test was defined by ischemic electrocardiogram changes and/or exertional chest pain.
Coronary physiology testing
Invasive physiology studies were performed using thermodilution techniques as previously described.14,19 Patients received 25 μg intravenous fentanyl and 1 mg intravenous midazolam before vascular access via the right radial approach. Intra-arterial verapamil (2.5 mg) was administered at the time of radial sheath insertion. Nitroglycerin (100 μg) was administered through the guide catheter into the left main artery at the beginning of the procedure. A PressureWire X 0.014-inch pressure-temperature sensor-tipped guidewire (Abbott Vascular, Santa Clara, California) was advanced to the distal third of the left anterior descending artery. The PressureWire measured simultaneously coronary pressure at a proximal (Pa) and distal (Pd) arterial segment. Coronary flow was quantified by averaging the transit time of three 3 cc aliquot injections of room temperature heparinized saline, to obtain the mean transit time (Tmn). IMR (IMR = Pd × Tmn) and CFR (CFR = baseline Tmn/hyperaemic Tmn) were subsequently calculated.
During the first phase, intravenous adenosine (140 μg/kg/min) was used as the sole hyperaemic agent to assess endothelial-independent microvascular function; however, these patients were excluded because of the primary objective of comparing IMR responses with multiple hyperaemic agents. During the second phase, intracoronary acetylcholine was added to the protocol to assess endothelial-dependent microvascular function. Acetylcholine injections were performed a minimum of 3 minutes after adenosine, to ensure that systemic hemodynamics returned to baseline and patient symptoms (if any) dissipated. Acetylcholine testing consisted of a 20 μg test dose, followed by 100 μg slow injection over 20 seconds, followed by slow infusion of 20 cc normal saline for a total of 90 seconds, similar to published protocols.20, 21, 22 After 90 seconds, a coronary angiogram was performed using a power injector to assess for epicardial vasomotion. Coronary flows were then measured using the standard thermodilution technique, completed within 1 minute of the acetylcholine bolus. Only microvascular responses to acetylcholine are reported given a priori aims to evaluate microvascular function. During the third phase, dobutamine was added as an additional hyperaemic agent.23 Dobutamine was performed at least 3 minutes after the acetylcholine injection, to ensure that systemic hemodynamics returned to baseline and patient symptoms (if any) dissipated. The dobutamine protocol was an accelerated protocol with 3-minute stages of 10, 20, 30, and 40 μg/kg/min infusions. Dobutamine IMR responses at 40 μg/kg/min were used for analyses. The intraclass correlation coefficients for the 3 measures of transit time during adenosine, acetylcholine, and dobutamine were 0.85, 0.95, and 0.89, respectively. Furthermore, during the preliminary phase of the program, 10 patients underwent repeated measures of adenosine IMR, separated by 10 minutes. Strong reproducibility was observed (r = 0.97; P < 0.01), with a mean difference of 0.39 (95% confidence interval: −1.31 to 2.09), corresponding to previously published data on adenosine IMR reproducibility.24
Data and statistical analysis
The association between the adenosine, acetylcholine, and dobutamine IMR was evaluated using Spearman’s rank-order correlations. Strong correlations were classified as a ρ > 0.80. Bland-Altman plots assessed the fixed bias between agent-specific IMRs. A fixed bias was considered if the mean difference between IMR responses was significantly different from zero using a 1-sample t-test.25 The 95% confidence interval of the mean differences defined the limits of agreement. Heart rate, systolic blood pressure (BP), diastolic BP, and IMR during baselines were compared using a repeated measures analysis of variance in patients who completed all 3 hyperaemic agents.
Adenosine IMR > 25 defined elevated microvascular response as previously described.26 However, defined cutoffs for acetylcholine and dobutamine IMR are unknown. Therefore, elevated acetylcholine and dobutamine IMRs were defined as > 31 and > 29, respectively. These values were derived based on the 66th percentile of IMR responses. For reference, the 66th percentile for adenosine IMR was 21.
To investigate how clinical variables may predict agent-specific IMR outcomes, the association between adenosine, acetylcholine, and dobutamine IMRs and clinically obtained variables was assessed. Logistic regression analyses were conducted by dichotomizing the agent-specific IMR responses as normal or elevated, based on IMR cutoffs described above. All variables presented in Table 1 (except for medications) were assessed in the logistic regression analysis. Clinical predictors with a P ≤ 0.1 were considered for further inclusion in a multivariate forward regression model. Predictors with a P > 0.1 and a moderate-to-strong correlation (r > 0.6) with other variables in the model were removed from the forward logistic regression model to avoid multicollinearity.
Table 1.
Clinical characteristics
| Variables | All patients | Adenosine Acetylcholine | Adenosine Acetylcholine |
|---|---|---|---|
| Dobutamine | |||
| n | 128 | 44 | 84 |
| Age (y) | 57 ± 12 | 57 ± 14 | 58 ± 11 |
| Sex, female, n (%) | 84 (66) | 32 (73) | 52 (62) |
| BMI (kg/m2) | 28.9 ± 6.1 | 27.1 ± 5.5 | 29.5 ± 6.2 |
| Hypertension, n (%) | 67 (52) | 22 (50) | 45 (54) |
| Diabetes mellitus, n (%) | 18 (14) | 6 (14) | 12 (14) |
| Hyperlipidaemia, n (%) | 58 (45) | 22 (50) | 36 (43) |
| Family history, n (%) | 49 (38) | 20 (46) | 29 (35) |
| Current/former smoker, n (%) | 55 (43) | 19 (43) | 36 (43) |
| Previous MI, n (%) | 41 (32) | 11 (25) | 30 (36) |
| Previous PCI, n (%) | 28 (22) | 11 (25) | 17 (20) |
| Normal angiogram, n (%) | 79 (62) | 28 (64) | 51 (61) |
| Exercise data | |||
| Exertional chest pain, n (%) | 41 (32) | 20 (46) | 21 (25) |
| Duke Treadmill Score (au) | 0.9 ± 6.1 | 0.5 ± 6.9 | 1.1 ± 5.7 |
| Positive stress test, n (%) | 83 (65) | 33 (75) | 50 (60) |
| LV septal thickness (mm) | 9.3 ± 1.7 | 9.0 ± 1.6 | 9.5 ± 1.7 |
| LA volume index (mL/m2) | 26.4 ± 8.5 | 26.6 ± 7.8 | 26.3 ± 9.0 |
| Medications | |||
| Beta blocker, n (%) | 50 (39) | 17 (39) | 33 (39) |
| ACEI or ARB, n (%) | 52 (41) | 18 (41) | 34 (41) |
| Statin, n (%) | 78 (61) | 27 (61) | 51 (61) |
| Calcium channel blocker, n (%) | 39 (31) | 17 (39) | 22 (26) |
| Aspirin, n (%) | 74 (58) | 25 (57) | 49 (58) |
| Low-density lipoprotein (mmol/L) | 2.22 ± 0.91 | 2.19 ± 0.87 | 2.24 ± 0.94 |
| High-density lipoprotein (mmol/L) | 1.47 ± 0.52 | 1.48 ± 0.40 | 1.47 ± 0.58 |
| Triglycerides (mmol/L) | 1.39 ± 0.80 | 1.30 ± 0.67 | 1.43 ± 0.87 |
| C-reactive protein (μg/mL) | 2.63 ± 3.35 | 2.29 ± 3.24 | 2.83 ± 3.43 |
| Uric acid (μmol/L) | 308 ± 82 | 295 ± 96 | 316 ± 73 |
| Creatinine (μmol/L) | 79 ± 18 | 79 ± 19 | 79 ± 17 |
| Thyroid-stimulating hormone (mIU/L) | 1.80 ± 0.90 | 1.96 ± 0.92 | 1.71 ± 0.88 |
Mean ± SD or count (%). Normal angiogram indicates completely smooth coronary arteries. No differences were observed between groups.
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; au, arbitrary units; BMI, body mass index; LA, left atrial; LV, left ventricle; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Lastly, to assess whether the acetylcholine and dobutamine IMR provide additional information over adenosine IMR, a sensitivity analysis of patients with either an elevated acetylcholine and/or dobutamine IMR was compared against patients with normal microvascular responses. This analysis was completed after exclusion of patients with an abnormal adenosine IMR. Clinical variables between these patient groups were compared using an independent samples t-test or a χ2 test, for continuous and categorical variables, respectively. Analyses were performed using IBM SPSS Statistics 23 (Armonk, NY). Parameter data are presented as mean ± standard deviation, whereas nonparametric data are presented as count (%). Significance was defined as P < 0.05.
Results
Between February 2016 and July 2019, 198 coronary assessments were performed. In 26 patients, coronary assessments were performed without preceding clinic visits. Complications or technical difficulties occurred in 13 patients (severe vasospasm requiring repeated boluses of intracoronary nitroglycerin after 100 μg acetylcholine [n = 3], rapid atrial fibrillation after 20 μg acetylcholine [n = 1], significant wire drift noted after procedure [n = 1], fractional flow reserve < 0.80 during adenosine infusions [n = 7], and inadequate data documentation [n = 1]). In addition, 31 patients received adenosine exclusively. These 70 patients were therefore excluded. Of the remaining 128 patients, 44 received adenosine and acetylcholine, and 84 patients received adenosine, acetylcholine, and dobutamine. Further clinical characteristics are presented in Table 1. No differences were observed in all clinical variables between patient groups (all P > 0.05; Table 1).
The coronary microvascular responses are presented in Table 2. Overall, 23%, 34%, and 35% of patients demonstrated elevated adenosine, acetylcholine, and dobutamine IMR responses, respectively (Table 2). In patients with an adenosine IMR > 25, only 12 of 30 (40%) patients demonstrated concurrently elevated acetylcholine IMR (> 31) responses. Similarly, only 12 of 21 (57%) patients demonstrated concurrently elevated dobutamine IMR (> 31) responses. In 84 patients who underwent all 3 agents, systolic BP, diastolic BP, and heart rate were unchanged across baselines (all P > 0.05). A small decrease in baseline IMR was observed throughout the procedure (P < 0.01); however, baseline changes in IMR were unrelated to the acetylcholine IMR (ρ = 0.12; P = 0.19), or the dobutamine IMR (ρ = −0.07; P = 0.53).
Table 2.
Coronary microvascular findings
| Variables |
Baseline 1 |
Adenosine |
Baseline 2 |
Acetylcholine |
Baseline 3 |
Dobutamine |
|---|---|---|---|---|---|---|
| n | 128 | 128 | 84 | |||
| FFR | 0.94 ± 0.03 | 0.90 ± 0.04 | 0.95 ± 0.03 | 0.88 ± 0.07 | 0.95 ± 0.03 | 0.89 ± 0.06 |
| Tmn (s) | 0.77 ± 0.37 | 0.27 ± 0.14 | 0.71 ± 0.37∗ | 0.36 ± 0.20 | 0.69 ± 0.35∗ | 0.37 ± 0.22 |
| IMR | 68.2 ± 32.2 | 20.5 ± 11.2 | 63.8 ± 31.0∗ | 30.0 ± 17.3 | 62.9 ± 31.8∗ | 28.3 ± 16.3 |
| Abnormal IMR, n (%) | 30 (23) | 44 (34) | 29 (35) | |||
| CFR | 3.1 ± 1.5 | 2.3 ± 1.4 | 2.2 ± 1.1 | |||
| Systolic BP (mm Hg) | 129 ± 20 | 119 ± 21 | 132 ± 21 | 133 ± 26 | 132 ± 25 | 138 ± 22 |
| Diastolic BP (mm Hg) | 69 ± 10 | 61 ± 11 | 68 ± 11 | 69 ± 12 | 70 ± 11 | 61 ± 13 |
| Heart rate (bpm) | 76 ± 14 | 89 ± 17 | 78 ± 14 | 76 ± 18 | 73 ± 12 | 112 ± 22 |
Statistical analyses were completed in patients who received all 3 hyperaemic agents only (n = 84).
Mean ± SD or count (%).
BP, blood pressure; CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microvascular resistance; SD, standard deviation.
Significantly lower than baseline 1 (P < 0.05).
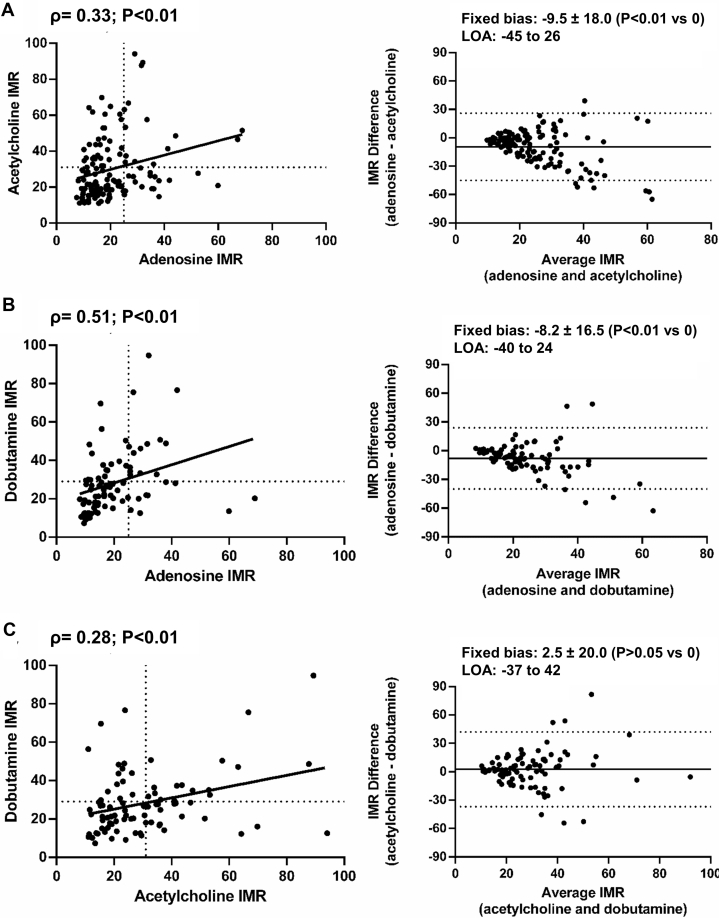
Correlations and agreement of microvascular responses between hyperaemic agents
In 128 patients, the correlation between adenosine and acetylcholine IMRs was ρ = 0.33 (P < 0.01), whereas Bland-Altman plots demonstrated significant fixed bias (−9.5 ± 18.0; P < 0.01; Fig. 1A). The correlation between CFRs was ρ = 0.15; P = 0.08. In 84 patients, the correlation between adenosine and dobutamine IMRs was ρ = 0.51 (P < 0.01), whereas a significant fixed bias was also observed (−8.2 ± 16.5; P < 0.01; Fig. 1B). The correlation between CFRs was ρ = 0.28; P = 0.01. Lastly, in 84 patients, the correlation between acetylcholine and dobutamine IMRs was ρ = 0.28 (P < 0.01); no fixed bias was observed (2.5 ± 20.0; P > 0.05; Fig. 1C). The correlation between CFRs was ρ = 0.05; P = 0.68.
Figure 1.
Correlations (left panel) and Bland-Altman plots (right panel) of the index of microvascular resistance (IMR) responses to (A) adenosine and acetylcholine, (B) adenosine and dobutamine, and (C) acetylcholine and dobutamine. Left panel: solid line indicates the regression slope, whereas the dashed lines indicate IMR cutoff values for adenosine (> 25), acetylcholine (> 31), and dobutamine (> 29). Right panel: solid line indicates the mean difference between IMR responses, whereas the dashed lines indicate the limits of agreement (LOA).
Clinical predictors of agent-specific IMR outcomes
Univariate and forward logistic regression analyses are presented in Table 3. Univariate predictors for an elevated adenosine IMR were hypertension, diabetes, and hyperlipidaemia, increasing age, body mass index, thyroid-stimulating hormone, creatinine, and LV septal thickness (all P < 0.1). An elevated adenosine IMR was independently associated with increasing age and LV septal thickness (both P < 0.05; Table 3). Univariate predictors for an elevated acetylcholine IMR were male sex, uric acid, creatinine, and LV septal thickness (all P < 0.1). An elevated acetylcholine IMR was independently associated with uric acid (P < 0.05; Table 3). Lastly, univariate and multivariable predictors for an elevated dobutamine IMR were hypertension and LA volume index (both P ≤ 0.1; Table 3). Linear regression analyses using the natural logarithm of the adenosine, acetylcholine, and dobutamine IMRs produced similar predictors as the logistic regression analyses (data not shown). Adenosine, acetylcholine, and dobutamine IMRs were not associated with previous Percutaneous Coronary Intervention or Myocardial Infarction, family history, smoking, exertional chest pain, Duke Treadmill Scores, C-reactive protein, or nonobstructive epicardial atherosclerotic disease (all P > 0.1).
Table 3.
Logistic regression analyses of clinical predictors associated with an elevated index of microvascular resistance (IMR) to adenosine, acetylcholine, and dobutamine
| Variable | Univariate |
Forward regression model |
|---|---|---|
| OR (95% CI), P-value | OR (95% CI), P-value | |
| Elevated adenosine IMR (>25) | ||
| Age (per 5 y) | 1.33 (1.10-1.61), P < 0.01 | 1.27 (1.03-1.59), P = 0.03 |
| BMI (per kg/m2) | 1.06 (0.99-1.13), P = 0.08 | |
| Hypertension | 3.24 (1.32-7.98), P < 0.01 | |
| Diabetes mellitus | 2.41 (0.84-6.90), P = 0.10 | |
| Hyperlipidaemia | 2.61 (1.12-6.09), P = 0.03 | |
| Thyroid-stimulating hormone (per mIU/L) | 1.64 (0.99-2.68), P = 0.05 | |
| Creatinine (per 10 μmol/L) | 1.39 (1.08-1.78), P = 0.01 | |
| LV septal thickness (per mm) | 1.49 (1.10-2.01), P = 0.01 | 1.58 (1.10-2.26), P = 0.01 |
| Elevated acetylcholine IMR (>31) | ||
| Sex (male) | 2.82 (1.31-6.06), P < 0.01 | |
| Uric acid (per 10 μmol/L) | 1.08 (1.03-1.15), P < 0.01 | 1.09 (1.03-1.16), P < 0.01 |
| Creatinine (per 10 μmol/L) | 1.31 (1.05-1.64), P = 0.02 | |
| LV septal thickness (per mm) | 1.33 (1.02-1.72), P = 0.03 | |
| Elevated dobutamine IMR (>29) | ||
| Hypertension | 2.67 (1.03-6.89), P = 0.05 | 3.99 (1.21-13.26), P = 0.03 |
| LA volume index (per mL/m2) | 1.07 (0.99-1.14), P = 0.07 | 1.07 (1.00-1.15), P = 0.04 |
BMI, body mass index; CI, confidence interval; LA, left atrial; LV, left ventricle; OR, odds ratio.
Sensitivity analysis excluding abnormal adenosine IMR
In 84 patients who received all 3 hyperaemic agents, 21(25%) patients had an adenosine IMR > 25, and were excluded from the subset analysis. Of the remaining 63 patients, 31 had an elevated acetylcholine and/or dobutamine IMR. Compared with patients with normal coronary microvascular responses, patients with an elevated acetylcholine and/or dobutamine IMR were more likely to have exertional chest pain, lower Duke Treadmill Scores, greater LA volume index (all P < 0.05; Table 4), and a trend towards increased LV septal thickness (P = 0.07). In addition, in the 21 patients who had an elevated adenosine IMR, only 4 (19%) patients had exertional chest pain. Conversely, 13 (42%) patients with a normal adenosine IMR, but elevated acetylcholine and/or dobutamine IMR, had exertional chest pain—the proportion of exertional chest pain between these groups was similar (P = 0.08). Lastly, similar results were observed after excluding patients with an abnormal adenosine IMR and/or CFR (< 2.0) (data not shown).
Table 4.
Clinical predictors of an elevated acetylcholine or dobutamine index of microvascular resistance (IMR) response, excluding patients with an abnormal adenosine IMR
| Variables |
High acetylcholine or dobutamine IMR |
No abnormalities |
P-value |
|---|---|---|---|
| n | 31 | 32 | |
| Exertional chest pain, n (%) | 13 (42) | 4 (13) | < 0.01 |
| Duke Treadmill Score (au) | −0.7 ± 5.5 | 2.7 ± 5.5 | 0.03 |
| LV septal thickness (mm) | 9.8 ± 1.2 | 8.9 ± 2.2 | 0.07 |
| LA volume index (mL/m2) | 29.7 ± 11.5 | 22.3 ± 4.4 | < 0.01 |
Mean ± SD or count (%).
au, arbitrary units; LA, left atrial; LV, left ventricle; SD, standard deviation.
Discussion
This study sought to characterize IMR responses to adenosine, acetylcholine, and dobutamine among patients referred for possible CMD. The principal findings of this study are 3-fold. First, IMR responses to each pharmacologic agent demonstrated weak-to-moderate association, the presence of fixed bias, and poor limits of agreement. Second, forward logistic regression analyses identified several clinical variables that were associated with agent-specific IMR responses. These clinical predictors were mostly distinct to each pharmacologic agent. Third, in patients with normal adenosine IMR responses, an elevated acetylcholine and/or dobutamine IMR was associated with cardiac and exercise abnormalities. Taken together, these data suggest that assessing the coronary microvasculature with multiple, mechanistically distinct, hyperaemic agents may provide insight towards microvascular control mechanisms. These findings also suggest that the use of adenosine in isolation may not adequately identify abnormal coronary microvascular responses.
An elevated adenosine IMR was observed in 23% of patients, corresponding with previously published data.26 Lee et al.26 also observed an association between an elevated adenosine IMR and traditional cardiovascular risk factors (ie, age, hypertension, diabetes mellitus, and body mass index). The current study supports the findings by Lee et al. and suggests that both age and LV septal thickness were independently associated with an elevated adenosine IMR. These data suggest that adenosine IMR may identify structural limits of microvascular dilatation rather than a functional abnormality. Abnormal microvascular structure has been documented in patients with hypertrophic cardiomyopathy,27 whereas microvascular rarefactions are commonly observed in patients with hypertension, obesity, and diabetes.28 Fujii et al.29 showed a drop in perfusion pressure from the epicardial arteries to the endocardial microvasculature in hypertensive dogs due to anatomic changes. To the extent that this is true in humans, the prognostic significance of adenosine IMR may fundamentally be structural in origin.
The current study observed poor agreement between the microvascular responses to adenosine and acetylcholine, supporting previous work.30 In addition, clinical predictors associated with elevated IMR responses differed between agents. Particularly, acetylcholine IMR was independently associated with uric acid, supporting the relevance of uric acid in the control of acetylcholine-induced vasoreactivity.31 The authors speculate that this association may reflect abnormalities with xanthine oxidase, which may facilitate excessive free radical production and drive endothelial dysfunction. In support of this, allopurinol and oxypurinol, xanthine oxidase inhibitors, have been shown to improve brachial artery flow-mediated dilation32 (a strong correlate to the acetylcholine IMR19) and acetylcholine-mediated coronary flow responses in patients undergoing angiography or PCI.33 Whether xanthine oxidase inhibitors can improve coronary microvascular function in patients with an abnormal acetylcholine IMR should be further investigated.
Moderate association between adenosine and dobutamine IMR was observed in the current study. Stronger agreement between coronary flow velocity responses to adenosine and dobutamine has been previously observed;34 however, no study to our knowledge has applied thermodilution techniques to quantify the IMR responses to both hyperaemic agents in patients with suspected CMD. In addition, the current study observed independent associations between dobutamine IMR, hypertension, and LA volume index. These associations likely relate to abnormal sympathetic activation. Hypertension is associated with elevated cardiac norepinephrine spillover,35 whereas experimentally upregulating cardiac sympathetic activity in animal models can cause adverse atrial remodelling.36 These data raise the possibility that the coronary microvascular response to dobutamine reflects abnormal cardiac sympathetic nerve activity.
Given the well-established utility of adenosine-mediated coronary vasodilation, it is reasonable to question the relevance of the microvascular responses to acetylcholine and dobutamine. It is possible that the prognostic significance of these responses is limited to those patients with anatomically abnormal microvasculature, which would be identified using adenosine. However, when excluding patients who demonstrated an abnormal adenosine IMR, patients with an elevated acetylcholine and/or dobutamine IMR presented with functional abnormalities including greater burden of exertional chest pain, higher risk graded exercise tests, and increased LA volume index. We hypothesized that this patient group would also be at increased risk of adverse cardiovascular events, and would benefit from targeted therapies.
Clinical relevance
Standardizations set by the Coronary Vasomotion Disorders International Study Group have recently defined CMD as abnormal coronary microvascular responses to either adenosine and/or acetylcholine37—only when acetylcholine provokes epicardial vasospasm or ischemic symptoms is the classification of microvascular dysfunction altered.37 Furthermore, similar pharmacologic therapies are used for patients with abnormal adenosine-mediated microvascular responses and/or acetylcholine-mediated microvascular spasms,38 suggesting that these coronary abnormalities are pathologically similar. The poor association between hyperaemic agents in the current study challenges previous observations and suggests that interrogating the coronary microvasculature with multiple mechanistically distinct hyperaemic agents may provide information related to a wider range of microvascular control mechanisms.
Limitations
First, our data suggest weak-to-moderate correlations between hyperaemic agents; however, our patient volumes are not yet large enough to clearly delineate discrete phenotypes. Second, there are good clinical data providing cutoff values for adenosine IMR,13,15,16 but no such data exist for acetylcholine or dobutamine, important when interpreting the logistic regression analyses in the current study. However, linear regression modelling produced similar findings. In addition, we do not present long-term clinical outcome data. It is unclear whether the mathematically derived cutoffs for abnormal acetylcholine or dobutamine responses predict clinically relevant events. Larger studies with long-term follow-ups are required to determine whether the acetylcholine and dobutamine IMR cutoffs used in this study are of clinical utility. Third, we did not randomize the order of adenosine, acetylcholine, and dobutamine. We observed a minor reduction in baseline IMRs, likely due to patient discomfort during the procedure. Nonetheless, we cannot exclude the possibility of a minor persistent effect from the preceding hyperaemic agent; however, any residual effect would serve to increase, not decrease, the correlation between agents. In addition, no relationship between changes in baseline IMR and subsequent agent-specific IMR responses was observed. Fourth, persistent symptoms of ischemic chest pain were a primary inclusion criterion in the current study. Unfortunately, we were unable to obtain objective measures of ischemia or angina burden in our patient cohort. Lastly, valid measures of IMR during all hyperaemic agents are reliant on the quality of saline injections, sensor wire position, and adequate guide seating without damping. However, despite these inherit limitations, thermodilution is superior relative to Doppler-derived coronary flow velocities.14,39 Furthermore, it is unclear whether epicardial responses to acetylcholine and/or dobutamine can influence the validity of IMR responses. However, large reductions in the fractional flow reserve, which were not observed in the current study, are likely required in order for epicardial vasomotion to significantly influence acetylcholine and/or dobutamine IMR responses. To assess for the effect of epicardial vasomotion, the data were analyzed both with the observed IMR and corrected IMR using Yong’s correction for epicardial stenosis.40 No significant differences were seen between these analyses (data not shown).
Conclusions
In conclusion, the coronary microvascular responses to adenosine, acetylcholine, and dobutamine demonstrated weak-to-moderate association, suggesting that multiple mechanistically distinct pharmacologic agents may help interrogate different microvascular control mechanisms. In addition, adenosine in isolation may not adequately identify abnormal coronary microvascular responses, as adenosine failed to identify a subset of patients with exertional chest pain, higher risk exercise stress tests, and altered cardiac structure. Future studies should therefore determine if a comprehensive coronary microvascular assessment can improve patient classification and subsequent treatment strategies in patients with suspected CMD.
Funding Sources
M.N. was supported by a CIHR Fredrick Banting and Charles Best Canadian Graduate Scholarship. The Cardiovascular Integrated Physiology Program received an unrestricted grant from Abbott Vascular Canada, for the development of the database used for this research.
Disclosures
S.E.S.M. reports receiving research support from Abbott Vascular Canada. The rest of the authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This research was approved by the Research Ethics Board at Southlake Regional Health Centre and York University and was conducted in compliance with the tri-council policy statement on the ethical conduct for research involving humans.
See page 140 for disclosure information.
References
- 1.Pepine C.J., Anderson R.D., Sharaf B.L. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGeoch R.J., Oldroyd K.G. Pharmacological options for inducing maximal hyperaemia during studies of coronary physiology. Catheter Cardiovasc Interv. 2008;71:198–204. doi: 10.1002/ccd.21307. [DOI] [PubMed] [Google Scholar]
- 3.Ali Raza J., Reeves W.C., Movahed A. Pharmacological stress agents for evaluation of ischemic heart disease. Int J Cardiol. 2001;81:157–167. doi: 10.1016/s0167-5273(01)00536-8. [DOI] [PubMed] [Google Scholar]
- 4.Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol. 2010;105:1–5. doi: 10.1007/s00395-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 5.Schelbert H.R. Anatomy and physiology of coronary blood flow. J Nucl Cardiol. 2010;17:545–554. doi: 10.1007/s12350-010-9255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knaapen P., Camici P.G., Marques K.M. Coronary microvascular resistance: methods for its quantification in humans. Basic Res Cardiol. 2009;104:485–498. doi: 10.1007/s00395-009-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Badri A., Bairey Merz C.N., Johnson B.D. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693. doi: 10.1016/j.jacc.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmari S.A.L., Modesto K., Bunch J. Doppler derived coronary flow reserve during dobutamine stress echocardiography further improves detection of myocardial ischemia. Eur J Echocardiogr. 2006;7:134–140. doi: 10.1016/j.euje.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Kelle S., Chiribiri A., Vierecke J. Long-term prognostic value of dobutamine stress CMR. JACC Cardiovasc Imaging. 2011;4:161–172. doi: 10.1016/j.jcmg.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Marinescu M.A., Löffler A.I., Ouellette M. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8:210–220. doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britten M.B., Zeiher A.M., Schächinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15:259–264. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 12.Murthy V.L., Naya M., Foster C.R. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng M.K.C., Yong A.S.C., Ho M. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ Cardiovasc Interv. 2012;5:515–522. doi: 10.1161/CIRCINTERVENTIONS.112.969048. [DOI] [PubMed] [Google Scholar]
- 14.Ng M.K.C., Yeung A.C., Fearon W.F. Invasive assessment of the coronary microcirculation: Superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 15.Fearon W.F., Low A.F., Yong A.S. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention: clinical perspective. Circulation. 2013;127:2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fearon W.F., Shah M., Ng M. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;51:560–565. doi: 10.1016/j.jacc.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 18.Mark D.B., Shaw L., Harrell F.E. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325:849–853. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 19.Nardone M., Miner S., McCarthy M., Ardern C.I., Edgell H. Noninvasive microvascular indices reveal peripheral vascular abnormalities in patients with suspected coronary microvascular dysfunction. Can J Cardiol. 2020;36:1289–1297. doi: 10.1016/j.cjca.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Ong P., Athanasiadis A., Borgulya G. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. J Am Coll Cardiol. 2012;59:655–662. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Ong P., Athanasiadis A., Hill S. Coronary microvascular dysfunction assessed by intracoronary acetylcholine provocation testing is a frequent cause of ischemia and angina in patients with exercise-induced electrocardiographic changes and unobstructed coronary arteries. Clin Cardiol. 2014;37:462–467. doi: 10.1002/clc.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong P., Athanasiadis A., Sechtem U. Intracoronary acetylcholine provocation testing for assessment of coronary vasomotor disorders. J Vis Exp. 2016:54295. doi: 10.3791/54295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collste O., Tornvall P., Alam M., Frick M. Coronary flow reserve during dobutamine stress in Takotsubo stress cardiomyopathy. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-007671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne A.R., Berry C., Doolin O. Microvascular resistance predicts myocardial salvage and infarct characteristics in ST-elevation myocardial infarction. J Am Heart Assoc. 2012;1 doi: 10.1161/JAHA.112.002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol. 2002;29:527–536. doi: 10.1046/j.1440-1681.2002.03686.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee B.-K., Lim H.-S., Fearon W.F. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartzkopff B., Mundhenke M., Strauer B.E. Alterations of the architecture of subendocardial arterioles in patients with hypertrophic cardiomyopathy and impaired coronary vasodilator reserve: a possible cause for myocardial ischemia. J Am Coll Cardiol. 1998;31:1089–1096. doi: 10.1016/s0735-1097(98)00036-9. [DOI] [PubMed] [Google Scholar]
- 28.Agabiti-Rosei E., Rizzoni D. Microvascular structure as a prognostically relevant endpoint. J Hypertens. 2017;35:914–921. doi: 10.1097/HJH.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 29.Fujii M., Nuno D.W., Lamping K.G. Effect of hypertension and hypertrophy on coronary microvascular pressure. Circ Res. 1992;71:120–126. doi: 10.1161/01.res.71.1.120. [DOI] [PubMed] [Google Scholar]
- 30.Sara J.D., Widmer R.J., Matsuzawa Y. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Prasad M., Matteson E.L., Herrmann J. Uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension. 2017;69:236–242. doi: 10.1161/HYPERTENSIONAHA.116.08436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cicero A.F.G., Pirro M., Watts G.F. Effects of allopurinol on endothelial function: a systematic review and meta-analysis of randomized placebo-controlled trials. Drugs. 2018;78:99–109. doi: 10.1007/s40265-017-0839-5. [DOI] [PubMed] [Google Scholar]
- 33.Baldus S., Köster R., Chumley P. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radic Biol Med. 2005;39:1184–1190. doi: 10.1016/j.freeradbiomed.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meimoun P., Sayah S., Tcheuffa J.C. Transthoracic coronary flow velocity reserve assessment: comparison between adenosine and dobutamine. J Am Soc Echocardiogr. 2006;19:1220–1228. doi: 10.1016/j.echo.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Grassi G., Ram V.S. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–466. doi: 10.1016/j.jash.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Kiriazis H., Du X.-J., Feng X. Preserved left ventricular structure and function in mice with cardiac sympathetic hyperinnervation. Am J Physiol Heart Circ Physiol. 2005;289:H1359–H1365. doi: 10.1152/ajpheart.01010.2004. [DOI] [PubMed] [Google Scholar]
- 37.Ong P., Camici P.G., Beltrame J.F. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 38.Ford T.J., Stanley B., Good R. Stratified medical therapy using invasive coronary function testing in angina. J Am Coll Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Fearon W.F., Farouque H.M.O., Balsam L.B. Comparison of coronary thermodilution and doppler velocity for assessing coronary flow reserve. Circulation. 2003;108:2198–2200. doi: 10.1161/01.CIR.0000099521.31396.9D. [DOI] [PubMed] [Google Scholar]
- 40.Yong A.S., Layland J., Fearon W.F. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6:53–58. doi: 10.1016/j.jcin.2012.08.019. [DOI] [PubMed] [Google Scholar]