Abstract
Vascular inflammation is linked with the pathogenesis of vasospastic angina (VSA). Recent studies reported the potential of pericoronary adipose tissue attenuation as shown on coronary computed tomography angiography to detect coronary inflammation. This report presents a case of myocardial infarction with nonobstructive coronary arteries potentially complicated with VSA that demonstrated reduction of inflammation after symptom improvement with calcium channel–blocker treatment as assessed by serial examination of pericoronary adipose tissue attenuation. This case highlighted the feasibility of a noninvasive assessment of pericoronary adipose tissue attenuation to evaluate the disease activity of VSA and guide patient management.
Résumé
L’inflammation vasculaire est liée à la pathogenèse de l’angine vasospastique (AVS). De récentes études ont révélé le potentiel de l’atténuation du tissu adipeux péricoronaire qui était observée à l’angiographie coronarienne par tomodensitométrie pour détecter l’inflammation coronarienne. Le présent rapport porte sur un cas d’infarctus du myocarde sans obstruction coronaire potentiellement compliqué par l’AVS qui selon l’examen en série de l’atténuation du tissu adipeux péricoronaire a démontré une réduction de l’inflammation après l’amélioration des symptômes à l’aide d’un traitement par bloqueurs des canaux calciques. Ce cas a illustré la faisabilité d’un examen non effractif de l’atténuation du tissu adipeux péricoronaire pour évaluer l’activité de l’AVS et orienter la prise en charge du patient.
Case
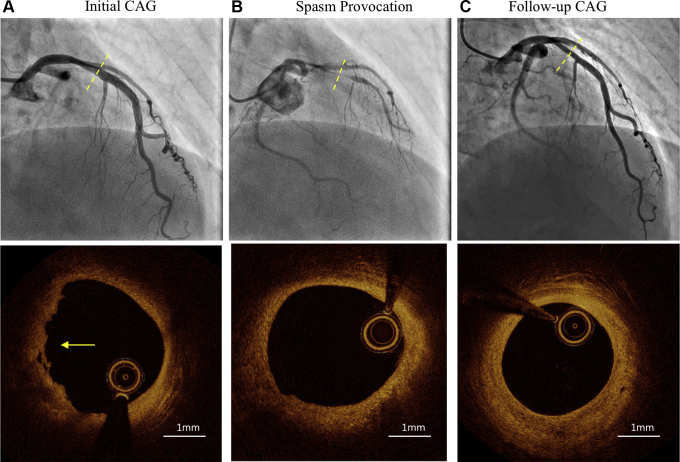
A 49-year-old woman presented to our emergency department with severe chest pain. She also reported having had a few episodes of atypical chest pain prior to presentation. An electrocardiogram exhibited biphasic T waves in the precordial leads. Laboratory data showed an elevated high-sensitivity troponin-I level of 653 pg/mL. She was diagnosed with non-ST-segment-elevation myocardial infarction and underwent urgent coronary angiography. Coronary computed tomography angiography (CTA) revealed an intermediate stenosis in the proximal left anterior descending (LAD) coronary artery. Subsequent coronary angiography after intracoronary administration of nitroglycerin showed mild stenosis in the proximal LAD artery. Optical coherence tomography (OCT) of the LAD artery revealed intracoronary thrombus overlying a fibrous plaque with an intact fibrous cap suggestive of OCT-defined plaque erosion as the culprit plaque phenotype1 (Fig. 1A). Given the preserved minimal lumen area of 5.12 mm2 with thrombolysis in myocardial infarction (TIMI) 3 flow, no stent was implanted, and dual antiplatelet therapy, with prasugrel and aspirin, was administrated.
Figure 1.
Serial coronary angiogram (upper panel) and optical coherence tomography images (lower panel). (A) Initial coronary angiography (CAG) revealed mild stenosis (dashed line) in the left anterior descending (LAD) coronary artery. Optical coherence tomography (OCT) revealed intracoronary thrombus (arrow) overlying a fibrous plaque with an intact fibrous cap, which was suggestive of OCT-defined plaque erosion as the culprit plaque phenotype. The minimal lumen area was preserved (5.12 mm2). (B) On the acetylcholine provocation test, subtotal stenosis was provoked after intracoronary administration of 100 μg of acetylcholine into the left coronary artery. OCT showed reduction of the thrombus volume in the proximal LAD coronary artery. (C) Follow-up CAG confirmed no significant progression of stenosis in the LAD coronary artery. OCT exhibited formation of a layered plaque.
One week later, she underwent a provocation test for coronary spasm using acetylcholine. After intracoronary administration of 100 μg of acetylcholine, subtotal stenosis, accompanied by severe chest pain and ST-segment elevation in the leads V1 to V3, was induced at the proximal LAD artery, which corresponded to the culprit site. Stenosis resolved after intracoronary administration of nitroglycerin. OCT performed after the provocation test showed a decrease in the thrombus volume at the proximal LAD artery compared with that observed at the acute phase (Fig. 1B). She was diagnosed with myocardial infarction with nonobstructive coronary arteries (MINOCA) potentially caused by vasospastic angina (VSA) and was prescribed with calcium-channel blockers (4 mg of benidipine taken twice a day). Dual antiplatelet therapy was terminated after 2 weeks. She was deemed to be intolerant to statin therapy, which was subsequently discontinued. During the first 6 months after discharge, she experienced a few episodes of light angina, mainly during rest, but she was free from angina after that.
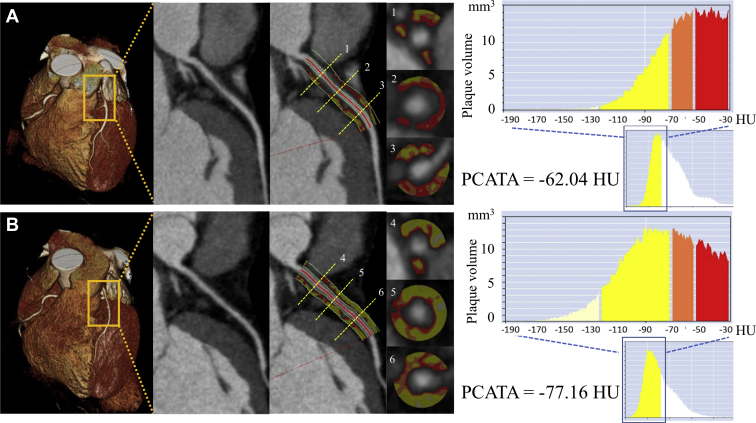
Two years later, she underwent follow-up coronary CTA and subsequent coronary angiography. Coronary CTA showed mild stenosis at the proximal LAD artery. OCT revealed formation of a layered plaque with no significant progression of the stenosis, suggesting that a healing process of the coronary plaque was underway2 (Fig. 1C). Serial coronary CTA revealed that the elevated pericoronary adipose tissue attenuation surrounding the LAD artery of –62.04 Hounsfield units detected at the acute phase decreased to –77.16 Hounsfield units at the 2-year follow-up (Fig. 2).
Figure 2.
Analysis of pericoronary inflammation by coronary computed tomography angiography (CTA). Pericoronary adipose tissue attenuation (PCATA), defined as the mean computed tomography attenuation value of the pericoronary adipose tissue within a radial distance equal to the reference diameter of the vessel along the segments (40 mm in length), was evaluated. (A) Coronary CTA on admission showed PCATA of –62.04 Hounsfield units (HU). (B) Coronary CTA performed 2 years later showed PCATA of –77.16 HU.
Discussion
Controversy still exists regarding the pathogenesis of VSA. Endothelial dysfunction and hyperreactivity of smooth muscle cells have been suggested to play key roles in the pathogenesis of coronary spasm, but the importance of underlying pericoronary inflammation has only been addressed recently.3 Coronary artery spasm serves as an important factor in the pathogenesis of not only chronic coronary artery disease such as VSA but also acute coronary syndrome.4
Ohyama et al. have reported the association between VSA and the inflammation of pericoronary adipose tissue, as assessed by 18F-fluorodeoxyglucose (FDG) uptake on positron emission tomography.5 The referenced study reported that coronary perivascular FDG uptake was decreased after treatment of VSA with mainly calcium channel–blockers, and there was a significant trend toward an association between the extent of symptom improvement and the reduction of FDG uptake. Pericoronary adipose tissue computed tomography attenuation has been introduced recently as a novel imaging biomarker that captures coronary artery inflammation and predicts clinical outcome.6 We also reported the feasibility of coronary CTA to demonstrate higher levels of pericoronary adipose tissue inflammation in patients with VSA.7 To our knowledge, this is the first report to indicate a reduction of inflammation after a course of calcium channel–blocker treatment, as assessed by serial examination of pericoronary adipose tissue attenuation on coronary CTA, in a patient presenting with MINOCA potentially complicated with VSA. The CTA finding was compatible with the symptom relief and serial OCT assessments of the initial acute coronary syndrome–culprit site, exhibiting both a decrease in the disease activity of VSA and the healing process of OCT-defined erosion.
Herein, we illustrated the potential of a comprehensive assessment of coronary CTA including pericoronary adipose tissue attenuation to evaluate the disease activity of VSA, as well as epicardial stenosis, and guide patient management.
Novel Teaching Points.
-
•
Recent studies reported the potential of pericoronary adipose tissue attenuation, as shown on coronary CTA, to detect coronary inflammation.
-
•
We present a case of MINOCA potentially complicated with vasospastic angina (VSA) that demonstrated reduction of inflammation after symptom improvement with calcium channel–blocker treatment as assessed by serial examination of pericoronary adipose tissue attenuation on coronary CTA.
-
•
This case highlighted the feasibility of a noninvasive assessment of attenuation of the pericoronary adipose tissue, as shown on computed tomography, to evaluate the disease activity of VSA and guide patient management.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study was conducted in compliance with the Declaration of Helsinki and approved by the institutional review board of Tsuchiura Kyodo General Hospital.
See page 206 for disclosure information.
References
- 1.Jia H., Abtahian F., Aguirre A.D. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimokado A., Matsuo Y., Kubo T. In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis. 2018;275:35–42. doi: 10.1016/j.atherosclerosis.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities—from bench to bedside. Eur Heart J. 2014;35:3180–3193. doi: 10.1093/eurheartj/ehu427. [DOI] [PubMed] [Google Scholar]
- 4.Ong P., Athanasiadis A., Hill S. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008;52:523–527. doi: 10.1016/j.jacc.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 5.Ohyama K., Matsumoto Y., Takanami K. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol. 2018;71:414–425. doi: 10.1016/j.jacc.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Oikonomou E.K., Marwan M., Desai M.Y. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno H., Hoshino M., Sugiyama T. Pericoronary adipose tissue inflammation on coronary computed tomography in patients with vasospastic angina. JACC Cardiovasc Imaging. 2021;14:511–512. doi: 10.1016/j.jcmg.2020.08.002. [DOI] [PubMed] [Google Scholar]