Abstract
Environmental contamination by pharmaceuticals is global, substantially altering crucial behaviours in animals and impacting on their reproduction and survival. A key question is whether the consequences of these pollutants extend beyond mean behavioural changes, restraining differences in behaviour between individuals. In a controlled, two-year, multigenerational experiment with independent mesocosm populations, we exposed guppies (Poecilia reticulata) to environmentally realistic levels of the ubiquitous pollutant fluoxetine (Prozac). Fish (unexposed: n = 59, low fluoxetine: n = 57, high fluoxetine: n = 58) were repeatedly assayed on four separate occasions for activity and risk-taking behaviour. Fluoxetine homogenized individuals' activity, with individual variation in populations exposed to even low concentrations falling to less than half that in unexposed populations. To understand the proximate mechanism underlying these changes, we tested the relative contribution of variation within and between individuals to the overall decline in individual variation. We found strong evidence that fluoxetine erodes variation in activity between but not within individuals, revealing the hidden consequences of a ubiquitous contaminant on phenotypic variation in fish—likely to impair adaptive potential to environmental change.
Keywords: animal personality, behavioural plasticity, behavioural types, ecotoxicology, individuality, pharmaceuticals
1. Introduction
In the last two decades, the variety and concentration of pharmaceuticals in the environment has grown substantially [1,2]. Most pharmaceuticals—including psychoactive drugs such as antipsychotics, anxiolytics and antidepressants—are only partially absorbed after ingestion [2], and once excreted are not fully removed by wastewater treatment [3]. Such pollutants make their way into the environment via wastewater [4], remaining biologically active in aquatic ecosystems [5]. As a result, pollution by psychoactive drugs is now ubiquitous in aquatic ecosystems around the world, entering food webs and accumulating in living organisms [2,6,7].
Collateral effects of psychoactive pollutants on wildlife are of increasing concern [8,9]. Psychoactive drugs target receptors of the human brain that are evolutionarily conserved throughout the animal kingdom; so it is perhaps not surprising that they can affect non-target species [10]. There is an accumulating body of evidence that even low concentrations of these compounds have the capacity to alter the physiology, reproduction and ultimately the survival of animals, especially fish (reviewed in [7,11,12]). Such bioactive contaminants can alter fundamental behaviours of wild fish [13]—including activity, sociality and feeding levels—raising serious questions about the ecological and evolutionary consequences of psychoactive pollution [7–9]. For instance, pollution-induced changes in activity levels can compromise dispersal and migration patterns, and disrupt the balance between securing resources (e.g. food, potential mates) and minimizing risks (e.g. predators, diseases). Yet the vast majority of research has considered only mean cohort effects of psychoactive pollution on animal behaviour [8], while neglecting intraspecific (within-species) variation. Such an approach ignores that behavioural variation among individuals has profound population- and community-level implications, since conspecifics are not ecologically equivalent to one another [14–16]. So it remains largely unknown whether pollution by psychoactive drugs impacts some individuals more than others [8,17] and attenuates individual differences in wildlife as it does in humans [18].
Different behavioural strategies among individuals—statistically captured as between-individual differences in behaviour that are consistent over time and across contexts [14]—are ubiquitous in the animal kingdom and essential for animal populations to thrive [14,16,19–21]. A common view is that such variation increases the power of selection through ecological and evolutionary processes, ranging from intra-species competition to anti-predatory responses and mate choice [14,15,22]. For example, more active and risk-prone individuals have been found to secure more resources and enjoy greater reproductive success relative to less active and more risk-averse conspecifics, but at the cost of higher mortality [23]. Such behavioural specialization is a major driver of reproductive isolation within lineages [24] and precedes changes in gene frequency [25]. As a result, intra-species behavioural variation fuels resilience [26], providing the adaptive potential for animal populations to survive in a changing [27] and increasingly polluted [28] world. In species as diverse as ants (Temnothorax longispinosus [29]) and salmonids (Oncorhynchus tshawytscha [30]), populations with higher degrees of behavioural variation have higher population growth and persist longer than less diverse populations in the face of environmental change. Conversely, the risk of extinction rises with reduced behavioural differences between individuals, as proven in wild populations of sockeye salmon (Oncorhynchus nerka) monitored over five decades [31]. The ecological importance of intra-population variation in behaviour requires a clear understanding of the effects of psychoactive pollutants at the individual level [8,17]. Yet empirical research explicitly testing whether and how psychoactive contaminants, and pharmaceuticals more broadly, modulate behavioural variation between individuals has received very little attention. While a few studies have shown that behavioural changes do occur at the individual level in animals exposed to contaminants [32,33], none have mounted a comprehensive, systematic investigation of the ecological and evolutionary effects of psychoactive contaminants on individual variation in animal behaviour.
Here, we examine whether and how long-term exposure to a common psychoactive contaminant alters behavioural variation in guppies (Poecilia reticulata)—a model species in ecological and evolutionary research, including the study of behavioural variation [34], and a known inhabitant of freshwater environments exposed to anthropogenic pollution [35,36]. We tested 12 independent mesocosm populations, descendants of 3600 wild fish, maintained in large mesocosms that simulated their shallow and vegetated aquatic habitats. Over 2 years (corresponding to up to six generations [37]), fish were exposed to targeted concentrations of fluoxetine (Prozac), one of the world's most widely prescribed psychotherapeutic drugs [32] and a ubiquitous environmental contaminant in sewage effluent and surface waters [38,39]. Four independent mesocosm populations were exposed to each of three treatments: freshwater control (unexposed), low fluoxetine (40 ng l−1) and high fluoxetine (366 ng l−1). The low-fluoxetine treatment reflects concentrations repeatedly detected in aquatic environments around the globe, while the high treatment represents sites heavily contaminated by wastewater outflow [38].
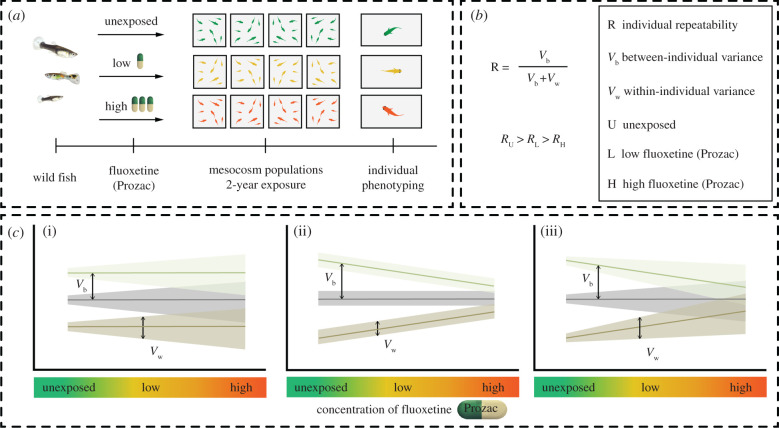
After exposure, we repeatedly measured each fish for its activity levels and propensity to take risks (figure 1a and electronic supplementary material, figure S1)—both ecologically relevant and well-established behavioural traits [14]. For each mesocosm population, we assessed the overall behavioural variation at the individual level (repeatability [21]) and the relative contribution of behavioural variation within and between individuals [40] (figure 1b). The impacts of psychoactive drugs can be best evaluated by isolating the effects within and between individuals—both critical factors determining vulnerability to extinction [26,41] that rely on different underlying mechanisms. Variation within individuals (plasticity) allows for rapid behavioural adjustments to cope with novel challenges, while differences between individuals are behavioural fingerprints that rely on slower evolutionary processes acting at phenotypic and genetic levels [40,42]. So we might expect a fish to adjust its individual behaviour when challenged by exposure to fluoxetine pollution (increasing within-individual variation; figure 1ci). Alternatively, we might expect fish to behave more similarly to each other because fluoxetine is formulated to narrow behavioural extremes [18] (decreasing between-individual variation; figure 1cii) or a combination of both scenarios (increasing within-individual variation and decreasing between-individual variation; figure 1ciii). In general, exposure to antidepressants is likely to reduce individual variation in behaviour [8], especially when interfering with the natural development of behaviour over the lifetime of an individual [43,44] and when affecting the genetic composition of its population [28,45].
Figure 1.
Schematic of the experimental design. (a) Wild fish (juveniles, and adult males and females) from a rainforest-fed stream in northern Australia—free from fluoxetine contamination—were split into 12 independent mesocosm populations. After 5 months fluoxetine exposure started, and lasted for 24 months. Coloured guppy symbols represent each treatment group (mean ± s.e.): green (unexposed), yellow (low fluoxetine, 40 ± 3 ng l−1) and red (high fluoxetine, 366 ± 28 ng l−1). For all treatments, we assessed individual behavioural phenotypes. (b) Repeatability of activity levels and risk-taking was calculated as the proportion of within- and between-individual variance. We expected repeatability to be inversely associated with the level of fluoxetine exposure. (c) Behavioural responses of three individuals over an environmental gradient (fluoxetine concentration): behavioural variation within and between individuals is represented as the thickness of dark regions surrounding each line and the distance between lines, respectively. The predicted decline in repeatability with increasing fluoxetine concentration can be explained through three possible scenarios: (i) an increase in within-individual differences, (ii) a decrease in between-individual differences and (iii) a simultaneous decrease in between-individual differences and increase in within-individual differences. (Online version in colour.)
2. Methods
(a). Multigenerational exposure
We collected wild guppies in November 2016 from Alligator Creek, a pristine rainforest-fed stream in Australia's Bowling Green Bay National Park (19°23'50.3″ S, 146°56'56.5″ E) that is free from fluoxetine contamination [33,46]. A total of 300 adults of balanced sex ratio were randomly assigned to each of 12 independent mesocosms (180 × 60 × 60 cm, length × width × height; water depth 30 cm) with a capacity of 648 l each. Mesocosms were maintained in a temperature-controlled greenhouse facility (23.4 ± 1.0°C) under a 12 : 12 h light : dark cycle, filled with carbon-filtered freshwater, aerated with oxygen probes and containing gravel substrates and natural vegetation (Java moss; Taxiphyllum barbieri) that simulated their natural habitat and provided shelter to fry. Every week, a 20% water change was performed on each mesocosm to maintain high water quality. Fish were fed ad libitum with commercial pellets every second day (Aquasonic Nutra Xtreme C1; 0.8 mm).
Exposure to fluoxetine started after the fish had acclimated to the mesocosms for five months and lasted two consecutive years (24 months). Four mesocosm populations were randomly allocated to one of three exposure regimes (mean ± s.e.): unexposed (0 ± 0 ng l−1), low fluoxetine (40 ± 3 ng l−1) and high fluoxetine (366 ± 28 ng l−1). Both the low- and high-fluoxetine treatments are reflective of concentrations repeatedly detected in freshwater habitats, with the former representing common surface water concentrations in fluoxetine-contaminated systems and the latter being typical of heavily effluent-dominated waterbodies [38]. Details of the dosing and analytical verification of fluoxetine treatment levels are provided in the electronic supplementary material.
(b). Behavioural assays
Fish were sampled from the mesocosm populations randomly, but in equal number—we tested 180 fish (n = 60 per treatment, n = 15 per mesocosm). Each fish was visually inspected to determine its life stage. Females over 15 mm and males displaying coloration and a fully developed gonopodium were considered adults [47]. We individually transferred each fish into a circular glass holding tank (12 × 23 cm, diameter × height) filled with 2 l of treatment water from its native mesocosm population. Each holding tank was aerated, contained a gravel substrate (2 cm layer) and live vegetation (Java moss), and was covered on all sides to control for external disturbance. Water temperature was maintained at 24 ± 1.0°C with a 12 : 12 h light : dark cycle and monitored daily. Fish were fed ad libitum as in the mesocosm populations, and a 50% water change was performed twice weekly.
Fish were habituated to the holding tanks for 48 h before behavioural measurements started. We then individually phenotyped each fish according to standard protocols for studying individual variation in animal behaviour [14] and validated for guppies [34]. We chose activity level (distance moved in cm) and risk-taking (use of the refuge in seconds, and consequently not exploring open spaces that are unfamiliar and potentially dangerous) as reference traits [48,49]. Individual variation in these traits is a key target of selection in all non-sessile animals [14] and is known to have both ecological and evolutionary implications [15]. We performed four behavioural trials on all individuals in random order, with 3 days between each trial.
In each trial, a fish was placed into an open-field arena (25 × 15 × 15 cm) with a white background and filled with treatment water from its native mesocosm population; that is, exposure to treatment water was maintained throughout the study. We adapted a standard protocol successfully used to investigate these behavioural traits [48–50]: a squared dark region (7.5 × 7.5 cm) was positioned in a bottom corner of the open-field arena as the refuge (electronic supplementary material, figure S1). Before each trial, the fish was placed into an opaque plastic cylinder and allowed to acclimate for 2 min, then was carefully released into the arena to freely explore for 20 min. During this time, its behaviour was filmed from above (Panasonic HC-V180) and scored, blind to treatment, using the automated video-tracking software EthoVision XT version 14.0.1326 (Noldus Information Technologies). The fish was returned to its individual holding tank soon after the trial ended. We replaced the water after every trial to exclude the possibility that released chemicals would affect subsequently tested fish. To further reduce contamination risk, 12 identical arenas were used (four dedicated to each treatment). The experiments were conducted within a 2 h window each day (10 : 00–12 : 00) to minimize environmental confounds.
The standard body size of each fish (±0.01 mm)—from the tip of the snout to the caudal peduncle—was measured after the four behavioural trials were completed.
(c). Statistical analysis
A final sample size of 174 fish (unexposed: n = 59, low fluoxetine: n = 57, high fluoxetine: n = 58) out of 180 completed all four behavioural trials (six fish were lost due to early mortality), giving a total of 703 trials and 235 h of observations. The number of animals and repeated measures per individual gave the statistical power needed to detect repeatability and estimate variance within and between individuals [51].
Data analysis was performed in R v. 3.5.3 [52], using the packages lme4, lmerTest, MCMCglmm and Emmeans [53–56]. Our risk-taking measure (refuge use) was square-root transformed to meet assumptions of normality and homogeneity of variance. We assumed Gaussian error distribution, which was confirmed for all response variables after visual inspection of model residuals. The significance level was set at α < 0.05.
We wanted to test whether long-term exposure to fluoxetine reduced behavioural variation within fish mesocosm populations (repeatability) and, if present, the extent to which this effect was driven by changes in behavioural variation within and/or between individuals. Accordingly, we fitted linear mixed-effects models (LMMs), with activity and risk-taking as dependent variables, separately for each treatment. Each model included individual identities (random intercepts) in the random structure to account for repeated measures, while the fixed effects were class (juveniles, males, females), mesocosm population (n = 12; four per treatment), trial (four repeated measures per individual) and time of day (10 : 00–12 : 00).
We tested whether random intercepts explained a significant portion of the variation observed; that is, whether individual variation in activity and risk-taking was repeatable at each treatment [51]. To do this, we used both likelihood ratio tests (LRTs) and Akaike information criteria (ΔAIC) to compare a full model, in which individual identities (random intercepts) were present, with a null model, in which random intercepts were excluded; we chose models with the best likelihood ratio, and lower AIC. In preliminary analyses, we also tested for the presence of heterogeneous variance between treatments, classes (age and sex) and trials (that is, random slopes/regression). We did this by running two separate models for each behavioural trait in which we included random intercepts and allowed individual slopes to either vary between treatments, classes, or trials. There was no evidence that inclusion of class and trial as random slopes increased model fit—no differences were detected between models with random intercepts and slopes or only intercepts via LRTs and ΔAIC—and we did not retain these terms in our final models (electronic supplementary material, table S1). Instead, comparisons between models identified heterogeneous individual variance across treatments in activity, but not in risk-taking (electronic supplementary material, table S1).
Since behavioural repeatability does not necessarily reflect variation in behaviour between individuals [50], partitioning variation within (residual) and between individuals was necessary to understand their relative contribution to overall individual variance. To determine whether variance within (residual) and between individuals changed across exposure treatments, we used Markov Chain Monte Carlo methods to obtain posterior distributions for the univariate LMMs above. We also used bivariate LMMs to discover whether within- and between-individual variation in activity and risk-taking were correlated across treatments. Within- and between-individual variances and repeatability were estimated using a non-informative prior (expected variance V = 1; degree of belief nu = 0.002) as recommended by [51], with 1 500 000 resamplings, 500 000 burn-ins, and 100 thinnings. Effective sample sizes above 1000 were obtained and we visually checked the posterior density plots to ensure proper model mixing and convergence. For univariate models only, a linear regression was fitted to both variance components and repeatability scores that had been randomly sampled from the posterior distributions using treatment (unexposed, low fluoxetine, high fluoxetine) as a continuous predictor. We also tested treatment as a categorical predictor to check for, and confirm, the consistency of results from the linear regressions (see electronic supplementary material, table S2). We repeated this procedure 10 000 times. Statistical significance was inferred from the empirical distribution of the 10 000 slopes. In addition, we performed pairwise comparisons between variance components and repeatability estimates across treatments, as suggested by [57], and assumed significant differences when the 95% credible intervals did not overlap with zero.
We tested whether fish from different treatments (unexposed, low fluoxetine, high fluoxetine) and classes (juveniles, males, females) differed on average in their activity, risk-taking and body size, by fitting a comprehensive LMM separately for each of these traits. LMMs for activity and risk-taking included individual identity as the random effect (random intercepts) and as fixed effects, treatment, class, treatment × class interaction, trial, time of day and mesocosm population nested in treatment. The LMM for body size included the identity of mesocosm populations as the random effect (random intercepts) nested in treatment, and as fixed effects, treatment, class and treatment × class interaction. Accordingly, a significant treatment × class interaction in the fixed-factor structure of the models would indicate that increasing fluoxetine concentrations had different effects on the mean behaviour and/or body size of fish depending on their sex and age. We ran pairwise comparisons with the conservative Bonferroni method for significant predictors and accounted for the variation explained by other predictors.
3. Results
(a). Behavioural variation diminishes in mesocosm populations exposed to fluoxetine
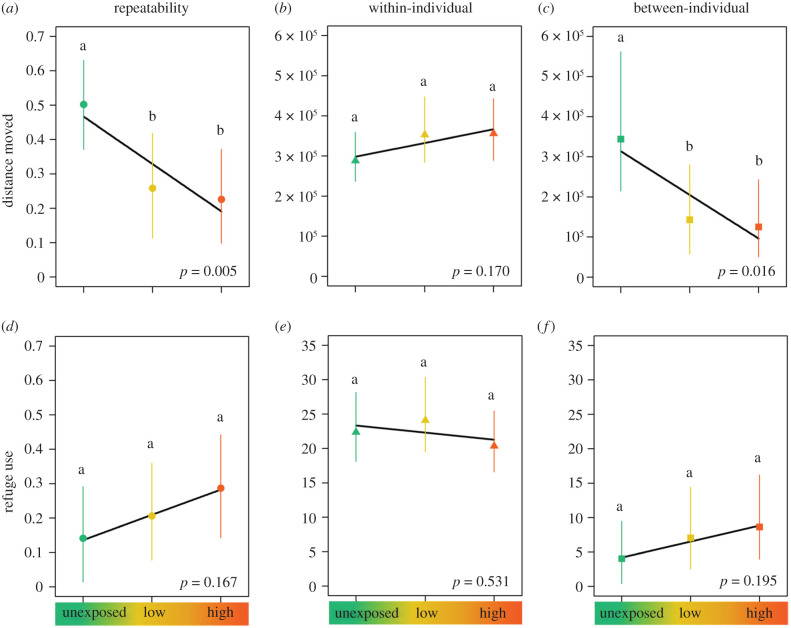
According to the prediction that individual variation in the behaviour of animals should be repeatable and explain a significant portion of the overall behavioural variation observed, we found substantial individual variation in activity and risk-taking that was repeatable over time and across treatments (table 1). We also found that repeatability of activity levels in the fluoxetine-exposed mesocosm populations was less than half that in the unexposed populations: consistent and structured differences in individuals' activity levels explained 50% of the total behavioural variation in unexposed populations, but only 24% and 22% in populations from the low- and high-fluoxetine treatments, respectively (table 2; electronic supplementary material, table S3; figure 2a). By contrast, repeatability of risk-taking did not vary across treatments (table 2; electronic supplementary material, table S3; figure 2d). Additionally, allowing individual slopes to vary between treatments improved the model fit for activity—confirming heterogeneous variance across treatments—but not risk-taking, while individual adjustments were comparable over time and across age and sex in both behavioural traits (electronic supplementary material, table S1).
Table 1.
Results from the LMMs with activity (distance moved) and risk-taking (refuge use) as dependent variables. Treatment (unexposed, low fluoxetine, high fluoxetine), class (juveniles, males, females), mesocosm population (four per treatment), trial (four repeated measures per individual) and time of day are included as fixed effects in both models. Random intercepts are also included for each individual, which allowed accounting for repeated measures and variance partitioning: intercepts (Vbetween), residuals (Vwithin) and repeatabilities. Test statistics and significant levels of the random effects (intercepts) were estimated using LRTs (p) and Akaike information criteria (ΔAIC) between the LM and LMM for each dependant variable. Analysis of variance was performed with Satterthwaite's method. Significance was α < 0.05 and significant results are in italics. Because of the nature of the risk-taking variable, high values represent low risk-taking.
| model | ||||
|---|---|---|---|---|
| activity: distance moved | ||||
| fixed effects | mean sq. | d.f. | F | p |
| treatment | 419 306 | 2, 175 | 1.302 | 0.275 |
| class | 443 948 | 2, 173 | 1.378 | 0.255 |
| treatment × class | 662 210 | 4, 173 | 2.056 | 0.089 |
| mesocosm | 556 453 | 6, 173 | 1.727 | 0.117 |
| trial | 4 214 929 | 3, 521 | 13.085 | <0.001 |
| time of day | 140 342 | 4, 173 | 0.436 | 0.783 |
| random effects | estimate | ΔAIC | p | |
| Vbetween | 177 568 | — | — | — |
| Vwithin | 322 119 | — | — | — |
| repeatability | 0.355 | 118.040 | 116.04 | <0.001 |
| risk-taking: refuge use | ||||
| fixed effects | mean sq. | d.f. | F | p |
| treatment | 89.588 | 2, 176 | 4.117 | 0.018 |
| class | 68.322 | 2, 174 | 3.140 | 0.046 |
| treatment × class | 6.358 | 4, 174 | 0.292 | 0.883 |
| mesocosm | 27.525 | 6, 174 | 1.265 | 0.276 |
| trial | 112.239 | 3, 523 | 5.158 | 0.002 |
| time of day | 16.248 | 4, 174 | 0.747 | 0.561 |
| random effects | estimate | ΔAIC | p | |
| Vbetween | 5.493 | — | — | — |
| Vwithin | 21.760 | — | — | — |
| repeatability | 0.202 | 45.081 | 43.081 | <0.001 |
Table 2.
Results from the random factor structure of LMMs with activity (distance moved) and risk-taking (refuge use) for each treatment as dependent variables. Random intercepts are included for each individual, which allowed variance partitioning: variation between (Vbetween) and within (Vwithin) individuals, and overall measure of repeatability are shown for each trait. Class (juveniles, males, females), mesocosm population (four per treatment), trial (four repeated measures per individual) and time of day are fixed effects in all models. Test statistics and significant levels of the random effects (intercepts) were estimated using LRTs (p) and the Akaike information criterion (ΔAIC) between the full and the null model. ΔAIC refers to the difference in AIC between the null and full models. Significance was α < 0.05 and significant results are in italics.
| variance components | ΔAIC | p | Vbetween ± s.e. | Vwithin ± s.e. | repeatability | |
|---|---|---|---|---|---|---|
| activity: distance moved | ||||||
| unexposed | 72.329 | 74.329 | <0.001 | 281 015 ± 68 | 281 681 ± 69 | 0.499 |
| low fluoxetine | 16.455 | 18.455 | <0.001 | 110 578 ± 43 | 344 092 ± 76 | 0.243 |
| high fluoxetine | 15.855 | 17.854 | <0.001 | 98 230 ± 40 | 345 699 ± 76 | 0.221 |
| risk-taking: refuge use | ||||||
| unexposed | 5.395 | 7.395 | 0.006 | 3.040 ± 0.225 | 21.560 ± 0.600 | 0.124 |
| low fluoxetine | 11.478 | 13.479 | <0.001 | 5.377 ± 0.299 | 23.433 ± 0.625 | 0.187 |
| high fluoxetine | 19.713 | 21.713 | <0.001 | 6.667 ± 0.333 | 19.840 ± 0.575 | 0.251 |
Figure 2.
Change in the overall measure of repeatability (expressed as a proportion) and its variance components (within and between individuals) for activity (distance moved in cm) and risk-taking (refuge use in seconds, square-root transformed) across treatments (unexposed, low fluoxetine, high fluoxetine). Graphs show changes in the overall measure of repeatability (a and d; medians as circles), and in behavioural variation within (b and e; medians as triangles) and between (c and f; medians as squares) individuals. Vertical lines represent 95% credible intervals, and estimates not sharing a common superscript are significantly different. (Online version in colour.)
(b). Lower variation between individuals drives the overall repeatability reduction
Partitioning the behavioural variation within and between individuals allowed us to disentangle their relative contribution to the overall estimates of repeatability. We observed a decline in behavioural differences between fish in activity levels at increasing concentrations of pharmaceutical pollution, with dramatic reductions in the behavioural expression of fish already observed at the lowest concentration of the pollutant (figure 2c; electronic supplementary material, table S3). By contrast, the variation in activity levels did not differ within individuals across treatments (figure 2b). Neither variation within nor between individuals differed across treatments for risk-taking (figure 2e,f, and electronic supplementary material, table S3). Activity and risk-taking were not correlated across treatments within or between individuals, except for negative between-individual covariation in the low-fluoxetine treatment (electronic supplementary material, table S4).
(c). Mean effects of fluoxetine pollution
Varying levels of exposure to fluoxetine did not result in changes in mean activity levels, irrespective of age and sex of the fish (table 1; electronic supplementary material, figure S2). By contrast, risk-taking varied on average between treatments (table 1): fluoxetine-exposed fish tended to spend more time in the refuge—indicating lower risk-taking—than unexposed fish, but we did not detect significant pairwise differences (electronic supplementary material, figure S2). We found mean differences in risk-taking across classes (table 1), where adult females took less risks than juveniles (estimate ± s.e.: 1.532 ± 0.612; d.f.175; p = 0.039). No other predictor included in the analysis—mesocosm population (n = 4 per treatment) or time of day—apart from trial significantly explained mean activity levels or risk-taking (table 1; electronic supplementary material, table S5).
4. Discussion
Our results provide a novel perspective on the effects of psychoactive contaminants on animals, revealing until-now unknown consequences of a ubiquitous environmental pollutant for behavioural variation within and between individuals. We report the first experimental evidence that consistent and highly structured differences in the activity levels of fish in unpolluted waters diminish substantially when they are exposed to environmentally realistic concentrations of fluoxetine: behavioural repeatability declines with increasing concentrations of the pollutant, and a substantial drop in repeatability already occurs at the lowest dose. Specifically, long-term exposure to fluoxetine pollution erodes behavioural variation between but not within individuals, uncovering the evolutionary process behind the overall decline.
The key finding is that chronic exposure to even very low concentrations of fluoxetine erodes variation in activity levels between individuals, but not plasticity within individuals; reduced variation between individuals drives decline in overall behavioural variation (repeatability). This contrasts with the view that behavioural diversity within populations is preserved through feedback loops alone [58,59]. Our results strongly suggest that bioactive pollution suppresses between-individual variation in the behaviour of chronically exposed mesocosm populations. This is crucial given that fluoxetine is continually discharged in the effluent from wastewater treatment plants [38] and is relatively stable [60], resulting in long-term ‘pseudopersistent’ contamination of aquatic ecosystems—as is also true for numerous pharmaceutical pollutants. Our 2-year exposure duration therefore represents an environmentally realistic scenario for chronically exposed populations around the globe. It remains unknown whether these effects are permanent or reversible when the contaminant is withdrawn. Previous studies indicate that chronic exposure to fluoxetine can lead to changes in neuronal morphology and activity which impair behaviour [61] and transcend generations [62], suggesting that even temporary contamination of habitats might have long-lasting effects not only on guppies [33], but on fish more generally.
In any case, unexpressed behavioural differences between individuals are likely to diminish their fitness benefits and, over time, reduce the magnitude of variation observed at the population level, as observed in fish populations under different selective regimes [63–65] and predicted for wildlife under anthropogenic disturbance [66]. For instance, risk-prone individuals would secure no more resources than risk-averse ones if their behavioural variation is consistently suppressed by fluoxetine, reducing the intensity of selection for maintaining such variation. By collapsing the diversity in behavioural strategies among individuals, chronic exposure to even very small concentrations of this psychoactive drug has the potential of reducing resilience [66] and compromising the adaptive capacity [61] of animal populations to survive in a rapidly changing world [26–28].
Two independent but not mutually exclusive mechanisms might explain the long-term effects of fluoxetine on the variation in individual behaviour, with lower levels likely stemming from permanent environmental effects and/or genetic adaptation to contaminated environments [40,42]. First, chronic exposure to antidepressants early in life is likely to restrain natural variation in emotional states and anxiety [8], with permanent effects on behavioural variation between individuals later in life [43,44]. Recent evidence suggests that differences in behaviour between individuals can increase over their lifetime (see [50] and references therein), relying on positive feedback loops between behavioural tendencies early in life and the environment [42]. For example, individuals that are more active, explorative, and risk-prone early in life should be more competitive in securing resources and should grow faster [67]—but see [68]—with cumulative life experiences over time (e.g. winning or losing when competing for resources) resulting in larger differences in behavioural strategies in adulthood [69]. Chronic exposure to fluoxetine early in life might curtail the natural development of behavioural differences over the lifetime, alleviating the efficacy of positive feedback loops that fuel behavioural diversity at the individual level.
It is also possible that long-term exposure to fluoxetine (equivalent to six overlapping generations in this study [37]) might have evolutionary effects on the genetic composition of populations, as observed in wild populations of Atlantic killifish (Fundulus heteroclitus) adapted to polluted waters from urban estuaries [28]. Indeed, behavioural diversity within populations largely depends on underlying genetic variation [42]. Genetic-based behavioural variation occurs between populations of wild guppies that are chronically exposed to anthropogenic pollution [70]. So with genetic variation shrinking in response to the adaptation to contaminated environments [45], diversity in the behavioural strategies of those individuals is also likely to diminish [66]. While identifying the exact mechanism was beyond the scope of the current study, our findings nevertheless reveal that between-individual behavioural variation may be compromised in animal populations exposed to realistic levels of psychoactive pollution—with potential consequences for adapting to future environmental stressors [28]. This evidence adds to a growing body of literature, in which exposure to environmental contaminants has been suggested to alter the behavioural expression in animals through multiple routes [71], and even strengthen behavioural variation (e.g. when the probability to encounter contaminated environments varies between individuals with different activity levels, furtherly increasing their difference in the future).
Studies have shown that environmentally relevant concentrations of antidepressants can alter mean activity levels and risk-taking of wild fish [13,46], interfering with their movement patterns and migrations [72] and their antipredator responses [73]. But mean effects of fluoxetine on risk-taking are not always consistent across species and dosages [74] and can have repercussions on the activity levels of fish (e.g. immobility or ‘freezing', is typically associated with fear and anxiety [46,73]). Nevertheless, in this study, the two behavioural traits—distance moved and refuge use—were largely uncorrelated at the individual level, confirming their independence (i.e. the activity level of a fish did not predict risk-taking). Risk-taking was lower on average in fluoxetine-exposed than unexposed fish, supporting previous evidence that fluoxetine amplifies mean antipredator responses in guppies [46]. Our juveniles took on average more risks than adult females because they had less to lose (their residual reproductive value is lower) and their smaller size offers a lower energy gain to potential predators (electronic supplementary material, table S6). In contrast with our findings on individual-level variation, the effects of fluoxetine on activity were less visible if looking at only mean variation across exposed and unexposed fish [33] and were independent of age and sex. This suggests that the hidden effects of psychoactive pollution at the individual level are extensive and can potentially overshadow the known consequences for mean phenotypes—as observed for jumping spiders (Eris militaris) exposed to insecticides [57].
Over the last decade, there has been increasing exploration of the consequences of psychoactive contaminants on wildlife, deepening our understanding of the changes in mean behaviours often observed in animals inhabiting polluted ecosystems. This study is the first mechanistic investigation of how the ubiquitous pollutant fluoxetine disrupts behavioural variation within animal populations. Where neither physiological tolerance nor behavioural compensation has evolved as a buffer, such effects in the wild can result in drastic population declines, impacting entire ecosystems [26,27]. Investigating the intra-population effects of psychoactive pollution on non-target species might help in addressing environmental challenges, leading to opportunities for improving wildlife conservation. Future research should aim to determine whether the pollution-induced decline of behavioural differences between individuals is consistent across animal species, and associated with concurrent declines in the individual variation of other ecologically relevant traits (e.g. physiology, growth, reproduction).
Supplementary Material
Supplementary Material
Acknowledgements
We thank David Williams and Envirolab Services for analytical testing of water samples, and Raphaël Royauté and Mathilde Tissier for their valuable input during the revision process.
Ethics
Experiments complied with Australian law and were approved by the Monash University Animal Ethics Committee (BSCI/2016/06 and BSCI/2018/11).
Data accessibility
All data and R codes are available as part of the electronic supplementary material.
Authors' contributions
G.P. and B.B.M.W. conceived the research question; G.P., J.M.M., M.G.B. and B.B.M.W. designed the study. All authors performed the experiments. G.P. analysed the data. G.P., J.M.M., M.G.B. and B.B.M.W. wrote the manuscript and all authors discussed it before submission.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Australian Research Council (DP130100385, DP160100372 and FT190100014 to B.B.M.W.), the Forrest Research Foundation (to G.P. and V.R.S), a University of Western Australia Fellowship Support grant (12104502 to G.P.), two Australian Government Research Training Program Scholarships (to J.M.M. and J.A.B.) and a Monash University Postgraduate Publications Award and a Kempe Foundations Research Grant (SMK-1954 to M.G.B.).
References
- 1.Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, Von Gunten U, Wehrli B. 2006. The challenge of micropollutants in aquatic systems. Science 313, 1072–1077. ( 10.1126/science.1127291) [DOI] [PubMed] [Google Scholar]
- 2.Monteiro SC, Boxall ABA. 2010. Occurrence and fate of human pharmaceuticals in the environment. In Rev. Environ. Contam. Toxicol. 202, 53–154. [DOI] [PubMed] [Google Scholar]
- 3.Klaminder J, Jonsson M, Fick J, Sundelin A, Brodin T. 2014. The conceptual imperfection of aquatic risk assessment tests: highlighting the need for tests designed to detect therapeutic effects of pharmaceutical contaminants. Environ. Res. Lett. 9, 084003 ( 10.1088/1748-9326/9/8/084003) [DOI] [Google Scholar]
- 4.Kümmerer K, Dionysiou DD, Olsson O, Fatta-Kassinos D. 2018. A path to clean water. Science 361, 222–224. ( 10.1126/science.aau2405) [DOI] [PubMed] [Google Scholar]
- 5.Klaminder J, Brodin T, Sundelin A, Anderson NJ, Fahlman J, Jonsson M, Fick J. 2015. Long-term persistence of an anxiolytic drug (oxazepam) in a large freshwater lake. Environ. Sci. Technol. 49, 10 406–10 412. ( 10.1021/acs.est.5b01968) [DOI] [PubMed] [Google Scholar]
- 6.Richmond EK, Rosi EJ, Walters DM, Fick J, Hamilton SK, Brodin T, Sundelin A, Grace MR. 2018. A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nat. Commun. 9, 4491 ( 10.1038/s41467-018-06822-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodin T, Piovano S, Fick J, Klaminder J, Heynen M, Jonsson M. 2014. Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations. Phil. Trans. R. Soc. B 369, 20130580 ( 10.1098/rstb.2013.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saaristo M, et al. 2018. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. R. Soc. B. 285, 20181297 ( 10.1098/rspb.2018.1297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold KE, Brown AR, Ankley GT, Sumpter JP. 2014. Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems. Phil. Trans. R. Soc. B 369, 20130569 ( 10.1098/rstb.2013.0569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fent K, Weston AA, Caminada D. 2006. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 76, 122–159. ( 10.1016/j.aquatox.2005.09.009) [DOI] [PubMed] [Google Scholar]
- 11.Ford A 2014. From gender benders to brain benders (and beyond!). Aquat. Toxicol. 151, 1–3. ( 10.1016/j.aquatox.2014.02.005) [DOI] [PubMed] [Google Scholar]
- 12.Brooks BW 2014. Fish on Prozac (and Zoloft): ten years later. Aquat. Toxicol. 151, 61–67. ( 10.1016/j.aquatox.2014.01.007) [DOI] [PubMed] [Google Scholar]
- 13.Brodin T, Fick J, Jonsson M, Klaminder J. 2013. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 339, 814–815. ( 10.1126/science.1226850) [DOI] [PubMed] [Google Scholar]
- 14.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 15.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. ( 10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 16.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacquin L, Petitjean Q, Côte J, Laffaille P, Jean S. 2020. Effects of pollution on fish behavior, personality and cognition: some research perspectives. Front. Ecol. Evol. 8, 86 ( 10.3389/fevo.2020.00086) [DOI] [Google Scholar]
- 18.Wong DT, Perry KW, Bymaster FP. 2005. The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 4, 764–774. ( 10.1038/nrd1821) [DOI] [PubMed] [Google Scholar]
- 19.Gosling SD, John OP. 1999. Personality dimensions in nonhuman animals: a cross-species review. Curr. Dir. Psychol. Sci. 8, 69–75. ( 10.1111/1467-8721.00017) [DOI] [Google Scholar]
- 20.Sih A, Bell AM, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 21.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith BR, Blumstein DT. 2008. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455. ( 10.1093/beheco/arm144) [DOI] [Google Scholar]
- 23.Stamps JA 2007. Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363. ( 10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- 24.Rundle HD, Boughman JW. 2010. Behavioral ecology and speciation. In Evolutionary behavioral ecology (eds Westneat DF, Fox CW), pp. 471–487. New York, NY: Oxford University Press. [Google Scholar]
- 25.West-Eberhard MJ 2003. Developmental plasticity and evolution. New York, NY: Oxford University Press. [Google Scholar]
- 26.McCann KS 2000. The diversity–stability debate. Nature 405, 228 ( 10.1038/35012234) [DOI] [PubMed] [Google Scholar]
- 27.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid NM, et al. 2016. The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wild fish. Science 354, 1305–1308. ( 10.1126/science.aah4993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modlmeier AP, Liebmann JE, Foitzik S. 2012. Diverse societies are more productive: a lesson from ants. Proc. R. Soc. B 279, 2142–2150. ( 10.1098/rspb.2011.2376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson SM, Satterthwaite WH. 2011. Weakened portfolio effect in a collapsed salmon population complex. Can. J. Fish. Aquat. Sci. 68, 1579–1589. ( 10.1139/f2011-084) [DOI] [Google Scholar]
- 31.Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS. 2010. Population diversity and the portfolio effect in an exploited species. Nature 465, 609 ( 10.1038/nature09060) [DOI] [PubMed] [Google Scholar]
- 32.Bean TG, Boxall ABA, Lane J, Herborn KA, Pietravalle S, Arnold KE. 2014. Behavioural and physiological responses of birds to environmentally relevant concentrations of an antidepressant. Phil. Trans. R. Soc. B 369, 20130575 ( 10.1098/rstb.2013.0575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan H, Polverino G, Martin JM, Bertram MG, Wiles SC, Palacios MM, Bywater CL, White CR, Wong BBM. 2020. Chronic exposure to a pervasive pharmaceutical pollutant erodes among-individual phenotypic variation in a fish. Environ. Pollut. 263, 114450 ( 10.1016/j.envpol.2020.114450) [DOI] [PubMed] [Google Scholar]
- 34.Burns JG 2008. The validity of three tests of temperament in guppies (Poecilia reticulata). J. Comp. Psychol. 122, 344 ( 10.1037/0735-7036.122.4.344) [DOI] [PubMed] [Google Scholar]
- 35.Araújo FG, Peixoto MG, Pinto BCT, Teixeira TP. 2009. Distribution of guppies Poecilia reticulata (Peters, 1860) and Phalloceros caudimaculatus (Hensel, 1868) along a polluted stretch of the Paraíba do Sul River, Brazil. Braz. J. Biol. 69, 41–48. ( 10.1590/S1519-69842009000100005) [DOI] [PubMed] [Google Scholar]
- 36.Widianarko B, Van Gestel CAM, Verweij RA, Van Straalen NM.. 2000. Associations between trace metals in sediment, water, and guppy, Poecilia reticulata (Peters), from urban streams of Semarang, Indonesia. Ecotoxicol. Environ. Saf. 46, 101–107. ( 10.1006/eesa.1999.1879) [DOI] [PubMed] [Google Scholar]
- 37.Reznick D, Buckwalter G, Groff J, Elder D. 2001. The evolution of senescence in natural populations of guppies (Poecilia reticulata): a comparative approach. Exp. Gerontol. 36, 791–812. ( 10.1016/S0531-5565(00)00241-2) [DOI] [PubMed] [Google Scholar]
- 38.Mole RA, Brooks BW. 2019. Global scanning of selective serotonin reuptake inhibitors occurrence and hazards in aquatic systems. Environ. Pollut. 250, 1019–1031. ( 10.1016/j.envpol.2019.04.118) [DOI] [PubMed] [Google Scholar]
- 39.Shareef A, Tjandraatmadja G, Kookana R, Williams M. 2010. Determination of potential organic contaminants of emerging environmental concern in domestic wastewater from urban sources. Clayton, Australia: CSIRO. [Google Scholar]
- 40.Dingemanse NJ, Kazem AJ, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 41.Ducatez S, Sol D, Sayol F, Lefebvre L. 2020. Behavioural plasticity is associated with reduced extinction risk in birds. Nat. Ecol. Evol. 4, 788–793. ( 10.1038/s41559-020-1168-8) [DOI] [PubMed] [Google Scholar]
- 42.Dochtermann NA, Schwab T, Sih A. 2015. The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B 282, 20142201 ( 10.1098/rspb.2014.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stamps JA, Groothuis TG. 2010. Developmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Phil. Trans. R. Soc. B 365, 4029–4041. ( 10.1098/rstb.2010.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fawcett TW, Frankenhuis WE. 2015. Adaptive explanations for sensitive windows in development. Front. Zool. 12, S3 ( 10.1186/1742-9994-12-S1-S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R. 2000. Effects of chemical contaminants on genetic diversity in natural populations: implications for biomonitoring and ecotoxicology. Mutat. Res./Rev. Mutat. Res. 463, 33–51. ( 10.1016/S1383-5742(00)00004-1) [DOI] [PubMed] [Google Scholar]
- 46.Saaristo M, McLennan A, Johnstone CP, Clarke BO, Wong BB.M. 2017. Impacts of the antidepressant fluoxetine on the anti-predator behaviours of wild guppies (Poecilia reticulata). Aquat. Toxicol. 183, 38–45. ( 10.1016/j.aquatox.2016.12.007) [DOI] [PubMed] [Google Scholar]
- 47.Houde A 2019. Sex, color, and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 48.Polverino G, Santostefano F, Díaz-Gil C, Mehner T. 2018. Ecological conditions drive pace-of-life syndromes by shaping relationships between life history, physiology and behaviour in two populations of Eastern mosquitofish. Sci. Rep. 8, 14673 ( 10.1038/s41598-018-33047-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krause J, Loader SP, McDermott J, Ruxton GD. 1998. Refuge use by fish as a function of body length–related metabolic expenditure and predation risks. Proc. R. Soc. Lond. B 265, 2373–2379. ( 10.1098/rspb.1998.0586) [DOI] [Google Scholar]
- 50.Polverino G, Cigliano C, Nakayama S, Mehner T. 2016. Emergence and development of personality over the ontogeny of fish in absence of environmental stress factors. Behav. Ecol. Sociobiol. 70, 2027–2037. ( 10.1007/s00265-016-2206-z) [DOI] [Google Scholar]
- 51.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 52.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 53.Hadfield JD 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 54.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1–7.
- 55.Kuznetsova A, Brockhoff PB, Christensen RH.B. 2013. lmerTest package: tests in linear mixed effect models. J. Stat. Softw. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 56.Lenth R, Singmann H, Love J, Buerkner P, Herve M. 2018. Emmeans: estimated marginal means, aka least-squares means. R package version 1.1.3.
- 57.Royauté R, Buddle CM, Vincent C. 2015. Under the influence: sublethal exposure to an insecticide affects personality expression in a jumping spider. Funct. Ecol. 29, 962–970. ( 10.1111/1365-2435.12413) [DOI] [Google Scholar]
- 58.Wolf M, van Doorn GS, Leimar O, Weissing FJ.. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 59.Sih A, Mathot KJ, Moiron M, Montiglio PO, Wolf M, Dingemanse NJ. 2015. Animal personality and state-behaviour feedbacks: a review and guide for empiricists. Trends Ecol. Evol. 30, 50–60. ( 10.1016/j.tree.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 60.Kwon JW, Armbrust KL. 2006. Laboratory persistence and fate of fluoxetine in aquatic environments. Environ. Toxicol. Chem. 25, 2561–2568. ( 10.1897/05-613R.1) [DOI] [PubMed] [Google Scholar]
- 61.Puścian A, et al. 2020. Chronic fluoxetine treatment impairs motivation and reward learning by affecting neuronal plasticity in the central amygdala. Br. J. Pharmacol. 178, 672–688. ( 10.1111/bph.15319. [DOI] [PubMed] [Google Scholar]
- 62.Vera-Chang MN, St-Jacques AD, Gagné, R., Martyniuk CJ, Yauk CL, Moon TW, Trudeau VL.. 2018. Transgenerational hypocortisolism and behavioral disruption are induced by the antidepressant fluoxetine in male zebrafish Danio rerio. Proc. Natl Acad. Sci. 115, E12435–E12442. ( 10.1073/pnas.1811695115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bell AM 2005. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473. ( 10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- 64.Bell AM, Sih A. 2007. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834. ( 10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- 65.Dingemanse NJ, Wright J, Kazem AJ, Thomas DK, Hickling R, Dawnay N. 2007. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138. ( 10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- 66.Geffroy B, Alfonso S, Sadoul B, Blumstein DT. 2020. A world for reactive phenotypes. Front. Conserv. Sci. 1, 611919 ( 10.3389/fcosc.2020.611919) [DOI] [Google Scholar]
- 67.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 68.Niemelä PT, Dingemanse NJ. 2018. Meta-analysis reveals weak associations between intrinsic state and personality. Proc. R. Soc. B 285, 20172823 ( 10.1098/rspb.2017.2823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Krüger A, Sachser N, Lindenberger U, Kempermann G. 2013. Emergence of individuality in genetically identical mice. Science 340, 756–775. ( 10.1126/science.1235294) [DOI] [PubMed] [Google Scholar]
- 70.Jacquin L, Dybwad C, Rolshausen G, Hendry AP, Reader SM. 2017. Evolutionary and immediate effects of crude-oil pollution: depression of exploratory behaviour across populations of Trinidadian guppies. Anim. Cogn. 20, 97–108. ( 10.1007/s10071-016-1027-9) [DOI] [PubMed] [Google Scholar]
- 71.Montiglio PO, Royauté R. 2014. Contaminants as a neglected source of behavioural variation. Anim. Behav. 88, 29–35. ( 10.1016/j.anbehav.2013.11.018) [DOI] [Google Scholar]
- 72.Hellström G, Klaminder J, Finn F, Persson L, Alanärä A, Jonsson M, Fick J, Brodin T. 2016. GABAergic anxiolytic drug in water increases migration behaviour in salmon. Nat. Commun. 7, 1–7. ( 10.1038/ncomms13460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin JM, Saaristo M, Bertram MG, Lewis PJ, Coggan TL, Clarke BO, Wong BBM. 2017. The psychoactive pollutant fluoxetine compromises antipredator behaviour in fish. Environ. Pollut. 222, 592–599. ( 10.1016/j.envpol.2016.10.010) [DOI] [PubMed] [Google Scholar]
- 74.Martin JM, et al. 2019. Antidepressants in surface waters: fluoxetine influences mosquitofish anxiety-related behavior at environmentally relevant levels. Environ. Sci. Technol. 53, 6035–6043. ( 10.1021/acs.est.9b00944) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and R codes are available as part of the electronic supplementary material.