Abstract
Induced prey defences against consumers are conspicuous in microbes, plants and animals. In toxigenic prey, a defence fitness cost should result in a trade-off between defence expression and individual growth. Yet, previous experimental work has failed to detect such induced defence cost in toxigenic phytoplankton. We measured a potential direct fitness cost of grazer-induced toxin production in a red tide dinoflagellate prey using relative gene expression (RGE) of a mitotic cyclin gene (cyc), a marker that correlates to cell growth. This approach disentangles the reduction in cell growth from the defence cost from the mortality by consumers. Treatments where the dinoflagellate Alexandrium catenella were exposed to copepod grazers significantly increased toxin production while decreasing RGE of cyc, indicating a defence-growth trade-off. The defence fitness cost represents a mean decrease of the cell growth rate of 32%. Simultaneously, we estimate that the traditional method to measure mortality loss by consumers is overestimated by 29%. The defence appears adaptive as the prey population persists in quasi steady state after the defence is induced. Our approach provides a novel framework to incorporate the fitness cost of defence in toxigenic prey–consumer interaction models.
Keywords: prey defence fitness cost, inducible defence, toxic dinoflagellate, paralytic shellfish toxin production, cell growth gene expression, toxin production gene expression
1. Introduction
Prey defences against consumers are conspicuous in plants, animals and microbes [1,2]. These defences can affect population dynamics of both consumers and prey [1–5], biodiversity and ecosystem function [6] and eco evolutionary dynamics of predator–prey systems [7–9].
Defences can be either constitutive (always present) or inducible (expressed by the threat of consumption) [1]. Inducible defences are common in aquatic plankton [10–13]. Notorious among unicellular aquatic prey are dinoflagellates and prokaryotic cyanobacteria, which produce a suite of powerful neurotoxins (saxitoxin and its congeners), referred as paralytic shellfish toxins (PST) whose blooms (popularly known as red tides) result in accumulation in shellfish and finfish, and represent a threat to foodwebs, public health and local economies [14]. Grazer-induced toxin production has been well documented in the dinoflagellate genus Alexandrium exposed to a multitude of metazoan grazers or their waterborne cues [15–22].
Inducible defences are understood as a cost-saving strategy, in which resources can be allocated to fitness components (growth and reproduction) instead of defence in the absence of consumers [1,23,24]. By definition, Darwinian fitness is the average production of viable offspring by a group of individuals in a population, or by a genotype. In unicellular organisms like phytoplankton, the net growth rate is a balance of cell division rate and mortality rate owing to grazing and other loss terms. Hence, the reduction in cell division rate that arises from an increase in defence such as toxin production is labelled a direct fitness cost, or tax paid by metabolic cost [25,26], heretofore labelled the fitness cost.
Maximizing all organismal functions cannot occur simultaneously [27]. Therefore, a trade-off between anti-grazing defence and cell growth is, theoretically, manifested as a fitness cost. However, trade-offs and defence fitness cost of inducible defence are not always apparent [24–26]. One important limitation is the difficulty of disentangling the decrease in prey numbers resulting from a decrease in the prey growth rate from mortality by consumers. To avoid this issue, fitness cost has typically been measured comparing prey growth in the absence and presence of consumer ‘cues’ (e.g. waterborne chemical cues, auditory cues, predators with disable feeding appendages, etc.) that induce expression of defensive traits while not allowing predators to consume the prey [28–30]. However, the expression of the defence can be much larger when prey are directly consumed than when exposed to consumer cues [15,20]. Further, a fitness defence cost in dinoflagellates in the presence of only consumer cues in short-term assays has not been detected [15,16,19,30]. Thus, both the fitness cost and benefit of the defence, which are critical information for properly conceptualizing and modelling consumer–prey interactions and understanding the evolution of prey defence, could be underestimated if not measured in the presence of grazers.
While evidence for a cost of constitutive toxin production in dinoflagellates is not universal, trade-offs of growth versus constitutive toxin production have been experimentally demonstrated [31,32] and modelled [33]. Thus, inducible costs of defence should also be expected. Here we use a novel approach to quantify a fitness cost of prey defence by measuring gene expression of cell growth, assuming that resource allocation is enzymatically modulated and must ultimately be gene-specific. The differential prey cell-growth gene expression in the presence and absence of consumers solves the problem of disentangling the fitness cost of induced defence from cell loss owing to consumption, thus providing a mean of determining trade-off and defence fitness cost.
Saxitoxin (STX) biosynthesis genes have been identified in multiple species of cyanobacteria and dinoflagellates, including Alexandrium spp. [34–36]. The abundance of sxtA, a unique starting gene of STX synthesis, was positively related to the number of gene copies and sxtA transcripts [35,37,38]. Murray et al. [39] also quantified sxtA4, one of the variants of sxtA, using quantitative polymerase chain reaction (qPCR) in toxigenic Alexandrium. A second core gene of STX biosynthesis, sxtG has also been used to confirm the gene expression level of STX transcripts [40]. Moreover, a mitotic cyclin B gene (cyc) in Alexandrium catenella has been suggested as biomarker for cell growth rate [41], and we posit that it can be used to quantify the defence fitness cost. While the transcript analyses of the first group of STX biosynthesis (e.g. sxtA, sxtG, sxtB) could be used to document the expression of Alexandrium anti-grazing defence, they may not, on their own, be used to quantify defence because they only represent the initiation of the steps to STX synthesis. Hence, traditional quantification of cell toxin content by high-performance liquid chromatography (HPLC) is still needed to establish a trade-off between defence and cell growth.
To determine the relationship between prey growth and grazer-induced toxin production of the dinoflagellate A. catenella, we investigated the change of target genes, determined by relative quantitation of gene expression using reverse transcription-quantitative PCR (RT-qPCR) of STX and cell growth (cyc), as well as toxin production measured by HPLC. We hypothesized that: (i) relative gene expression (RGE) of STX (sxtA and sxtG) of A. catenella increases in the presence of grazers, whereas RGE of cell growth (cyc) decreases; (ii) there is a defence-growth trade-off between toxin production and RGE of cyc; and (iii) differential RGE of cyc in the presence and absence of grazers quantifies a significant fitness cost owing to grazer-induced toxin production.
2. Material and methods
(a). Sample collection and culture
The toxigenic dinoflagellate A. catenella (formerly known as Alexandrium fundyense, strain BF-5, isolated from the Bay of Fundy, Canada), was grown in batch cultures at excess nutrients (F/2 medium without silicate [42]), and maintained in the exponential growth phase. All experiments were conducted in an environmental chamber kept at 18°C, and illuminated with fluorescent lighting (approx. 100 µM m−2 s−1) set to a 12 h : 12 h light : dark photoperiod. The grazer, the calanoid copepod Acartia hudsonica historically co-occurring with toxic A. catenella, was collected from Casco Bay, Maine, USA (43°39′ N, 74°47′ W), a location in which blooms of A. catenella are common [43]. Unlike some species which lose their defence capability over time under laboratory conditions [44], our BF-5 strain has maintained its grazer-induced toxin production for years. Triplicate copepod cultures were maintained with a mixed diet of Rhodomonas sp., Tetraselmis sp. and Thalassiosira weissflogii [43]. Copepods were cultured for at least three generations (approx. three months) prior to experiments to remove maternal and environmental effects. Prior to assay, copepods were starved in 0.22 µm filtered seawater for 24 h to ensure grazers had completely voided their guts [45].
(b). Grazer-induced toxin production assay
Alexandrium catenella increases toxin production within 48 h of exposure to grazing copepods [20,21]. Thus, we carried out measurements of time-dependent gene expression of stxA, stxG and cyc, along with cell concentrations and cell toxin content measured by HPLC. Alexandrium catenella cells were placed in 500 ml bottles (250 cells ml−1) either without copepods (controls: constitutive toxin production) or with 20 adult female A. hudsonica (treatments) for a period of 96 h. The excess nutrients in cultures, and the low ratio of consumer to prey (3–4 orders of magnitude lower than during bloom conditions) were intended to avoid possible biases on cell growth rate or toxin production caused by overabundance of consumers [22]. Experiments were done in quadruplicate sets for both control and treatment, and carried out at 18°C. During the experiment, cells were harvested at 0, 4, 8, 24, 48, 72 and 96 h, then cells were gently separated by wet-sieving onto a 63 µm mesh to remove copepods, nauplii, eggs and faecal pellets. Two aliquots from each bottle were filtered onto 5 µm pore size polycarbonate membranes; one for toxin analysis and another for RNA extraction. For the former, the filter was washed off with 1 ml of 0.1 M acetic acid, and for the latter 1 ml of TRIzol Reagent (Invitrogen). Filters were immediately frozen at −80°C. An aliquot of 10 ml was also taken from each bottle for cell counts (500 µl) and was preserved in 0.5% acid Lugol's solution made with filtered seawater.
(c). Toxin analysis
Cell samples that had been preserved in 0.1 M acetic acid were thawed at room temperature and homogenized on a Fastprep-24 Tissue and Cell Homogenizer (MP Biomedicals) using Lysing Matrix C silica beads (approx. 1 mm in diameter) at speed of 6 m s−1 for three 40 s cycles. Complete disruption of the cells was confirmed by microscopic examination. The cells were centrifuged, and the supernatant was filtered through 0.45 mm ultra-filtration centrifuge cartridges (Millipore). Concentrations of PST were determined by reverse-phase ion-pairing HPLC using the post-column oxidative fluorescence method described by Oshima et al. [46]. Toxin groups including gonyautoxins 1, 2, 3 and 4 (GTX1-4), STX, neosaxitoxin (NEO) and C toxins (C1 and C2) were separated on an Alltech C8 column (150 mm × 4.6 mm). The post-column derivatization system consisted of a peristaltic pump to deliver oxidant and acid. Fluorescent PST derivatives were detected using an online Waters 474 fluorescence detector (excitation: 330 nm and emission: 390 nm). Certified toxin standards from the National Research Council of Canada (Halifax) were used. The toxin concentration was calculated from HPLC chromatograms using software that recorded the chromatograms and integrated peak areas (Empower, Waters).
(d). Growth rate and toxin production rate
Alexandrium catenella cells were counted on an inverted microscope (Olympus IX70) at 10× or 20× magnification from the same sub-sample preserved in Lugol's solution. The growth rate (μ, d−1) was calculated, assuming exponential growth, as
| 2.1 |
In this equation, N0 is the cell concentration (cells ml−1) at the beginning (t0) and Nt is the concentration at the conclusion of each exposure time (tt) in days; μ represents the cell division rate in the control bottles, and the net growth rate in the treatment bottles, respectively.
The specific toxin production rate (μtox: d−1) was determined as
| 2.2 |
In equation (2.2), T is the total toxin concentration in the culture (fmol ml−1) and other terms are as defined in equation (2.1).
(e). RNA extraction and complementary DNA synthesis
Cells from the samples preserved in Trizol reagent were thawed in ice and homogenized as described above, then centrifuged at 10 000g for 1 min, and the supernatant transferred to a clean tube for subsequent RNA extraction. Total RNA, treated by DNase, was isolated as described previously [47] and quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Complementary DNA (cDNA) was synthesized from 180 to 280 ng of the total RNA using iScript cDNA Synthesis Kit (Promega) and modified oligo dT [48]. The resultant first-strand cDNA was purified using Zymo DNA Clean and Concentrator (Zymo Research).
(f). Gene expression analysis
Gene expression analyses were conducted using RT-qPCR. From previous studies several pairs of primers were designed and tested for specificity and PCR efficiency by RT-qPCR (see the electronic supplementary material, appendix S1). All qPCRs were performed on a StepOnePlus real-time PCR system (Applied Biosystmes) and run in 10 µl reactions with Fast SYBR Green Master Mix (Applied Biosystmes) with the following amplification conditions: 95°C for 10 s, 45 cycles of 15 s at 95°C and 30 s at 60°C. 15 s at 72°C for extension followed by a melting curve analysis. RGE levels of the target genes were compared to those in the reference gene according to the Pfaffl method corrected by standard curves to estimate the primer efficiency [49]. The reference genes, lbp (luciferin-binding protein) and cob (cytochrome b), were used to normalize the gene expression level [41,50]. Here, we show results normalized by lbp because this gene was more stable than cob (electronic supplementary material, appendix S1C).
(g). Fitness cost and grazer ingestion rate
The grazing rate (g, d−1) is traditionally calculated as
| 2.3 |
In equation (2.3), μgross is the cell division rate in the absence of copepods (control bottle) and μnet is the net growth rate in the copepod treatment (cell division rate minus copepod-linked losses). Although it has been assumed that μgross is the same in control and treatment bottles, this assumption may not hold true if there is a trade-off between grazer-induced toxin production and cell division rate. The loss term in g effectively represents both cell losses owing to grazing and reduced cell division rate arising from the trade-off. Thus, the two losses must be disentangled. The growth rate in toxigenic A. catenella can be expressed as having a constitutive (μcontrol = μc) and a grazer-induced (μtreatment = μt) component, and the change in growth rate (Δμ) can be expressed as
| 2.4 |
The fitness cost (d−1) is the loss of growth from committing resources for toxin production. A modified grazing rate (g′) and the changes in prey concentration accounting for grazer-induced toxin production can be measured and re-expressed using equation (2.4), where g′ is calculated by
| 2.5 |
and
| 2.6 |
A significant and positive correlation between relative expression of cyclin gene (cyc) and cell growth rate in the absence of grazers (figure 3a), implies that we can use the former as a proxy of cell division rate during the experiment. This assumes that grazing losses do not intrinsically affect the cell cycle, and thus the cell division rate as demonstrated earlier [51]. As mentioned earlier, we avoided grazing-mediated nutrient regeneration in the treatment by adding excess nutrients to cultures and keeping the grazer-prey ratio low. Thus, fitness cost is derived from the proportion of relative expression of cyclin gene (cyc) between cells in the control (constitutive toxin production) and the copepod treatment (induced toxin production):
| 2.7 |
The ingestion rate, I (cells copepod−1 d−1) was calculated according to Frost [52] and compared to the modified ingestion rate (I′) as
| 2.8 |
where is the mean cell concentration during the experiment calculated as
| 2.9 |
In equations (2.8) and (2.9), V is the volume of the bottle, n is the number of copepods, Δt is the elapsed time in days and N0 is the initial concentration of A. catenella.
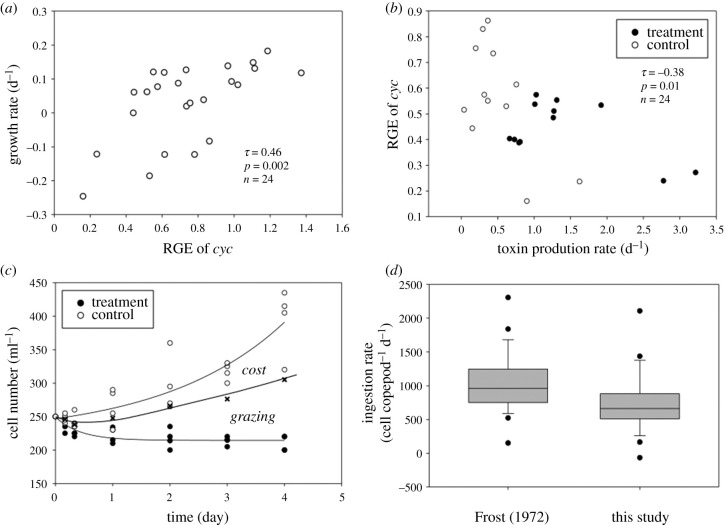
Figure 3.
Relative gene expression (RGE) of growth-related gene (cyc) versus cell growth rate in the control (a), and between toxin production rate (μtox) and growth rate calculated by the regression of figure 3a (b), the changes in cell concentration owing to fitness cost and grazing (c), and copepod ingestion rates (d) during the grazing assay. In (c), the difference between the upper and middle line is the cell loss owing to the defence fitness cost, and the difference between the middle and lower line represents the cell loss owing to the grazing. (d) compares copepod ingestion rates estimated by the traditional grazing equation [52] versus the modified ingestion rate that accounts for the reduction in cell growth rate owing to toxin production (this study). Boxplot represents the second and third quartile of data, line in the box is the median, dots are outliers and the error bar represents the range of observations. Lines are regression fits.
(h). Statistical analysis
In the case of non-normal distributed data, a Kruskal–Wallis one-way ANOVA on ranks was used and all pairwise comparisons among controls/treatments were assessed with the Student–Newman–Keul's (SNK) post hoc procedure. The effects of day (hours in the experiment), presence of grazer, and their interaction on cell concentration, toxin content, toxin profile, growth rate and RGE (four independent replicates per test and two samples for PST analysis) were tested by two-way ANOVA (electronic supplementary material, appendix S2). Time-dependent changes in these variables were also tested separately by regression analysis for the controls and treatments. Ingestion rates were tested with a Mann–Whitney U-test to compare the Frost equation versus our modified equation. All statistical analyses were performed using SigmaPlot v.11.0 and SPSS v.26 software.
3. Results
(a). Cell growth and toxin content
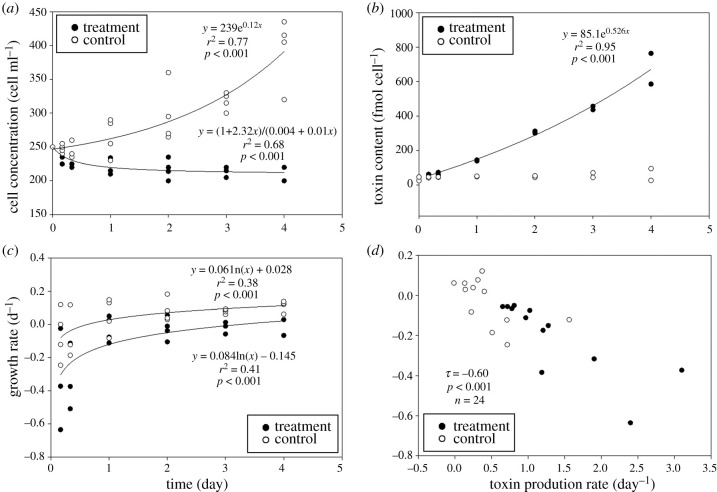
The patterns of cell concentration, toxin content and net growth rate in A. catenella were different between the controls (no grazers) and treatments (with grazers) (F1,42 = 207, p < 0.001, F1,14 = 28.2, p < 0.001 and F1,36 = 26.9, p < 0.001, respectively). Cells in the controls showed an exponential (r2 = 0.78, p < 0.001, n = 24) increase from an initial mean value of 250 to a maximum of 394 cells ml−1 after 96 h (figure 1a; ANOVA, F6,21 = 14.8, p < 0.001). By contrast, cells in the treatments declined relatively quickly for the first 24 h (r2 = 0.69, p < 0.001, n = 24), and then slowly for the remainder of the experiment (non-parametric ANOVA, H6 = 18.5, p = 0.005), achieving quasi steady state at approximately 225 cells ml−1. There was an interaction between treatment and time on cell concentration (two-way ANOVA, F6,42 = 16.3, p < 0.001). From beginning to end, toxin content in the treatments increased 20-fold from 35 to 674 fmol cell−1 (r2 = 0.95, p < 0.001, n = 14) (figure 1b; non-parametric ANOVA, H6 = 12.6, p = 0.049). By contrast, there was no difference in cell toxin content versus time among the controls (non-parametric ANOVA, H6 = 2.97, p = 0.812), and the interaction between treatment and time was not significant (two-way ANOVA, F6,14 = 1.13, p = 0.395). The net growth rate by day differed within treatments only (figure 1c; ANOVA, F5,18 = 3.07, p = 0.036), and there was no interaction between treatment and time (two-way ANOVA, F5,36 = 0.169, p = 0.972). In addition, net growth rates in the treatments were low, with negative values when toxin production rates were high, suggesting a trade-off between the net growth rate and the specific toxin production rate (μtox) (figure 1d; Kendall's tau correlation coefficient (τ): τ = −0.60, p < 0.001, n = 24). Most toxin profiles in treatments showed a significant increase relative to the controls (electronic supplementary material, appendices S2 and S3); GTX3 increased 41-fold, GTX4 increased 5-fold, NEO increased 11-fold and STX increased from zero to 51 fmol cell−1. C2 was the only one to significantly increase at day 4 (electronic supplementary material, appendix S3).
Figure 1.
Cell concentration (a), cell toxin content (b), cell growth rate versus time (c) and toxin production rate (μtox) versus cell growth rate (d) during the grazing assay. The growth rate in the copepod treatment refers to the net growth rate (μnet). Lines are regression fits.
(b). Gene expression analysis
The apparent trade-off between grazer-induced toxin production and net growth rate (figure 1d) is biased by the loss of cells owing to grazing. This is where RGE analysis disentangles grazing loss from the fitness cost of toxin production. Genes involved in the first step of STX biosynthesis (sxtA and sxtG) and cell growth (cyc) were used in the analysis. RGE of sxtA4 and sxtG was significantly higher in the treatment (with copepods) than in the control (without copepods) (two-way ANOVA, F1,36 = 36.5, p < 0.001 and F1,36 = 133, p < 0.001), whereas RGE of the same genes in the control did not vary significantly with time. In the copepod treatments, RGE of STX genes peaked within the first 48 h and decreased afterwards (figure 2a,b; sxtA4 and sxtG: SNK post hoc; p < 0.001). However, toxin gene expression was not correlated to STX production measured by HPLC, which confirms that STX genes are not necessarily quantitative proxies for toxin content in real-time. Consistent with the fitness cost hypothesis, RGE of cyc in the treatments was significantly lower than in the controls (two-way ANOVA, F1,36 = 33.7, p < 0.001). RGE of cyc varied with time (figure 2c; cyc: SNK post hoc; p < 0.001), and the pattern was similar for the treatment and the control. Because of the differential expression of STX genes early in the experiment (4–24 h), there was no correlation between RGE of sxt and cyc.
Figure 2.
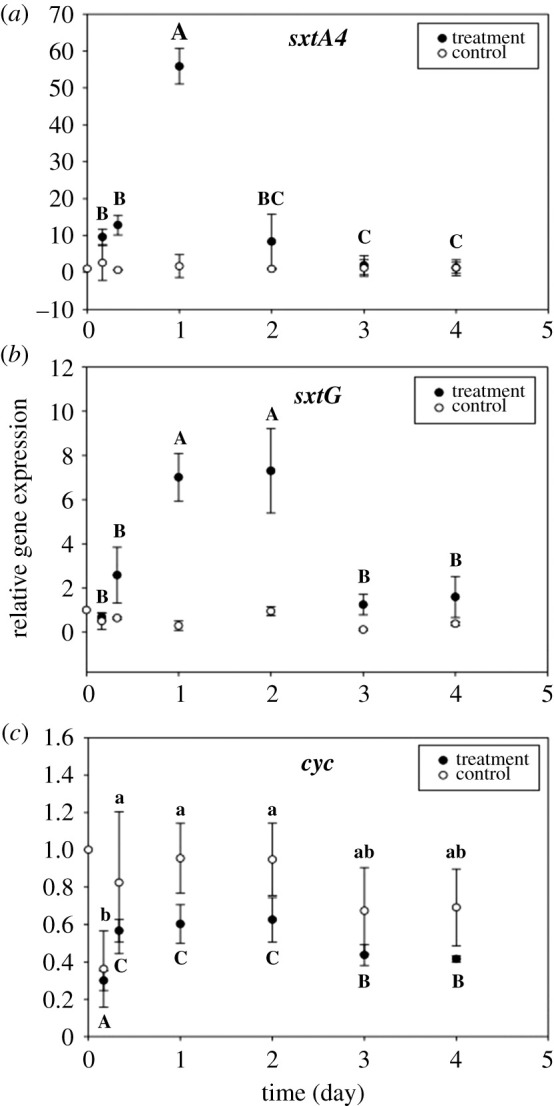
Relative expression of STX genes (sxtA4 and sxtG, a,b) and cell growth-related gene (cyc, c). The reference gene (lbp) was used to normalize the gene expression levels. Letters above/below bars represent significant differences among mean values of groups in the control (lowercase) and treatment (uppercase). Error bars represent ± 1 standard deviation of the mean (n = 4).
(c). Fitness cost and ingestion rate
Relative gene expression of cyc was positively correlated to cell growth rate in the control (figure 3a; τ = 0.46, p = 0.002, n = 24), which suggests that cyc is transcriptionally regulated with cell growth. Moreover, a trade-off was confirmed by the significantly negative correlation between toxin production rate measured by HPLC and RGE of cyc (figure 3b, τ = −0.38, p = 0.01, n = 24). Thus, we used equation (2.7) to calculate the fitness cost incurred by grazer-induced defence. The consequences of the defence fitness cost are shown in figure 3c; electronic supplementary material, appendix S4. The upper and lower lines in figure 3c represent mean cell concentrations in the control and the copepod treatment, respectively. The midline represents the estimated cell concentration accounting for the reduction in specific growth rate (fitness cost) owing to grazer-induced toxin production. Thus, the difference between the upper and middle line is the cell loss owing to the defence fitness cost, equivalent to a reduction of the cell growth rate of 17–39% (mean = 32%, s.d. = 8, n = 6) during the experiment. The difference between the middle and lower line represents the actual cell loss owing to grazing. A comparison of the grazing rates by the Frost equation and equation (2.8) of this study, which accounts for the defence fitness cost, shows a corresponding overestimation of grazing losses by the Frost equation of 17–35% (mean = 29%, s.d. = 6, n = 6) (figure 3d; electronic supplementary material, appendix S4, Mann–Whitney U-test, U = 151, p = 0.005, n = 48). Thus, the fitness cost is balanced by the reduction of the grazing mortality rate (T-test, F = 0.27, p = 0.44).
4. Discussion
This study tests a hypothesis regarding a trade-off between grazer-induced defence and cell growth, and presents a novel approach involving RGE of cell growth to quantify the fitness cost of chemical defence. A 20-fold increase in grazer-induced toxin production in A. catenella was evident by measured cell toxin content using HPLC (figure 1b), consistent with increases of RGE of STX genes using RT-qPCR (figure 2a,b). By contrast, RGE of cell growth gene (cyc) was significantly lower in the presence of grazers (figure 2c). These observations are consistent with the trade-off hypothesis (figure 3b), and the existence of a significant defence fitness cost (figure 3c), equivalent to a mean reduction of the cell division rate of 32%. Further, failure to account for the fitness cost would have led to a mean overestimation of grazing loss during the experiment of 29% (figure 3d).
While costs of constitutive defence in phytoplankton have been documented [8,9,31,32], to our knowledge, the approach presented here represents a novel demonstration and quantification of a direct fitness cost of inducible defence (toxin production) in phytoplankton prey. This approach avoids the conflation of the fitness loss owing to toxin production with the fitness loss owing to consumption, and the consequent overestimation of grazing loss. Further, in our study, induced toxin production is an adaptive defence in which the resulting defence fitness cost (32%) is nearly balanced by a reduction (29%) of the grazing mortality rate, resulting in quasi steady state of the prey population once the defence is deployed.
The increase in grazer-mediated toxin production observed in this study is consistent with previous observations on the genus Alexandrium [15,16,20–22,53]. This inducible response may be advantageous because resources associated with a costly defence (toxin production) are used when needed, and otherwise allocated to other functions like growth and reproduction [1,23,24].
The genes sxtA and sxtG are directly connected to the biosynthesis of STX in A. catenella. In this study, the RGE of sxtA and sxtG did not vary significantly with time in the control, whereas in the copepod treatment all genes peaked at 24 h and decreased after 48 h (figure 2). The 33-fold increase on RGE of sxtA4 is consistent with an observed 51-fold elevation of sxtA RGE during an Alexandrium bloom in Long Island relative to laboratory cultures [54]. Comparing RGE of sxtA and sxtG genes with toxin content by HPLC, shows that STX was only induced after 24 h (electronic supplementary material, appendix S3D). This suggests that the initiators, sxtA and sxtG were predominantly expressed early in the experiments, and then protein biosynthesis followed. Similarly, gene expression in a terrestrial plant defence system showed peaks at approximately 18 h after pathogenic infection [55]. Thus, future studies employing these gene markers ensure observations are made during early exposure periods.
Other studies showed that the sxtA (sxtA1 and sxtA4) copy number was well-correlated with cell abundance and toxin content in Alexandrium species [35,39,56,57]. However, PST production appears absent in other Alexandrium strains, even if they have sxtA4 [35,58]. Another toxin related gene, sxtG, was also sequenced in the genomic DNA from both toxic and non-toxic species [40]. It is unclear whether the reported lack of PST production in the presence of STX genes is owing to a detection limit problem, more complex PST biosynthesis in dinoflagellates than in cyanobacteria, duplication events in the evolution of these genes, or that the genes are not expressed in cultures where there is no selective pressure to produce toxin [58]. At this stage, both methods for STX gene expression and conventional PST measurement should be used to understand molecular controls of toxin production.
Universal cell-cycle regulators, cyclin-dependent kinases and their specific regulatory subunit (i.e. cyclin B) have been reported from the dinoflagellate cell cycle [59–62]. More recently Zhuang et al. [41] suggested that the mitotic cyclin gene might be a biomarker for monitoring the growth rates of A. catenella. We investigated the differential expression of the cell growth-related gene (cyc) between constitutive and grazer-induced A. catenella in time-course experiments. The RGE of cyc in all copepod treatments was significantly lower than in the controls, supporting the hypothesis that expressed cyc of A. catenella decreases in the presence of grazers.
In time-dependent experiments, both the actual toxin content measured by HPLC and the relative expression of STX genes were significantly increased in response to grazers, whereas both of these measurements remained unchanged in cells not exposed to grazers (figures 1b and 2a,b). Moreover, cell division rate calculated from the relative expression of the cell growth gene (cyc) was significantly lower in cells exposed to grazers than unexposed cells (figure 3b), confirming the trade-off between toxin production rate and cell division rate suggested by figure 1d. The reduced growth rate of induced cells (μt) compared to control cells (μc) has been suggested as a way to measure a fitness cost of toxin production [15,28–30], but traditional grazing experiments typically ignore the possible defence fitness cost, and assume that the cell division rate is the same in the presence and absence of grazers. Here, we suggest that this assumption is incorrect, and that there is a significant fitness cost of toxin production. Failure to account for this cost would lead, in this instance, to a significant overestimation of the cell loss owing to grazing.
Our approach to calculating the defence fitness cost (equation (2.7)) requires further validation, particularly to test the assumption that grazers do not affect the prey cell division rate [51], except indirectly through induced toxin production, leading to the cost of defence. That is, both the control and the treatment fall along a common line in the relationship between growth rate and RGE of cyc. Indirect evidence supports this assumption. RGE of cyc and toxin production rate are independent of grazing pressure in two A. catenella strains in which toxin production is not grazer-induced [63]. Yet, both downregulation and upregulation of cyclin genes in a diatom species was observed [64], although a correlation to cell growth rate was not determined. Simultaneous upregulation of both toxin production and RGE of cyc by grazers is difficult to envision in light of resource allocation theory. However, Alexandrium species display additional forms of defence such as reduction in cell size [65] and bioluminescence [66]. If the expression of these defences also involves additional downregulation of the RGE of cyc, then equation (2.7) calculates the fitness cost of all defence forms, and the cost of toxin production alone is overestimated. Future studies can better discern the mechanistic nature of defences and their fitness costs by measuring RGE of cyc in the presence and absence of grazers (as done in our study), and the frequency of cells in the different phases of the cell cycle and the phase durations in a diel cycle to independently calculate cell division rates (equation 3 in ref. [51]). Use of other growth-related genes, i.e. RuBisco (rbcL), might prove useful. It would also be instructive to see if our approach can detect a defence fitness cost when cells are exposed only to grazer cues in cage experiments. Finally, further assays should compare RGE of cyc along a resource gradient (e.g. deplete to replete nutrients or light) for both control and grazer treatments to test for similar responses to the resource.
Harmful algal bloom (HAB) species have on average lower reported growth rates than non-HAB species [67]. It has been hypothesized that K-selected species are capable of compensating for their low cell growth rates by reducing their losses owing to grazing, either by being toxic or via other defence mechanisms [67,68]. Yet, properly modelling consumer-toxic prey interactions requires knowing the fitness cost of toxin production to avoid the error of determining herbivore impact only by reduction in fitness caused by herbivory loss [69–71]. Thus, we developed grazing equations that account for the defence fitness cost (equations (2.6) and (2.7)). The modified ingestion rates were significantly lower than Frost [52] ingestion rates at all time points, indicating grazing losses were overestimated on average by 29%. Altogether, the findings in this study suggest a revision of the conceptual model of grazer-toxic prey interaction and of the control on cell growth and toxicity. Otherwise, the grazing losses may be overestimated because of the assumption that cell division rate is the same in the presence and absence of grazers is invalid, and overestimating grazing would, in turn, bias conclusions on energy predators derive from prey and trophic transfer efficiency.
If a defence trait persists despite its cost, it must also accrue a benefit to the prey. This study suggests that while inducible toxin production in A. catenella is costly, it may also be adaptive because the realized decrease in cell division rate is nearly balanced by a reduction in cell mortality rate owing to grazing, allowing the prey to persist at quasi steady state after the defence is induced. Hence, future studies should examine the fitness benefit of toxin production among genotypes that vary in their degree of toxin production, including strains that do not produce toxin in the presence of grazers. The approach presented here represents a novel means of quantifying the fitness cost of chemical defence, which is a key issue in understanding the effect of nonconsumptive effects of consumers on prey [71,72], the role of inducible defence on fluctuations of prey populations [4], and advancing understanding of the coevolutionary arms race between consumers and prey in the plankton [8,9,73,74].
Supplementary Material
Acknowledgements
We thank Senjie Lin, Ann Bucklin, Catherine Matassa, Ewaldo Leitao Jr., Jeng Chang and three anonymous reviewers for comments and suggestions on an earlier draft of the manuscript, and Claudia Koerting for help with the HPLC system.
Data accessibility
Data are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.nzs7h44q0 [75].
Authors' contributions
G.P. and H.G.D. designed the study. G.P. performed research and collected data. G.P. and H.G.D. analysed output data and wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
Research supported by the US National Science Foundation (grant no. NSF OCE-1130284) and the National Oceanic and Atmospheric Administration National Centers for Coastal Ocean Science Competitive Research Program (award NA18NOS4780173 to University of Connecticut). This is an ECOHAB contribution.
References
- 1.Tollrian R, Harvell CD. 1999. The ecology and evolution of inducible defenses. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Brönmark C, Hansson L-A. 2012. Chemical ecology in aquatic systems. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Karban R, Baldwin IT. 1997. Induced responses to herbivory. Chicago, IL: University of Chicago Press. [Google Scholar]
- 4.Verschoor AM, Vos M, van der Stap I.. 2004. Inducible defences prevent strong population fluctuations in bi- and tritrophic food chains. Ecol. Lett. 7, 1143–1148. ( 10.1111/j.1461-0248.2004.00675.x) [DOI] [Google Scholar]
- 5.Pavia H, et al. 2012. Chemical defences against herbivores. In Chemical ecology in aquatic systems (eds Bronmark C, Hansson L-A), pp. 210–235. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Rasher DB, Hoey AS, Hay ME. 2013. Consumer diversity interacts with prey defense to drive ecosystem function. Ecology 94, 1347–1358. ( 10.1890/12-0389.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodie ED III, Brodie ED Jr. 1999. Predator-prey arms races: asymmetrical selection on predators and prey may be reduced when prey are dangerous. Bioscience 49, 557–558. ( 10.2307/1313476) [DOI] [Google Scholar]
- 8.Meyer JR, Ellner SP, Hairston NG Jr, Jones LE, Yoshida T. 2006. Prey evolution on the time scale of predator-prey dynamics revealed by allele-specific quantitative PCR. Proc. Natl Acad. Sci. USA 103, 10 690–10 695. ( 10.1073/pnas.0600434103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasada M, Yamamichi M, Yoshida T. 2014. Form of an evolutionary tradeoff affects eco-evolutionary dynamics in a predator–prey system. Proc. Natl Acad. Sci. USA 111, 16 035–16 040. ( 10.1073/pnas.1406357111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMott WR, Moxter F. 1991. Foraging cyanobacteria by copepods: responses to chemical defense and resource abundance. Ecology 72, 1820–1834. ( 10.2307/1940981) [DOI] [Google Scholar]
- 11.Lürling M, Van Donk E. 2000. Grazer-induced colony formation in Scenedesmus: are there costs to being colonial? Oikos 88, 111–118. ( 10.1034/j.1600-0706.2000.880113.x) [DOI] [Google Scholar]
- 12.Yoshida T, Hairston NG, Ellner SP. 2004. Evolutionary trade–off between defence against grazing and competitive ability in a simple unicellular alga, Chlorella vulgaris. Proc. R. Soc. B 271, 1947–1953. ( 10.1098/rspb.2004.2818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long JD, Smalley GW, Barsby T, Anderson JT, Hay ME. 2007. Chemical cues induce consumer-specific defenses in a bloom-forming marine phytoplankton. Proc. Natl Acad. Sci. USA 104, 10 512–10 517. ( 10.1073/pnas.0611600104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M. 2012. The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 14, 10–35. ( 10.1016/j.hal.2011.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selander E, Thor P, Toth G, Pavia H. 2006. Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc. R. Soc. B 273, 1673–1680. ( 10.1098/rspb.2006.3502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergkvist J, Selander E, Pavia H. 2008. Induction of toxin production in dinoflagellates: the grazer makes a difference. Oecologia 156, 147–154. ( 10.1007/s00442-008-0981-6) [DOI] [PubMed] [Google Scholar]
- 17.Van Donk E, Ianora A, Vos M.. 2011. Induced defences in marine and freshwater phytoplankton: a review. Hydrobiologia 668, 3–19. ( 10.1007/s10750-010-0395-4) [DOI] [Google Scholar]
- 18.Selander E, et al. 2019. Copepods drive large-scale trait-mediated effects in marine plankton. Sci. Adv. 5, eaat5096 ( 10.1126/sciadv.aat5096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevett A, Lindström J, Xu J, Karlson B, Selander E. 2019. Grazer-induced bioluminescence gives dinoflagellates a competitive edge. Curr. Biol. 29, R564–R565. ( 10.1016/j.cub.2019.05.019) [DOI] [PubMed] [Google Scholar]
- 20.Senft-Batoh CD, Dam HG, Shumway SE, Wikfors GH, Schlichting CD. 2015. Influence of predator–prey evolutionary history, chemical alarm-cues, and feeding selection on induction of toxin production in a marine dinoflagellate. Limnol. Oceanogr. 60, 318–328. ( 10.1002/lno.10027) [DOI] [Google Scholar]
- 21.Senft-Batoh CD, Dam HG, Shumway SE, Wikfors GH. 2015. A multi-phylum study of grazer-induced paralytic shellfish toxin production in the dinoflagellate Alexandrium fundyense: a new perspective on control of algal toxicity. Harmful Algae 44, 20–31. ( 10.1016/j.hal.2015.02.008) [DOI] [Google Scholar]
- 22.Griffin JE, Park G, Dam HG. 2019. Relative importance of nitrogen sources, algal alarm cues and grazer exposure to toxin production of the marine dinoflagellate Alexandrium catenella. Harmful Algae 84, 181–187. ( 10.1016/j.hal.2019.04.006) [DOI] [PubMed] [Google Scholar]
- 23.Karban R 2011. The ecology and evolution of induced resistance against herbivores. Funct. Ecol. 25, 339–347. ( 10.1111/j.1365-2435.2010.01789.x) [DOI] [Google Scholar]
- 24.Brönmark C, Lakowitz T, Nilsson PA, Ahlgren J, Lennartsdotter C, Hollander J. 2012. Costs of inducible defence along a resource gradient. PLoS ONE 7, e30467 ( 10.1371/journal.pone.0030467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Donk E, Lürling M, Lampert W. 1999. Consumer-induced changes in phytoplankton: inducibility, costs, benefits, and the impact on grazers. In The ecology and evolution of inducible defenses (eds Tollrian R, Harvell CD), pp. 89–104. Princeton, NJ: Princeton University Press. [Google Scholar]
- 26.Strauss SY, Rudgers JA, Lau JA, Irwin RE. 2002. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 17, 278–285. ( 10.1016/S0169-5347(02)02483-7) [DOI] [Google Scholar]
- 27.Townsend CR, Calow P. 1981. Physiological ecology: an evolutionary approach to resource use. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 28.Relyea RA 2002. Costs of phenotypic plasticity. Am. Nat. 159, 272–282. ( 10.1086/338540) [DOI] [PubMed] [Google Scholar]
- 29.Boeing WJ, Wissel B, Ramcharan CW. 2005. Costs and benefits of Daphnia defense against Chaoburus in nature. Can. J. Fish. Aquat. Sci. 62, 1286–1294. ( 10.1139/f05-043) [DOI] [Google Scholar]
- 30.Selander E, Kubanek J, Hamberg M, Andersson MX, Cervin G, Pavia H. 2015. Predator lipids induce paralytic shellfish toxins in bloom-forming algae. Proc. Natl Acad. Sci. USA 112, 6395–6400. ( 10.1073/pnas.1420154112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardison DR, Sunda WG, Shea D, Litaker RW. 2013. Increased toxicity of Karenia brevis during phosphate limited growth: ecological and evolutionary implications. PLoS ONE 8, e58545 ( 10.1371/journal.pone.0058545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blossom HE, Markussen B, Daugbjerg N, Krock B, Norlin A, Hansen PJ. 2019. The cost of toxicity in microalgae: direct evidence from the dinoflagellate Alexandrium spp. Front. Microbiol. 10, 1065 ( 10.3389/fmicb.2019.01065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborty S, Pančić M, Andersen KH, Kiørboe T. 2019. The cost of toxin production in phytoplankton: the case of PST producing dinoflagellates. ISME J. 13, 64–75. ( 10.1038/s41396-018-0250-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellmann R, Mihali TK, Jeon YJ, Pickford R, Pomati F, Neilan BA. 2008. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 74, 4044–4053. ( 10.1128/AEM.00353-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stüken A, Orr RJ, Kellmann R, Murray SA, Neilan BA, Jakobsen KS. 2011. Discovery of nuclear-encoded genes for the neurotoxin saxitoxin in dinoflagellates. PLoS ONE 6, e20096 ( 10.1371/journal.pone.0020096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hackett JD, Wisecaver JH, Brosnahan ML, Kulis DM, Anderson DM, Bhattacharya D, Plumley FG, Erdner DL. 2013. Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol. Biol. Evol. 30, 70–78. ( 10.1093/molbev/mss142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stüken A, Dittami SM, Eikrem W, Mcnamee S, Campbell K, Jakobsen KS, Edvardsen B. 2013. Novel hydrolysis-probe based qPCR assay to detect saxitoxin transcripts of dinoflagellates in environmental samples. Harmful Algae 28, 108–117. ( 10.1016/j.hal.2013.06.003) [DOI] [Google Scholar]
- 38.Stüken A, Riobó P, Franco J, Jakobsen KS, Guillou L, Figueroa RI. 2015. Paralytic shellfish toxin content is related to genomic sxtA4 copy number in Alexandrium minutum strains. Front. Microbiol. 6, 404 ( 10.3389/fmicb.2015.00404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray SA, Wiese M, Brett S, Kellmann R, Hallegraeff G, Neilan BA. 2011. sxtA-based quantitative molecular assay to identify saxitoxin-producing harmful algal blooms in marine waters. Appl. Environ. Microbiol. 77, 7050–7057. ( 10.1128/AEM.05308-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orr RJ, Stüken A, Murray SA, Jakobsen KS. 2013. Evolutionary acquisition and loss of saxitoxin biosynthesis in dinoflagellates: the second ‘Core’ gene, sxtG. Appl. Environ. Microbiol. 79, 2128–2136. ( 10.1128/AEM.03279-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuang Y, Zhang H, Lin S. 2013. Cyclin B gene and its cell cycle-dependent differential expression in the toxic dinoflagellate Alexandrium fundyense Atama group I/Clade I. Harmful Algae 26, 71–79. ( 10.1016/j.hal.2013.04.002) [DOI] [Google Scholar]
- 42.Guillard RR 1975. Culture of phytoplankton for feeding marine invertebrates. In Culture of marine invertebrate animals (eds Smith WL, Chanley MH), pp. 29–80. New York, NY and London, UK: Plenum Press. [Google Scholar]
- 43.Colin SP, Dam HG. 2007. Comparison of the functional and numerical responses of resistant versus non-resistant populations of the copepod Acartia hudsonica fed the toxic dinoflagellate Alexandrium tamarense. Harmful Algae 6, 875–882. ( 10.1016/j.hal.2007.05.003) [DOI] [Google Scholar]
- 44.Demott WR, Mckinney EN. 2015. Use it or lose it? Loss of grazing defenses during laboratory culture of the digestion-resistant green alga Oocystis. J. Plankton Res. 37, 399–408. ( 10.1093/plankt/fbv013) [DOI] [Google Scholar]
- 45.Dam HG, Peterson WT. 1988. The effect of temperature on the gut clearance rate constant of planktonic copepods. J. Exp. Mar. Biol. Ecol. 123, 1–14. ( 10.1016/0022-0981(88)90105-0) [DOI] [Google Scholar]
- 46.Oshima Y 1995. Post-column derivatization HPLC methods for paralytic shellfish poisons. In Manual on harmful marine microalgae (eds Hallegraeff GM, Anderson DM, Cembella AD, Enevoldsen HO), pp. 81–94. IOC manuals and guides no. 33. Paris, France: UNESCO. [Google Scholar]
- 47.Lin S, Zhang H, Zhuang Y, Tran B, Gill J. 2010. Spliced leader–based metatranscriptomic analyses lead to recognition of hidden genomic features in dinoflagellates. Proc. Natl Acad. Sci. USA 107, 20 033–20 038. ( 10.1073/pnas.1007246107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Hou Y, Miranda L, Campbell DA, Sturm NR, Gaasterland T, Lin S. 2007. Spliced leader RNA trans-splicing in dinoflagellates. Proc. Natl Acad. Sci. USA 104, 4618–4623. ( 10.1073/pnas.0700258104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaffl MW 2001. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45 ( 10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiese M, Murray SA, Alvin A, Neilan BA. 2014. Gene expression and molecular evolution of sxtA4 in a saxitoxin producing dinoflagellate Alexandrium catenella. Toxicon 92, 102–112. ( 10.1016/j.toxicon.2014.07.003) [DOI] [PubMed] [Google Scholar]
- 51.Chang J, Dam HG. 1993. The influence of grazing on the estimation of phytoplankton growth rate via cell cycle analysis: experimental and modeling evidence. Limnol. Oceanogr. 38, 202–212. ( 10.4319/lo.1993.38.1.0202) [DOI] [Google Scholar]
- 52.Frost BW 1972. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 17, 805–815. ( 10.4319/lo.1972.17.6.0805) [DOI] [Google Scholar]
- 53.Wohlrab S, Iversen MH, John U. 2010. A molecular and co-evolutionary context for grazer induced toxin production in Alexandrium tamarense. PLoS ONE 5, e15039 ( 10.1371/journal.pone.0015039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhuang Y, Zhang H, Hannick L, Lin S. 2015. Metatranscriptome profiling reveals versatile N-nutrient utilization, CO2 limitation, oxidative stress, and active toxin production in an Alexandrium fundyense bloom. Harmful Algae 42, 60–70. ( 10.1016/j.hal.2014.12.006) [DOI] [Google Scholar]
- 55.Windram O, et al. 2012. Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell 24, 3530–3557. ( 10.1105/tpc.112.102046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Penna A, et al. 2015. The sxt gene and paralytic shellfish poisoning toxins as markers for the monitoring of toxic Alexandrium species blooms. Environ. Sci. Technol. 49, 14 230–14 238. ( 10.1021/acs.est.5b03298) [DOI] [PubMed] [Google Scholar]
- 57.Murray SA, Ruvindy R, Kohli GS, Anderson DM, Brosnahan ML. 2019. Evaluation of sxtA and rDNA qPCR assays through monitoring of an inshore bloom of Alexandrium catenella Group 1. Sci. Rep. 9, 1–12. ( 10.1038/s41598-019-51074-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang DZ, Zhang SF, Zhang Y, Lin L. 2016. Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: a molecular overview. J. Proteom. 135, 132–140. ( 10.1016/j.jprot.2015.08.008) [DOI] [PubMed] [Google Scholar]
- 59.Barbier M, Leighfield T, Soyer-Gobillard MO, Van Dolah FM.. 2003. Permanent expression of a cyclin B homologue in the cell cycle of the dinoflagellate Karenia brevis. J. Eukaryot. Microbiol. 50, 123–131. ( 10.1111/j.1550-7408.2003.tb00246.x) [DOI] [PubMed] [Google Scholar]
- 60.Bertomeu T, Morse D. 2004. Isolation of a dinoflagellate mitotic cyclin by functional complementation in yeast. Biochem. Biophys. Res. Commun. 323, 1172–1183. ( 10.1016/j.bbrc.2004.09.008) [DOI] [PubMed] [Google Scholar]
- 61.Eschbach E, John U, Reckermann M, Cembella AD, Edvardsen B, Medlin LK. 2005. Cell cycle dependent expression of toxicity by the ichthyotoxic prymnesiophyte Chrysochromulina polylepis. Aquat. Microb. Ecol. 39, 85–95. ( 10.3354/ame039085) [DOI] [Google Scholar]
- 62.Cembella A, John U. 2006. Molecular physiology of toxin production and growth regulation in harmful algae. In Ecology of harmful algae (eds Granéli E, Turner JT), pp. 215–227. Berlin, Germany: Springer. [Google Scholar]
- 63.Park G 2018. Costs and benefits of putatively anti-grazing defenses in the marine dinoflagellate Alexandrium catenella. Ph.D. dissertation, University of Connecticut, Storrs, CT, USA. [Google Scholar]
- 64.Amato A, et al. 2018. Grazer-induced transcriptomic and metabolomic response of the chain-forming diatom Skeletonema marinoi. ISME J. 12, 1594–1604. ( 10.1038/s41396-018-0094-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryderheim F, Selander E, Kiørboe T. 2020. Costs and benefits of toxin production in a dinoflagellate. bioRxiv, 199380 ( 10.1101/2020.07.12.199380) [DOI] [Google Scholar]
- 66.Cusick KD, Widder EA. 2020. Bioluminescence and toxicity as driving factors in harmful algal blooms: ecological functions and genetic variability. Harmful Algae 98, 101850 ( 10.1016/j.hal.2020.101850) [DOI] [PubMed] [Google Scholar]
- 67.Smayda TJ 1997. What is a bloom? A commentary. Limnol. Oceanogr. 42, 1132–1136. ( 10.4319/lo.1997.42.5_part_2.1132) [DOI] [Google Scholar]
- 68.Stolte W, Garcés E. 2006. Ecological aspects of harmful algal in situ population growth rates. In Ecology of harmful algae (eds Granéli E, Turner JT), pp. 139–152. Berlin, Germany: Springer. [Google Scholar]
- 69.Janzen DH 1981. Evolutionary physiology of personal defence. In Physiological ecology: an evolutionary approach to resource use (eds Townsend CR, Calow P), pp. 145–164. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 70.Creel S, Christianson D. 2008. Relationship between direct predation risk and risk effects. Trends Ecol. Evol. 23, 194–201. ( 10.1016/j.tree.2007.12.004) [DOI] [PubMed] [Google Scholar]
- 71.Peacor SD, Peckarsky BL, Trussell GC, Vonesh JR. 2013. Costs of predator-induced phenotypic plasticity: a graphical model for predicting the contribution of nonconsumptive and consumptive effects of predators on prey. Oecologia 171, 1–10. ( 10.1007/s00442-012-2394-9) [DOI] [PubMed] [Google Scholar]
- 72.Sheriff MJ, Peacor SD, Hawlena D, Thaker M. 2020. Non-consumptive predator effects on prey population size: a dearth of evidence. J. Anim. Ecol. 89, 1302–1316. ( 10.1111/1365-2656.13213) [DOI] [PubMed] [Google Scholar]
- 73.Roberts EC, Legrand C, Steinke M, Wootton EC. 2011. Mechanisms underlying chemical interactions between predatory planktonic protists and their prey. J. Plankton Res. 33, 833–841. ( 10.1093/plankt/fbr005) [DOI] [Google Scholar]
- 74.Pančić M, Kiørboe T. 2018. Phytoplankton defence mechanisms: traits and trade-offs. Biol. Rev. 93, 1269–1303. ( 10.1111/brv.12395) [DOI] [PubMed] [Google Scholar]
- 75.Park G, Dam HG. 2021. Cell-growth gene expression reveals a direct fitness cost of grazer-induced toxin production in red tide dinoflagellate prey Dryad Digital Repository. ( 10.5061/dryad.nzs7h44q0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Park G, Dam HG. 2021. Cell-growth gene expression reveals a direct fitness cost of grazer-induced toxin production in red tide dinoflagellate prey Dryad Digital Repository. ( 10.5061/dryad.nzs7h44q0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.nzs7h44q0 [75].