Introduction
Ketamine, a nonbarbiturate general anaesthetic drug, has been proposed, off-label, as a second-line agent for maintenance sedation of patients with acute respiratory distress syndrome (ARDS) requiring mechanical ventilation, including those with Coronavirus infectious disease 2019 (COVID-19).1 Cholangiopathy is reported after chronic exposure to ketamine, including in drug users and in burned patients.2 , 3 We observed 5 COVID-19 patients from 5 distinct tertiary centers (1 in Germany, and 4 in France) with cholangiopathy after exposure to intravenous ketamine, between March 20, 2020 and April 6, 2020, during the first pandemic wave in Europe. Liver injury was dose-dependent, progressive, and total ketamine exposure correlated with outcome, including liver-related death.
Patients
The median (range) age of these patients was 59 (35–65) years, and 3 (60%) were males. Underlying conditions included: uncomplicated hypertension (3 patients), uncomplicated diabetes mellitus (2 patients), kidney transplantation (1 patient with a 53 mL/min/1.73 m2 glomerular filtration rate), HBV infection under pre-emptive antiviral treatment with entecavir (1 patient), resolved hepatitis B (1 patient), smoking (1 patient). All patients had normal liver function and none had cirrhosis. The median body mass index was 28 (21–33) kg/m2. The initial median serum C-reactive protein (CRP) level was 160 (65–641) mg/L. All patients underwent mechanical ventilation for 40 (39–59) days. Nadir partial pressure of oxygen (PaO2) and arterial oxygen saturation (SaO2) were 50 (42–62) mmHg and 81 (71–93) percent, respectively. All patients received norepinephrine for 10 (2–15) days with a maximum dose of 0.4 (0.1–0.6) μg/kg/min. Maximum arterial lactate level was 2.4 (1.5–4.9) mmol/L. All patients developed acute kidney injury, and 3 (60%) required renal replacement therapy. Intravenous ketamine was given to all patients during 16 (6–26) days at a dose of 0.6 (0.1-2.2) mg/kg/h, totaling a cumulative dose of 9.5 (3.8–95.9) grams.
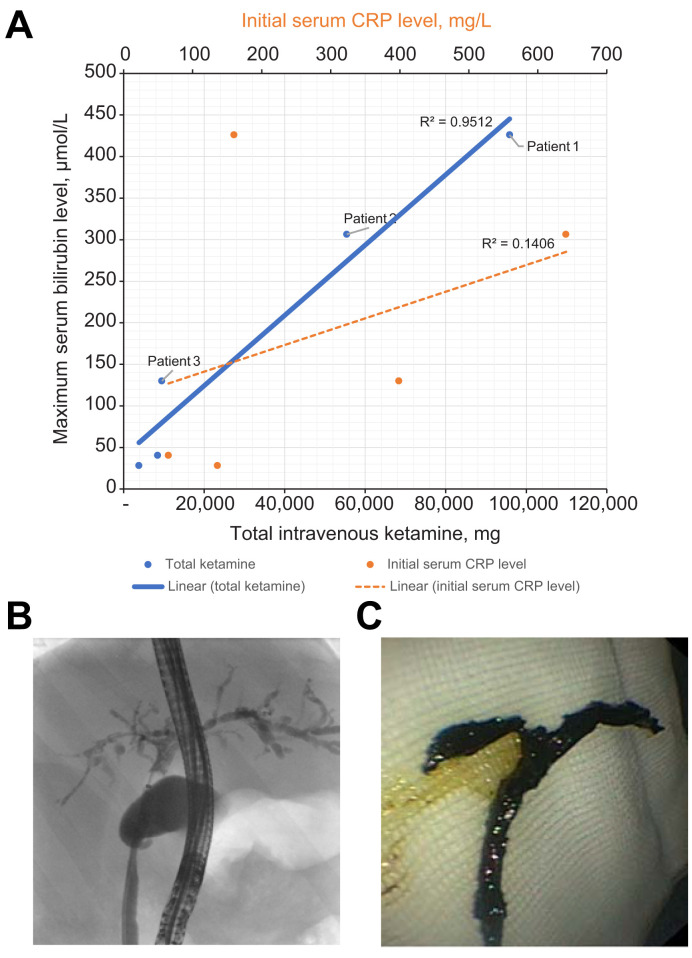
Initial serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), and total bilirubin levels were 2.3 (1–22.5), 1.7 (0.4–6.0), 0.7 (0.3–6.8), 0.9 (0.3–1.2), and 0.6 (0.3–1.0) times the upper limit of normal (ULN) range, respectively, and peaked, under ketamine, at 9.0 (5.7–22.6), 8.0 (3–12), 30.7 (18.6–248), 9.2 (4.9–11.5), and 18.0 (1.6–27.5) times the ULN, respectively. All liver screens, including for hepatotropic viruses, were negative. The relationships between total ketamine exposure, initial CRP and maximum serum bilirubin levels in the intensive care unit are presented in the Fig. 1 A.
Fig. 1.
Liver injury and bile duct obstruction with ketamine treatment.
(A) Maximum total serum bilirubin distribution by total ketamine dose exposure and by initial C-reactive protein level. (B) Cholangiogram 15 weeks after SARS-CoV-2 infection. (C) Biliary cast extracted during the first endoscopic retrograde cholangiopancreatography at week 15. (This figure appears in color on the web.)
At the end of follow-up, 6 (4–8) months after ketamine withdrawal, serum AST, ALT, GGT, ALP, and total bilirubin levels had decreased to 1.4 (0.6–2.1), 1.4 (0.7–4.3), 8.8 (5.3–37.6), 3.9 (1.6–11.0), and 1.0 (0.7–17.5) times the ULN, respectively, and all patients had cholangiopathy. One patient died with progressive sclerosing cholangitis, and decompensated cirrhosis; 1 died on the waiting liver for a liver transplant with jaundice, pruritus, biliary sepsis, portal hypertension, and a median (interquartile range) liver stiffness of 64.1 (4.5) kPa; 1 had pruritus without jaundice. The other 2 patients had recurrent biliary sepsis.
The 2 patients with end-stage liver disease were those with the most pronounced jaundice in the intensive care unit (patients one and two, Fig. 1A), who had been exposed to ketamine at a mean dose of 2.2 mg/kg/h for 14 and 16 days, respectively. The third patient also had jaundice in the intensive care unit (patient 3, Fig. 1A), and had received lower doses of ketamine (0.16 mg/kg/h) for 26 days, which corresponded to the longest exposure in the series.
One patient had endoscopic retrograde cholangiopancreatography (ERCP) that showed contrast medium filling defects in the common bile duct and rarefication of the intrahepatic biliary tract (Fig. 1B). A complete cast of the bile duct was removed during ERCP (Fig. 1C). MRI showed aspects of sclerosing cholangitis, with strictures and dilatations of intrahepatic bile ducts, peribiliary cysts and multiple biliary casts (appendix). Histological figures of cholangitis compatible with biliary obstructions, including cholangiolar proliferation, biliary plugs, portal inflammation with neutrophil infiltrates, extensive biliary fibrosis and cirrhosis (in the 2 most severe patients), were observed in the 4 patients with a liver biopsy (supplementary information).
Discussion
The characteristics of liver injury observed in this series were consistent with all previous descriptions of ketamine cholangiopathies, including dilatations and strictures of the intrahepatic and extrahepatic bile ducts, biliary sepsis and decompensated cirrhosis.3 Liver injury was dose-dependent and progressive, despite drug withdrawal, and was poorly related to initial CRP level, a surrogate of COVID-19 severity. The ECRP and MRI characteristics suggested diffuse intrahepatic biliary obstructions. Ketamine undergoes extensive metabolism in the liver, initially via nitrogen demethylation to norketamine, a water-insoluble by-product. Forensic studies have shown that norketamine is present in human bile after ketamine poisoning.4 We speculate that our patients were overexposed to ketamine, leading to biliary precipitations of norketamine, biliary strictures, cholangitis, secondary biliary cirrhosis, and end-stage liver disease; but there is no experimental proof yet.
The mechanism of ketamine toxicity could be, however, more complex. Ketamine could act as a second hit in a previously injured biliary tract, either by SARS-CoV-2, (there is little, inconsistent, evidence that Sars-CoV-2 can infect biliary cells);5 by other medications; by the systemic inflammatory response syndrome; and/or by hypoxia, although none of our patients had profound shock or profound hypoxemia. Critical illness cholangiopathy is the main differential diagnosis. While our observations suggest that ketamine toxicity was dose-dependent, an idiosyncratic reaction is also possible, as for most drug-induced liver injuries. Whether ketamine contributed, or not, to acute kidney injury in our series is a very important question but is beyond the scope of this study.
In conclusion, intravenous ketamine at the recommended doses for maintenance sedation of patients undergoing mechanical ventilation for ARDS, including COVID-19 patients, can be associated with biliary obstructions, cholestatic liver injury, biliary cirrhosis, and end-stage liver disease. It is notable that all patients received intravenous ketamine within the recommended doses for maintenance sedation of intensive care unit patients undergoing mechanical ventilation for ARDS.1 COVID-19 patients who underwent maintenance sedation with ketamine, especially those who developed jaundice in the intensive care unit, should be screened for long-term liver injury, including cholangiopathy. The guidelines for maintenance sedation of patients with ARDS,1 regardless of COVID-19, should not include ketamine, especially if a prolonged, or a higher dose sedation, is expected. Further studies, including biliary casts analyses and case control studies, should be conducted to identify the contributing factors to secondary sclerosing cholangitis after COVID-19.
Financial support
The study did not receive any external funding.
Authors’ contributions
VM: conception of the study, acquisition, analysis and interpretation of the data, draft of the manuscript; KB, HW, PDM, ND, JDR, PI, JPDVH, JMC, SP: acquisition, analysis and interpretation of the data, edition of the manuscript. The authors declare they have seen and approved the final version of the manuscript. All members of the keta-cov group facilitated the study or took care of the reported patients.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.02.007.
Contributor Information
The Keta-Cov research group:
Vincent Mallet, Kilian Bock, Paul Doumbe Mandengue, Nicolas Dufour, Torsten Voigtlaender, Jean-Damien Ricard, Pierre Isnard, Vincent Frochot, Emmanuel Letavernier, Lucile Moga, Amandine Landrieux, Pierre-Emmanuel Rautou, Nathalie Pons-Kerjean, Anaïs Vallet-Pichard, Laurent Chouchana, Coralie Gernez, Carole Burger, Anne Scemla, Christian de Tymoski, François Depret, Emmanuel Dudoignon, Lionel Lamhaut, Rémi Flicoteau, Géraldine Rousseau, Jean-Paul Duong Van Huyen, Jean-Michel Correas, Heiner Wedemeyer, and Stanislas Pol
Supplementary data
The following is the supplementary data to this article:
References
- 1.Chanques G., Constantin J.M., Devlin J.W., Ely E.W., Fraser G.L., Gelinas C., et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020;46(12):2342–2356. doi: 10.1007/s00134-020-06307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agence nationale de sécurité du médicament et des produits de santé (ANSM) June 20, 2017. Ketamine: risk of serious liver damage during prolonged use and/or at high doses–Information Point.https://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Ketamine-risque-d-atteintes-hepatiques-graves-lors-d-utilisations-prolongees-et-ou-a-doses-elevees-Point-d-Information Available from: [Google Scholar]
- 3.Seto W.K., Mak S.K., Chiu K., Vardhanabhuti V., Wong H.F., Leong H.T., et al. Magnetic resonance cholangiogram patterns and clinical profiles of ketamine-related cholangiopathy in drug users. J Hepatol. 2018;69(1):121–128. doi: 10.1016/j.jhep.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Licata M., Pierini G., Popoli G. A fatal ketamine poisoning. J Forensic Sci. 1994;39(5):1314–1320. [PubMed] [Google Scholar]
- 5.Han D., Fang Q., Wang X. SARS-CoV-2 was found in the bile juice from a patient with severe COVID-19. J Med Virol. 2021;93:102–104. doi: 10.1002/jmv.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.