Abstract
The COVID-19 pandemic has led to a rapid escalation in use of home monitoring and video consultations in children with a variety of chronic respiratory conditions. Our department set up a home spirometry service from scratch once it became evident that we needed to keep patients away from hospital clinics whenever possible. We faced a number of challenges but now have around 400 children using home spirometers. There are a number of portable spirometers available, some with online platforms. The technology, particularly the software/apps interface, has been improved by the companies in response to issues that have arisen. We believe the use of home monitoring is here to stay.
Keywords: Home spirometry, Adherence, Technology, Telemedicine, Asthma, Suppurative lung disease
Introduction
Our tertiary respiratory centre has a large cohort of patients seen regularly in a clinic setting. By March 2020 in London, the COVID-19 pandemic meant our patients could no longer all be seen for standard face to face appointments [1], [2], so telephone and a month later, video consultations were introduced [3]. Spirometry is the pulmonary function test most often carried out, with its internationally agreed standards to determine quality [4]. Conducting spirometry during clinic visits is an important objective parameter in the clinical monitoring of patients, especially those with asthma and chronic suppurative lung disease - including cystic fibrosis (CF) and primary ciliary dyskinesia (PCD). Therefore, with the rapid shift to remote consultations, a way to monitor lung function at home was urgently needed. We essentially set up a home spirometry service from scratch, and present here how we did it, our experience with data for the 9 months of April to December 2020, and practical tips for other centres. We have left out trade names of all spirometers in this review due to commercial sensitivities.
Adult studies
Remote spirometry methods have been available for some years, and the growing use of smart phones and tablets has increased demand for portable medical devices, such as spirometers, that can pair with apps via Bluetooth. Handheld turbine spirometers are now readily available and there have been a number of comparison studies with hospital-based equipment; although mostly in adults carried out in a hospital setting, and these generally find good agreement [5], [6], [7], [8].
In terms of practical use, a large study of 437 patients post bone marrow transplant carried out weekly home spirometry for one year, and found acceptable limits of agreement for FEV1 (forced expiratory volume in 1 second) but there was a tendency to under-read at home; nevertheless, they felt this was useful for self-monitoring of early post-transplant lung complications [9]. In a pilot study 10 adult patients with idiopathic pulmonary fibrosis used real-time wireless spirometry daily, and found good agreement between the home and hospital FVC (forced vital capacity) readings with some lower readings at home, but they concluded that home spirometry was acceptable [10]. A study of 40 adults with motor neurone disease found that FVC and maximal inspiratory pressure measurements guided by a respiratory therapist at home were tightly correlated with standard measures, and were positively accepted by the patients and carers [11]. In a larger study, 111 adults with amyotrophic lateral sclerosis and 30 healthy controls were able to perform acceptable spirometry at home following instruction with web-based videos and online training [12].
A multicentre study of 267 young people and adults (aged 14 years or above) with CF (eICE study) had an intervention arm with patients measuring spirometry at home twice a week (as well as electronically recorded symptoms) [13]. Adherence (defined as doing tests > 80% of the time) to once weekly spirometry was 50% and twice weekly just 19%. They did detect more exacerbations compared to usual care but there was no difference in decline in lung function over one year.
Paediatric studies
Again, comparison studies of portable vs conventional spirometers have been carried out. One recent study in 76 patients with CF under 18 years of age compared supervised use of handheld spirometers and conventional laboratory spirometry, both measured in the clinic [14]. They found that whilst there was a linear correlation, the limits of agreement for FEV1 were wide (+9% to −14%), and 15% readings were ≥10% less on the handheld spirometers, so the authors concluded the devices could not be used interchangeably. A study of 48 patients aged 12–18 years with asthma found they were able to successfully use a portable spirometer with a high degree of agreement between the two devices, but the measurements were obtained in a hospital setting and supervised by a technician [15]. Another study compared hand vs conventional spirometry after exercise in 122 children aged 6–12 years being screened for asthma (with 40 subsequently diagnosed with asthma); they found reasonable agreement but the devices were not interchangeable, with FEV1 on the handheld, a mean of 2% lower both at baseline and after exercise [16]. These results were similar to an earlier study in 50 asthmatic children aged 5–15 years having an exercise challenge and bronchodilator responsiveness (BDR) measured [17]. The pocket-sized turbine spirometer gave responsiveness similar but lower results and was not interchangeable with conventional flow-volume spirometry; it also missed some of the cases with positive exercise induced bronchospasm (4/16) and positive BDR (2/8). Another study in 70 children with stable asthma and CF found the portable spirometer read higher than the standard spirometer, unusually as most studies find the portable spirometer gives lower measurements [18].
Finally, the CLIMB-CF multicentre study has been presented at the 2020 North American CF Conference [19]. They assessed paired spirometry readings taken unsupervised at home with the handheld spirometer and then later the same day supervised using the clinic spirometer. There were 67 children with CF, median age 10 years, and although the devices correlated as expected, there was substantial bias with the home readings being lower in 78% participants, by a mean difference of FEV1 6.5%. The bias remained but was less in children ≥ 12 years (FEV1 3.8% mean difference). They also found that spirometry was one of the home monitoring measures that teenagers found least acceptable [20].
In terms of actual clinical practice, in Melbourne, Australia, they studied ‘telehealth spirometry’ in 22 patients aged 7–17 years with CF, either during a ‘home admission’ for intravenous antibiotics, or for ongoing monitoring [21]. The family needed a home computer or tablet, internet connection and a microphone and video camera. Spirometry was successful in 55/59 (93%) sessions according to standard ATS/ERS criteria, using a web-based video call with a respiratory scientist who shared the family’s computer screen each time. Spirometry was not to be carried out without direct supervision which took on average 22 min (range 15–47 min). The family then encrypted a pdf of the results and emailed it to the CF centre. The barriers to success were principally technical, with incompatible operating systems, inconsistent internet connections or unsuitable home computers. Only doing spirometry with a technician present would certainly be problematic outside of a trial setting in our centre, due to the sheer number of patients being seen in video clinics each week. Also, we suspect not all families would wish to share personal computer access with members of the healthcare team.
Home spirometry has been assessed in 39 young people with CF aged 12–21 years who were asked to do weekly tests for one year [22]. The home measures correlated well with those done in clinic, but mean adherence to weekly spirometry was 59%, and only 30% people used the device for more than 80% of the study. Using regular home spirometry led to a small increase in medication adherence but no change in exacerbation rate or rate of decline in lung function over the year. In a similar study, 49 children aged 5–19 years with CF were asked to do home spirometry 3 times a week for a year [23]. Good adherence (defined as making > 70% requested measures) was found in 54% of the children, with median completed data of 77%. They did not see any deterioration in lung function in the 4 weeks prior to a pulmonary exacerbation.
Spirometer selection
After some initial research into home spirometers, five spirometry supply companies were approached for further information on their products. Only those products with a CE (Conformité Européenne) mark were considered, meaning they conformed to health, safety, and environmental protection standards for products sold within the European Economic Area. Features that were key requirements for a paediatric home spirometer were identified (see Table 1 ).
Table 1.
Key requirements for home spirometer.
Manufacturer factors
Spirometer factors
|
Global lung Function Initiative.
In total six spirometers were considered. Along with financial quotes, each company was asked to provide information on the following: the current availability of their product (including despatch and delivery time); which reference equations were used to generate predicted values; whether an app was utilised or devices were standalone; how the end report was generated and presented; what ongoing support we would be given; and future plans for development of the product. Due to concerns surrounding time and availability during the pandemic, companies were asked to demonstrate their products via video link so that ease of use could be assessed, rather than going through the usual process of testing spirometers within our department. Two spirometers met our criteria and were ordered.
Process of roll out to patients
We are not going into financial details but were grateful for NHS England who funded spirometers for half our children with CF, and our children with PCD.
The roll out process is summarised in Table 2 . The multidisciplinary teams (MDT) for each of the patient groups with chronic suppurative lung disease (CSLD) including CF and PCD, as well as asthma were asked to provide a priority list for sending out home spirometers. All patients aged 6 years and above were eligible although we started with those aged 8 and above. Priority was based on clinical need as well as factors such as previous spirometry technique to judge how likely they would perform it successfully at home. Following this, the most suitable product for that family was identified. Spirometers were ordered and dispatched in batches according to the priority list.
Table 2.
Roll out process.
|
At first one of the spirometers did not give a flow volume loop so these were used for children we knew had reproducible technique; this was soon rectified.
In order to get a baseline and compile trend data, we asked patients to perform spirometry monthly. In addition, the intention was for patients to perform spirometry within the 24 hours before a planned video consultation. Our nurse specialists or physiotherapist would also ask for spirometry if the child was symptomatic and the parents had contacted us. Spirometry reports were also requested by shared centres and sent securely via nhs.net. Finally, we would sometimes plan to check the lung function response to an intervention such as starting a mucoactive agent or oral antibiotics. We did not want routine weekly spirometry, particularly in the light of the published literature on lack of adherence.
Royal Brompton Hospital figures
In the 9 month period April to December 2020, we sent out 391 spirometers -
-
•
CF – 199
-
•
PCD – 95
-
•
Asthma – 77
-
•
Other CSLD – 20
The number of video calls made to help with technique/setting up totalled 153 (39%) which was time-consuming, taking between 10 and 30 min.
In 67 (17%) patients, they had entered their details (such as height, weight, age, and ethnicity) incorrectly the first time, requiring a call to correct this. This happened despite up to date details having been sent in writing with the spirometer.
We had to chase up 58 (15%) of patients to set up their spirometer in the first place (some parents had to be called up to 8 times to remind them).
National figures
In October 2020, the UKCFMA (United Kingdom Cystic Fibrosis Medical Association) sent out a survey to all adult and paediatric centres about their use of home spirometry during the pandemic [personal communication Dr Caroline Elston]. Data were returned from 42 CF centres; 95% had provided home spirometers and 88% felt it provided useful data to improve quality of care. Inevitably there had been some challenges which were similar to those we had encountered.
Challenges we encountered
As well as advantages, there are a number of drawbacks to remote consultations and telemedicine [3]. Once established, the home spirometry service has presented unique challenges not previously encountered carrying out spirometry in the outpatient and inpatient setting. Aside from the time spent on procurement, identifying patients and mailing devices, the process of setting up families with their spirometer has been more time consuming then first anticipated. Many families required assistance to install the app, enter correct demographic data and correctly pair their smartphone/tablet with the spirometer. We found a video call to be the most successful way of resolving these issues. Since families were by then familiar with the videocall platform (Attend Anywhere) that had been provided by NHS England for remote consultations, we were able to take advantage of this technology to provide on the spot troubleshooting.
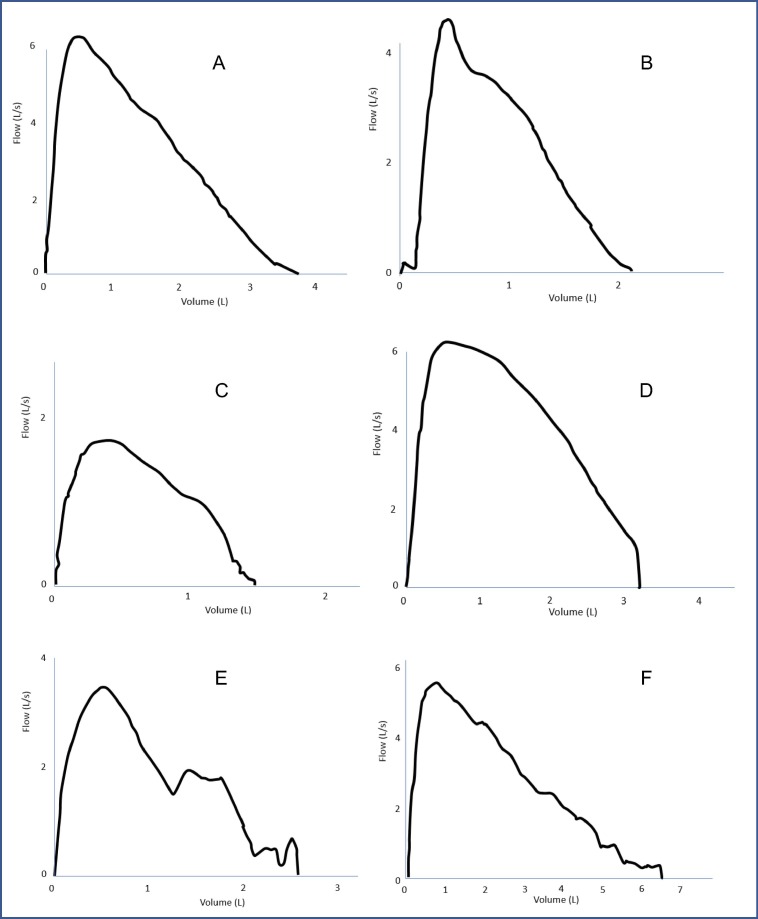
We also encountered poor technical quality on many initial tests submitted (see fig. 1 for examples), even from those patients whose technique in clinic was known to be good. For this reason, we ensure that all tests received are manually checked by a specialist paediatric respiratory physiologist prior to being uploaded to a patients’ electronic record. Correcting these difficulties often required additional coaching for the patient and education for their families in technically acceptable manoeuvres. The Melbourne study [21] had the respiratory scientist help the family online with every measurement taken, but this would be even more time consuming, especially as we have found that, with time and practice, many of our patients now do perfectly acceptable spirometry unsupervised.
Fig. 1.
Real examples of flow volume loops received from our paediatric patients using home spirometry. A – Normal flow volume curve. A deep breath to maximal inspiration has been taken with maximum expiration. B – Delayed start. There is a slight pause/delay prior to expiration after total inspiration. C – Submaximal effort. Total inspiration has not been achieved and expiration is a poor effort, as seen by lack of peak in the flow volume curve. D – Early termination. Maximal expiration has not been achieved as seen by the sharp cut off in the flow volume curve. E – Multiple blows. Following inspiration, there is more than a single blow during expiration as shown with multiple peaks, a common way some children try to ‘cheat’ to get a better result. F – Incorrect mouthpiece position. The end of the mouthpiece must be behind the teeth with lips sealed around it, as the tongue or teeth can obstruct air flow during maximal expiration. In this example, the malposition is causing higher expiratory volumes.
Even when patients were attending the hospital in person for clinic, or were an inpatient, providing spirometry training in person was difficult. Since spirometry is considered an aerosol generating procedure, staff are required to wear full personal protective equipment (PPE), including a mask and visor. This is a barrier to communication with the child, and also makes demonstration of techniques, such as correct positioning for the mouthpiece, impossible. For this reason, we found a video call preferable to face to face teaching.
We also found a number of technical issues with the spirometry and software (Table 3 ), although most have since been corrected. We found the companies to be responsive in a timely manner when problems were identified.
Table 3.
Technical issues to be aware of.
|
We know that adherence to regular home spirometry 1–3 times a week is poor in adults and children [13], [22], [23]. We were not requesting regular spirometry, but found adherence to a pre-clinic measurement was also lower than expected, and despite reminders, we quite often have to ask again during the remote consultation.
There is also a concern that patients from socially disadvantaged families are potentially at further disadvantage - not everyone can afford an up to date smartphone or tablet which meant the current apps could not be loaded; some have poor internet connection at home; and when English is not the family’s first language this presents further difficulties for training.
Advice for other centres
We have compiled a list of top tips for other centres setting up a home spirometry service, based on our experiences (Table 4 ).
Table 4.
Tips for setting up a home spirometry service.
| Tips | Rationale |
|---|---|
| Patient/parent related | |
| Offer a video call | Early detection of poor technique is an important factor for valid home spirometry. Identifying issues early on and arranging video calls allows time to rectify any problems and provide appropriate coaching to the patients. Video calling has proven extremely beneficial and has improved technique and reproducibility. Patient/parent engagement is also improved as a more personal service is provided |
| Virtual may be better than face to face training | Virtual calls enable technique to be demonstrated with a mouthpiece in the correct position, which is not possible wearing full PPE |
| Produce videos and teaching presentations | Videos produced with the hospital Communications team showcase good technique when using devices. They can be included in a teaching presentation for the MDT and parents/patients. Live Question & Answer sessions via webinar for parents can also be useful |
| Patient contact list | Ensure patient/parent contact details (including email and mobile phone) are up to date |
| Group emails | Sending group emails to patients enables the relaying of important information and new updates within the service efficiently. Ensure the whole address list is not visible to individuals |
| Dedicated work mobile phone | This enables the team to communicate with patients when working from home |
| Staff related | |
| Priority list | Early clinical prioritisation by the MDT for roll out i.e., who should receive the first batch of devices |
| Physiologist’s team email | A dedicated Home Spirometry team email allows efficient communication |
| MDT teaching and troubleshooting | Sending a troubleshooting guide to other members of the MDT helps them answer common queries and problems with the devices. The physiologists also provided teaching and training on using the app to members of the MDT |
Conclusions
Early in the COVID-19 pandemic, we realised we needed a new way of working, to maintain the health of a large cohort of children with chronic lung conditions, but at the same time keep them away from hospital clinics wherever possible. This particularly applied to children with chronic suppurative lung disease and significant asthma who were used to having regular clinic checks including lung function monitoring. The pandemic accelerated the need for technological advances and provided a unique opportunity to create a service that is patient centred and forward thinking. Telemedicine and home spirometry is not a new concept [3], and we found the rapid implementation on such a large scale challenging but rewarding. Home monitoring was necessary to compliment video consultations. Our home spirometry service was set up quickly, so inevitably we had to learn and improve the service as we went along. However, we believe the roll out has been successful and has been appreciated by most families. We believe this practice will continue beyond the pandemic and that home monitoring is here to stay. We continue to gather data that will allow robust auditing of this service, and also plan to collect patient reported outcome measures on families’ experiences of remote monitoring, to inform future service developments.
Educational aims
The reader will be able to:
-
•
Understand the challenges of setting up a home spirometry service.
-
•
Help centres set up their own service using our experiences.
-
•
Demonstrate pitfalls in the current systems.
Directions for future research
-
•
How to improve remote training of patients in home spirometry.
-
•
How to improve patient adherence to use of home monitoring.
-
•
How to improve the technology.
Declarations of interest
None.
Funding
None.
References
- 1.NICE guidelines [NG166]. COVID-19 rapid guidelines: severe asthma. https://www.nice.org.uk/guidance/ng166/. Published 3.4.20 (accessed 17.1.21).
- 2.NICE guidelines [NG170]. COVID-19 rapid guidelines: cystic fibrosis. https://www.nice.org.uk/guidance/ng170. Published 9.4.20 (accessed 17.1.21).
- 3.Dixon E., Dick K., Ollosson S., Jones D., Mattock H., Bentley S., et al. Telemedicine and cystic fibrosis: do we still need face-to-face clinics? Paediatr Respir Rev. 2021 doi: 10.1016/j.prrv.2021.05.002. In press. [DOI] [PubMed] [Google Scholar]
- 4.Graham B.L., Steenbruggen I., Miller M.R., Barjaktarevic I.Z., Cooper B.G., Hall G.L., et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Exarchos K.P., Gogali A., Sioutkou A., Chronis C., Peristeri S., Kostikas K. Validation of the portable Bluetooth® Air Next spirometer in patients with different respiratory diseases. Respir Res. 2020;21:79. doi: 10.1186/s12931-020-01341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos Hernández C., Núñez Fernández M., Pallares Sanmartín A., Mouronte Roibas C., Cerdeira Domínguez L., Botana Rial M.I., et al. Validation of the portable Air-Smart Spirometer. PLoS ONE. 2018;13:e0192789. doi: 10.1371/journal.pone.0192789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr R.G., Stemple K.J., Mesia-Vela S., Basner R.C., Derk S.J., Henneberger P.K., et al. Reproducibility and validity of a handheld spirometer. Respir Care. 2008;53:433–441. [PMC free article] [PubMed] [Google Scholar]
- 8.Du Plessis E., Swart F., Maree D., Heydenreich J., Van Heerden J., Esterhuizen T.M., et al. The utility of hand-held mobile spirometer technology in a resource-constrained setting. S Afr Med J. 2019;109:219–222. doi: 10.7196/SAMJ.2019.v109i4.13845. [DOI] [PubMed] [Google Scholar]
- 9.Cheng G.S., Campbell A.P., Xie H., Stednick Z., Callais C., Leisenring W.M., et al. Correlation and agreement of handheld spirometry with laboratory spirometry in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2016;22:925–931. doi: 10.1016/j.bbmt.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moor C.C., Wapenaar M., Miedema J.R., Geelhoed J.J.M., Chandoesing P.P., Wijsenbeek M.S. A home monitoring program including real-time wireless home spirometry in idiopathic pulmonary fibrosis: a pilot study on experiences and barriers. Respir Res. 2018;19:105. doi: 10.1186/s12931-018-0810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geronimo A., Simmons Z. Evaluation of remote pulmonary function testing in motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:348–355. doi: 10.1080/21678421.2019.1587633. [DOI] [PubMed] [Google Scholar]
- 12.Rutkove S.B., Qi K., Shelton K., Liss J., Berisha V., Shefner J.M. ALS longitudinal studies with frequent data collection at home: study design and baseline data. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:61–67. doi: 10.1080/21678421.2018.1541095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechtzin N., Mayer-Hamblett N., West N.E., Allgood S., Wilhelm E., Khan U., et al. eICE Study Team. Home monitoring of patients with cystic fibrosis to identify and treat acute pulmonary exacerbations. eICE Study Results. Am J Respir Crit Care Med. 2017;196:1144–1151. doi: 10.1164/rccm.201610-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avdimiretz N., Wilson D., Grasemann H. Comparison of a handheld turbine spirometer to conventional spirometry in children with cystic fibrosis. Pediatr Pulmonol. 2020;55:1394–1399. doi: 10.1002/ppul.v55.610.1002/ppul.24743. Epub 2020 Mar 23. [DOI] [PubMed] [Google Scholar]
- 15.Ring B., Burbank A.J., Mills K., Ivins S., Dieffenderfer J., Hernandez M.L. Validation of an app-based portable spirometer in adolescents with asthma. J Asthma. 2019;1–8 doi: 10.1080/02770903.2019.1702201. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harri K., Tapani R.S., Senja K., Matti K. Hand-held turbine spirometer: agreement with the conventional spirometer at baseline and after exercise. Pediatr Allergy Immunol. 2005;16:254–257. doi: 10.1111/j.1399-3038.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 17.Kannisto S., Vanninen E., Remes K., Korppi M. Use of pocket-sized turbine spirometer in monitoring exercise-induced bronchospasm and bronchodilator responses in children. Pediatr Allergy Immunol. 1999;10:266–271. doi: 10.1034/j.1399-3038.1999.00037.x. [DOI] [PubMed] [Google Scholar]
- 18.Bastian-Lee Y., Chavasse R., Richter H., Seddon P. Assessment of a low-cost home monitoring spirometer for children. Pediatr Pulmonol. 2002;33:388–394. doi: 10.1002/ppul.10085. [DOI] [PubMed] [Google Scholar]
- 19.Edmondson C., Westrupp N., Wallenburg J., Brownlee K., Alton E.W., Bush A., et al. Monitoring lung function of young people with CF: is it reliable? Results from the CF-CLIMB study. Pediatr Pulmonol. 2020;55(Suppl 2):S290. [Google Scholar]
- 20.Edmondson C., Westrupp N., Wallenburg J., Brownlee K., Alton E.W., Bush A., et al. What is feasible when it comes to monitoring young people with cystic fibrosis at home? Results from the CF-CLIMB study. Pediatr Pulmonol. 2020;55(Suppl 2):S297. [Google Scholar]
- 21.Logie K., Welsh L., Ranganathan S.C. Telehealth spirometry for children with cystic fibrosis. Arch Dis Child. 2020;105:1203–1205. doi: 10.1136/archdischild-2019-317147. Epub 2019 Nov 6. [DOI] [PubMed] [Google Scholar]
- 22.Shakkottai A., Kaciroti N., Kasmikha L., Nasr S.Z. Impact of home spirometry on medication adherence among adolescents with cystic fibrosis. Pediatr Pulmonol. 2018;53:431–436. doi: 10.1002/ppul.23950. [DOI] [PubMed] [Google Scholar]
- 23.van Horck M., Winkens B., Wesseling G., van Vliet D., van de Kant K., Vaassen S., et al. Early detection of pulmonary exacerbations in children with Cystic Fibrosis by electronic home monitoring of symptoms and lung function. Sci Rep. 2017;7 doi: 10.1038/s41598-017-10945-3. [DOI] [PMC free article] [PubMed] [Google Scholar]