Abstract
The amygdala is a subcortical structure implicated in both the expression of conditioned fear and social fear recognition. Social fear recognition deficits following amygdala lesions are often interpreted as reflecting perceptual deficits, or the amygdala's role in coordinating responses to threats. But these explanations fail to capture why amygdala lesions impair both physiological and behavioural responses to multimodal fear cues and the ability to identify them. We hypothesized that social fear recognition deficits following amygdala damage reflect impaired conceptual understanding of fear. Supporting this prediction, we found specific impairments in the ability to predict others' fear (but not other emotions) from written scenarios following bilateral amygdala lesions. This finding is consistent with the suggestion that social fear recognition, much like social recognition of states like pain, relies on shared internal representations. Preserved judgements about the permissibility of causing others fear confirms suggestions that social emotion recognition and morality are dissociable.
Keywords: amygdala, lesion, fear, emotion recognition, moral judgment
1. Introduction
The amygdala is a subcortical structure implicated in a wide array of social and affective processes but essential for relatively few [1–5]. Among its essential functions are coordinating the acquisition and expression of conditioned fear. Acquired amygdala damage reliably impairs fear conditioning, and behavioural, physiological and (in humans and perhaps other species, subjective) responses to threats [6–9]. These impairments are typically paralleled by deficits in social recognition of others’ fear (but typically not other emotions) across multiple cues and modalities, including facial expressions, vocal tones, body postures and musical compositions [10–17]. Various theories aimed at explaining these paired deficits in fear responding and social fear recognition do not capture some portion of the observed findings. We hypothesize that acquired amygdala lesions impair the ability to generate representations of internal fearful states, thus impeding recognition of those states. We show, consistent with this hypothesis, that established social fear recognition deficits in a patient with acquired bilateral amygdala lesions extend to failures to predict which of a series of written statements would cause fear. These results contradict hypotheses that social fear recognition deficits following amygdala damage result solely from impaired threat detection or perceptual deficits and suggest a role for the amygdala in maintaining internal representations of fear. Moreover, preserved judgements of the morality of causing others fear following amygdala lesions confirm that judgements of social emotion and morality are dissociable.
Fear is the multimodal state that accompanies the anticipation of aversive outcomes and promotes adaptive responses, including avoidance and escape [18–20]. The amygdala is thought to play a key causal role in generating this state [21] because lesions to the amygdala consistently impair various facets of conditioned fear expression, experience and learning, including cognitive, physiological and behavioural responses to threats [6,7,22,23]. Such lesions also reliably impair the ability to recognize others' fear across visual and auditory modalities [13–15,23]. These deficits are yoked, such that patients with amygdala lesions who do retain coordinated fear responses also show preserved recognition of others’ fear [17].
Some have suggested that these correlated deficits in fear responding and social fear recognition simply reflect the amygdala's role in coordinating responses to threats [18,24–26]. But this explanation requires that fearful expressions are interpreted primarily as threats, whereas behavioural evidence shows that among typical respondents, fearful expressions are viewed as appetitive, not aversive [27,28]. This explanation also does not capture why amygdala lesions impair not just physiological and behavioural responses to social fear cues, but the ability to identify them as fearful. An alternate theory holds that the amygdala supports social fear recognition by directing spontaneous attention to salient, diagnostic perceptual features of faces, like the eyes of fearful expressions, thus facilitating their recognition [29–32]. But this theory struggles to explain why amygdala lesions also impair recognition of fearful stimuli without any single diagnostic cue, like fearful voices and music [10,11,15]. A third explanation posits that the amygdala coordinates the generation of a multimodal internal representation—or central state—of fear [11,18,33,34]. According to this theory, amygdala damage impairs social fear recognition because identifying fear in a face, voice, body or musical composition is typically performed by linking external perceptual information to an internal representation of fear, which is coordinated by the amygdala. This hypothesis would be supported by evidence that amygdala damage impairs the ability to predict whether others would experience fear in response to written scenarios, which do not represent a threat to the respondent and carry no low-level perceptual cues. Instead, correctly predicting which of a series of written scenarios would elicit fear requires prospection, or generating a representation of the internal state likely to result from the scenario.
To test the hypothesis that the amygdala plays such a role in social fear recognition, we tested the ability of a patient with bilateral amygdala damage to infer which of a series of written hypothetical statements would cause the target of the statement to experience fear. This patient and matched comparison participants read 100 short written statements (for example: ‘I could easily hurt you’, ‘I don't want to be friends anymore’) and were asked to predict how someone would feel if another person were to make that statement to them. Task stimuli were generated to elicit one of five possible emotions (anger, disgust, fear, happiness and sadness; see electronic supplementary material, appendix A for a full set of statements). Prior testing confirms that healthy individuals reliably predict each statement will primarily elicit the target emotion [35]. Neuroimaging also confirms that accurately evaluating the fear-eliciting statements in the set preferentially recruits the amygdala [36,37], paralleling the amygdala's preferential recruitment in response to nonverbal expressions of fear relative to other emotions [38]. Further, individuals with psychopathic traits, who exhibit functional and structural amygdala anomalies, are selectively impaired in identifying only the fear-eliciting statements [35,39], and show reduced amygdala responses to them [36,37].
Following prior work, participants selected which of five possible emotional states (anger, disgust, fear, happiness, sadness) would be most likely to follow each statement. Also following prior work [35], participants also rated how morally permissible it would be to make each statement, using a 4-point scale. We predicted that, unlike judgements of specific emotions, moral judgements would be spared following amygdala lesions that are not congenital or acquired in early childhood. Judgements of moral permissibility rely on a neural network [40] that becomes progressively refined and distributed during early and middle childhood [41,42]. As a result, lesions to the amygdala acquired in late childhood or adulthood are not consistently associated with impaired moral judgement [43] whereas childhood-onset lesions to regions such as prefrontal cortex that are implicated in moral judgements result in greater deficits than adult-onset brain lesions [44]. The predicted results would confirm that judgements of emotion and morality are dissociable and would be consistent with theories that moral judgements are more strongly affected by amygdala abnormalities that are congenital or emerge in early childhood (as in the case of psychopathy) than by amygdala lesions acquired in adolescence or adulthood.
Participants included SM, a 49-year-old caucasian female patient with complete bilateral amygdala lesions, and 45 neurologically healthy comparisons (HCs) matched with her in terms of sex (all female), race (caucasian) and age (mean age 45.31, s.d. = 11.17). This sample size was generated from a power analysis conducted using STATA that found a sample size of 45 would provide a statistical power of 0.80 to detect an effect size r = 0.41 obtained in previously published work [35]. SM is one of the best-characterized human cases of bilateral amygdala damage [7]. She does not exhibit typical subjective, physiological or behavioural conditioned fear responses, and shows severe and selective impairment in social fear recognition from facial expressions, music and when drawing representations of fearful faces [45]. She can, however, identify vocal tones and complex visual scenes associated with fear. This discrepancy could indicate that she is selectively impaired in processing only some social fear cues but that her social fear understanding is otherwise intact. Alternatively, she may possess semantic knowledge regarding fear-linked perceptual cues (for example, that fearful voices are high-pitched) that she can use to identify certain stimuli despite impaired internal representations of fear. The present task enabled us to adjudicate between these possibilities by testing SM's ability to predict social fear from written statements free of diagnostic perceptual cues. SM completed the task twice approximately three months apart to establish reliability, and comparisons completed it once.
2. Results
(a). Emotion recognition
Relative to comparisons, SM was selectively impaired in inferring others' fear. Whereas her accuracy for recognizing anger-disgust- and happiness-eliciting statements was comparable to comparisons’ (table 1), she achieved only 45.92% accuracy for correctly identifying fear-eliciting statements, 2.90 s.d. below the comparison mean of 84.30% (CI95 [80.44, 88.17]). Her fear recognition accuracy was consistently poor across the first (55.00%) and second (36.84%) testing session. SM was also impaired in recognizing sadness-eliciting statements, achieving 62.50% accuracy, 2.64 s.d. below the comparison mean of 86.56% (CI95 [83.90, 89.21]). But her performance inferring sadness rose substantially from the first test (45.00% accuracy) to the second (80.00%), resulting in a CI95 [28.20, 96.80] that overlapped the mean of comparisons, as did her CI95 for other emotion categories. Only SM's CI95 for fear-eliciting statements did not overlap with comparisons (table 1 and figure 1).
Table 1.
Descriptive statistics for behavioural responses to written statements for healthy controls and SM.
| SM |
HC |
|||
|---|---|---|---|---|
| mean (s.d.D) | 95% CI | mean (s.d.) | 95% CI | |
| emotion predictiona | ||||
| anger | 50.00 (14.14) | 30.40–69.60 | 56.00 (21.04) | 49.85–62.15 |
| disgust | 90.00 (7.07) | 80.20–99.80 | 94.66 (5.88) | 92.94–96.38 |
| fear | 45.92 (12.84)c | 28.13–63.72 | 84.30 (13.23) | 80.44–88.17 |
| happiness | 100.00 (0) | 100.00–100.00 | 99.43 (1.97) | 98.86–100.00 |
| sadness | 62.50 (24.75)c | 28.20–96.80 | 86.56 (9.10) | 83.90–89.21 |
| moral permissibilityb | ||||
| anger | 1.15 (0.14) | 0.95–1.35 | 1.48 (0.30) | 1.40–1.57 |
| disgust | 1.25 (0.35) | 0.76–1.74 | 2.17 (0.68) | 1.97–2.37 |
| fear | 1.15 (0.14) | 0.95–1.34 | 1.54 (0.30) | 1.45–1.63 |
| happiness | 3.85 (0.07) | 3.75–3.95 | 3.81 (0.26) | 3.73–3.88 |
| sadness | 1.45 (0.42)c | 0.86–2.04 | 2.36 (0.42) | 2.24–2.48 |
aPercentage of correct responses for emotion prediction trials.
bAverage rating of moral permissibility in moral judgement trials.
cgreater than 2 s.d. diff. from HC mean (using HC s.d.).
Figure 1.
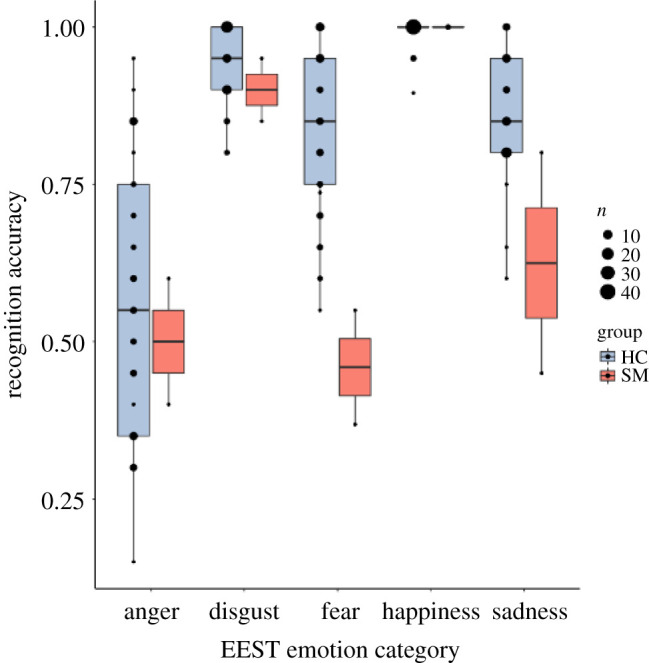
Comparison of SM and matched healthy comparisons (HCs) on recognition accuracy during emotional state inference. (Online version in colour.)
The statistical significance of these results was confirmed using a Bayesian approach for comparing a single case to a comparison sample [46,47] implemented using the psycho package in R [48]. Results confirmed the pattern described above, such that SM's average recognition accuracy for fear and sadness, but no other emotion, was significantly different from the comparison group's (p < 0.01). Further investigation of each of SM's testing sessions separately revealed consistent poor emotion recognition performance for fear-eliciting statements, with her performance in the first testing session (55.00% accuracy) lower than 98.30% (CI95 [95.98, 99.87]) of the comparison population (p < 0.05) and in the second testing session (36.84% accuracy) lower than 99.95% (CI95 [99.82, 100]) of the comparison population (p < 0.05). For sadness-eliciting statements, SM's performance in the first testing session (45.00% accuracy) was lower than 100% (CI95 [99.99, 100]) of the comparison population (p < 0.05), but in the second testing session her performance (80.00% accuracy) was lower than only 75.99% (CI95 [65.51, 85.73]) of the comparison population (p > 0.05).
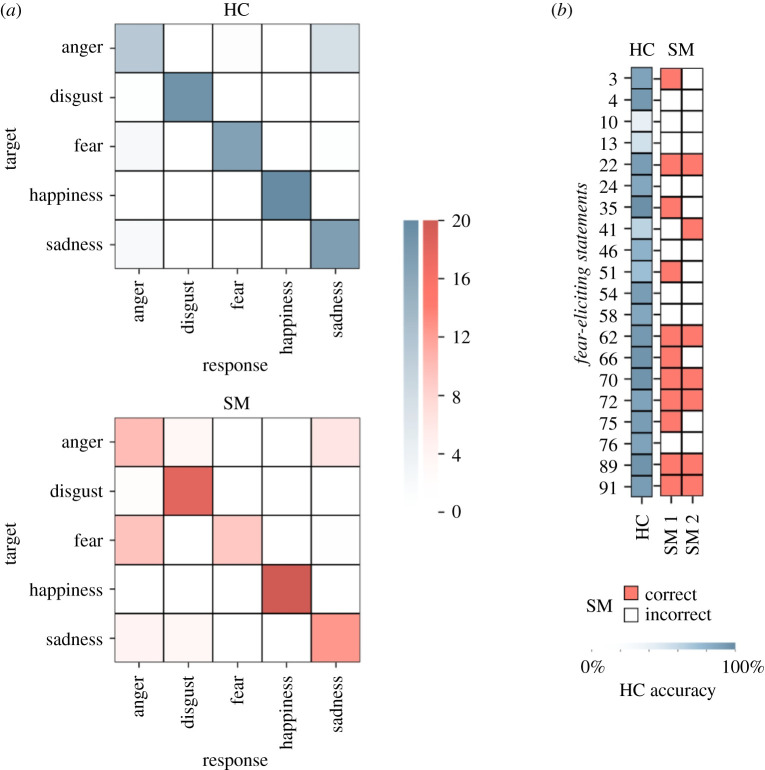
SM also showed a notable bias away from selecting fear as a response option. All comparisons selected each emotion a minimum of 16 and a maximum of 26 trials (were choices distributed equally, each option would be chosen 20 times). SM largely neglected the fear option, choosing it only 11 times on the first session and 8 times on the second session. Considering responses to only the 20 fear-eliciting statements, SM preferentially rated these statements as anger-eliciting (figure 2), an option she chose in 47.50% of these trials. These trials include fear-eliciting statements that did not involve direct interpersonal threat but rather helpful warnings of potential threat in one's environment (e.g. ‘I think something moved behind you’, ‘I think you are being followed’ and ‘I don't think you are safe here’) for which SM also predominantly picked anger (5 out of 6 responses across the two testing sessions) as the elicited emotional response. She showed no comparable bias away from other emotional options (electronic supplementary material, figure S1). Given the apparent bias towards anger responses, we conducted a chi-squared test to examine whether distributions of error responses for fearful expressions differed between SM and HCs. No significant difference emerged, , p = 0.27, confirming that while SM demonstrated a proportional increase in anger error responses (90.48% of error responses to fear-eliciting statements) it was not significantly different than that observed in HCs (70.92% of error responses).
Figure 2.
(a) Comparison of SM and matched healthy comparisons (HCs) on emotional state inference errors. Darker colour on the diagonal indicates accurate responses. SM frequently mislabels fear-eliciting target statements as anger-eliciting. (b) Item-wise accuracy for the fear-eliciting target statements averaged across HC subjects (blue) and for each SM session (red). SM's errors persist across time points. Row numbers indicate EEST item number. (Online version in colour.)
(b). Moral permissibility
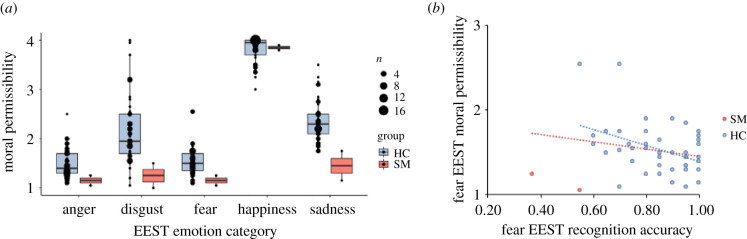
Consistent with prior findings [35,39], comparisons judged anger- (mean = 1.48, s.d. = 0.30) and fear-eliciting (mean = 1.49, s.d. = 0.29) statements, which generally consisted of threats and provocation, as least morally permissible. Controlling for multiple comparisons (Bonferroni corrected p = 0.005), judgements of these categories did not differ significantly, t44 = −0.19, p = 0.85, but both were more severe than judgements of any other emotional category (for the remaining emotions, disgust and sadness < happiness, all p < 0.001). By contrast to SM's difficulty predicting the affective outcomes of fear-eliciting statements, her judgements of the moral consequences of such statements were preserved. Across all categories, SM's moral permissibility ratings were within 2 s.d. of comparisons (with the exception of sadness; table 1), although she generally judged all negative emotion-eliciting statements as less permissible than comparisons did, and as impermissible to a similar degree. Pairwise comparisons across SM's item-level responses indicated that judgements of moral permissibility of anger-, fear- and disgust-eliciting statements did not differ from one another (all p > 0.10). Her judgements of sadness-eliciting statements did not differ from disgust-eliciting statements (t38 = −1.57, p = 0.13), but they were judged as more morally permissible than anger- (t38 = −2.62, p = 0.01) and fear-eliciting statements (t38 = −2.39, p = 0.02). Neither of these pairwise comparisons remain significant after correcting for multiple comparisons (p < 0.005). Finally, SM rated happiness-eliciting statements as more morally permissible than all other emotions (all p < 0.001). This pattern of findings emerged when examining judgements of moral permissibility regardless of whether the emotional labels were predetermined by the emotionally evocative statements task or were the labels she herself chose when responding to the emotion recognition portion of the task (electronic supplementary material, table S1 and figure S2).
As observed previously [35,39], comparisons who more accurately identified statements that cause fear also judged the permissibility of these statements more severely (rτ45 = −0.25, p = 0.03). Both Cook's D criteria and estimations of Mahalanobis distance indicated SM's responses to be multivariate outliers (table 2) and thus inclusion of SM's scores in these analyses reduced correlations to non-significance (rτ47 = −0.15, p = 0.18; figure 3).
Table 2.
Influence of SM on association between prediction and perceived permissibility of causing fear in others.
| SM |
||
|---|---|---|
| time 1 | time 2 | |
| leverage (h)a | 0.07 | 0.20* |
| studentized residual (R)b | −2.10 | −1.78 |
| Cook's Dc | 0.22* | 0.44* |
| Mahalanobis distance (M–D)d | 3.29 (p = 0.007) | 9.04 (p < 0.001)** |
aObservations with values larger than 2(k + 1)/n are considered to be potentially highly influential, where k is the number of predictors and n is the sample size.
bObservations with values larger than 3 in absolute value are considered outliers.
cRecommended thresholds for significance include D > 1, D > 4/n and D > 4/(n − k − 1). *These values exceed the second two recommendations.
dp < 0.001 indicates a significant multivariate outlier.
Figure 3.
(a) Comparison of SM and matched healthy comparisons (HCs) on ratings of moral permissibility. (b) Correlations between individual differences in emotion recognition performance and ratings of moral permissibility for fear statements. (Online version in colour.)
3. Discussion
We find that bilateral damage to the amygdala is associated with consistent impairments in predicting others' fear. When considering statements such as ‘I want to punch you’ or ‘I could kill you if I wanted to’, SM was less likely than neurologically healthy individuals to predict that someone who was the target of threats of physical harm would experience fear, suggesting for the first time conceptual (rather than simply perceptual) social fear recognition deficits. SM preferentially responded that such statements would result in anger, which is more commonly predicted to follow insults or provocation, and often accompanies behavioural approach rather than avoidance of threat. These responses are consistent with SM's own self-report; she has been held at gunpoint and physically assaulted in the past, and reports feeling calm, not afraid, during those experiences [6]. The observed deficits are consistent with prior evidence that the amygdala is recruited specifically when evaluating fear-eliciting statements [36,37], and closely mirrors social fear recognition deficits observed when individuals with amygdala lesions evaluate facial, vocal, postural and musical expressions of emotion [10,11,15,16,49,50]. Together, these findings support the amygdala's hypothesized role in coordinating internal representations of fear, which incorporate episodic, sensory and interoceptive information. Damage to the amygdala therefore impedes the ability to predict whether fear would result from written scenarios that provide no low-level fear-relevant perceptual cues, such that correctly identifying them requires conceptual understanding of fear.
At the same time, SM did not differ from comparisons when making moral judgements about eliciting fear. Moral judgements rely upon a distributed network of regions that becomes progressively refined during childhood and adolescence, such that we predicted amygdala lesions acquired in adulthood or adolescence, as in the case of SM, would spare these judgements. This stands in contrast to consistently aberrant moral judgements about causing fear we observe in adults with psychopathic traits, who are affected by structural and functional amygdala deficits that emerge early in development [35,39]. The present findings are consistent with theories that amygdala abnormalities more strongly affect moral judgements when they are congenital or emerge early in development (as in the case of psychopathy) compared to those acquired in adolescence or adulthood (as in the case of SM).
These findings contribute to answering the question: how do people make predictions about others' internal states? Consistent evidence from lesion and neuroimaging studies indicate that the amygdala plays a key role in recognizing others’ fear (but not other emotions) from facial expressions. Early hypotheses that fearful expressions signal a threat that is interpreted via the amygdala are contradicted by behavioural evidence that fearful expressions are primarily interpreted by observers as appeasing and appetitive (fearful expressions may also serve other functions that are relevant in non-social situations, such as widening the field of view) [14,27,28]. More recently, an inventive experimental task seemed to indicate that the amygdala aids in recognizing facial fear through its role in directing attention to the eyes of fearful faces. Prompts to focus on the eyes of fearful faces normalized SM's recognition of fearful expressions—albeit only temporarily [30]. But patients with amygdala lesions show deficits recognizing a broader array of social fear cues, including vocal tones, body postures and music, to which this explanation does not easily apply.
An alternative explanation for the observed pattern of deficits is that the amygdala plays a key role in coordinating the generation of a multimodal internal representation of fear that incorporates episodic, sensory and interoceptive information [18,45]. Recruitment of the amygdala following the detection of social cues linked to fear—including facial, postural, vocal or even olfactory cues—may reflect the generation of an internal representation of fear that enables relevant social cues to be interpreted and the likely goals and behaviours of the communicator predicted. According to this explanation, interpreting others' fear follows similar processes as interpreting other basic states like pain, which relies upon empathic simulation coordinated by regions, such as the insula and anterior cingulate cortex, that represent affective and motivational features of experienced pain [51–54]. This explanation is most consistent with the totality of the available data, including the emerging literature on social fear transmission in non-human species, which finds that detection of others’ fear results in empathic simulation of that state [55–57]. It also explains why, in a pair of monozygotic twins with amygdala lesions following Urbach–Wiethe disease (the same disease affecting patient SM), the twin who shows no physiological fear responses is also the one who cannot accurately interpret others' fear [17].
Both SM and comparisons misjudged fear-eliciting statements as anger a large majority of the time. Proportionally, but not significantly, more of SM's errors in response to fear were ‘anger’ responses. This outcome has several potential interpretations. One relates to the fact that SM's reactions to real-life fear-eliciting stimuli are dominated by approach-based responses. For example, SM responds to various real-life non-human threats—such as snakes, spiders and humans in monster costumes—with interest and curiosity [6]. Thus, SM may have selected anger because it is an approach-based (if negative) emotion.
A less likely possibility is that her selecting anger reflects SM simulating how she would feel in response to the statements; when her life was actually threatened in the past by a knife-wielding stranger who made a statement similar to those used in this study (‘I'm going to cut you, bitch!’) she reported she ‘remained calm, did not panic, and did not feel afraid’, and told the man, ‘If you're going to kill me, you're gonna have to go through my God's angels first’ (for the full description see electronic supplemental material of Feinstein et al. [6]). It also does not appear to reflect her potentially simulating the state of the speaker. We specifically considered SM's responses to the small subset of fear-eliciting statements that do not involve direct threats but rather warnings of external threats (e.g. ‘I don't think you are safe here’). Here, SM also predominantly selected anger for 5 out of 6 responses. This provides some support that SM's impaired fear recognition in the current task more likely results from difficulty simulating the fearful state that would result from such a statement. This is also consistent with data from experience sampling studies of SM's real-time emotional experiences in everyday life, which revealed that she endorsed feeling ‘fearless’ most across the three month period, but no tendency towards increased anger, suggesting a deficit of fear rather than excess of anger [6]. Although our task did not provide a ‘calm’ or ‘interested’ answer option in order to simulate the format of facial emotion recognition paradigms, future work allowing these response options or a free choice option would allow for direct examination of whether she would select these choices over ‘anger’.
At the same time, SM did not differ significantly from comparisons when making moral judgements about fear-eliciting statements, consistent with the emerging agreement that empathic processes—such as simulating others' affective states—are dissociable from moral judgements [58]. Consistent with prior findings [35,39], healthy adult women in this study who had more difficulty predicting others’ fear also judged causing fear to be more permissible. By contrast, SM consistently judged causing fear to be impermissible despite difficulty correctly inferring the emotional state of fear. This stands in notable contrast to individuals with psychopathy, who are affected by early emerging amygdala dysfunction and structural abnormalities and who are consistently impaired in predicting others' fear and in evaluating the moral permissibility of fear-eliciting statements, which they judge leniently. This may reflect the fact that in psychopathy, amygdala abnormalities are developmental in nature and emerge early in childhood [37,59,60] in contrast to SM whose lesions occurred during late childhood/adolescence. However, the bulk of this work relies on cross-sectional data and thus the directionality of the relationship between psychopathic or callous traits and developmental amygdala abnormalities is not clear.
This distinction may relate to the amygdala's role in the development of distributed cortical networks involved in moral judgements [60,61]. Input from the amygdala probably tunes various components of this network to evaluate the risks and rewards of outcomes for the self and others during development [41,42,62]. In psychopathic populations, insufficient input from the amygdala in early childhood may result in this network developing abnormally, leaving affected individuals with serious moral deficits [34,63–67]. Later in development, however, the transmission of amygdala-cortical signals shifts, with regions such as the prefrontal cortex and anterior cingulate cortex exerting more top-down inhibitory control of the amygdala. Thus, the amygdala's contributions to moral judgements in adolescence and adulthood may become less critical, leaving moral judgements in patients with acquired amygdala lesions less affected. This is consistent with evidence that SM is not psychopathic and her moral judgements, behaviour and capacity for care and compassion are intact [7,68]. However, it should be noted that SM's moral judgements were less nuanced than those of comparisons. SM made similar judgements about causing any form of negative affect, whereas comparisons tended to judge causing anger or fear to be less morally permissible than causing sadness or disgust. This pattern of findings persisted regardless of whether the emotional labels in question were predetermined or the labels she herself chose. Thus, it does not appear that SM views causing any one particular emotion (including anger or fear) as particularly unacceptable. Instead, this suggests that acquired amygdala damage may lead to more heuristic or less nuanced evaluations of various moral violations such that causing unpleasant states of any kind is immoral.
The current study is limited in several respects. Our findings do not provide any insight into lateralization of amygdala function, as our amygdala lesioned sample consists of a single individual (SM) with complete bilateral damage. We therefore cannot determine whether similar patterns would be observed in patients with damage to only the left or right amygdala—although previous neuroimaging studies assessing responses to fear-eliciting statements have implicated both right and left amygdala function in judgements of these stimuli [36,37]. It also cannot be determined why SM struggled to predict others' sadness the first time she completed the task, but not the second time. This outcome was not hypothesized, making it difficult to draw strong conclusions. Finally, the early developmental trajectory of Urbach–Wiethe and amygdala function is relatively unclear, thus whether SM exhibited a healthy early neurodevelopmental trajectory cannot be stated conclusively.
Despite limitations, the present study furthers our understanding of how people interpret and predict the emotions of others. Our results link complete bilateral amygdala damage to consistent impairments in predicting how others would respond to threats of harm, which are typically judged to elicit fear, whereas her predictions of responses to insults (anger), comments about contamination (disgust) and compliments (happiness) were intact. That these impairments were observed in response to purely semantic information suggest that the amygdala's role in social fear recognition cannot result purely from its influence on perceptual processes, nor from its role in responding to threats to the self. Rather, amygdala lesions may impair the ability to generate coordinated internal representations of fear, and therefore the ability to ‘put oneself in the shoes' of a person under threat and to understand the emotion that person is most likely to experience.
4. Methods
(a). Participants
(i). SM
SM is one of the best-characterized human cases of bilateral amygdala damage [7]. SM suffered complete bilateral amygdala lesions resulting from Urbach–Wiethe disease, a rare genetic disorder which caused calcification of SM's amygdalae late in childhood. At the time of testing, SM's lesions were relatively selective to the amygdala and include all nuclei of the bilateral amygdala, plus a small region of the adjacent entorhinal cortex, the anterior cingulate and striatum, but all other subcortical and cortical structures were spared. There is some evidence of changes in her cortical morphology including increased cortical thickness in the ventral medial prefrontal cortex and anterior cingulate cortex [69]. Her neuropsychological profile has remained stable for the past two decades. She has no history of other neurological damage or psychiatric diagnoses and her performance on standardized tests of IQ, memory, language and perception is within the normal range. All testing procedures with SM were approved by the University of Iowa Institutional Review Board.
(ii). Healthy comparison
A comparison sample of 45 caucasian female participants (mean age = 45, s.d. = 11) were recruited from the Washington, DC metropolitan area. Only caucasian women from the United States who reported English as their primarily language were included. Additional exclusion criteria included: current psychiatric diagnosis, drug use within 24 h of completing the study, the reported experience of emotionally distressing symptoms at time of completing the study and failure to complete all questionnaires included in the study. An a priori power analysis, conducted in STATA and based on an observed effect size of r = 0.41 in previously published work using the emotionally evocative statements task in an adult community sample [35], confirmed that a sample size of 45 would provide a statistical power of 0.80 at an alpha of 0.05. All testing procedures with HC participants were approved by the Georgetown University Review Board.
(b). Emotionally evocative statements task
The emotionally evocative statements task consists of 100 previously validated short statements that were created to elicit anger (‘I told you to shut up’), disgust (‘I never wash my hands’), fear (‘You better watch your back’), happiness (‘You always make me smile’) or sadness (‘You really let me down’) [35]. Statements were presented on paper (see electronic supplementary material, appendix A) in randomized order and participants were instructed to read each statement and first evaluate how morally acceptable it would ever be for someone to make that statement to another person. Participants indicated the degree of perceived moral permissibility for each statement using a 4-point scale (1 = never acceptable, 2 = rarely acceptable, 3 = usually acceptable and 4 = always acceptable). Next, participants read all the statements again, and this time evaluated what emotion the target would be most likely to experience if that statement were made to them using a forced-choice format in which the response options included anger, disgust, fear, happiness and sadness. SM completed the task twice approximately three months apart.
Supplementary Material
Supplementary Material
Ethics
All testing procedures with SM were approved by the University of Iowa Institutional Review Board. All testing procedures with HC participants were approved by the Georgetown University Review Board.
Data accessibility
All data supporting the current manuscript can be found as an electronic supplementary material.
Authors' contributions
E.M.C., P.E.T., D.T., T.W.B. and A.A.M. conceived and designed the study. E.M.C. and J.R. collected experimental data. E.M.C. and K.O. carried out the statistical analyses. E.M.C., J.R., K.O., P.E.T., D.T., T.W.B. and A.A.M. contributed materials and analysis tools. E.M.C., K.O. and A.A.M. drafted the manuscript. All authors provided critical feedback on the manuscript and gave final approval for publication. All authors agree to be held accountable for the work.
Competing interests
We declare we have no competing interests.
Funding
NIH T32 GM108540 (PI: D.T.). 1 P50 MH094258-04A1 (PI: D.T.). R01DC014960 (PI: P.E.T.)
References
- 1.Phelps EA, LeDoux JE. 2005. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187. ( 10.1016/j.neuron.2005.09.025) [DOI] [PubMed] [Google Scholar]
- 2.Amaral DG 2006. The amygdala, social behavior, and danger detection. Ann. N Y Acad. Sci. 1000, 337–347. ( 10.1196/annals.1280.015) [DOI] [PubMed] [Google Scholar]
- 3.Hariri AR 2011. The amygdala: inside and out. F1000 Biol. Rep. 3, 2 ( 10.3410/B3-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adolphs R 2001. The neurobiology of social cognition. Curr. Opin Neurobiol. 11, 231–239. ( 10.1016/S0959-4388(00)00202-6) [DOI] [PubMed] [Google Scholar]
- 5.Boll S, Gamer M, Gluth S, Finsterbusch J, Büchel C. 2013. Separate amygdala subregions signal surprise and predictiveness during associative fear learning in humans. Eur. J. Neurosci. 37, 758–767. ( 10.1111/ejn.12094) [DOI] [PubMed] [Google Scholar]
- 6.Feinstein JS, Adolphs R, Damasio A, Tranel D. 2011. The human amygdala and the induction and experience of fear. Curr. Biol. 21, 34–38. ( 10.1016/j.cub.2010.11.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinstein JS, Adolphs R, Tranel D. 2016. The amygdala, social behavior, and danger detection. In Living without an amygdala (eds DG Amaral, R Adolphs), pp. 1–38. New York, NY: Guilford. [Google Scholar]
- 8.Klumpers F, Morgan B, Terburg D, Stein DJ, van Honk J. 2015. Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Soc. Cogn. Affect. Neurosci. 10, 1161–1168. ( 10.1093/scan/nsu164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phelps EA, Labar KS, Anderson AK, O'connor KJ, Fulbright RK, Spencer DD. 1998. Specifying the contributions of the human amygdala to emotional memory: a case study. Neurocase 4, 527–540. ( 10.1080/13554799808410645) [DOI] [Google Scholar]
- 10.Gosselin N, Peretz I, Johnsen E, Adolphs R. 2007. Amygdala damage impairs emotion recognition from music. Neuropsychologia 45, 236–244. ( 10.1016/j.neuropsychologia.2006.07.012) [DOI] [PubMed] [Google Scholar]
- 11.Gosselin N, Peretz I, Hasboun D, Baulac M, Samson S. 2011. Impaired recognition of musical emotions and facial expressions following anteromedial temporal lobe excision. Cortex 47, 1116–1125. ( 10.1016/j.cortex.2011.05.012) [DOI] [PubMed] [Google Scholar]
- 12.Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. 1998. Neural structures associated with recognition of facial expressions of basic emotions. Proc. R. Soc. B 265, 1927–1931. ( 10.1098/rspb.1998.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolphs R, Tranel D, Damasio H, Damasio A. 1994. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672. ( 10.1038/372669a0) [DOI] [PubMed] [Google Scholar]
- 14.Anderson AK, Phelps EA. 2000. Expression without recognition: contributions of the human amygdala to emotional communication. Psychol. Sci. 11, 106–111. ( 10.1111/1467-9280.00224) [DOI] [PubMed] [Google Scholar]
- 15.Scott SK, Young AW, Calder AJ, Hellawell DJ, Aggleton JP, Johnson M. 1997. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature 385, 254–257. ( 10.1038/385254a0) [DOI] [PubMed] [Google Scholar]
- 16.Pishnamazi M, Tafakhori A, Loloee S, Modabbernia A, Aghamollaii V, Bahrami B, Winston JS. 2016. Attentional bias towards and away from fearful faces is modulated by developmental amygdala damage. Cortex 81, 24–34. ( 10.1016/j.cortex.2016.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker B, et al. 2012. Fear processing and social networking in the absence of a functional amygdala. Biol. Psychiatry 72, 70–77. ( 10.1016/j.biopsych.2011.11.024) [DOI] [PubMed] [Google Scholar]
- 18.Adolphs R 2013. The biology of fear. Curr. Biol. 23, R79–R93. ( 10.1016/j.cub.2012.11.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeDoux JE 2007. The amygdala. Curr. Biol. 17, R868–R874. ( 10.1016/j.cub.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 20.Marsh AA 2013. What can we learn about emotion by studying psychopathy? Front. Hum. Neurosci. 7, 181 ( 10.3389/fnhum.2013.00181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adolphs R 2016. How should neuroscience study emotions? by distinguishing emotion states, concepts, and experiences. Soc. Cogn. Affect. Neurosci. 12, 24–31. ( 10.1093/scan/nsw153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. 1995. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269, 1115–1118. ( 10.1126/science.7652558) [DOI] [PubMed] [Google Scholar]
- 23.Sprengelmeyer R, Young AW, Schroeder U, Grossenbacher PG, Federlein J, Buttner T, Przuntek H. et al. 1999. Knowing no fear. Proc. R. Soc. B 226, 2451–2456. ( 10.1098/rspb.1999.0945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox AS, Oler JA, Tromp DPM, Fudge JL, Kalin NH. 2015. Extending the amygdala in theories of threat processing. Trends Neurosci. 38, 319–329. ( 10.1016/j.tins.2015.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. 2012. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 35, 121–143. ( 10.1017/S0140525X11000446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelps EA 2006. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 57, 27–53. ( 10.1146/annurev.psych.56.091103.070234) [DOI] [PubMed] [Google Scholar]
- 27.Marsh AA, Ambady N, Kleck RE. 2005. The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion 5, 119–124. ( 10.1037/1528-3542.5.1.119) [DOI] [PubMed] [Google Scholar]
- 28.Hammer JL, Marsh AA. 2015. Why do fearful facial expressions elicit behavioral approach? Evidence from a combined approach-avoidance implicit association test. Emotion 15, 223–231. ( 10.1037/emo0000054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, Solak E. 2006. Attention to the eyes and fear-recognition deficits in child psychopathy. Br. J. Psychiatry 189, 280–281. ( 10.1192/bjp.bp.105.018150) [DOI] [PubMed] [Google Scholar]
- 30.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. 2005. A mechanism for impaired fear recognition after amygdala damage. Nature 433, 68–72. ( 10.1038/nature03086) [DOI] [PubMed] [Google Scholar]
- 31.Gamer M, Buchel C. 2009. Amygdala activation predicts gaze toward fearful eyes. J. Neurosci. 29, 9123–9126. ( 10.1523/JNEUROSCI.1883-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamer M, Schmitz AK, Tittgemeyer M, Schilbach L. 2013. The human amygdala drives reflexive orienting towards facial features. Curr. Biol. 23, R917–R918. ( 10.1016/j.cub.2013.09.008) [DOI] [PubMed] [Google Scholar]
- 33.Aubé W, Angulo-Perkins A, Peretz I, Concha L, Armony JL. 2015. Fear across the senses: brain responses to music, vocalizations and facial expressions. Soc. Cogn. Affect. Neurosci. 10, 399–407. ( 10.1093/scan/nsu067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsh AA 2016. Understanding amygdala responsiveness to fearful expressions through the lens of psychopathy and altruism: amygdala responses to fearful expressions. J. Neurosci. Res. 94, 513–525. ( 10.1002/jnr.23668) [DOI] [PubMed] [Google Scholar]
- 35.Marsh AA, Cardinale EM. 2012. Psychopathy and fear: specific impairments in judging behaviors that frighten others. Emotion 12, 892–898. ( 10.1037/a0026260) [DOI] [PubMed] [Google Scholar]
- 36.Marsh AA, Cardinale EM. 2014. When psychopathy impairs moral judgments: neural responses during judgments about causing fear. Soc. Cogn. Affect. Neurosci. 9, 3–11. ( 10.1093/scan/nss097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardinale EM, Breeden AL, Robertson EL, Lozier LM, Vanmeter JW, Marsh AA. 2018. Externalizing behavior severity in youths with callous–unemotional traits corresponds to patterns of amygdala activity and connectivity during judgments of causing fear. Dev. Psychopathol. 30, 191–201. ( 10.1017/S0954579417000566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fusar-Poli P, et al. 2009. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 34, 418–432. [PMC free article] [PubMed] [Google Scholar]
- 39.Cardinale EM, Marsh AA. 2015. Impact of psychopathy on moral judgments about causing fear and physical harm. PLoS ONE 10, e0125708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skuse D, Morris J, Lawrence K. 2003. The amygdala and development of the social brain. Ann. N Y Acad. Sci. 1008, 91–101. ( 10.1196/annals.1301.010) [DOI] [PubMed] [Google Scholar]
- 41.Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, Monk CS, Phan KL. 2016. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood: amygdala-frontal connectivity development. Hum. Brain Mapp. 37, 1684–1695. ( 10.1002/hbm.23129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. 2013. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 33, 4584–4593. ( 10.1523/JNEUROSCI.3446-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. 2007. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature 446, 908–911. ( 10.1038/nature05631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taber-Thomas BC, Asp EW, Koenigs M, Sutterer M, Anderson SW, Tranel D. 2014. Arrested development: early prefrontal lesions impair the maturation of moral judgement. Brain 137, 1254–1261. ( 10.1093/brain/awt377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adolphs R 1995. Fear and the human amygdala. Neurocase 3, 267–274. ( 10.1093/neucas/3.4.267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crawford JR, Garthwaite PH. 2007. Comparison of a single case to a control or normative sample in neuropsychology: development of a Bayesian approach. Cogn. Neuropsychol. 24, 343–372. ( 10.1080/02643290701290146) [DOI] [PubMed] [Google Scholar]
- 47.Crawford JR, Garthwaite PH. 2012. Single-case research in neuropsychology: a comparison of five forms of t-test for comparing a case to controls. Cortex 48, 1009–1016. ( 10.1016/j.cortex.2011.06.021) [DOI] [PubMed] [Google Scholar]
- 48.Makowski D 2018. The psycho package: an efficient and publishing-oriented workflow for psychological science. J. Open Source Softw. 3, 470 ( 10.21105/joss.00470) [DOI] [Google Scholar]
- 49.Broks P, et al. 1998. Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia 36, 59–70. ( 10.1016/S0028-3932(97)00105-X) [DOI] [PubMed] [Google Scholar]
- 50.Adolphs R, Russell JA, Tranel D. 1999. A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychol. Sci. 10, 167–171. ( 10.1111/1467-9280.00126) [DOI] [Google Scholar]
- 51.Lamm C, Decety J, Singer T. 2011. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502. ( 10.1016/j.neuroimage.2010.10.014) [DOI] [PubMed] [Google Scholar]
- 52.Corradi-Dell'Acqua C, Tusche A, Vuilleumier P, Singer T. 2016. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat. Commun. 7, 10904 ( 10.1038/ncomms10904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rütgen M, Seidel E-M, Pletti C, Riečanský I, Gartus A, Eisenegger C, Lamm C. 2017. Psychopharmacological modulation of event-related potentials suggests that first-hand pain and empathy for pain rely on similar opioidergic processes. Neuropsychologia 116, 5–14. ( 10.1016/j.neuropsychologia.2017.04.023) [DOI] [PubMed] [Google Scholar]
- 54.Mischkowski D, Crocker J, Way BM. 2016. From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain. Soc. Cogn. Affect. Neurosci. 11, 1345–1353. ( 10.1093/scan/nsw057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Waal FBM, Preston SD. 2017. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 18, 498–509. ( 10.1038/nrn.2017.72) [DOI] [PubMed] [Google Scholar]
- 56.Meyza KZ, Bartal IB-A, Monfils MH, Panksepp JB, Knapska E. 2017. The roots of empathy: through the lens of rodent models. Neurosci. Biobeh. Rev. 76, 216–234. ( 10.1016/j.neubiorev.2016.10.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Debiec J, Olsson A. 2017. Social fear learning: from animal models to human function. Trends Cogn. Sci. 21, 546–555. ( 10.1016/j.tics.2017.04.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Decety J, Cowell JM. 2014. Friends or foes: is empathy necessary for moral behavior? Perspect. Psychol. Sci. 9, 525–537. ( 10.1177/1745691614545130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White SF, VanTieghem M, Brislin SJ, Sypher I, Sinclair S, Pine DS, Hwang S, Blair RJ. 2016. Neural correlates of the propensity for retaliatory behavior in youths with disruptive behavior disorders. Am. J. Psychiatry 173, 282–290. ( 10.1176/appi.ajp.2015.15020250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blair RJR 2007. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn. Sci. 11, 387–392. ( 10.1016/j.tics.2007.07.003) [DOI] [PubMed] [Google Scholar]
- 61.Fumagalli M, Priori A. 2012. Functional and clinical neuroanatomy of morality. Brain 135, 2006–2021. ( 10.1093/brain/awr334) [DOI] [PubMed] [Google Scholar]
- 62.Tottenham N, Gabard-Durnam LJ. 2017. The developing amygdala: a student of the world and a teacher of the cortex. Curr. Opin. Psychol. 17, 55–60. ( 10.1016/j.copsyc.2017.06.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kochanska G, Gross JN, Lin M-H, Nichols KE. 2002. Guilt in young children: development, determinants, and relations with a broader system of standards. Child Dev. 73, 461–482. ( 10.1111/1467-8624.00418) [DOI] [PubMed] [Google Scholar]
- 64.Blair R 1995. A cognitive developmental approach to morality: investigating the psychopath. Cognition 57, 1–29. ( 10.1016/0010-0277(95)00676-P) [DOI] [PubMed] [Google Scholar]
- 65.Blair R, Jones L, Clark F, Smith M. 1997. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology 34, 192–198. ( 10.1111/j.1469-8986.1997.tb02131.x) [DOI] [PubMed] [Google Scholar]
- 66.Ciucci E, Baroncelli A, Franchi M, Golmaryami FN, Frick PJ. 2014. The association between callous-unemotional traits and behavioral and academic adjustment in children: further validation of the inventory of callous-unemotional traits. J. Psychopathol. Behav. Assess. 36, 189–200. ( 10.1007/s10862-013-9384-z) [DOI] [Google Scholar]
- 67.Blair R 2006. The emergence of psychopathy: implications for the neuropsychological approach to developmental disorders. Cognition 101, 414–442. ( 10.1016/j.cognition.2006.04.005) [DOI] [PubMed] [Google Scholar]
- 68.Lilienfeld SO, Sauvigné KC, Reber J, Watts AL, Hamann S, Smith SF, Patrick CJ, Bowes SM, Tranel D. 2018. Potential effects of severe bilateral amygdala damage on psychopathic personality features: a case report. Pers. Disord.: Theory Res. Treat. 9, 112–121. ( 10.1037/per0000230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boes AD, Mehta S, Rudrauf D, Van Der Plas E, Grabowski T, Adolphs R, Adolphs R, Nopoulos P. 2012. Changes in cortical morphology resulting from long-term amygdala damage. Soc. Cogn.Affect. Neurosci. 7, 588–595. ( 10.1093/scan/nsr047) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the current manuscript can be found as an electronic supplementary material.