Abstract
Background
Clinical laboratory testing has been an essential part of COVID-19 management. Serology can provide valuable information regarding a patient’s exposure to virus, and may have a larger role to play as vaccines becomes available. Limited data is available on the serological response in pediatric patients. Here we investigate the use of one manufacturer’s commercial assays for detecting IgM and IgG in an exclusively pediatric population.
Methods
Abbott SARS-CoV-2 IgM and IgG assays were performed on an Abbott ARCHITECT i1000. For specificity studies, we tested 78 patient specimens collected before the COVID-19 pandemic, and 66 specimens from patients who tested negative for SARS-CoV-2 nucleic acid amplification test (NAAT) during the COVID-19 pandemic. For sensitivity we tested 181 specimens from 41 patients with a positive NAAT result. Precision data was acquired for 20 days.
Results
For IgM, the highest qualitative positive agreement with molecular results was observed to be 15–30 days after a positive NAAT result or after symptom onset. For IgG, the highest positive agreement was 31–60 days after a positive NAAT result or 61–90 days after the start of symptoms. IgM started to decline 30 days after NAAT results and faded by 90 days. IgG started to decrease 60 days after a positive NAAT result.
Conclusion
The Abbott IgM and IgG assays have negative agreements of 98.7–100% relative to NAAT results. The IgM and IgG levels assayed by these methods start to decline months after positive molecular results and onset of symptoms in a pediatric population.
Abbreviations: NAAT, Nucleic Acid Amplification Tests; COVID-19, Coronavirus Disease-19; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; S, Spike protein; N, Nucleocapsid protein; RT-PCR, Reverse Transcriptase Polymerase Chain Reaction
Highlights
-
•
In this pediatric cohort, the Abbott SARS-CoV-2 IgM and IgG assays have negative agreements of 98.7-100% with NAAT.
-
•
The Abbott SARS-CoV-2 IgM had the highest positive agreement with NAAT results 15-30 days after results and symptom onset.
-
•
The Abbott SARS-Co-V-2 IgG had the highest positive agreement 31-60 days after results or 61-90 days after symptom onset.
-
•
IgM started to decline 30 days after NAAT results and faded by 90 days after.
-
•
IgG against the nucleocapsid protein started to decrease 60 days after results, but could still be detected months after.
1. Introduction
The recent pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) causing Coronavirus disease-19 (COVID-19) in 2020 has revealed many novel characteristics previously not seen with other coronavirus caused illnesses. Among patients, the symptoms and degree of illness can vary significantly. One particular feature is the discrepancy in how COVID-19 affects the pediatric population as compared to other groups. Children are traditionally more affected by coronavirus infections, however SARS-CoV-2 does not follow that pattern [1].
Clinical laboratory testing has played a crucial role in managing this illness, from molecular assays used to diagnose acute infections to serological assays that can inform past exposure. At present time, there is much more to learn about this virus, the disease it causes, and the individual responses to it.
Although the clinical utility of these serological assays, beyond indicating a past exposure to the virus, are not well established yet, there is limited knowledge about the serological response of the pediatric population to the infection. Several studies have looked at the performance of clinical chemistry/immunology automated analyzers based methods [[2], [3], [4], [5], [6], [7], [8]] on mostly adult patient specimens. Depending on the method, the assays detect either IgM only, IgG only or Total Ig (IgM + IgG).
Emerging studies have focused on the immunological response to SARS-CoV-2 in the pediatric population, with an emphasis on Multisystem Inflammatory Syndrome in Children (MIS-C). Rostad et al. [9] compared the serological responses of 10 patients with MIS-C to 10 symptomatic COVID-19 patients and 4 hospitalized controls. Yonker et al. also compared IgM and IgG concentrations of a larger cohort of children with acute COVID-19 to children with mild and severe cases of MIS-C [10]. Dingens et al. screened samples for anti-SARS-CoV-2 IgG from pediatric patients visiting the hospital for a period of 2 months and found a seroprevalence of about 1% [11]. Weisberg et al. compared IgA, IgG and IgM concentrations of adult vs. pediatric COVID-19 patients [12].
In this article, we evaluated the performance of two serological assays made by Abbott, to detect anti-SARS-CoV-2 IgM and IgG in children. Furthermore, we profiled the longitudinal antibody response to SARS-CoV-2 infection with these methods. To our knowledge, this is a unique study looking at the longitudinal serological response to SARS-CoV-2 infection in a pediatric population using the Abbott methods.
2. Materials and methods
2.1. Patient population
All samples tested were residual samples left over from clinical testing. Our presumptive negative samples were derived from 2 groups. One consisted of 78 samples from the pre-COVID-19 era. These specimens were collected from children in the Atlanta area prior to February of 2020. The second group consisted of 66 specimens collected in April and May of 2020 from patients who had a negative RT-PCR result for SARS-CoV-2.
A third group of samples was collected from patients who had a positive NAAT result for SARS-CoV-2. For this group, date of symptom onset was derived from information from the medical record.
This study was approved by the institutional review board (IRB) at Children’s Healthcare of Atlanta.
2.2. Clinical laboratory assays
Serology testing was performed by the Abbott ARCHITECT i1000 instrument using the Abbott SARS-CoV-2 IgG and the AdviseDx SARS-CoV-2 IgM assays (Abbott Park, IL). Both assays are chemiluminescent microparticle immunoassays that give a qualitative result of “Positive” or “Negative” based on a numerical index value and a cutoff based on the sample result divided by calibrator result (S/C). The IgG assay targets the nucleocapsid protein and has a cutoff value of 1.4. The IgG assay was authorized by the FDA under Emergency Use Authorization (EUA) in April 2020. The IgM assay targets the spike protein and has a cutoff value of 1.0 and was authorized by the FDA under an EUA in October 2020. Nucleic Acid Amplification Test (NAAT) detection of SARS-CoV-2 from nasopharyngeal swabs was performed using the Simplexa COVID-19 Direct assay on the Diasorin Liason MDX thermocycler system (Cypress, CA) targeting the ORF1ab and S genes, for the majority of samples. An alternate assay was used for 2 of the NAAT positives: the Hologic Aptima SARS-CoV-2 (Marlborough, MA) assay targeting the ORF1ab gene.
3. Results
3.1. Precision of IgM and IgG assays
To study the precision of the assay, we ran 2 levels of controls for each assay for 20 days. The results are presented in Table 1. The negative and positive controls gave the expected results each time and the precision statistics were similar to the manufacturer’s data [13,14].
Table 1.
Precision studies of Abbott SARS-CoV-2 IgM and IgG assays.
| IgM | ||
|---|---|---|
| N | 29 | |
| Days | 20 | |
| Cutoff (S/C) |
1 |
|
| Control L1 |
Control L2 |
|
| Mean (S/C) | 0.02 | 2.73 |
| SD (S/C) | 0.01 | 0.10 |
| CV | 20.8% | 3.8% |
| Range | 0.02 to 0.03 | 2.59 to 2.99 |
| IgG | ||
| N | 25 | |
| Days | 20 | |
| Cutoff (S/C) |
1.4 |
|
| Control L1 |
Control L2 |
|
| Mean (S/C) | 0.05 | 3.40 |
| SD (S/C) | 0.004 | 0.08 |
| CV | 7.2% | 2.2% |
| Range | 0.04 to 0.05 | 3.27 to 3.59 |
3.2. Specificity of IgM and IgG assays
To assess the specificity of the assays we first assayed 78 samples that were collected prior to the COVID-19 era (before February of 2020) from pediatric patients at our institution. Forty four (56%) were specimens from female patients and 34 (44%) from male patients. The median age of this cohort was 12.5 years old, ranging from 11 days to 19 years old. One specimen was from September of 2017; eleven of the specimens were from January and March of 2018; 50 of the specimens were from March, April, and June to December of 2019, and 2 specimens were from January of 2020. Results are presented in Table 2. Of the 78 specimens none tested positive for IgG, but one did test positive for IgM. The Index (S/C) for the sample was 1.12 (cutoff is ≥ 1), and the sample was collected 6/25/2019, from a patient visiting the ED with GI symptoms and non-reactive hepatitis B and HIV serology.
Table 2.
Specificity of Abbott SARS-CoV-2 IgM and IgG assays on Pre-Covid19 samples.
| Group Characteristics | Results |
|||
|---|---|---|---|---|
| IgM | IgG | |||
| N | 78 | Positive | 1 | 0 |
| Median Age | 12.5 Y | Negative | 77 | 78 |
| Age Range | 11 d to 19 Y | NPAa | 98.70% | 100% |
| Male | 34 (44%) | |||
| Female | 44 (56%) | Median Index (S/C) | 0.03 | 0.04 |
| Average Index (S/C) | 0.06 | 0.06 | ||
| Collection Period | 9/30/17 to 1/24/20 | Range of Index (S/C) | 0.01 to 0.21 | 0.01 to 0.29 |
IgM Index (S/C) ≥ 1.00 is Positive.
IgG Index (S/C) ≥ 1.4 is Positive.
NPA = Negative Percent Agreement.
Specificity of the serology assays was tested on a second cohort of samples from patients who had a negative RT-PCR result for SARS-CoV-2. The leftover samples used for serology from these patients corresponded from same day to 3 days after collection of the NAAT sample. We tested samples from 66 patient samples, consisting of 40 males and 26 females. This cohort had a median age of 5.5 years old ranging from 28 days old to 20 years old, and samples were collected in April and May of 2020. Results are presented in Table 3. Of the 66 specimens tested in this group, all results were negative for both IgM and IgG.
Table 3.
Specificity of Abbott SARS-CoV-2 IgM and IgG assays on NAAT Negative patients.
| Group Characteristics |
Results |
|||
|---|---|---|---|---|
| IgM | IgG | |||
| N | 66 | Positive | 0 | 0 |
| Median Age | 5.5 Y | Negative | 66 | 66 |
| Age Range | 28 D to 20 Y | NPAa | 100% | 100% |
| Male | 40 (61%) | |||
| Female | 26 (39%) | Median Index (S/C) | 0.05 | 0.02 |
| Average Index (S/C) | 0.03 | 0.05 | ||
| Collection Period | 4/14/20 to 5/12/20 | Range of Index (S/C) | 0.01 to 0.27 | 0 to 0.73 |
IgM Index (S/C) ≥ 1.00 is Positive.
IgG Index (S/C) ≥ 1.4 is Positive.
The Index (S/C) is the result unit fo rthe assays and is the sample result divided by the calibrator result.
The Index (S/C) is the result unit fo rthe assays and is the sample result divided by the calibrator result.
NPA = Negative Percent Agreement.
3.3. Longitudinal IgM and IgG results in SARS-CoV-2 positive patients
To determine assay performance, we analyzed SARS-CoV-2 IgM and IgG reactivity on samples from pediatric patients who tested positive for SARS-CoV-2 RNA by a NAAT. This cohort was composed of samples from 41 patients with the characteristics presented in Table 4. The median age of this group was 14 years, ranging from 12 days to 19 years. A total of 181 samples were analyzed for both IgM and IgG. Four of the 41 patients were asymptomatic for COVID-19. Most were samples from inpatients (80%), including the COVID-19 asymptomatic patients. The remaining 8 were outpatients, with 5 of those coming from emergency department visits.
Table 4.
Characteristics of SARS-CoV-2 RT-PCR positive patients.
| Group Characteristics | |
|---|---|
| n samples | 181 |
| n patients | 41 |
| Asymptomatic | 4 |
| Median Age | 14 Y |
| Age Range | 12 D to 19 Y |
| Male | 24 (59%) |
| Female | 17 (41%) |
| Inpatients | 33 (80%) |
| Outpatientsa | 8 (20%) |
| Collection Period | 4/6/20 to 10/05/20 |
5 outpatient visits were Emergency Department only.
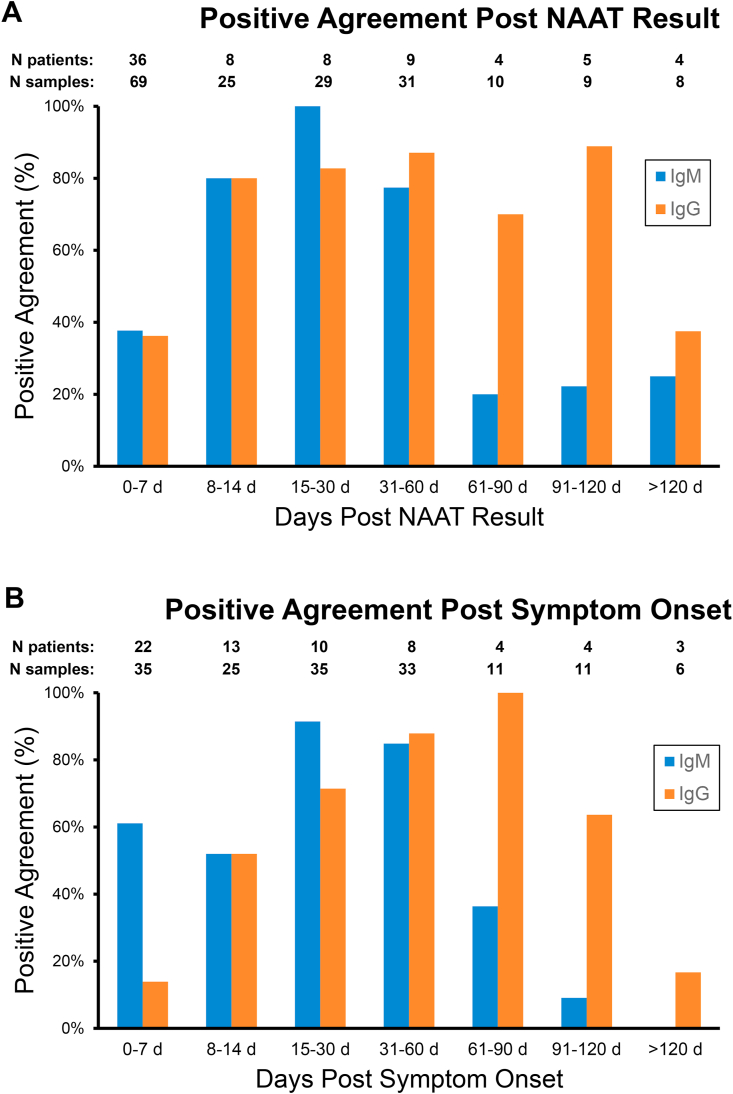
We first looked at the positive percent agreement (PPA) between the serologic assays and the molecular NAAT results (Fig. 1), based on the serologic qualitative readout of positive or negative. The data was examined as days following a positive NAAT result (Fig. 1A) or days after COVID-19 symptom onset (Fig. 1B) (data from 4 COVID-19 asymptomatic patients was excluded). The average difference between symptom onset and NAAT result was 6 days. The time periods were divided into the following groups: 0–7 d, 8–14 d, 15–30 d, 31–60 d, 61–90 d, 91–120 d or greater than 120 days. When analyzing the data as days after a positive NAAT result (Fig. 1A), PPA was 38% and 36% for IgM and IgG, respectively, at 0–7 days, and 80% for both IgM and IgG at 8–14 days. For the time period of 15–30 days after a positive NAAT result, PPA peaked at 100% for IgM and 83% for IgG. PPA values for IgM then decreased to 77% for the time between 31 and 60 days and decreased significantly after 60 days to values of 25% or less. Agreement numbers for IgG stayed between 80% and 89% for the time periods between 8 and 120 days after a positive NAAT result (80%, 83%, 87%, 70% and 89% respectively) and declined to 38% after 120 days after a positive NAAT result. Alternatively the serologic data based on days after symptom onset (Fig. 1B) showed that IgM’s PPA peaked in the time period of 15–30 days at 91%, decreased to 85% at 31–60 days and rapidly declined to 36%, 9% and 0% in the three time periods after 60 days. For IgG, the PPA increased steadily from 0 to 7 days after symptom onset (14%) to 8–14 days (52%) to 15–30 days (71%) to 31–60 days (88%), and peaked at 100% at 61–90 days. The PPA for IgG started to decline after day 90, first to 64% at 91–120 days and then to 17% after 120 days.
Fig. 1.
Qualitative Positive Agreement of Abbott SARS-CoV2 IgM and IgG assays to Molecular Result. Residual blood samples from 41 patients with positive molecular results for SARS-CoV-2 were tested for IgM or IgG. Data was analyzed based on time period that sample was collected post PCR result (A) or after symptom onset (B). N patients indicates the number of patients for each time period and N samples indicate the total samples tested for each time period.
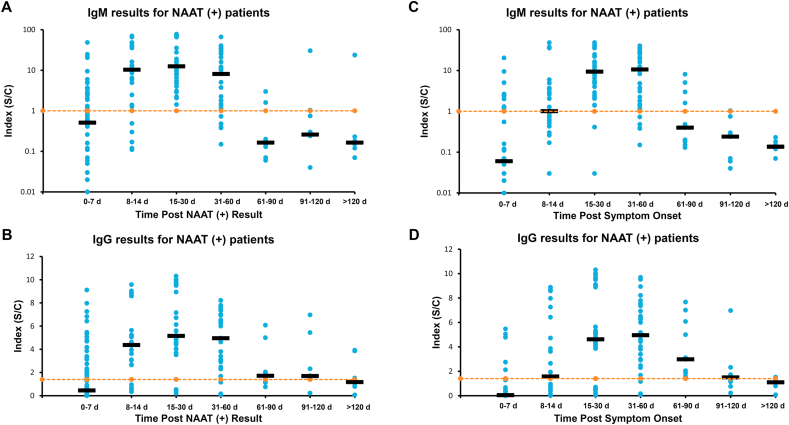
Next we analyzed the data based on the quantitative index output (Fig. 2). Although the output of both tests is a qualitative result, there is an associated numerical index that increases with higher antibody concentration. We analyzed these numerical indices for the same time groups described above after a positive molecular result (Fig. 2A–B) and after onset of symptoms (Fig. 2C–D). When the data is categorized by time after positive NAAT result (Fig. 2A), the median index of IgM results progressed from 0.5 for 0–7 days to 10.3 for 8–14 days. The median IgM peaked at 12.5 for 15–30 days, and started to decrease to 8.1 during the second month (31–60 days), then to 0.2 to 0.3 after 60 days. When the data was divided by time after symptom onset (Fig. 2C), the IgM median index was 0.1 at 0–7 days, 1.0 at 8–14 days, and peaked at 9.4 and 10.7 at days 15–30 and 31–60 respectively, and then started to decline to 0.4 and less 60 days after symptom onset. IgG data post positive molecular result (Fig. 2B), showed the median index started at 0.5 at 0–7 days, increased to 4.4 at 8–14 days, reached its peak at 5.2 at 15–30 days, was 5.0 at 31–60 days, and decreased to 1.7 and less after 60 days. For IgG post symptom onset data (Fig. 2D), the index average went from 0.04 at days 0–7, to 1.6 at days 8–14, increased to 4.6 at days 15–30, peaked at 5.0 at 31–60 days, and began to decline to 3.0 and below 60 days after start of symptoms.
Fig. 2.
Longitudinal Results of Abbott SARS-Co-V2 IgM and IgG in Molecular Positive Pediatric Patients. Data is shown for IgM results, after NAAT positive result (A) and after symptom onset (C). Data for IgG after NAAT positive result (B) and after symptom onset (D). Five asymptomatic patients were excluded from C and D IgM vertical axis is in log scale. Orange dotted line represents cutoffs, IgM (1.0) and IgG (1.4). Dark horizontal bars represent the median value for that time period. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
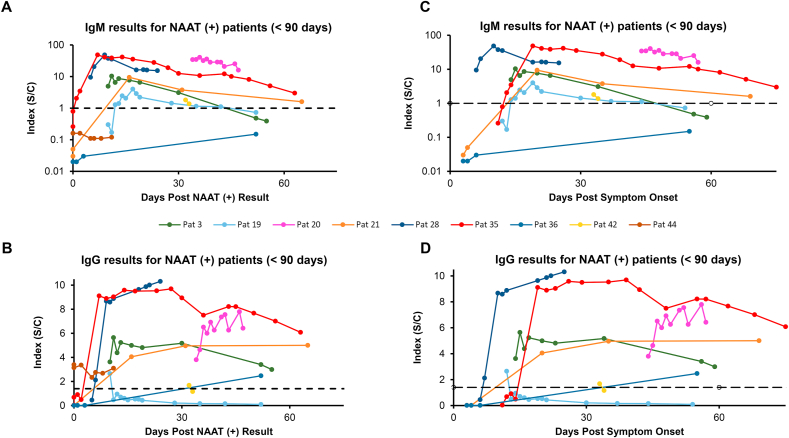
Data was teased out for individual patients. Two groups of patients were examined: patients with samples that spanned less than 3 months (Fig. 3), and patients with samples that spanned more than 3 months (Fig. 4). The <90 days group was composed of 9 patients post NAAT results, and 8 patients post symptoms; the >90 days group had 6 patients post NAAT results and 5 patients post symptoms. For the <90 days post positive NAAT result group (Figure 3A), 2 patients (36 and 44) never had a positive IgM. In addition, 2 other patients [[3], [19]] initially had positive IgM results but were IgM negative by 60 days. Other patients in this group showed a downward trend for IgM by 60 days after positive NAAT result. Looking at days post symptom onset (Fig. 3C), all patients showed a downward trend for IgM or were negative by day 60, again except for patient 36, who was IgM negative. For IgG post positive NAAT result (Fig. 3B), patient 19 went from positive to negative IgG before 30 days, as did patient 42 at just over 30 days. Patients 3 and 35 showed a downward trend for IgG by day 60. Similar patterns were observed for the patients post symptom onset (Fig. 3C).
Fig. 3.
Longitudinal IgM and IgG Results from Distinct Patients less than 90 days. Individual patient’s IgM and IgG concentrations based on the test index are plotted. Patients with samples <90 days from PCR result are plotted for IgM (A) or IgG (B). Patients with samples <90 days from start of symptoms are shown for IgM (C) or IgG (D). Black dotted lines represent the cutoffs for each assay. IgM vertical axis is log scale.
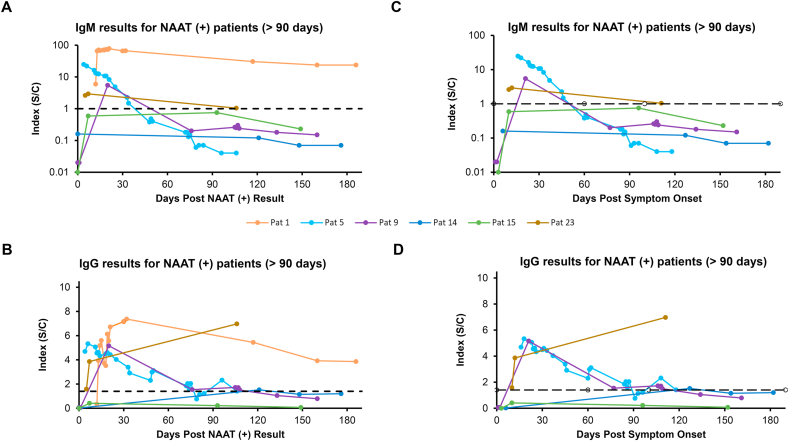
Fig. 4.
Longitudinal IgM and IgG Results from Distinct Patients greater than 90 days. Individual patient’s IgM and IgG concentrations based on the test index are plotted. Patients with samples >90 days from PCR result are plotted for IgM (A) or IgG (B). Patients with samples <90 days from start of symptoms are shown for IgM (C) or IgG (D). Black dotted lines represent the cutoffs for each assay. IgM vertical axis is log scale.
For the greater than 3 months post positive NAAT result group (Figure 4A), 2 patients [[14], [15]] were negative for IgM, although both lacked specimens in the period when IgM may have been present, so it is possible they mounted an IgM response that had waned by 90 days after molecular results. Patients 5 and 9 went from IgM positive to negative before 60 and 90 days, respectively. Patients 1 and 23 still demonstrated positive IgM by 90 days but were trending downward, although patient 1 still had detectable IgM after 180 days. Similar patterns were observed post symptom onset (Fig. 4C), except for patient 1, who was asymptomatic. For IgG post positive NAAT result (Fig. 4B), patients 1, 5 and 9 showed decreasing indexes with time. Patients 5 and 9 indexes approached near or under the cutoff by 90 days. Patient 1 still showed an index of 3.8 after day 180, but was trending downward. Patient 23 showed a rise in the last timepoint, with an index of 7, 100 days after positive NAAT result. Patient 14 went from positive to negative, although there is gap of about 120 days between the first and second samples, with the second sample still being positive, suggesting that the IgG level was fading after 4 months. Patient 15 did not show a positive IgG, although a gap of about 90 days is missing after his positive result, so it is unknown if he mounted an IgG response that was negative by 90 days. Post symptom trends of IgG indices (Fig. 4D) were similar to those post positive NAAT result.
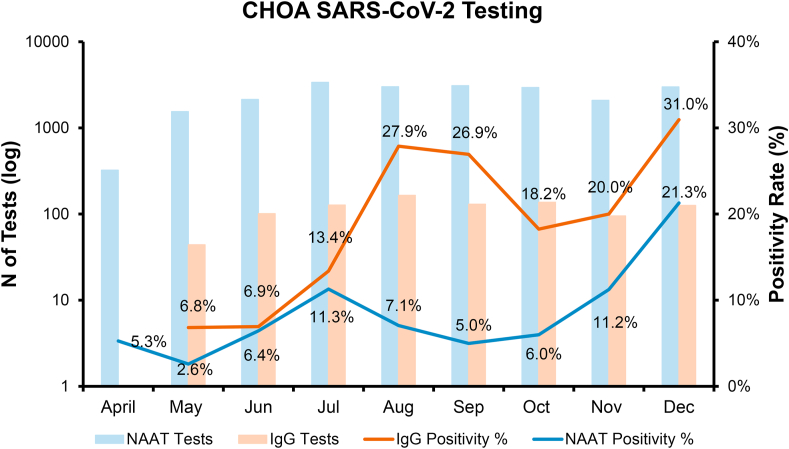
Finally, we looked at the positivity rate of SARS-CoV-2 IgG compared to positivity of the molecular test (Fig. 5). The IgG test went live for clinical use at our institution at the end of May 2020, and we tracked the percentage of positive results monthly. Molecular tests to detect SARS-CoV-2 were offered clinically starting in April of 2020. The positivity rate of IgG testing reached a first peak of about 27% in the months of August and September and a second peak of 31% in December. The positivity rate for molecular tests reached a first peak of about 11% in July and a second peak of 21.3% in December.
Fig. 5.
Positivity rated of SARS-CoV-2 Clinical IgG an NAAT Tests in Pediatric Population. Total number of tests are plotted in the primary axis (log scale) for IgG (light orange columns) and NAAT (light blue columns). Positivity rate of IgG test (dark orange line) and NAATs (dark blue line) are plotted monthly for April to December of 2020 on the secondary vertical axis. IgG test went live clinically in May 2020. NAAT=Nucleic Acid Amplification Test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion and conclusion
We present here our evaluation of Abbott’s anti-SARS-CoV-2 IgM and IgG tests with exclusively pediatric samples. Our precision study results yielded results similar to those obtained from the manufacturer based on their package insert information [13,14]. Our specificity studies showed that on samples from patients with a negative molecular result for SARS-CoV-2 our negative agreement was 100% for both IgM and IgG. When testing samples collected previous to COVID-19 we found that one specimen collected on 6/25/2019 tested positive for IgM. The Index (S/C) of this specimen was 1.12, just above the cutoff of 1 for the IgM assay. Due to the timing of the specimen, this is likely a false positive result. Our NPA was 98.7% for the IgM assay based on the pre-COVID-19 cohort and NPA of 99.3% for the overall negative cohort, slightly lower than the 99.56% reported by the manufacturer in their package insert [13]. Pre-COVID-19 specimens showed 100% negative agreement with IgG.
Next, we looked at samples from patients who tested positive to SARS-CoV-2 by NAAT. When looking at qualitative positive agreement, for IgM the highest PPA was 15–30 days after positive molecular result or symptom onset. Of note, it is important to consider that there may be some false positives in this group, since we saw one false positive in the negative cohort. With IgG, the highest PPA was 31–60 days after positive molecular test or between 30 and 90 days after symptom onset. Since the IgG and IgM antibodies against SARS-CoV-2 detected in the Abbott and AdviseDX assays recognize different targets (nucleocapsid and Spike proteins, respectively), it is possible that the difference in positivity maybe due to differences in immunogenicity of targets with the Spike protein being more immunogenic. Median IgM indexes were above the cutoff between 8 and 61 days after molecular results or 15 and 60 days after symptom onset. Median IgG were above cutoff between 8 and 120 days after NAAT results or symptoms.
When tracking the results for individual patients we observed that most patients started trending down for IgM and IgG after 30 days (post NAAT or post symptom). For IgM, most patients had either a negative or close to cutoff result by 90 days. Most patients had detectable IgG after 60 days and was declining after 90 days, although some still had positive IgG about 100 and 180 days after NAAT results. In addition, we calculated that median seroconversion for IgM was 2 days post molecular result and 9 days post symptom start, and for IgG it was 3.5 days after molecular result and 9 days post symptom. Similar results from previous reports that found median seroconversion for IgM and IgG was 5 and 4 days after PCR result [15] or 11.9 days and 10.7 days post symptom onset [16] respectively.
Our study does have various limitations, including a limited number of samples and patients. Samples were limited to residual specimens, excluding the possibility of a randomized study. For the specificity cohort, we could not evaluate cross reactivity of the assays to other coronaviruses, as we didn’t know if any of the specimens had antibodies to non-novel coronaviruses in their systems, especially for the pre-COVID-19 group. We only obtained test results from one manufacturer that targets the nucleocapsid (N) for IgG and spike (S) for IgM, so the concentrations of antibodies to other SARS-CoV-2 antigens is unknown (i.e. IgG against spike protein). Finally, as the majority of positive patients in this study were inpatient (80%), and due to limited clinical information, it is possible that some patients had medical co-morbidities that could affect their immunological response.
Comparison of the positivity rate of the NAAT and IgG assays at our institution showed that a first peak in IgG positivity in August followed the first peak in NAAT results in July, and then IgG had a second peak concurrent with NAAT positives in December. Of importance is that the NAAT positive population is not the same as the IgG positive population, as the NAAT group is much larger and would include pre surgical and other outpatients, whereas the IgG positive group is inpatient and much smaller. However, it is noteworthy that there is a temporal relationship between the positivity rate of both tests.
When looking at duration of IgM detection in our study group, it starts to decrease by 30 days and is almost gone by 90 days after. IgG against nucleocapsid protein starts to decline between 30 and 60 days and lasts to about 90 days after symptoms, although in some patients it can last longer. Our results are similar to other studies in adults, where antibodies start to decline by 90 days, but could still be detected by 4 months after symptoms. Ibarrondo et al. observed that IgG against the spike receptor-binding domain started to decline by 90 days [16]. Long et al. saw a decrease 2–3 months after infection with IgM and IgG against nucleoprotein and spike [17]. Iyer et al. showed decreases in IgA, IgM and IgG against the spike receptor-binding domain, but still detectable IgG up to 4 months after [18]. Isho et al. observed detectable anti-spike and anti-spike receptor-binding domain IgG up to 105 days after symptom onset, although in a declining trend as well [19]. From our limited set of data it appears that the pediatric immunological response follows a similar trend to that seen in adult studies.
Our results could be helpful in assessing the serological response of children to the COVID-19 infection and to define immunity trends to help design preventive measures for outbreak policies for school children [20]. One interesting unknown is what the relationship is between antibody levels detected with the assays used in this study to actual neutralization of the virus. Recent reports have shown that results of antibody concentrations by commercial platforms may have varied viral neutralization capacities [21,22]. Other future directions would include assessing the role of these serological assays in assessing the efficacy of SARS-CoV-2 vaccines. However, the IgG assay in this study would not be effective for recipients of vaccines targeting the Spike protein, because it detects antibodies against the nucleocapsid protein.
Credit author statement
Cristina Interiano: Conceptualization, Investigation, Methodology, Data curation, Writing- Reviewing and Editing.
Sheicho Muze: Investigation, Methodology.
Brian Turner: Methodology.
Mark Gonzalez: Conceptualization, Writing-Reviewing and Editing.
Beverly Rogers: Supervision, Writing-Reviewing and Editing.
Robert Jerris: Conceptualization, Writing-Reviewing and Editing.
Elizabeth Weinzierl: Writing-Reviewing and Editing.
Mohamed Elkhalifa: Writing-Reviewing and Editing.
Van Leung-Pineda: Conceptualization, Investigation, Supervision, Data curation, Visualization, Writing- Original draft preparation, Reviewing and Editing.
Declaration of competing interest
Cristina Interiano: None to declare.
Sheicho Muze: None to declare.
Brian Turner: None to declare.
Mark Gonzalez: None to declare.
Beverly Rogers: None to declare.
Robert Jerris: None to declare.
Elizabeth Weinzierl: None to declare.
Mohamed Elkhalifa: None to declare.
Van Leung-Pineda: None to declare.
Acknowledgements
This study was supported by a grant from Abbott Laboratories. The authors wish to thank Kevin Pannell and Wilfred Morales for collecting residual specimens and Randal Schneider for coordinating reagent procurement.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2021.e00208.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan A., Pepper G., Wener M.H., Fink S.L., Morishima C., Chaudhary A., Jerome K.R., Mathias P.C., Greninger A.L. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in boise, Idaho. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. e00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang M.S., Hock K.G., Logsdon N.M., Hayes J.E., Gronowski A.M., Anderson N.W., Farnsworth C.W. Clinical performance of two SARS-CoV-2 serologic assays. Clin. Chem. 2020;66:1055–1062. doi: 10.1093/clinchem/hvaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang M.S., Hock K.G., Logsdon N.M., Hayes J.E., Gronowski A.M., Anderson N.W., Farnsworth C.W. Clinical performance of the roche SARS-CoV-2 serologic assay. Clin. Chem. 2020;66:1107–1109. doi: 10.1093/clinchem/hvaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theel E.S., Harring J., Hilgart H., Granger D. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01243-20. e01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naaber P., Hunt K., Pesukova J., Haljasmägi L., Rumm P., Peterson P., Hololejenko J., Eero I., Jõgi P., Toompere K., Sepp E. Evaluation of SARS-CoV-2 IgG antibody response in PCR positive patients: comparison of nine tests in relation to clinical data. PLoS. 2020;15(2020) doi: 10.1371/journal.pone.0237548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harb R., Remaley A.T., Sacks D.B. Evaluation of three commercial automated assays for the detection of anti-SARS-CoV-2 antibodies. Clin. Chem. 2020;66:1351–1353. doi: 10.1093/clinchem/hvaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suhandynata R.T., Hoffman M.A., Kelner M.J., McLawhon R.W., Reed S.L., Fitzgerald R.L. Multi-platform comparison of SARS-CoV-2 serology assays for the detection of COVID-19. J. Appl. Lab. Med. 2020;5:1324–1336. doi: 10.1093/jalm/jfaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostad C.A., Chahroudi A., Mantus G., Lapp S.A., Teherani M., Macoy L., Tarquinio K.M., Basu R.K., Kao C., Linam W.M., Zimmerman M.G., Shi P.Y., Menachery V.D., Oster M.E., Edupuganti S., Anderson E.J., Suthar M., Wrammert J., Jaggi P. Quantitative SARS-CoV-2 serology in children with Multisystem inflammatory Syndrome (MIS-C) Pediatrics. 2020;146 doi: 10.1542/peds.2020-018242. [DOI] [PubMed] [Google Scholar]
- 10.Yonker L.M., Neilan A.M., Bartsch Y., Patel A.B., Regan J., Arya P., Gootkind E., Park G., Hardcastle M., St John A., Appleman L., Chiu M.L., Fialkowski A., De la Flor D., Lima R., Bordt E.A., Yockey L.J., D’Avino P., Fischinger S., Shui J.E., Lerou P.H., Bonventre J.V., Yu X.G., Ryan E.T., Bassett I.V., Irimia D., Edlow A.G., Alter G., Li J.Z., Fasano A. Pediatric severe acute respiratory Syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J. Pediatr. 2020;227:45–52. doi: 10.1016/j.jpeds.2020.08.037. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingens A.S., Crawford K.H.D., Adler A., Steele S.L., Lacombe K., Eguia R., Amanat F., Walls A.C., Wolf C.R., Murphy M., Pettie D., Carter L., Qin X., King N.P., Veesler D., Krammer F., Dickerson J.A., Chu H.Y., Englund J.A., Bloom J.D. Serological identification of SARS-CoV-2 infections among children visiting a hospital during the initial Seattle outbreak. Nat. Commun. 2020;11:4378. doi: 10.1038/s41467-020-18178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisberg S.P., Connors T.J., Zhu Y., Baldwin M.R., Lin W.H., Wontakal S., Szabo P.A., Wells S.B., Dogra P., Gray J., Idzikowski E., Stelitano D., Bovier F.T., Davis-Porada J., Matsumoto R., Poon M.M.L., Chait M., Mathieu C., Horvat B., Decimo D., Hudson K.E., Zotti F.D., Bitan Z.C., La Carpia F., Ferrara S.A., Mace E., Milner J., Moscona A., Hod E., Porotto M., Farber D.L. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AdviseDx SARS-CoV-2 IgM for Use with Abbott Architect Package Insert. Created October. 2020. Abbott Laboratories. [Google Scholar]
- 14.SARS-CovV-2 IgG for Use with Abbott Architect Package Insert. Created April. 2020. (Abbott Laboratories) [Google Scholar]
- 15.Suhandynata R.T., Hoffman M.A., Kelner M.J., McLawhon R.W., Reed S.L., Fitzgerald R.L. Longitudinal monitoring of SARS-CoV-2 IgM and IgG seropositivity to detect COVID-19. J. Appl. Lab. Med. 2020;5:908–920. doi: 10.1093/jalm/jfaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., Ferbas K.G., Tobin N.H., Aldrovandi G.M., Yang O.O. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., Su K., Zhang F., Gong J., Wu B., Liu X.M., Li J.J., Qiu J.F., Chen J., Huang A.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 18.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., Astudillo M., Yang D., Miller T.E., Oliver E., Fischinger S., Atyeo C., Iafrate A.J., Calderwood S.B., Lauer S.A., Yu J., Li Z., Feldman J., Hauser B.M., Caradonna T.M., Branda J.A., Turbett S.E., LaRocque R.C., Mellon G., Barouch D.H., Schmidt A.G., Azman A.S., Alter G., Ryan E.T., Harris J.B., Charles R.C. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H., Li Z., Chao G., Rojas O.L., Bang Y.M., Pu A., Christie-Holmes N., Gervais C., Ceccarelli D., Samavarchi-Tehrani P., Guvenc F., Budylowski P., Li A., Paterson A., Yue F.Y., Marin L.M., Caldwell L., Wrana J.L., Colwill K., Sicheri F., Mubareka S., Gray-Owen S.D., Drews S.J., Siqueira W.L., Barrios-Rodiles M., Ostrowski M., Rini J.M., Durocher Y., McGeer A.J., Gommerman J.L., Gingras A.C. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abe5511. eabe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulyte A., Radtke T., Abela I.A., Haile S.R., Braun J., Jung R., Berger C., Trkola A., Fehr J., Puhan M.A., Kriemler S. Seroprevalence and immunity of SARS-CoV-2 infection in children and adolescents in schools in Switzerland: design for a longitudinal, school-based prospective cohort study. Int. J. Publ. Health. 2020;65:1549–1557. doi: 10.1007/s00038-020-01495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang M.S., Case J.B., Franks C.E., Chen R.E., Anderson N.W., Henderson J.P., Diamond M.S., Gronowski A.M., Farnsworth C.W. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa211. Online ahead of print, 10.1093/clinchem. /hvaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suhandynata R.T., Hoffman M.A., Huang D., Tran J.T., Kelner M.J., Reed S.L., McLawhon R.W., Voss J.E., Nemazee D., Fitzgerald R.L. Commercial serology assays predict neutralization activity against SARS-CoV-2. Clin. Chem. 2021 Jan 30;67(2):404–414. doi: 10.1093/clinchem/hvaa262. Online ahead of print, 10.1093/clinchem/ PMID: 33084854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.