Abstract
The global influenza pandemic that emerged in 1918 has become the event of reference for a broad spectrum of policymakers seeking to learn from the past. This article sheds light on multiple waves of excess mortality that occurred in the US state of Michigan at the time with insights into how epidemics might evolve and propagate across space and time. We analyzed original monthly data on all-cause deaths by county for the 83 counties of Michigan and interpreted the results in the context of what is known about the pandemic. Counties in Michigan experienced up to four waves of excess mortality over a span of two years, including a severe one in early 1920. Some counties experienced two waves in late 1918 while others had only one. The 1920 wave propagated across the state in a different manner than the fall and winter 1918 waves. The twin waves in late 1918 were likely related to the timing of the statewide imposition of a three-week social distancing order. Michigan’s experience holds sobering lessons for those who wish to understand how immunologically naïve populations encounter novel viral pathogens.
The 1918 influenza pandemic, one of the most devastating pandemics to affect humankind,1,2 affected nearly every inhabited part of the globe, killing an estimated 50 million people.3–5 The lack of attention paid to this pandemic over the subsequent century is surprising and earned it the title “the Forgotten Pandemic.”6 Yet the global and relatively recent nature of the pandemic make it a rich source for enhancing our understanding of pandemics, and knowledge about the 1918 pandemic has formed the basis of modern pandemic preparedness planning.7 The emergence of the COVID-19 pandemic has underscored the value of such knowledge.
A question of central importance to the COVID-19 pandemic is whether it will manifest as a single wave or multiple waves, and how severe and long these waves will be. As the unfolding experience is demonstrating, much will depend on human behavior and how effectively measures such as social distancing are implemented. The 1918 pandemic can provide insights into how respiratory viral pandemics evolve and propagate. The objective of this article is to analyze the dynamics of that pandemic in Michigan, a geographically diverse state in the Midwestern region of the United States.
In this article, we used monthly county-level data on deaths in Michigan to estimate excess deaths during the period 1918 to 1920. We identified the number and timing of waves of mortality and their geographic spread and examined the waves sequentially for evidence of patterns that may further elucidate the dynamics of these epidemics or, if they were part of the same pandemic, the pandemic in its entirety. This exercise produced three phenomena of note, including (1) a widespread and steep fourth wave of excess deaths in early 1920 (also seen in the US state of Arizona and other countries8–14), which, in some counties, was more severe than the better-known waves in late 1918 and early 1919; (2) variations in the timing and number of peaks in different counties in late 1918; and (3) notable differences in the way the 1920 wave and the late 1918 waves propagated across the state. Collectively these findings mean that Michigan experienced four waves of excess mortality over a span of two years, and not the two or three that earlier studies have identified.
METHODS
The data set contains monthly counts of all-cause deaths for the 83 counties of Michigan for the period 1914 to 1921 (n = 7968).15 We selected all-cause deaths because cause-specific data were not reported uniformly across all relevant years and the challenge of determining which reported causes of death should count as “influenza-attributable” for those waves that may have been caused by the pandemic influenza virus. For example, a contemporary report from Massachusetts identified 85 different conditions as possible causes of pandemic-related mortality.16 Furthermore, using seasonally unadjusted influenza, pneumonia, and broncho-pneumonia deaths produced a spatio-temporal picture that very closely mirrors the phenomena described here. It should be noted that, while all-cause mortality data may accurately identify the timing of mortality peaks, they are less accurate when used for the computation of actual mortality.17
We estimated excess deaths by seasonally adjusting county-level data using the additive mode of the PROC X12 module in SAS (SAS Institute Inc, Cary, NC) as follows18:
a trend moving average was computed from the original data on deaths,
the original time series was de-trended using the moving average mentioned in step 1,
outliers (including a winter epidemic in 1915 to 1916 and the pandemic peak[s] of 1918 to 1920) were identified and replaced with nonextremal estimates,
a month-wise average was computed from the series in step 3 to produce initial estimates of regular monthly (seasonal) components,
the monthly components were subtracted from the series in step 3 to obtain a preliminary seasonally adjusted series,
the trend was re-estimated using the series obtained in step 5, and
steps 1 to 6 were repeated twice more, but using the series generated in step 5 from the previous iteration.15,19
The outputs of this process consisted of three components: a trend or long-term component, a cyclical or seasonal component, and an irregular or residual component. These components can be added to reproduce the original time series. We examined the irregular component, which identifies excess deaths not explained by normal seasonal variations or long-term trends. We defined an episode of excess deaths as a “wave” if, for at least one month during the episode, the excess was large enough to be designated an outlier by the X12 algorithm and the episode occurred between January 1918 and July 1920. We interpolated the resulting monthly county-level time series to produce weekly estimates of excess deaths.
RESULTS
Figure 1 shows aggregate excess deaths in Michigan. The four prominent peaks occurred during
March to May 1918,
September to November 1918,
November 1918 to January 1919, and
January to March 1920.
FIGURE 1—
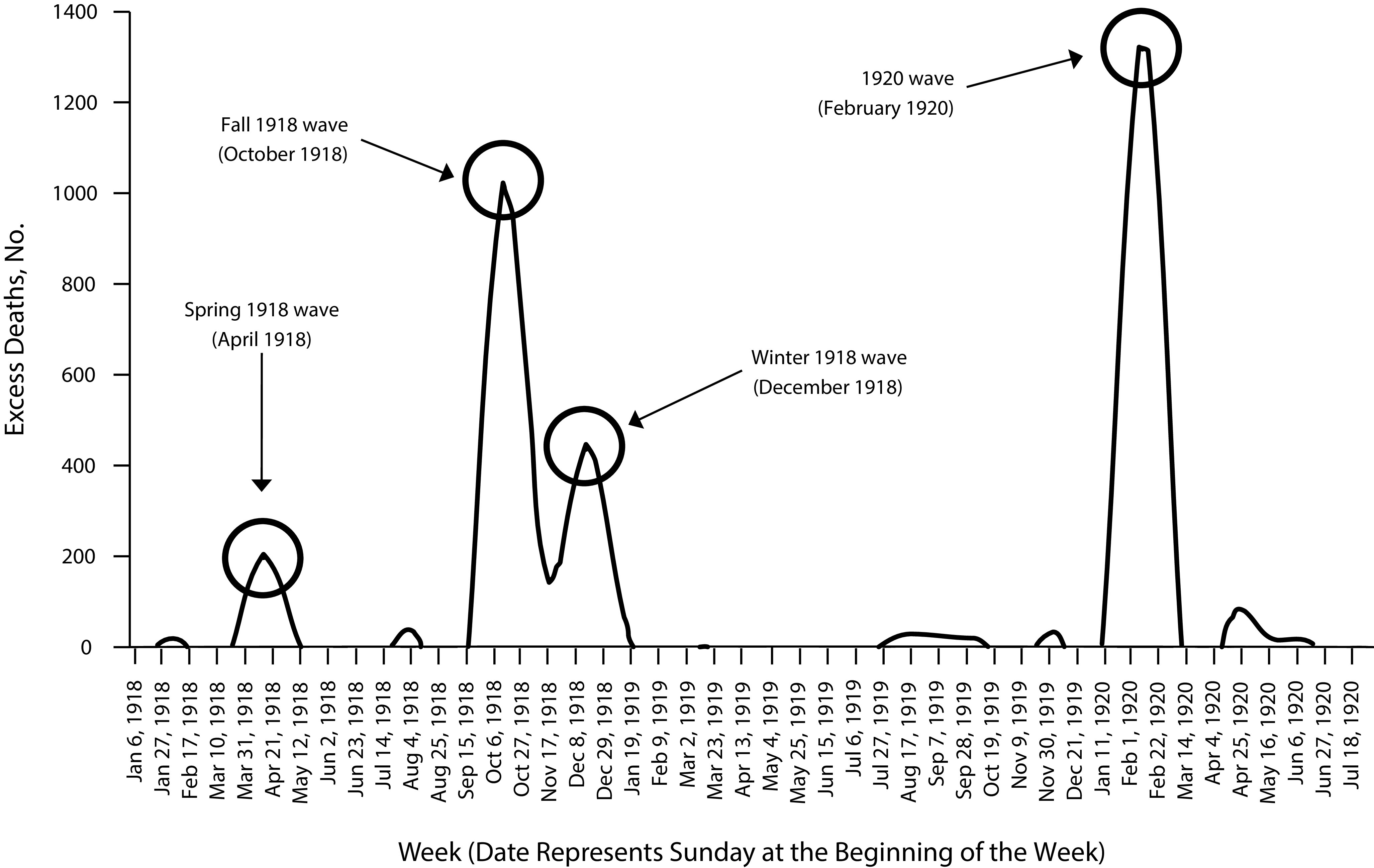
The Four Waves of Excess Deaths in Michigan: 1918–1920
Notably, the fourth peak, which was as severe as the fall 1918 peak, does not form part of the Centers for Disease Control and Prevention’s (CDC’s) characterization of the pandemic (Figure 2). In addition, the early wave in 1918, also seen in Figure 2, emerged in the vast majority of counties across the various regions of the state.
FIGURE 2—
A Screenshot From the Centers for Disease Control and Prevention’s Web Site Commemorating the Pandemic and Showing the Spring (Herald), Fall, and Winter 1918 “Waves”
Figure 3a compares the timing of the fall and winter 1918 peaks in four geographically dispersed counties, Washtenaw and Wayne in the east, Ingham in south–central Michigan, and Kent in the west.20 These counties were selected for their large populations and, thus, lower likelihood that outliers would affect the overall mortality patterns. Overlaid on this graph are two vertical lines marking the dates on which Michigan Governor Albert Sleeper issued (October 19) and subsequently lifted (November 7) a statewide order banning public gatherings.21,22
FIGURE 3—
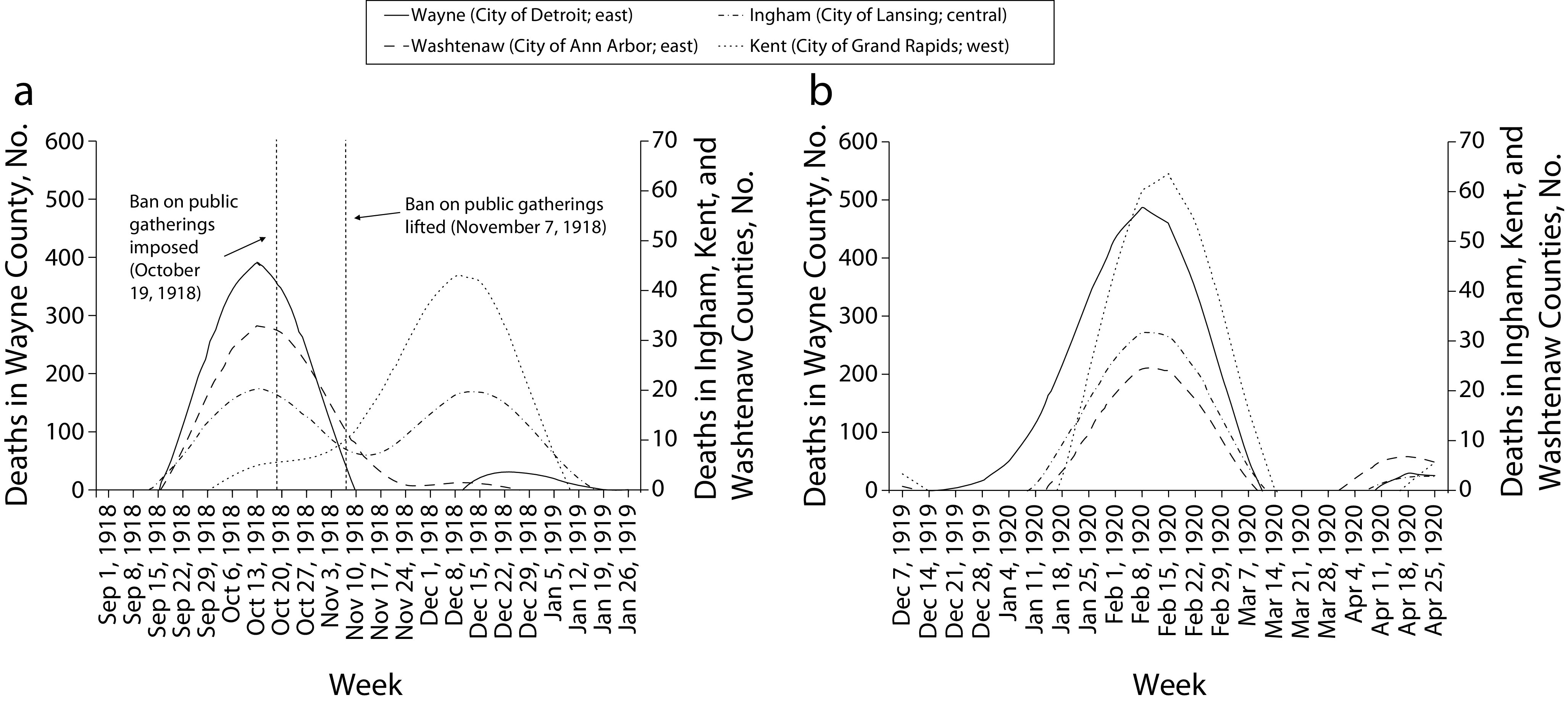
Excess Deaths During (a) the Second (Fall 1918) and Third (Winter 1918) Waves and (b) the Fourth (Spring 1920) Wave: Highly Populated Counties in Michigan
The two counties in eastern Michigan experienced peaks in October 1918 with virtually no subsequent excess mortality in 1918 to 1919. By contrast, Ingham County in south–central Michigan experienced two peaks of similar size, one each in October and December 1918. Kent County in the west experienced its only peak in December 1918. If in fact the deaths were caused by the same pathogen, the epidemic appears to have spread westward across the state, with a single early peak in the east, a single late peak in the west, and both an early and a late peak in the center. The pattern in the center of the state (Ingham County) conforms best to the CDC’s characterization of two separate waves in late 1918, but was not observed in all parts of Michigan. Indeed, an approximately even fraction of counties in Michigan experienced each of the two waves of late 1918, suggesting that the bimodal pattern of October and December 1918 for the entire state, seen in Figure 1, is as much the result of aggregation across counties as it is a reflection of the experiences of individual counties. The same observation likely applies to the pattern observed for the entire United States and other countries.
The fourth wave, by contrast, consisted of a single peak, occurring simultaneously across almost all the counties of Michigan during the weeks of February 8 and 15, 1920 (Figures 1 and 3b). For the rest of this article, therefore, we will refer to the two late-1918 peaks as two separate waves: the “Fall 1918” or “second” wave and the “Winter 1918” or “third” wave, respectively. The 1920 wave will be referred to as the “1920” or “fourth” wave.
In terms of duration, the Fall 1918, Winter 1918, and 1920 waves each spanned eight to nine weeks (Figures 1, 3a, and 3b). The 1920 wave shows a particularly marked concentration in the four weeks of February 1920, with excess death totals of 1322 and 1314 in the first two weeks alone. The highest excess deaths in any of the three earlier waves, in the second week of October 1918, totaled 1023.
Figures 4a and 4b show weekly spatial snapshots of excess deaths across the counties of Michigan during the weeks in the Fall and Winter 1918 waves when the largest numbers of counties were peaking. Figure 4c is a snapshot of the same phenomenon during the 1920 wave. The shades of gray represent the status of the epidemic during the week in question in each county, with dark gray signifying the peak week for the county, the next darkest gray signifying the week with the second-highest number of excess deaths, and lighter shades of gray signifying the third- and fourth-highest weeks, respectively.
FIGURE 4—
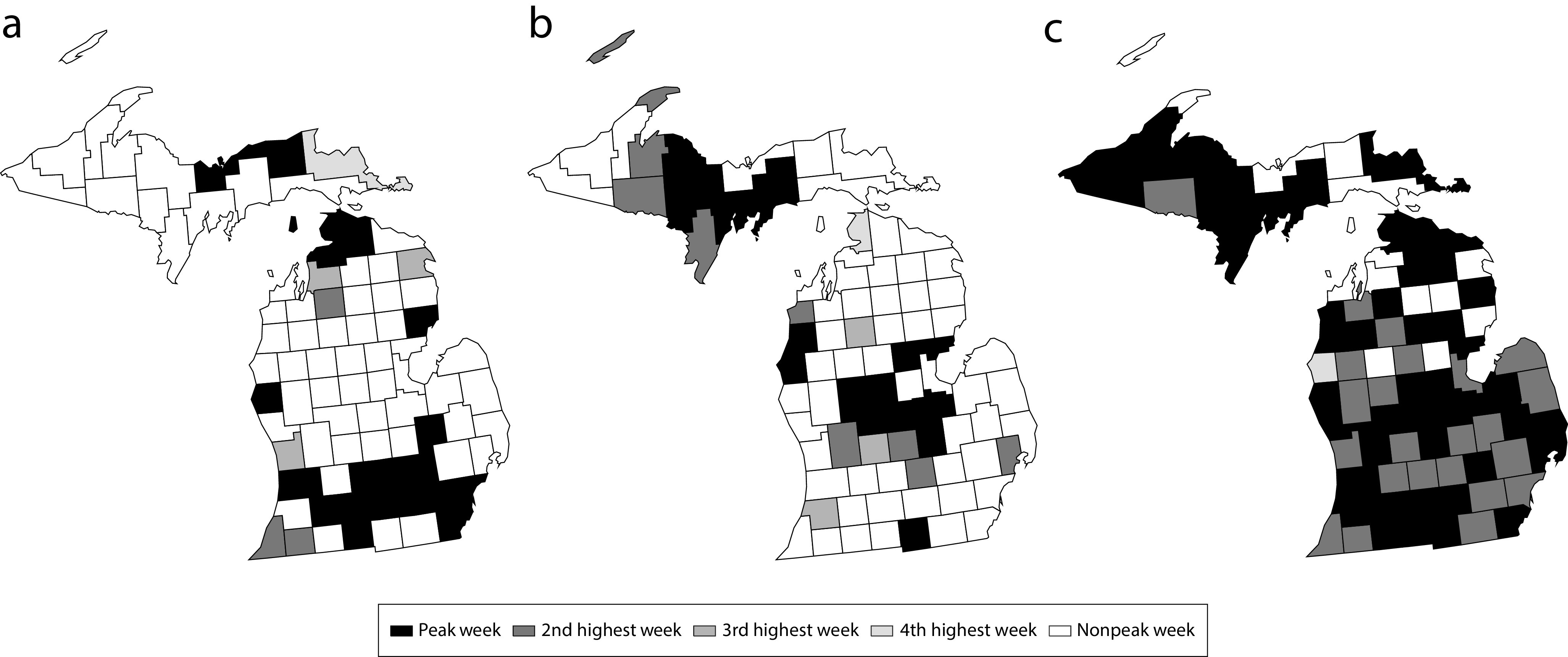
Peak Influenza Pandemic Weeks in Michigan Counties on the Weeks of (a) October 13, 1918, (b) December 15, 1918, and (c) February 15, 1920
Notes. Part a shows counties in which the Fall 1918 wave was higher than the Winter 1918 wave with their status the week of October 13, 1918. Part b shows counties in which the Winter 1918 wave was higher than the Fall 1918 wave with their status the week of December 15, 1918. Part c shows the status of the 1920 wave in Michigan counties the week of February 15, 1920. Note the simultaneous statewide peak in 1920 in contrast to the spatially distributed peaks in 1918 (a and b).
The Fall 1918 wave (Figure 4a) was more pronounced than the Winter 1918 wave (Figure 4b) in two regions of Michigan. The first region consisted of the counties in the main population centers of southern Michigan along the Detroit, Michigan, to Chicago, Illinois, transportation routes. These included the population centers of Detroit, Ann Arbor, and Jackson, Michigan. The second region consisted of the counties straddling two major shipping routes connecting the Great Lakes, the Straits of Mackinac between Lake Michigan with Lake Huron, and the St Mary’s River connecting Lake Superior with Lake Huron. The Winter 1918 wave was more pronounced than the Fall 1918 wave in the central part of the Lower Peninsula of Michigan and in the central and western parts of the Upper Peninsula.
The 1920 wave (Figure 4c) matched the combined Fall 1918 and Winter 1918 waves in terms of severity (Figure 1). Thirty-nine out of 83 counties (46%) experienced their absolute peak week (i.e., maximum excess deaths across all four waves) during the 1920 wave. The February 1920 volume of the Michigan Bulletin of Vital Statistics describes “a most notable increase” in the number of deaths from influenza compared with January of the same year, adding “In fact, there were 312 more deaths from pneumonia during the month than there were in the month of October 1918, the month of greatest mortality during the previous epidemic,”23(p17) pneumonia being a common and often fatal complication of influenza.4
DISCUSSION
The data show a robust wave of excess mortality in early 1920 in Michigan. It was as severe as the lethal Fall 1918 or Winter 1918 waves and, in its peak week, considerably more severe than either earlier wave. In addition, the 1920 wave was an isolated wave that propagated rapidly across the state and peaked simultaneously across the vast majority of counties in all regions of the state (Figure 4c). The Fall 1918 and Winter 1918 waves, by contrast, were consecutive waves that appeared with differing degrees of severity, singly or in a pair, across the different counties of Michigan, and the preceding Spring 1918 wave was the least pronounced of the four.
The question of whether these four waves were part of a single pandemic unfolding serially or separate epidemics caused by different pathogens remains an open one. Reasons to be cautious in interpreting the four waves as part of a single pandemic include (1) the absence of genetic evidence from Michigan that any of the four waves was caused by the same pathogen that caused any of the other waves, (2) the possibility of immunization effects across different influenza viruses that may have caused different waves,24 (3) the absence of cross-protection from infection across waves,25 and (4) the absence of proof that the unusual pattern of age-specific mortality during the late 1918 and early 1920 waves of excess mortality (Figures A and B, available as supplements to the online version of this article at http://www.ajph.org)4,10 were directly caused by a virus.26 On the other hand, findings consistent with different combinations of waves being part of a single pandemic include (1) the same unusual pattern of age-specific mortality across the late 1918 and 1920 waves (Figures A and B); (2) research from other locations showing cross-protection between the Spring 1918 and Fall 1918 waves, a possible indicator that the waves were caused by the same pathogen27; and (3) the classification of the waves in Michigan in both years as being caused by “influenza” (rather than some other disease). In sum, given the contradictory nature of the literature comparing the different waves of excess mortality, the possibility that the early 1918, late 1918, and 1920 waves were caused by a different pathogen or that different waves were caused by different mutations of the same virus cannot be ruled out.
A number of factors may have played a role in producing the four-wave pattern of excess deaths observed in Michigan. These include
Public health responses: Shortly after Governor Sleeper’s ban on public gatherings was imposed (October 19, 1918),18,19 excess deaths declined (Figure 3a). This nonpharmaceutical intervention could also account for the consecutive nature of the Fall and Winter 1918 waves, as was observed in several major US cities28—soon after the ban was lifted, cases began to increase again in some counties. Similar phenomena are now being observed in the context of the ongoing COVID-19 pandemic in Michigan and other US states.
Weather conditions: Influenza transmission is most efficient at approximately 40 degrees and low relative humidity.29,30 Weather conditions during the Fall 1918, Winter 1918, and 1920 waves were likely ideal for transmission of an influenza virus, whether or not it was the same one across the three waves. The lack of daily humidity data by county impedes detailed analysis of the connection between weather conditions and transmission. However, the Weather Bureau reported in January 1920 that “unusually cold weather of December continued with increasing severity during most of January,”31(p3) and the following month, February 1920, “was not nearly so cold” with temperatures returning to or slightly above the average.32(p15) It appears that favorable conditions for the rapid and efficient spread of influenza viruses, not present in January 1920, developed in February, perhaps contributing to the abruptness and severity of the 1920 wave if it was an influenza wave.
Short-term demographic changes: Some 8000 young men from the Michigan Guard who were in wartime Europe in 1918 had returned by February 1920. 33 This change in the composition of the population and its increased mobility could have facilitated the more rapid spread of the pathogen that caused the severe excess mortality in 1920.
Other behavioral and economic factors: more people spending time indoors in close proximity during the uncommonly cold winter of 1919 to 192031 and Michigan’s extensive shipping and rail systems at the time may also have facilitated the spread of the pathogen.34–37
The findings presented in the article suggest several opportunities for further research to better understand the sequence of four waves of excess deaths in Michigan and elsewhere in 1918 to 1920. These include
factors accounting for the difference in propagation between the Spring 1918, Fall 1918, Winter 1918, and 1920 waves;
possible cross-protection effects (or the absence thereof) between the various waves, which could illuminate whether they were caused by the same or a similar pathogen9,38,39;
the roles of transportation networks, climate and weather, nonpharmaceutical interventions, and demographic changes (troops returning from World War I during the summer of 1919) in accounting for the differences in propagation between the various waves;
analysis of county-level data from other states for the purposes of comparison and validation of our findings; and
genetic analysis of tissue samples taken from victims from the four different waves to identify the pathogen(s) underlying each one.
CONCLUSIONS
A central finding of this article is the emergence of four waves of infection and mortality in 1918 to 1920 in Michigan. In addition to the relatively mild wave in the spring of 1918, counties in Michigan experienced one or two waves of excess mortality in late 1918, depending on their locations and, very likely, on the timing of the governor’s statewide ban on public gatherings. A year later, the counties of the state were struck by another almost uniformly devastating wave of infections and mortality.
Michigan’s experience holds sobering lessons for those who wish to understand how immunologically naïve populations encounter novel viral pathogens. First, the timing of nonpharmaceutical interventions of the kind being applied to the COVID-19 pandemic today may play a role in the emergence and severity of “trailer” waves of infection and death.27,40 Second, the second and third waves identified by the CDC in Figure 2 may reflect the combination of three phenomena: (1) a delayed wave, with the early wave dominating in some parts of the United States and the late wave dominating in others; (2) the gradual movement of the same pathogen from its place of introduction to other areas; and (3) the effects of the timing of the adoption and subsequent relaxation of social distancing measures, giving rise to one or two waves depending on the timing of the measures. And third, if the 1920 wave was caused by the same virus that caused one or more of the three 1918 waves, the findings in this article raise the sobering possibility that, even after one or more severe rounds of infection and death have subsided, the pandemic may re-emerge with a vengeance months or years later when conditions—including weather, mobility of people, and the availability of susceptible hosts—are favorable for a resurgence.
ACKNOWLEDGMENTS
Portions of this research were presented in a course titled “Clinical Knowledge, Ethics, Epidemiology in Pandemics” offered at Michigan State University in the spring and summer of 2020.
The authors would like to thank students enrolled in the course for their feedback on this research.
CONFLICTS OF INTEREST
None of the authors has any conflicts of interest to report.
Footnotes
REFERENCES
- 1.Wagner DM, Klunk J, Harbeck M et al. Yersinia pestis and the plague of Justinian 541–543 AD: a genomic analysis. Lancet Infect Dis. 2014;14(4):319–326. doi: 10.1016/S1473-3099(13)70323-2. [DOI] [PubMed] [Google Scholar]
- 2.Haensch S, Bianucci R, Signoli M et al. Distinct clones of Yersinia pestis caused the black death. PLoS Pathog. 2010;6(10):e1001134. doi: 10.1371/journal.ppat.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson NPAS, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76(1):105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 4.Taubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potter CW. A history of influenza. J Appl Microbiol. 2001;91(4):572–579. doi: 10.1046/j.1365-2672.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- 6.Crosby AW. America’s Forgotten Pandemic: The Influenza of 1918. 2nd ed. New York, NY: Cambridge University Press; 2003. [DOI] [Google Scholar]
- 7.Moxnes JF, Christophersen OA. The Spanish flu as a worst case scenario? Microb Ecol Health Dis. 2008;20:1–26. doi: 10.1080/08910600701699067. [DOI] [Google Scholar]
- 8.Dahal S, Jenner M, Dinh L, Mizumoto K, Viboud C, Chowell G. Excess mortality patterns during 1918–1921 influenza pandemic in the state of Arizona, USA. Ann Epidemiol. 2018;28(5):273–280. doi: 10.1016/j.annepidem.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra S, Yu Y. The 1918 influenza pandemic and subsequent birth deficit in Japan. Demogr Res. 2015;33(11):313–326. doi: 10.4054/DemRes.2015.33.11. [DOI] [Google Scholar]
- 10.Erkoreka A. The Spanish influenza pandemic in occidental Europe (1918–1920) and victim age. Influenza Other Respir Viruses. 2010;4(2):81–89. doi: 10.1111/j.1750-2659.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowell G, Viboud C, Simonsen L et al. The 1918–1920 influenza pandemic in Peru. Vaccine. 2011;29(2):B21–B26. doi: 10.1016/j.vaccine.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh Y-H. Excess deaths and immunoprotection during 1918–1920 influenza pandemic, Taiwan. Emerg Infect Dis. 2009;15(10):1617–1619. doi: 10.3201/eid1510.080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richard SA, Sugaya N, Simonsen L, Miller MA, Viboud C. A comparative study of the 1918–1920 influenza pandemic in Japan, USA and UK: mortality impact and implications for pandemic planning. Epidemiol Infect. 2009;137(8):1062–1072. doi: 10.1017/S0950268809002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195(7):1018–1028. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 15.Annual Report on the Registration of Births and Deaths, Marriages and Divorces in Michigan. Lansing, MI: Wynkoop Hallenbeck Crawford Co, State Printers. Available at: https://catalog.hathitrust.org/Record/000054220/Home. Accessed July 20, 2018.
- 16.Office of the Secretary of the Division of Vital Statistics, The Commonwealth of Massachusetts. Seventy-Seventh Annual Report of the Vital Statistics of Massachusetts: Births, Marriages, Divorces, and Deaths for the Year 1918. Boston, MA: Wright & Potter Printing Co, State Printers; 1920. pp. 180–189. Available at: https://babel.hathitrust.org/cgi/pt?id=uc1.b5327610;view=1up;seq=5. Accessed January 14, 2021. [Google Scholar]
- 17.Andreasen V, Simonsen L. The perils of using annual all-cause mortality data to estimate pandemic influenza burden. Vaccine. 2011;29(2):B49–B55. doi: 10.1016/j.vaccine.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 18.SAS/ETS® 13.2 User’s Guide. Cary, NC: SAS Institute Inc; 2013. The Proc X12 procedure; pp. 2670–2792. Available at: https://support.sas.com/documentation/onlinedoc/ets/132/x12.pdf. Accessed June 14, 2020. [Google Scholar]
- 19.Dagum E. Essays Collection of Estela Bee Dagum in Statistical Sciences. The X-11-ARIMA seasonal adjustment method. Statistics Canada. 1980:697–675. Available at: https://www.census.gov/ts/papers/1980X11ARIMAManual.pdf. Accessed June 13, 2018. [Google Scholar]
- 20. Table 53: Population of cities by minor civil divisions: 1920, 1910, and 1900. Michigan. In: Fourteenth Census of the United States. Volume 1: Number and Distribution of Inhabitants. US Census. 1923–1925:458–468. Available at: https://www.census.gov/library/publications/1921/dec/vol-01-population.html. Accessed September 19, 2018.
- 21.City will obey influenza ban. Churches, theaters, and other meeting places close indefinitely Detroit Free Press 20. October19181 [Google Scholar]
- 22.Michigan Department of Health. Forty-Seventh Annual Report of the Commissioner of the Michigan Department of Health for the Fiscal Year Ending June 30, 1919. Michigan Department of Health. 1920. Available at: http://hdl.handle.net/2027/spo.9320flu.0014.239. Accessed August 1, 2018.
- 23.Petrie WF, editor. Michigan Bulletin of Vital Statistics. February 1920. Vol. XXIII. State Board of Health; 1920:18. Available at: https://hdl.handle.net/2027/chi.102184665. Accessed September 6, 2018. [Google Scholar]
- 24.Shanks GD, Milinovich G, Waller M, Clements A. Spatio-temporal investigation of the 1918 influenza pandemic in military populations indicates two different viruses. Epidemiol Infect. 2015;143(9):1816–1825. doi: 10.1017/S0950268814002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanks GD, MacKenzie A, Mclaughlin R et al. Mortality risk factors during the 1918–1919 influenza pandemic in the Australian Army. J Infect Dis. 2010;201(12):1880–1889. doi: 10.1086/652868. [DOI] [PubMed] [Google Scholar]
- 26.Shanks GD, Brundage JF. Pathogenic responses among young adults during the 1918 influenza pandemic. Emerg Infect Dis. 2012;18(2):201–207. doi: 10.3201/eid1802.102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barry JM, Viboud C, Simonsen L. Cross-protection between successive waves of the 1918–1919 influenza pandemic: epidemiological evidence from US army camps and from Britain. J Infect Dis. 2008;198(10):1427–1434. doi: 10.1086/592454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bootsma M, Ferguson N. The effect of public health measures on the 1918 influenza pandemic in U.S. cities. Proc Natl Acad Sci U S A. 2007;104(18):7588–7593. doi: 10.1073/pnas.0611071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowen AC, Steel J. Roles of humidity and temperature in shaping influenza seasonality. J Virol. 2014;88(14):7692–7695. doi: 10.1128/JVI.03544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider CF. Climatological data, Michigan January 1920. US Department of Agriculture, Weather Bureau. January, 1920. Available at: https://hdl.handle.net/2027/uiug.30112003018196?urlappend=%3Bseq=335. Accessed February 21, 2020.
- 32.Schneider CF. Climatological data, Michigan February 1920. US Department of Agriculture, Weather Bureau. February 1920. Available at: https://hdl.handle.net/2027/uiug.30112003018196?urlappend=%3Bseq=349. Accessed February 21, 2020.
- 33.Michigan Department of Military and Veterans Affairs. World War I. 2020. Available at: https://www.michigan.gov/dmva/0,9665,7-402-100108_3003_3009-17110--,00.html. Accessed April 23, 2020.
- 34.Reyes O, Lee E, Sah P, Viboud C, Chandra S, Bansal S. Spatio-temporal patterns and diffusion of the 1918 influenza pandemic in British India. Am J Epidemiol. 2018;187(12):2550–2560. doi: 10.1093/aje/kwy209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein J. Inland Water Transportation in the United States. Department of Commerce Bureau of Foreign and Domestic Commerce; 1923. pp. 23–25. Available at: https://www.google.com/books/edition/_/g04fb0Xx_wMC?hl=. Accessed September 18, 2018. [Google Scholar]
- 36.Woodford AM. The Michigan Companion: A Guide to the Arts, Entertainment, Festivals, Food, Geography, Geology, Government, History, Holidays, Industry, Institutions, Media, People, Philanthropy, Religion, and Sports of the Great State of Michigan. Detroit, MI: Omnigraphics; 2011. Steamer passenger trade; p. 634. [Google Scholar]
- 37.Michigan Department of Transportation. Michigan’s Railroad History: 1825–2014. 2015: 4. Available at: https://www.michigan.gov/documents/mdot/Michigan_Railroad_History_506899_7.pdf. Accessed September 19, 2018. [Google Scholar]
- 38.Mamelund SE, Haneberg B, Mjaaland S. A missed summer wave of the 1918–1919 influenza pandemic: evidence from household surveys in the United States and Norway. Open Forum Infect Dis. 2016;3(1):ofw040. doi: 10.1093/ofid/ofw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Økland H, Mamelund SE. Race and 1918 influenza pandemic in the United States: a review of the literature. Int J Environ Res Public Health. 2019;16(14):2487. doi: 10.3390/ijerph16142487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morse SS. Pandemic influenza: studying the lessons of history. Proc Natl Acad Sci U S A. 2007;104(18):7313–7314. doi: 10.1073/pnas.0702659104. [DOI] [PMC free article] [PubMed] [Google Scholar]


